Abstract
Dengue virus (DENV) is the most prevalent mosquito-borne viral pathogen in humans. Currently, there is no clinically approved vaccine or antiviral for DENV. Combination therapy is a common practice in antiviral treatment and a potential approach to search for new treatments for infectious pathogens. In this study, we performed a combination treatment in cell culture by using three distinct classes of inhibitors, including ribavirin (a guanosine analog with several antiviral mechanisms), brequinar (a pyrimidine biosynthesis inhibitor), and INX-08189 (a guanosine analog). The compound pairs were evaluated for antiviral activity by use of a DENV-2 luciferase replicon assay. Our result indicated that the combination of ribavirin and INX-08189 exhibited strong antiviral synergy. This result suggests that synergy can be achieved with compound pairs in which one compound suppresses the synthesis of the nucleoside for which the other compound is a corresponding nucleoside analog. In addition, we found that treatment of cells with brequinar alone could activate interferon-stimulated response elements (ISREs); furthermore, brequinar and NITD-982 (another pyrimidine biosynthesis inhibitor) potentiated interferon-induced ISRE activation. Compared to treatment with brequinar, treatment of cells with ribavirin alone could also induce ISRE activation, but to a lesser extent; however, when cells were cotreated with ribavirin and beta interferon, ribavirin did not augment the interferon-induced ISRE activation.
INTRODUCTION
Over 2.5 billion people worldwide are at risk of dengue virus (DENV) infection, with 390 million human infections and 96 million cases with disease manifestations each year (1). DENV is endemic throughout tropical and subtropical climates and is found mostly in urban and semiurban areas. This positive-sense single-stranded RNA virus is transmitted mainly by the Aedes aegypti mosquito and is classified under the genus Flavivirus in the family Flaviviridae. Other notable viruses in this group include yellow fever virus, Japanese encephalitis virus, West Nile virus, and tick-borne encephalitis virus. Currently, neither an antiviral nor a vaccine is approved for DENV. Care for hospitalized dengue patients is supportive, mainly through optimal replenishment of body fluids. Treatment is intensive for those who succumb to the severe forms of the disease, i.e., dengue shock syndrome (DSS) and dengue hemorrhagic fever (DHF).
Multiples approaches have been taken to identify inhibitors of DENV (2, 3). Small-molecule inhibitors have been reported to target various DENV proteins, including capsid (4, 5), envelope (6), protease (7, 8), nonstructural protein (NS) 4B (9, 10), methyltransferase (2, 11), and RNA-dependent RNA polymerase (12–16). Inhibition of host factors important for viral replication and of compounds with immunomodulation activities, including imino sugars (17), cholesterol inhibitors (18), chloroquine (19), and prednisolone (20), has also been pursued for potential treatment of DENV infections.
Ribavirin is a drug with a broad spectrum of antiviral activity. Ribavirin in combination with pegylated alpha interferon (PEG-IFN-α) in the past has been the standard treatment regimen for hepatitis C virus (HCV), a virus from the family Flaviviridae that is related to DENV. Besides HCV, ribavirin had also shown some success in the treatment of respiratory syncytial virus (21) and Lassa fever virus (22). Ribavirin is a guanosine analog with several antiviral mechanisms, one of which is to inhibit de novo biosynthesis of guanine nucleotides through direct binding to cellular IMP dehydrogenase (IMPDH) (23). Depletion of the intracellular pool of nucleoside triphosphates was proposed to be a major antiviral mechanism for ribavirin to inhibit flaviviruses (24). In addition, ribavirin could function as a mutagen to increase error catastrophe (25) and potentiated the antiviral activity of IFN-α/β by augmenting the expression of IFN-stimulated genes (ISGs) (26). Similar to ribavirin, brequinar also has a broad antiviral spectrum against both positive- and negative-strand RNA viruses (27, 28). Brequinar inhibits de novo biosynthesis of uracil nucleotides by inhibiting cellular dihydroxyorotate dehydrogenase (DHODH) (29). Depletion of intracellular pyrimidine triphosphates is the main antiviral mechanism for brequinar (27). Brequinar was first identified and developed as an antimetabolite in cancer and immunosuppression therapy; since tumor cells rely heavily on de novo nucleotide synthesis, lowering pyrimidine synthesis (by use of brequinar) may interfere with the rapid proliferation of lymphocytes (30).
Combination therapy is commonly practiced in anti-infective treatment to minimize drug resistance. Although there are no clinically approved antivirals for DENV, it is of interest to examine whether compounds that are in clinical use or in preclinical development for other viruses inhibit DENV and, if so, whether these compounds have synergistic effects against DENV when used in combination. In this study, we selected three clinical and preclinical compounds (brequinar, ribavirin, and INX-08189) with known anti-DENV activities and examined their combinatory antiviral activities in a cell culture system. The results showed that combination of the guanosine analog INX-08189 with the GTP pool-depleting drug ribavirin inhibited DENV in a synergistic manner. The observed synergy may potentially be used to reduce the doses and therefore to increase the safety margins of inhibitors to achieve a therapeutic window in vivo. In addition, we found that brequinar and another uridine biosynthesis inhibitor can potentiate interferon-stimulated response element (ISRE) activation induced by interferon.
MATERIALS AND METHODS
Cells and culture media.
Huh-7 cells stably expressing a luciferase-reporting DENV-2 (New Guinea C [NGC] strain) replicon were described previously (31). These cells were maintained at 37°C and 5% CO2 in Dulbecco's modified Eagle medium (DMEM) (high glucose; Life Technologies) supplemented with 2 mM l-glutamine (Life Technologies), 0.1 mM nonessential amino acids (NEAA; Life Technologies), 10 μg/ml puromycin (Clontech), 1% penicillin-streptomycin (Life Technologies), and 10% fetal bovine serum (FBS; HyClone). The replicon cells seeded for antiviral assay were maintained in phenol red-free DMEM (Life Technologies) supplemented with 1 mM sodium pyruvate (Life Technologies), 2 mM l-glutamine, 0.1 mM NEAA (Life Technologies), 1% penicillin-streptomycin, and 2% FBS. HEK 293T cells were maintained at 37°C and 5% CO2 in DMEM (low glucose) supplemented with 0.1 mM NEAA, 1% penicillin-streptomycin, and 10% FBS. HEK 293T cells seeded for assays were maintained with 2% FBS instead.
Compounds.
Ribavirin (CAS number 36791-04-5), brequinar sodium salt hydrate, and recombinant human IFN-β were purchased from Sigma-Aldrich. INX-08189 and NITD-982 (28) were synthesized in-house. All compounds were dissolved in dimethyl sulfoxide (DMSO).
DENV replicon antiviral assay.
The replicon assay was performed as described previously (31). Briefly, replicon cells were treated with 2- or 3-fold serial dilutions of test compounds. After incubation at 37°C for 48 h, a luciferase substrate (ViviRen; Promega) was added according to the manufacturer's protocol. Luminescence was measured using a Clarity luminescence reader (BioTek), with an integration time of 0.1 s. The concentrations of compounds that decreased luciferase expression by 50% (EC50) and 90% (EC90) were calculated by nonlinear regression analysis (GraphPad Prism). Compound synergy analysis was performed using Chalice Analyzer (Zalicus).
Cell viability assay.
Cell viability was measured using the CellTiter-Glo (CTG) luminescence cell viability assay (Promega) according to the manufacturer's protocol. Approximately 1.5 × 104 replicon cells or 2 × 104 HEK 293T cells were seeded in a 96-well plate in a total volume of 100 μl. After 16 h of incubation, the cells were treated with a test compound. After another 48 h, 25 μl of CTG was added to each well, and the cells were incubated at room temperature for 20 min. Luminescence was read with an integration time of 0.1 s, using a Clarity luminescence reader (BioTek).
Plasmids and transfection of HEK 293T cells for ISRE induction assay.
A construct containing a firefly luciferase reporter gene under the control of an ISRE gene promoter (pISRE-TA-Luc) and a construct containing a Renilla luciferase reporter gene under the control of the herpes simplex virus type 2 thymidine kinase gene (HSV-TK) promoter (pGL4.74-hRluc/TK) were purchased from Clontech and Promega, respectively. Batch transfection of HEK 293T cells was performed with jetPRIME (Polyplus). For one 96-well culture plate, 12 μg each of pISRE-TA-Luc and pGL4.74-hRluc/TK was diluted in 600 μl of jetPRIME transfection buffer. Forty-eight microliters of jetPRIME was then added, mixed, and incubated for 10 min at room temperature. This mixture was added to 2.4 × 106 cells in a final volume of 12 ml DMEM containing 0.1 mM NEAA, 1% penicillin-streptomycin, and 2% FBS. Finally, 100 μl of this cell suspension was added to each well of the microplate, containing 1 μl of compound. Cells were incubated for 48 h at 37°C in the presence of 5% CO2. Luciferase expression was assayed using the Dual-Glo Stop & Glo assay system (Promega) according to the manufacturer's recommendations. Briefly, medium was removed from the wells containing cells, and the cells were washed twice with phosphate-buffered saline (PBS) (Life Technologies). Cells were then lysed for 20 min at room temperature on an orbital shaker. Subsequently, a 20-μl aliquot of cell lysate from each well was pipetted into a well of a white-wall, white-bottom plate. Firefly luciferase expression was measured by injecting 100 μl firefly luciferase substrate into each well. Expression was measured 2 s later on a Clarity luminescence reader (BioTek), using a 10-s integration time. After the first 20-s reading, 100 μl of Stop & Glo reagent was injected into each well. Renilla luciferase expression was measured 2 s later, with an integration time of 10 s.
Statistical analysis.
All the EC50s were calculated using 4-point nonlinear regression curve fitting with a variable slope and no constraints on both the top and bottom values, using Prism software. Chalice Analyzer (32) was used to evaluate synergy. The theories of Loewe additivity, Bliss independence, and the highest single agent (HSA) were previously described in detail (33–35).
RESULTS
Antiviral activities of individual compounds.
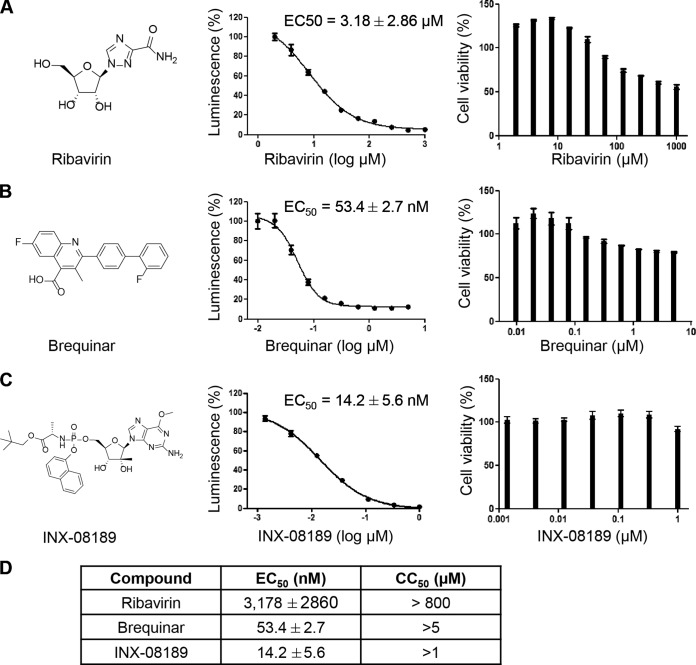
We selected three compounds (ribavirin, brequinar, and INX-08189) to test their combined antiviral activities (Fig. 1A to C, left panels). Each of the selected compounds has a distinct mode of action (see the introduction). A DENV-2 luciferase replicon assay was used to measure antiviral activity throughout the study. Prior to combination testing, we first examined the anti-DENV activities of the individual compounds (Fig. 1A to C, middle panels), as well as their cytotoxicities (right panels). Figure 1D summarizes the EC50, EC90, and CC50 (concentration of the compound leading to 50% cell death as measured by CellTiter-Glo) values of each compound. The results demonstrate that all selected compounds have anti-DENV activities with a good therapeutic window in cell culture.
FIG 1.
Host nucleoside inhibitors and preclinical nucleoside analogs exhibit potent anti-DENV-replication activities, with minimal cytotoxicities in cell culture. (A) The chemical structure of ribavirin, a well-characterized inhibitor of IMPDH, is shown. The EC50 of ribavirin for the Huh-7 NGC replicon is about 8.29 μM, and the EC90 is about 61.77 μM. The CC50 of ribavirin for the Huh-7 NGC replicon is >850 μM. (B) The structure of brequinar, a potent DHODH inhibitor, is shown. Its EC50 for the Huh-7 NGC replicon is about 51.5 nM, and the EC90 is about 248.8 nM. The CC50 of brequinar for the Huh-7 NGC replicon is >5 μM. (C) The chemical structure of the guanosine analog INX-08189 is shown. The EC50 of INX-08189 for the Huh-7 NGC replicon is about 14.48 nM, and the EC90 is about 131.5 nM. The CC50 of INX-08189 for the Huh-7 NGC replicon is >1 μM. (D) The EC50, EC90, and CC50 values of the respective compounds are summarized, demonstrating that all the selected compounds have anti-DENV activity, with a good therapeutic window, in cell culture. The data are averages for three to five independent experiments.
Antiviral activities of combination treatments.
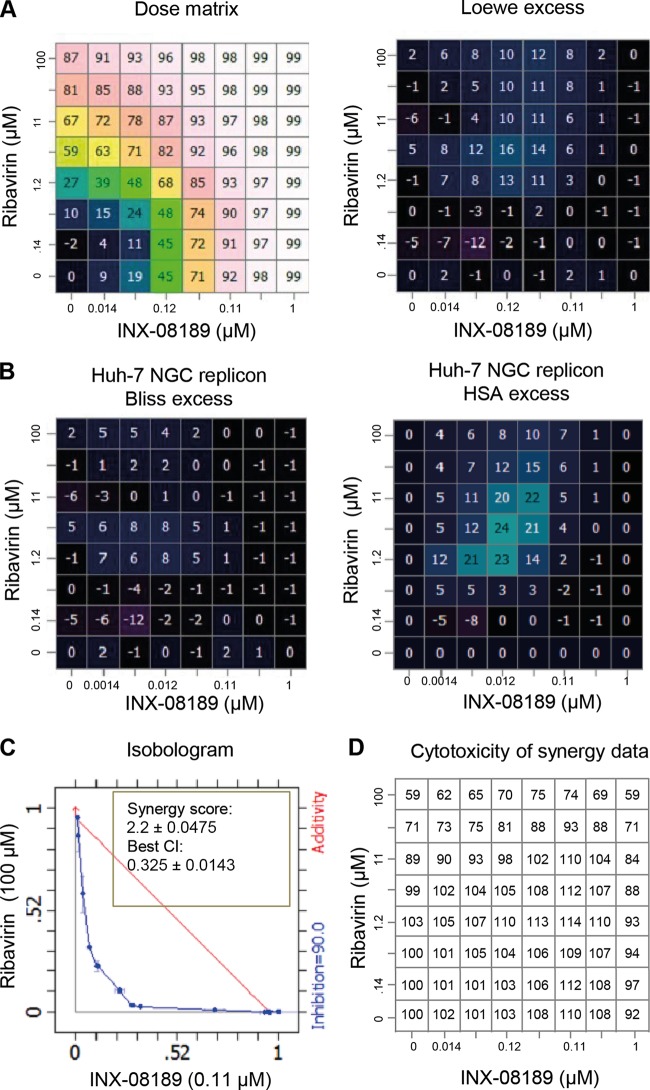
We measured antiviral activities of different compound pairs in which an inhibitor of the nucleoside pathway was paired with a nucleoside inhibitor, INX-08189. To examine whether combinatory treatments have synergistic or additive effects, we tested each compound at seven concentrations (excluding a control without compound), with a dose matrix centered on the EC50. Figure 2A, left panel, shows the percentages of replicon inhibition for each dose in the dose matrix. Chalice Analyzer was used to calculate the Loewe excess (Fig. 2A, right panel), Bliss excess (Fig. 2B, left panel), and HSA excess (Fig. 2B, right panel) values. These parameters are commonly used to indicate the excess percent inhibition; the excess percent inhibition is calculated by deducting the expected percent inhibition values of various combinations, assuming nonsynergy pairing in various models, from the experimental percent inhibition values. These data allowed us to calculate the isobologram, synergy score, and best combination index (CI) for each pair (Fig. 2C). In general, synergy scores of >1 and CI of <1 indicate that a combination treatment has a synergistic effect; a synergy score of 1 and a CI of 1 indicate that a combination treatment has only an additive effect (33). To assess whether synergy could be achieved at high inhibition levels, we set the isobologram level at 0.9 to capture meaningful synergy with a 90% viral reduction (equivalent to a 1-log10 reduction). Among the compound pairs, only ribavirin plus INX-08189 generated synergy, with a synergy score of 2.2 ± 0.0475 and a CI of 0.325 ± 0.0143 (Fig. 2C). The observed synergy was not due to cytotoxicity, as there was no significant cytotoxicity for all the combinations tested (Fig. 2D). The compound pair of a guanosine nucleoside analog and brequinar did not show any antiviral synergy (data not shown). Collectively, the results demonstrate that various compound pairs have different synergistic or additive antiviral effects.
FIG 2.
Chalice analysis of ribavirin and INX-08189 combination treatment of the NGC replicon. Luciferase activity was measured at 48 h posttreatment, with a dosing matrix centered on the EC50 of each compound for the Huh-7 NGC replicon. (A) Dose matrix-response values for the combination, showing inhibition of luciferase activity (1 − treated value/untreated value) for the serially diluted compounds. Inhibition values were analyzed by Chalice software to generate the combinations' excess values (the Loewe excess values are shown here). (B) Computed Bliss and HSA excesses for the compound combination. Values are averages for six experiments. (C) Isobologram, synergy score, and best combination index (CI) at 90% inhibition for ribavirin and INX-08189. Values shown are averages for eight experiments. (D) Cell viability with drug combination treatment. The concentrations of ribavirin and INX-08189 were identical to those used in the dose matrix for antiviral activity assay (see panel A). The synergy calculation was derived from averages for eight data sets.
Treatment of cells with brequinar or ribavirin alone activates ISRE.
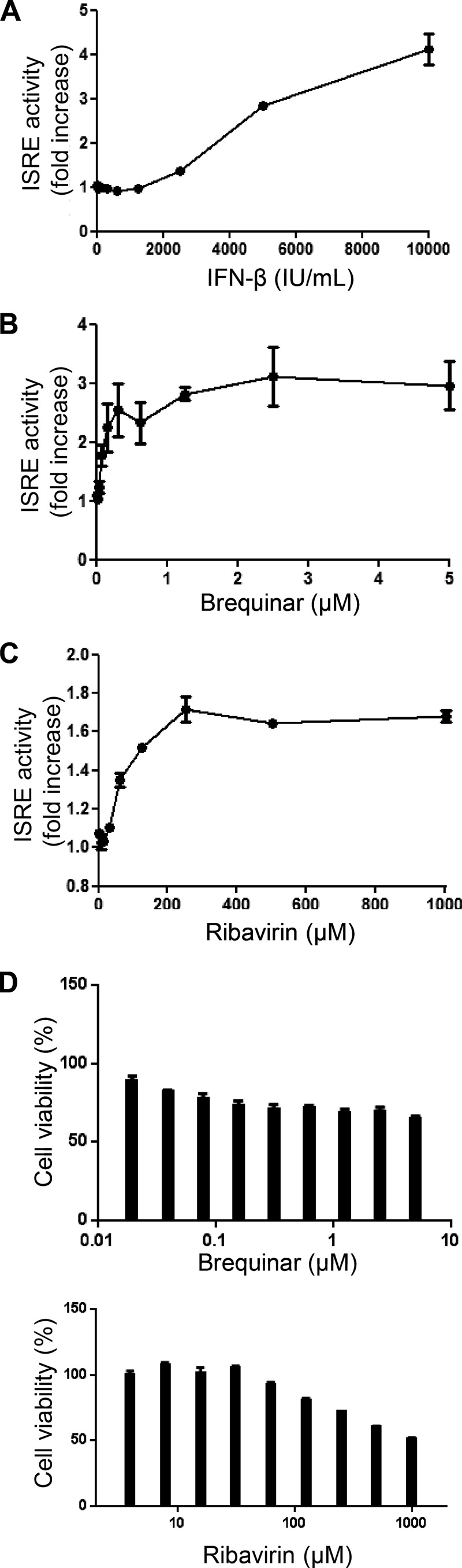
Recent studies suggested that inhibitors of IMPDH or DHODH induce a state of cell stress that potentiates (i.e., further increases the potency of) the type I IFN response (26, 36). The antiviral effect of these inhibitors may be contributed by both potentiation of the IFN response and depletion of intracellular nucleotides. We asked whether ribavirin- or brequinar-mediated potentiation of the IFN response could lead to the synergistic effect observed with the combination of ribavirin plus INX-08189. We cotransfected HEK 293T cells with the following two plasmids: (i) plasmid pISRE-TA-Luc contained the firefly luciferase gene under the control of an ISRE, and the expression of firefly luciferase was used to measure ISRE activation upon compound treatment; and (ii) plasmid pGL4.74-hRluc/TK contained the Renilla luciferase gene under the control of the HSV-TK promoter, and the expression of Renilla luciferase was used to normalize transfection efficiency. The transfected cells were treated with increasing doses of IFN-β (Fig. 3A), brequinar (Fig. 3B), or ribavirin (Fig. 3C) for 48 h before being assayed for luciferase expression. Fold induction of the ISRE activity was measured by dividing the individual treatment signal by the mock treatment (1% DMSO) signal. Although ISRE induction followed a dose-dependent response curve with all three inhibitors (Fig. 3A to C), the effect with ribavirin treatment was modest compared to that with IFN-β or brequinar treatment. Specifically, treatments with IFN-β and brequinar resulted in maximal 4-fold and 3-fold increases in ISRE activity, respectively. The effect of brequinar was quite pronounced even at low concentrations and started to plateau at around 300 nM (Fig. 3B). Ribavirin treatment induced ISRE activation by a maximum of 1.68-fold. The induction of ISRE activity by brequinar and ribavirin was not due to cytotoxicity, as their CC50 values in HEK 293T cells were >5 μM and >800 μM, respectively (Fig. 3D). The results indicate that in the absence of exogenous IFN, treatment of cells with brequinar or ribavirin alone can induce ISRE activation.
FIG 3.
Dose-dependent induction of ISRE by IFN-β and nucleoside synthesis inhibitors. HEK 293T cells batch transfected with pISRE-TA-Luc and pGL4.74-hRluc/TK were simultaneously treated with increasing concentrations of IFN-β, brequinar, or ribavirin in individual wells. (A and B) Treatment with IFN-β (A) caused a maximum 4-fold increase in ISRE activation, while treatment with brequinar (B) caused a maximum 3-fold increase in ISRE activation. (C) Activation of ISRE by ribavirin was modest, showing a maximum increase of 1.68-fold. (D) The induction of ISRE activity by either host nucleoside inhibitor was not due to cytotoxicity, as the CC50 values for HEK 293T cells were >5 μM. The average results for two experiments are shown.
DHODH inhibitors potentiate IFN-induced ISRE activation.
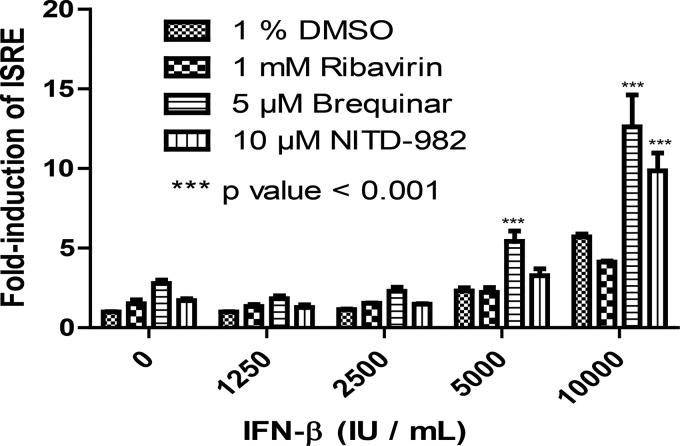
Since brequinar or ribavirin alone could induce ISRE activation, we examined whether these compounds could potentiate/enhance the ISRE activation when cells were coincubated with exogenous IFN. To this end, HEK 293T cells were coincubated with IFN-β and ribavirin or brequinar. As shown in Fig. 4, compared with the DMSO control, brequinar significantly potentiated ISRE activation when the cells were cotreated with 5,000 and 10,000 IU/ml IFN-β (P < 0.001); no potentiation was observed when the cells were cotreated with lower doses of IFN-β. In contrast, treatment with ribavirin plus IFN-β did not augment ISRE activation (Fig. 4). The results indicate that brequinar, a DHODH inhibitor, can enhance exogenous IFN-induced ISRE activation.
FIG 4.
Induction of ISRE by IFN-β is potentiated in the presence of DHODH inhibitors. HEK 293T cells were batch transfected with pISRE-TA-Luc and pGL4.74-hRluc/TK. Transfected cells were simultaneously treated with increasing concentrations of IFN-β and the indicated concentrations of the respective compounds. At 48 h posttreatment, luciferase activities were assayed using the Dual-Glo Stop & Glo reagent. Cotreatment of HEK 293T cells with brequinar and 5,000 or 10,000 IU/ml of IFN-β significantly potentiated ISRE activation compared to that with the DMSO control. There was no potentiation observed at lower doses of IFN-β. In contrast, cotreatment of cells with ribavirin and IFN-β did not augment ISRE activation. Treatment of cells with another pyrimidine biosynthesis inhibitor, NITD-982, with 10,000 IU/ml of IFN-β also significantly augmented the ISRE activation.
Next, we asked whether other pyrimidine biosynthesis inhibitors can also potentiate IFN-induced ISRE activation. To address this question, we used NITD-982, a compound that was recently reported to be a potent inhibitor of DHODH (28). Treatment with NITD-982 at a noncytotoxic concentration significantly augmented ISRE activation when cells were coincubated with 10,000 IU/ml IFN-β (P < 0.001) (Fig. 4). Taken together, the results demonstrate that DHODH inhibitors (brequinar and NITD-982) can potentiate IFN-induced ISRE activation.
DISCUSSION
In this study, we explored the possibility of a combinatorial therapy selected from three compounds that have anti-DENV activities. Since INX-08189 is a potent nucleoside inhibitor for DENV (Fig. 1C), we chose this compound to perform combination analysis and tested the hypothesis that cotreatment with INX-08189 plus another inhibitor that perturbs the synthesis of a natural triphosphate nucleotide(s) will lead to antiviral synergy. Our results showed that the compound pair ribavirin plus INX-08189 exhibited clear antiviral synergy, with a strong CI of 0.325 (Fig. 2C). The combination has the potential to lower the dose of either compound 3 times to achieve a level of viral inhibition similar to that with either compound alone (33); this may translate into a larger therapeutic window for the treatment of DENV with these two compounds. The synergy is not due to the cytotoxicity of the combination treatment (Fig. 2D). It should be noted that synergy was observed for compound pairs in which one compound suppressed the synthesis of the nucleoside for which the other compound is a corresponding nucleoside analog. This was expected, because reduction of the natural nucleoside pool (by the nucleoside synthesis inhibitor) increases the ratio of the nucleoside analog triphosphate versus the natural nucleoside triphosphate, making the nucleoside analog triphosphate more likely to be incorporated into the viral RNA, thus terminating viral RNA synthesis. Specifically, synergy was found between ribavirin (a guanosine synthesis inhibitor) and INX-08189 (a guanosine analog).
A similar combination antiviral approach was previously employed for HCV and HIV. For HCV, INX-08189 was tested in combination with ribavirin in the presence or absence of pegylated interferon (PEG-IFN) in HCV patients; unfortunately, adverse effects (heart and renal toxicity) were observed (37). This was not too surprising, as INX-08189 showed similar toxicological findings when administered as monotherapy (38). For HIV, a CTP synthase inhibitor (3′-deazauridine) strongly potentiated the anti-HIV-1 activity of a 5′-triphosphate of the cytidine analog lamivudine (3TC) and a 2,3′-dideoxycytidine (ddC) in cell culture (39). One explanation for drug synergy is the parallel pathway inhibition model, which suggests that two drugs will be synergistic if they inhibit two proteins in parallel pathways essential for an observed phenotype (40). Our synergy results seem to support the parallel pathway inhibition model. However, we could not exclude other possible mechanisms that may also contribute to the observed synergy.
It would be interesting to test whether the antiviral synergy of ribavirin plus INX-08189 observed in cell culture can be reproduced in the AG129 mouse model of dengue. Since a nucleoside inhibitor requires host kinases to convert to its active triphosphate form, caution should be taken in considering such an in vivo study. Due to the potential species difference in converting INX-08189 to the INX-08189 triphosphate between humans and mice, the relevance of the nonhuman animal model should be carefully validated experimentally in analyzing nucleoside inhibitors.
The potential involvement of the IFN pathway in the observed antiviral activity was investigated. For brequinar, besides inhibiting pyrimidine synthesis, our results showed that brequinar alone could activate ISRE in cell culture; furthermore, brequinar could enhance the exogenous IFN-induced ISRE activation. Such an enhancement was further validated with another pyrimidine biosynthesis inhibitor (NITD-982). The mechanisms of brequinar/NITD-982-mediated ISG activation and IFN signaling enhancement remain to be determined. Nevertheless, these observations may have clinical implications. When patients receive therapy, DENV has already established its replication, triggering an immune response to produce IFN and other cytokines (41, 42). If patients are treated with brequinar, its ability to augment IFN-induced ISRE activation should enhance the overall antiviral status of patients.
The synergy observed with the ribavirin-plus-INX-08189 treatment is unlikely to occur through ribavirin-mediated ISRE activation. This is because brequinar alone activated ISRE (Fig. 3B) but failed to synergize with INX-08189. In addition, the activation level of IRES was modest even at high concentrations of ribavirin.
There is an urgent unmet medical need to develop a safe and effective antiviral for DENV infection. Although no single compound has been approved for clinical use, the possibility of repurposing clinically tried or approved drugs for DENV is a tractable option. To this end, balapiravir, a cytidine nucleoside analog that was stopped for HCV clinical development, was tested in dengue patients; unfortunately, the compound did not show any efficacy in the dengue clinical trial (13). Similarly, celgosivir, a host alpha-glucosidase inhibitor (initially developed for HCV), also failed to show a significant difference compared to placebo (43). An alternative approach for repurposing clinically approved drugs for DENV is to search for a combination therapy. Such an approach has two conceptual advantages. First, synergy between two drugs would allow one to achieve efficacy at lower doses, leading to an increased therapeutic window for potentially toxic compounds (44). Theoretical and experimental studies have shown that drugs that exhibit synergy for a specific effect are usually not synergistic for side effects (32, 45). Indeed, toxicity experiments suggested that the observed antiviral synergy of the ribavirin-plus-INX-08189 combination was not due to cytotoxicity (Fig. 2). However, due to the unpredictable nature of toxicity associated with nucleoside analogs, caution should be taken in extrapolating in vitro toxicity results. Second, combination treatment would minimize the chance of resistance. In the absence of effective monotherapy for DENV, a combination of two moderately effective drugs may be needed. This is evident in the treatment of HIV, where only a combination of drugs effectively reduces viremia to an undetectable level. These considerations make synergistic drug pairs ideal candidates for treatment of infectious pathogens. The current study used DENV-2 as a model to achieve proof of concept. Future studies are needed to expand the current observation in cell culture to an appropriate in vivo study.
ACKNOWLEDGMENTS
We thank Chek Shik Lim, Christophe Leroy, and Joseph Lehar for data analysis of synergy. We thank colleagues at Novartis Institute for Tropical Diseases (NITD) for helpful discussions and help during the course of this study.
We are all employees of Novartis.
REFERENCES
- 1.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GRW, Simmons CP, Scott TW, Farrar JJ, Hay SI. 2013. The global distribution and burden of dengue. Nature 496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lim SP, Sonntag LS, Noble C, Nilar SH, Ng RH, Zou G, Monaghan P, Chung KY, Dong H, Liu B, Bodenreider C, Lee G, Ding M, Chan WL, Wang G, Jian YL, Chao AT, Lescar J, Yin Z, Vedananda TR, Keller TH, Shi P-Y. 2011. Small molecule inhibitors that selectively block dengue virus methyltransferase. J Biol Chem 286:6233–6240. doi: 10.1074/jbc.M110.179184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noble CG, Chen Y-L, Dong H, Gu F, Lim SP, Schul W, Wang Q-Y, Shi P-Y. 2010. Strategies for development of dengue virus inhibitors. Antiviral Res 85:450–462. doi: 10.1016/j.antiviral.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 4.Byrd CM, Dai D, Grosenbach DW, Berhanu A, Jones KF, Cardwell KB, Schneider C, Wineinger KA, Page JM, Harver C, Stavale E, Tyavanagimatt S, Stone MA, Bartenschlager R, Scaturro P, Hruby DE, Jordan R. 2013. A novel inhibitor of dengue virus replication that targets the capsid protein. Antimicrob Agents Chemother 57:15–25. doi: 10.1128/AAC.01429-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martins IC, Gomes-Neto F, Faustino AF, Carvalho FA, Carneiro FA, Bozza PT, Mohana-Borges R, Castanho MA, Almeida FC, Santos NC, Da Poian AT. 2012. The disordered N-terminal region of dengue virus capsid protein contains a lipid-droplet-binding motif. Biochem J 444:405–415. doi: 10.1042/BJ20112219. [DOI] [PubMed] [Google Scholar]
- 6.Wang Q-Y, Patel SJ, Vangrevelinghe E, Xu HY, Rao R, Jaber D, Schul W, Gu F, Heudi O, Ma NL, Poh MK, Phong WY, Keller TH, Jacoby E, Vasudevan SG. 2009. A small-molecule dengue virus entry inhibitor. Antimicrob Agents Chemother 53:1823–1831. doi: 10.1128/AAC.01148-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lai H, Sridhar Prasad G, Padmanabhan R. 2013. Characterization of 8-hydroxyquinoline derivatives containing aminobenzothiazole as inhibitors of dengue virus type 2 protease in vitro. Antiviral Res 97:74–80. doi: 10.1016/j.antiviral.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noble CG, Shi P-Y. 2012. Structural biology of dengue virus enzymes: towards rational design of therapeutics. Antiviral Res 96:115–126. doi: 10.1016/j.antiviral.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 9.van Cleef KWR, Overheul GJ, Thomassen MC, Kaptein SJF, Davidson AD, Jacobs M, Neyts J, van Kuppeveld FJM, van Rij RP. 2013. Identification of a new dengue virus inhibitor that targets the viral NS4B protein and restricts genomic RNA replication. Antiviral Res 99:165–171. doi: 10.1016/j.antiviral.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 10.Xie X, Wang Q-Y, Xu HY, Qing M, Kramer L, Yuan Z, Shi P-Y. 2011. Inhibition of dengue virus by targeting viral NS4B protein. J Virol 85:11183–11195. doi: 10.1128/JVI.05468-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luzhkov VB, Selisko B, Nordqvist A, Peyrane F, Decroly E, Alvarez K, Karlen A, Canard B, Qvist J. 2007. Virtual screening and bioassay study of novel inhibitors for dengue virus mRNA cap (nucleoside-2′O)-methyltransferase. Bioorg Med Chem 15:7795–7802. doi: 10.1016/j.bmc.2007.08.049. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y-L, Yin Z, Lakshminarayana SB, Qing M, Schul W, Duraiswamy J, Kondreddi RR, Goh A, Xu HY, Yip A, Liu B, Weaver M, Dartois V, Keller TH, Shi P-Y. 2010. Inhibition of dengue virus by an ester prodrug of an adenosine analog. Antimicrob Agents Chemother 54:3255–3261. doi: 10.1128/AAC.00397-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen NM, Tran CNB, Phung LK, Duong KTH, Huynh HLA, Farrar J, Nguyen QTH, Tran HT, Nguyen CVV, Merson L, Hoang LT, Hibberd ML, Aw PPK, Wilm A, Nagarajan N, Nguyen DT, Pham MP, Nguyen TT, Javanbakht H, Klumpp K, Hammond J, Petric R, Wolbers M, Nguyen CT, Simmons CP. 2013. A randomized, double-blind placebo controlled trial of balapiravir, a polymerase inhibitor, in adult dengue patients. J Infect Dis 207:1442–1450. doi: 10.1093/infdis/jis470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niyomrattanakit P, Chen Y-L, Dong H, Yin Z, Qing M, Glickman JF, Lin K, Mueller D, Voshol H, Lim JYH, Nilar S, Keller TH, Shi P-Y. 2010. Inhibition of dengue virus polymerase by blocking of the RNA tunnel. J Virol 84:5678–5686. doi: 10.1128/JVI.02451-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noble CG, Lim SP, Chen Y-L, Liew CW, Yap L, Lescar J, Shi P-Y. 2013. Conformational flexibility of the dengue virus RNA-dependent RNA polymerase revealed by a complex with an inhibitor. J Virol 87:5291–5295. doi: 10.1128/JVI.00045-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yin Z, Chen Y-L, Schul W, Wang Q-Y, Gu F, Duraiswamy J, Kondreddi RR, Niyomrattanakit P, Lakshminarayana SB, Goh A, Xu HY, Liu W, Liu B, Lim JYH, Ng CY, Qing M, Lim CC, Yip A, Wang G, Chan WL, Tan HP, Lin K, Zhang B, Zou G, Bernard KA, Garrett C, Beltz K, Dong M, Weaver M, He H, Pichota A, Dartois V, Keller TH, Shi P-Y. 2009. An adenosine nucleoside inhibitor of dengue virus. Proc Natl Acad Sci U S A 106:20435–20439. doi: 10.1073/pnas.0907010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watanabe S, Rathore APS, Sung C, Lu F, Khoo YM, Connolly J, Low J, Ooi EE, Lee HS, Vasudevan SG. 2012. Dose- and schedule-dependent protective efficacy of celgosivir in a lethal mouse model for dengue virus infection informs dosing regimen for a proof of concept clinical trial. Antiviral Res 96:32–35. doi: 10.1016/j.antiviral.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Whitehorn J, Van Vinh Chau N, Truong NT, Tai LTH, Van Hao N, Hien TT, Wolbers M, Merson L, Dung NTP, Peeling R, Simmons C, Wills B, Farrar J. 2012. Lovastatin for adult patients with dengue: protocol for a randomised controlled trial. Trials 13:203. doi: 10.1186/1745-6215-13-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tricou V, Minh NN, Van TP, Lee SJ, Farrar J, Wills B, Tran HT, Simmons CP. 2010. A randomized controlled trial of chloroquine for the treatment of dengue in Vietnamese adults. PLoS Negl Trop Dis 4:e785. doi: 10.1371/journal.pntd.0000785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tam DTH, Ngoc TV, Tien NTH, Kieu NTT, Thuy TTT, Thanh LTC, Tam CT, Truong NT, Dung NT, Qui PT, Hien TT, Farrar JJ, Simmons CP, Wolbers M, Wills BA. 2012. Effects of short-course oral corticosteroid therapy in early dengue infection in Vietnamese patients: a randomized, placebo-controlled trial. Clin Infect Dis 55:1216–1224. doi: 10.1093/cid/cis655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smee DF, Bray M, Huggins JW. 2001. Antiviral activity and mode of action studies of ribavirin and mycophenolic acid against orthopoxviruses in vitro. Antivir Chem Chemother 12:327–335. [DOI] [PubMed] [Google Scholar]
- 22.Haas WH, Breuer T, Pfaff G, Schmitz H, Köhler P, Asper M, Emmerich P, Drosten C, Gölnitz U, Fleischer K, Günther S. 2003. Imported Lassa fever in Germany: surveillance and management of contact persons. Clin Infect Dis 36:1254–1258. doi: 10.1086/374853. [DOI] [PubMed] [Google Scholar]
- 23.Malinoski F, Stollar V. 1981. Inhibitors of IMP dehydrogenase prevent Sindbis virus replication and reduce GTP levels in Aedes albopictus cells. Virology 110:281–289. doi: 10.1016/0042-6822(81)90060-X. [DOI] [PubMed] [Google Scholar]
- 24.Leyssen P, Balzarini J, De Clercq E, Neyts J. 2005. The predominant mechanism by which ribavirin exerts its antiviral activity in vitro against flaviviruses and paramyxoviruses is mediated by inhibition of IMP dehydrogenase. J Virol 79:1943–1947. doi: 10.1128/JVI.79.3.1943-1947.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crotty S, Cameron CE, Andino R. 2001. RNA virus error catastrophe: direct molecular test by using ribavirin. Proc Natl Acad Sci U S A 98:6895–6900. doi: 10.1073/pnas.111085598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas E, Feld JJ, Li Q, Hu Z, Fried MW, Liang TJ. 2011. Ribavirin potentiates interferon action by augmenting interferon-stimulated gene induction in hepatitis C virus cell culture models. Hepatology 53:32–41. doi: 10.1002/hep.23985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qing M, Zou G, Wang Q-Y, Xu HY, Dong H, Yuan Z, Shi P-Y. 2010. Characterization of dengue virus resistance to brequinar in cell culture. Antimicrob Agents Chemother 54:3686–3695. doi: 10.1128/AAC.00561-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Q-Y, Bushell S, Qing M, Xu HY, Bonavia A, Nunes S, Zhou J, Poh MK, Florez de Sessions P, Niyomrattanakit P, Dong H, Hoffmaster K, Goh A, Nilar S, Schul W, Jones S, Kramer L, Compton T, Shi P-Y. 2011. Inhibition of dengue virus through suppression of host pyrimidine biosynthesis. J Virol 85:6548–6556. doi: 10.1128/JVI.02510-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu S, Neidhardt EA, Grossman TH, Ocain T, Clardy J. 2000. Structures of human dihydroorotate dehydrogenase in complex with antiproliferative agents. Structure 8:25–33. doi: 10.1016/S0969-2126(00)00077-0. [DOI] [PubMed] [Google Scholar]
- 30.Batt DG. 1999. Inhibitors of dihydroorotate dehydrogenase. Expert Opin Ther Pat 9:41–54. doi: 10.1517/13543776.9.1.41. [DOI] [Google Scholar]
- 31.Ng CY, Gu F, Phong WY, Chen Y-L, Lim SP, Davidson A, Vasudevan SG. 2007. Construction and characterization of a stable subgenomic dengue virus type 2 replicon system for antiviral compound and siRNA testing. Antiviral Res 76:222–231. doi: 10.1016/j.antiviral.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 32.Lehár J, Krueger AS, Avery W, Heilbut AM, Johansen LM, Price ER, Rickles RJ, Short GF, Staunton JE, Jin X, Lee MS, Zimmermann GR, Borisy AA. 2009. Synergistic drug combinations tend to improve therapeutically relevant selectivity. Nat Biotechnol 27:659–666. doi: 10.1038/nbt.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chou T-C. 2006. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev 58:621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 34.Meletiadis J, Verweij PE, TeDorsthorst DT, Meis JF, Mouton JW. 2005. Assessing in vitro combinations of antifungal drugs against yeasts and filamentous fungi: comparison of different drug interaction models. Med Mycol 43:133–152. doi: 10.1080/13693780410001731547. [DOI] [PubMed] [Google Scholar]
- 35.Geary N. 2013. Understanding synergy. Am J Physiol Endocrinol Metab 304:E237–E253. doi: 10.1152/ajpendo.00308.2012. [DOI] [PubMed] [Google Scholar]
- 36.Pan Q, de Ruiter PE, Metselaar HJ, Kwekkeboom J, de Jonge J, Tilanus HW, Janssen HLA, van der Laan LJW. 2012. Mycophenolic acid augments interferon-stimulated gene expression and inhibits hepatitis C virus infection in vitro and in vivo. Hepatology 55:1673–1683. doi: 10.1002/hep.25562. [DOI] [PubMed] [Google Scholar]
- 37.Gentile I, Coppola N, Buonomo AR, Zappulo E, Borgia G. 2014. Investigational nucleoside and nucleotide polymerase inhibitors and their use in treating hepatitis C virus. Expert Opin Investig Drugs 23:1211–1223. doi: 10.1517/13543784.2014.921680. [DOI] [PubMed] [Google Scholar]
- 38.Vernachio JH, Bleiman B, Bryant KD, Chamberlain S, Hunley D, Hutchins J, Ames B, Gorovits E, Ganguly B, Hall A, Kolykhalov A, Liu Y, Muhammad J, Raja N, Walters CR, Wang J, Williams K, Patti JM, Henson G, Madela K, Aljarah M, Gilles A, McGuigan C. 2011. INX-08189, a phosphoramidate prodrug of 6-O-methyl-2′-C-methyl guanosine, is a potent inhibitor of hepatitis C virus replication with excellent pharmacokinetic and pharmacodynamic properties. Antimicrob Agents Chemother 55:1843–1851. doi: 10.1128/AAC.01335-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao WY, Johns DG, Mitsuya H. 2000. Potentiation of the anti-HIV activity of zalcitabine and lamivudine by a CTP synthase inhibitor, 3-deazauridine. Nucleosides Nucleotides Nucleic Acids 19:371–377. doi: 10.1080/15257770008033015. [DOI] [PubMed] [Google Scholar]
- 40.Yeh P, Tschumi AI, Kishony R. 2006. Functional classification of drugs by properties of their pairwise interactions. Nat Genet 38:489–494. doi: 10.1038/ng1755. [DOI] [PubMed] [Google Scholar]
- 41.Chen Y-L, Abdul Ghafar N, Karuna R, Fu Y, Lim SP, Schul W, Gu F, Herve M, Yokohama F, Wang G, Cerny D, Fink K, Blasco F, Shi P-Y. 2014. Activation of peripheral blood mononuclear cells by dengue virus infection depotentiates balapiravir. J Virol 88:1740–1747. doi: 10.1128/JVI.02841-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wong KL, Chen W, Balakrishnan T, Toh YX, Fink K, Wong S-C. 2012. Susceptibility and response of human blood monocyte subsets to primary dengue virus infection. PLoS One 7:e36435. doi: 10.1371/journal.pone.0036435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Low JG, Sung C, Wijaya L, Wei Y, Rathore APS, Watanabe S, Tan BH, Toh L, Chua LT, Hou Y, Chow A, Howe S, Chan WK, Tan KH, Chung JS, Cherng BP, Lye DC, Tambayah PA, Ng LC, Connolly J, Hibberd ML, Leo YS, Cheung YB, Ooi EE, Vasudevan SG. 2014. Efficacy and safety of celgosivir in patients with dengue fever (CELADEN): a phase 1b, randomised, double-blind, placebo-controlled, proof-of-concept trial. Lancet Infect Dis 14:706–715. doi: 10.1016/S1473-3099(14)70730-3. [DOI] [PubMed] [Google Scholar]
- 44.Fitzgerald JB, Schoeberl B, Nielsen UB, Sorger PK. 2006. Systems biology and combination therapy in the quest for clinical efficacy. Nat Chem Biol 2:458–466. doi: 10.1038/nchembio817. [DOI] [PubMed] [Google Scholar]
- 45.Owens CM, Mawhinney C, Grenier JM, Altmeyer R, Lee MS, Borisy AA, Lehár J, Johansen LM. 2010. Chemical combinations elucidate pathway interactions and regulation relevant to hepatitis C replication. Mol Syst Biol 6:375. doi: 10.1038/msb.2010.32. [DOI] [PMC free article] [PubMed] [Google Scholar]