Abstract
Introduction
Understanding clinical reasoning is essential for patient care and medical education. Dual-processing theory suggests that nonanalytic reasoning is an essential aspect of expertise; however, assessing nonanalytic reasoning is challenging because it is believed to occur on the subconscious level. This assumption makes concurrent verbal protocols less reliable assessment tools.
Methods
Functional magnetic resonance imaging was used to explore the neural basis of nonanalytic reasoning in internal medicine interns (novices) and board-certified staff internists (experts) while completing United States Medical Licensing Examination and American Board of Internal Medicine multiple-choice questions.
Results
The results demonstrated that novices and experts share a common neural network in addition to nonoverlapping neural resources. However, experts manifested greater neural processing efficiency in regions such as the prefrontal cortex during nonanalytical reasoning.
Conclusions
These findings reveal a multinetwork system that supports the dual-process mode of expert clinical reasoning during medical evaluation.
Keywords: Dual-process theory, expertise, functional MRI, medical education, neural efficiency, nonanalytical reasoning
Introduction
Clinical reasoning entails the cognitive processes that culminate in a diagnosis and treatment plan, and thus is central to almost everything a physician does in practice (Higgs et al. 2008). The “hidden” nature of clinical reasoning renders it difficult to assess through current methods in medical education (Higgs et al. 2008; Schuwirth 2009).Without the ability to directly observe clinical reasoning, a major emphasis of research in clinical reasoning has been the development and testing of theory. Presently, dual-process theory is the leading cognitive theory that has been applied to the construct of clinical reasoning (Norman and Eva 2010). This theory attributes expertise in clinical reasoning to greater use of nonanalytic reasoning, which is believed to be immediate, largely subconscious, and thus difficult or perhaps impossible for subjects to describe (e.g., fast thinking or pattern recognition; Norman and Eva 2010). While medical practitioners regularly use both analytic and nonanalytic reasoning in clinical reasoning tasks, nonanalytic reasoning is believed to correlate most strongly with expertise, yet it is also the more challenging to evaluate (Schmidt and Boshuizen 1993). However, novel neuroimaging techniques may be particularly well-suited to this task.
Cognitive expertise involves chunking of information, or assembling a string of perceptual cues into a more meaningful pattern (de Groot 1965), relying on processes such as working memory (Boreham 1994). Experts are able to generate better problem representation as well as better “next steps or moves” (Simon 1990) in order to select the best diagnostic option (Elstein et al. 1990). Thus, experts differ from novices in how they process information and arrive at an answer, such that experts do not choose more next steps or answers, but the quality of their answers or next steps are superior (Elstein et al. 1990).
In medical education research, think-aloud protocols are a commonly employed means for assessing thought processes while engaging in an activity such as clinical reasoning. Think-aloud method is thought to provide insight into the underpinnings of expertise in physicians (Boreham 1994). However, scholars disagree on the validity of verbally reporting one's thoughts (i.e., think-aloud protocols), because it may interfere with the very act of thinking (Russo et al. 1989; Ericsson 2006). Moreover, think-aloud protocols would be expected to perform better in the assessment of consciously accessible thought processes that are inherent to analytic reasoning (where one actively compares and contrasts options), as opposed to the subconscious processes of nonanalytic reasoning that are believed to be the mainstay of experts engaged in clinical problem solving.
One of the current “gold standards” for assessing the end result of clinical reasoning is multiple-choice questions (MCQs) from professional regulatory authorities such as the American Board of Internal Medicine (ABIM) and National Board of Medical Examiners (NBME). The scores from such high-stakes MCQ tests have evidence of high reliability and validity and allow sampling of a large number of topics during an examination session. MCQs can also isolate tasks such as identifying the most likely diagnosis or the next step in diagnosis or therapy, providing a useful assessment of clinical reasoning, particularly when the questions are vignette-based and require consideration of the optimal diagnosis or treatment. (Schuwirth et al. 2001). However, as MCQs do not allow investigators to observe the thought processes that lead to the final answer, an inability to elucidate the process of clinical reasoning can be viewed as a significant limitation.
Given that immediate vocalization of nonanalytic reasoning is difficult, if not impossible, there is a need for other investigative methods to understand this essential aspect of expertise. Functional magnetic resonance imaging (fMRI) is a particularly promising method for enhancing the understanding of nonanalytic reasoning and development of medical expertise, especially when viewed in conjunction with educational theory. fMRI can elucidate otherwise invisible patterns of regional brain activation, acting like a flashlight allows us to see the brain areas and pathways that are inherent to clinical problem solving. Similar assessments in other fields indicate that regions such as the caudate and precuneus appear to play an important role in generating and utilizing perception units or “chunks” when determining the next “best move” in board games, for example, (Wan et al. 2011). There is also some evidence that more skilled or experienced individuals (e.g., experts) demonstrate more efficient neuronal utilization than novices confronted with the same tasks (Neubauer and Fink 2009).
Thus, taking into account both dual-process theory and relevant neuroimaging experience, we compared brain activation patterns for novice and expert physicians in order to discern whether clinical reasoning expertise correlates with distinct activation patterns on functional neuroimaging. We hypothesized that experts and novices would display a shared network of clinical reasoning expertise, as expertise is an adaption built on the foundation developed while one is a novice. Second, extrapolating from other fields, we hypothesized that neural areas such as the precuneus and caudate would demonstrate greater activation in experts as opposed to novices during nonanalytic reasoning. Lastly, the notion of neural efficiency is reportedly a hallmark of skill and expertise (Neubauer and Fink 2009); thus, we hypothesized that experts would display less overall brain activation (more efficient networks activated to accomplish the task) than novices.
Material and Methods
Participants
Following completion of written informed consent, board-certified internal medicine attending physicians (experts) and internal medicine interns (novices) with faculty appointments at the Uniformed Services University (USU) participated in the study. Board certification represents the culmination of expertise in medicine and is the culmination of years of medical school and residency education. Hence, we defined board-certified physicians as experts in this study; whereas internal medicine interns, who just completed medical school and were several years away from board certification, were defined as novices. There were several exclusion criteria: presence of shrapnel or surgical metal devices, inability to complete an fMRI due to anxiety or claustrophobia, taking calcium channel blockers (which can impact regional blood flow), or pregnancy. The study protocol and procedures employed were approved by the Institutional Review Boards of the USU and Walter Reed Army Medical Center. The procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Demographics
The mean age of the experts was 39.5 ± 7 (range = 32–51 years), including 15 men and two women. For the novices, the mean age was 29.6 ± 2 (range = 28–35 years), including seven men and three women. Experts were significantly older, and had significantly greater years of clinical experience, than novices (P < 0.05).
Measurements
Multiple-choice questions
We used validated MCQs from the ABIM and NBME to assess physician performance. These organizations are responsible for certifying or licensing physicians in the United States, and they conduct validity studies to assess the appropriateness of their items by subjecting them to a rigorous internal content review and performance analysis.
We selected cardiology and rheumatology questions for the study, as they represent core domains in internal medicine. The MCQs ask “What is the most likely diagnosis?”, necessitating integration and synthesis of data to answer (i.e., the examination items assessed clinical reasoning). Each participant answered 32 questions: 16 NBME items (United States Medical Licensing Examination [USMLE] Step 2 Clinical Knowledge items) and 16 ABIM items (Maintenance of Certification [MOC]). We selected questions that fit on a single screen and contained only words (i.e., no chest X-rays or other images). In addition, the MCQ format (participants pushed handheld buttons for answer options “A” to “E”) made them ideal for use in the fMRI scanner, eliminating the need for participants to speak, as jaw motion impairs fMRI image interpretation.
fMRI Data acquisition
Subjects were scanned on a 3T 750 MRI scanner (General Electric, Milwaukee, WI) with a 32-channel head coil. Acquisitions were performed using an echo-planar imaging (EPI) sequence of 40 contiguous sagittal slices per brain volume (TR = 2000 ms, TE = 25 ms, flip angle = 60° slice thickness = 4.0 mm). In-plane resolution was 3.75 × 3.75 mm (64 × 64 voxels). An fMRI task presentation of the 32 questions was created using E-Prime software (Psychology Software Tools, Inc.) and displayed via a goggle system (Nordic NeuroLab Inc., Milwaukee, WI) while each participant was in the fMRI scanner. The questions were presented in random order for each subject over the course of four fMRI acquisition runs, with eight questions per run. The mean run length (± standard deviation) was 392 ± 62 sec. During the same imaging session, a high-resolution T1-weighted image was acquired for anatomical reference (three dimensional GRE; TR = 6.6 ms, TE = 2.5 ms, flip angle = 12°). This image consisted of 312 sagittal slices with a slice thickness of 0.6 mm and an in-plane resolution of 0.468 × 0.468 mm (512 × 512 voxels). For voxel-wise analysis on whole brain data, we controlled false positive rates per map at alpha=0.05, using random-effects models and consistent with prior work.
Procedure
Before entering the fMRI scanner, participants were formally trained in procedures for answering MCQs in the scanner. Each MCQ was projected in three phases. In the first phase, the stem (question) appeared (“reading” phase), ending with “what is the most likely diagnosis?” or a related diagnostic question, but not displaying answer options “A” to “E”. Each participant was given a maximum of 60 sec to read the stem, or could push any button to move on to the answer options (the second or “answering” phase) more quickly. Participants were then given 7 sec to choose an answer option using the finger response items. The final “reflection” phase ensued, in which participants were instructed to silently reflect on how they arrived at the diagnosis utilizing analytical reasoning processes (“how did you establish the diagnosis for this item?”), which they did for 14 sec, before the next question was presented. The reflection phase thus was characterized by analytic thinking about how they chose the answer they did (e.g., actively comparing and contrasting alternatives). Before entering the scanner, participants received training on how to analyze their thinking (a think-aloud procedure).
fMRI Data analysis
All fMRI data were processed using the AFNI software package in accordance with previously published methods (Cox 1996; Durning et al. 2012). The participant's EPI scans were preprocessed by first removing the three volumes (6 sec) from each 4D time series. Next the scans were corrected for slice timing and motion then coregistered to the T1 anatomical image (anatomic scans were registered to Talairach space). The images were spatially smoothed using 8-mm full-width at half-maximum Gaussian kernel and converted to percent-change-from-mean. For the first level analysis, the four datasets for each subject were concatenated. The “answer” times varied from question to question (depending on how quickly the participant answered) and were modeled with a gamma-variate function with variable duration and variable relative amplitude (amplitude variation was based on duration variation). The “reflection” time was constant at 14 sec and was modeled with a nonvariable gamma-variate. The GLM analysis determined the significance of these model time courses, along with head motion parameters, to generate β coefficients and t statistics for each voxel, for the contrast of interest: answer phase relative to reflection (answer > reflection) which isolates nonanalytical reasoning: answering (utilizing both analytical and nonanalytical reasoning)—reflection (analytical reasoning)(Chen et al. 2012). Second-level analysis across all subjects was then performed using linear mixed-effects modeling conducted on the individual contrast for experts and novices separately. These comparisons were used in the conjunction analysis (Price and Friston 1997) to examine brain regions with similar levels of activation, versus those with significantly different levels of activation, between the two groups for answer > reflection. Results of the second-level analyses were corrected for multiple comparisons using family wise error (FWE) correction (from a Monte Carlo simulations using AFNI's 3dClustSim) to achieve corrected P values (P < 0.05) based on cluster size.
Results
fMRI Conjunction analysis
Whole brain analysis revealed a common network, with similar levels of activation, between the two groups involving the bilateral precentral gyrus, bilateral middle frontal gyrus, bilateral dorsomedial prefrontal cortex (DMPFC), left dorsolateral prefrontal cortex (DLPFC), bilateral postcentral gyrus, bilateral inferior parietal lobule, left superior parietal lobule, left precuneous, left middle temporal gyrus, and left fusiform gyrus (Table 1, Fig.1). Areas of significantly greater activation in experts were the left ventrolateral prefrontal cortex (VLPFC), left lateral orbitofrontal cortex (OFC), right superior parietal lobule, right inferior occipital gyrus, bilateral middle occipital gyrus, bilateral insula, bilateral lentiform nucleus, bilateral dorsal anterior cingulate cortex (ACC), bilateral cerebellum, bilateral thalamus, and bilateral parahippocampal gyrus. The sole area in which novices demonstrated significantly greater activation than experts was the ventral anterior cingulate cortex (Table 1, Fig.1).
Table 1.
Peak activations during nonanalytic reasoning
| Region |
Experts |
Novices |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ans>Refl | Brod. no. | Hemi | x | y | z | t score | Hemi | x | y | z | t score |
| Precentral Gyrus* | BA4 | L | −36 | −17 | 60 | 5.8 | L | −37 | −23 | 59 | 3.0 |
| R | 40 | −19 | 54 | 8.1 | R | 37 | −20 | 54 | 4.8 | ||
| Middle Frontal Gyrus* | BA6 | L | −21 | −3 | 56 | 7.0 | L | −28 | −7 | 57 | 5.1 |
| R | 30 | −9 | 58 | 6.7 | R | 26 | −9 | 56 | 4.5 | ||
| Medial Frontal Gyrus (DMPFC)* | BA8 | L | −2 | 16 | 48 | 6.7 | L | −11 | 4 | 48 | 4.8 |
| R | 1 | 5 | 51 | 5.9 | R | 11 | 15 | 43 | 3.0 | ||
| DLPFC* | BA9 | L | −47 | 28 | 29 | 9.8 | L | −49 | 6 | 37 | 3.9 |
| DLPFC* | BA46 | L | −43 | 30 | 23 | 8.2 | L | −40 | 20 | 23 | 3.2 |
| IFG(VLPFC) | BA45 | L | −52 | 7 | 23 | 6.2 | |||||
| R | 54 | 10 | 24 | 5.4 | |||||||
| OFC (lateral) | BA47 | L | −31 | 28 | −4 | 7.1 | |||||
| Postcentral Gyrus* | BA3 | L | −40 | −22 | 55 | 6.3 | L | −41 | −27 | 56 | 2.9 |
| R | 47 | −23 | 48 | 9.1 | R | 39 | −27 | 54 | 4.3 | ||
| Inferior Parietal Lobule* | BA40 | L | −36 | −39 | 48 | 6.7 | L | −40 | −40 | 44 | 4.0 |
| R | 32 | −42 | 50 | 6.8 | R | 33 | −42 | 50 | 4.0 | ||
| Superior Parietal Lobule* | BA7 | L | −28 | −53 | 58 | 5.5 | L | −28 | −63 | 55 | 5.5 |
| R | 27 | −55 | 51 | 4.2 | |||||||
| Precuneus* | L | −28 | −68 | 37 | 5.9 | L | −23 | −60 | 48 | 4.9 | |
| Middle Temporal Gyrus* | BA39 | L | −29 | −65 | 30 | 5.1 | L | −28 | −58 | 28 | 3.4 |
| Fusiform* | BA37 | L | −40 | −56 | −8 | 6.3 | L | −46 | −50 | −10 | 3.2 |
| R | 29 | −41 | −15 | 4.1 | |||||||
| Inferior Occipital Gyrus | BA19 | R | 43 | −77 | −4 | 4.8 | |||||
| Middle Occipital Gyrus | BA39 | L | −44 | −76 | 14 | 4.0 | |||||
| R | 32 | −82 | 14 | 3.7 | |||||||
| Insula | BA13 | L | −29 | 16 | 14 | 5.3 | |||||
| R | 29 | 25 | 10 | 5.9 | |||||||
| Lentiform Nucleus | L | −17 | 5 | 3 | 5.7 | ||||||
| R | 11 | 4 | 0 | 5.7 | |||||||
| Dorsal ACC | BA32 | L | −3 | 20 | 40 | 5.6 | |||||
| R | 5 | 19 | 40 | 5.3 | |||||||
| Ventral ACC | BA24 | L | −22 | 6 | 43 | 3.6 | |||||
| Cerebellum | L | −17 | −54 | −28 | 5.8 | ||||||
| R | 26 | −61 | −30 | 4.9 | |||||||
| Thalamus | L | −7 | −17 | −7 | 4.8 | ||||||
| R | 6 | −17 | −8 | 4.9 | |||||||
| Parahippocampal Gyrus | L | −34 | −36 | −10 | 3.4 | ||||||
| R | 33 | −38 | −9 | 3.9 | |||||||
Nonanalytic reasoning (Answering > Reflection) in experts (left panel) and novices (right panel). Asterisk (*) denotes regions of similar levels of activation as revealed by conjunction analysis. Brodmann's areas and laterality (hemisphere) are provided in addition to coordinates given in Talairach space. All results are based on FWE correction P < 0.05 and t scores are indicated.
Figure 1.
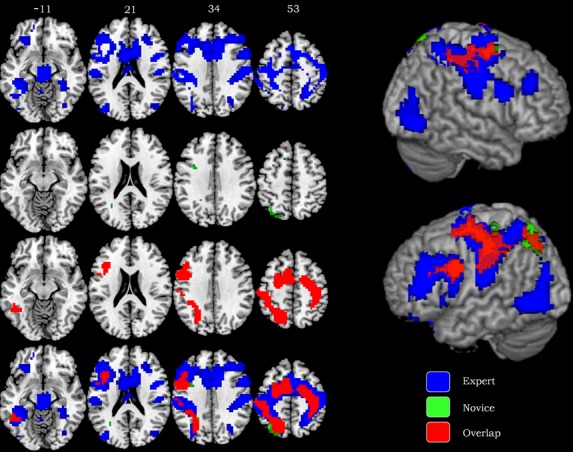
Whole brain analysis of experts and novices during nonanalytic clinical reasoning (answering > reflection). Axial slices with corresponding Talairach coordinates indicate unique and shared activation patterns for experts and novices. The results demonstrate nonoverlapping activations for experts in blue and nonoverlapping activation for novices in green. The activations shared by both groups are represented in red. All results are thresholded at FWE corrected P < 0.05.
fMRI Direct group comparisons
When directly comparing experts and novices for the magnitude of differences between the two groups, experts demonstrated significantly less activation relative to novices in the right postcentral gyrus, bilateral DLPFC, DMPFC, bilateral ventromedial prefrontal cortex, bilateral lateral OFC, bilateral medial OFC, ventral ACC, and dorsal ACC. Significantly greater activation in experts compared to novices was evident in the rostrolateral prefrontal cortex and cuneus. (Table 2, Fig.2).
Table 2.
Direct comparison between experts and novices (answer > reflection)
| Expert |
Novice |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Region | Brod. no. | Hemi | x | y | z | t score | Hemi | x | y | z | t score |
| Ans > Refl | |||||||||||
| Postcentral Gyrus | BA1 | R | 56 | −16 | 48 | −2.95 | |||||
| DLPFC | BA9 | L | −48 | 28 | 28 | −3.030 | |||||
| DLPFC | BA46 | L | −40 | 36 | 10 | −2.66 | R | 41 | 35 | 10 | −3.699 |
| DMPFC | BA9 | L | −8 | 50 | 36 | −4.295 | R | 15 | 44 | 27 | −3.037 |
| vmPFC | BA10 | L | −18 | 47 | 7 | −3.099 | R | 19 | 50 | 1 | −3.717 |
| Ventral ACC | BA24 | L | −8 | 17 | 22 | −3.500 | R | 4 | 29 | 13 | −3.77 |
| lOFC | BA47 | L | −28 | 30 | −4 | −3.110 | R | 26 | 30 | −2 | −3.05 |
| mOFC | BA11 | L | −16 | 46 | −10 | −2.460 | R | 24 | 27 | −12 | −3.179 |
| Dorsal ACC | BA32 | L | −8 | 22 | 20 | −2.96 | R | 6 | 28 | 22 | −2.76 |
| RLPFC | BA10 | R | 46 | 43 | −6 | 3.55 | |||||
| Cuneus | BA18 | R | 17 | −84 | 25 | 2.82 | |||||
Negative t scores are indicative of areas demonstrating significantly lesser activation for experts than for novices, while positive t scores reveal areas of greater activation in experts compared to novices. Brodmann's areas and laterality (hemisphere) are provided in addition to coordinates given in Talairach space. All results are based on FWE correction P < 0.05.
Figure 2.
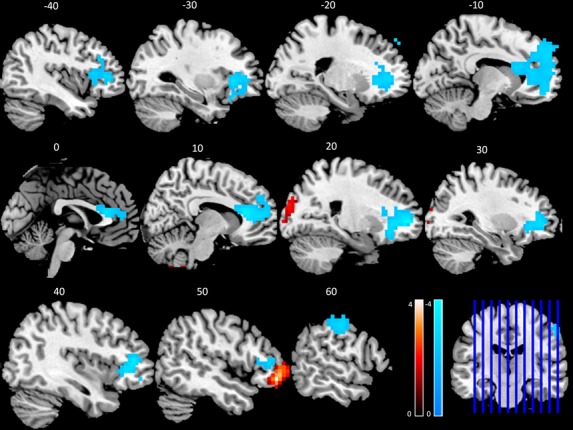
Direct comparisons between experts and novices (Expert > Novice) during nonanalytic clinical reasoning. Warm colors denote regions where experts demonstrate greater responses and cooler colors reveal regions where novices demonstrate greater responses during nonanalytic clinical reasoning (answering > reflection). Sagittal slices are presented with corresponding Talairach coordinates and all results are thresholded at FWE corrected P < 0.05. The color bars indicate t scores and the coronal slice presents orientation of sagittal results.
Discussion
To our knowledge, this is the first study to utilize functional neuroimaging to study nonanalytic reasoning during evaluation in the field of medicine. We explored the functional neuroimaging of expert and novice performance using the current gold standard for competency assessment, validated, vignette-based MCQs. We report that novices and experts share a common neural network, but also show some significant differences in regional brain activation, during nonanalytical reasoning (Fig.1). Experts demonstrate neural-processing efficiency in regions such as the prefrontal cortex (Fig.2), which may buttress dual-process theory, and help to elucidate neural networks that represent expertise. This may ultimately enable the identification of fMRI biomarkers of effective clinical reasoning, which could facilitate educational interventions to improve desired regional brain activation in order to reduce cognitive errors.
Shared network
The results support our hypothesis that experts and novices share a common network of activation. Experts and novices demonstrate similar levels of activation in the motor (BA4) and premotor (BA6) regions postulated to be critical to clinical reasoning (Fletcher and Carruthers 2012). The DMPFC and left lateral DLPFC both also showed similar activation levels for both groups. The former is reportedly involved in self-referential evaluation (Northoff and Bermpohl 2004), which may be critical in understanding and making inferences (Amodio and Frith 2006), in turn contributing to nonanalytical information processing. The latter is thought to be involved in attention shifting and control, selection (Sylvester et al. 2003) modulation of self-control (Figner et al. 2010), and cognitive flexibility (Braver et al. 2009).
We also found some posterior brain regions that were active in both groups. The inferior parietal lobule is involved in the validation of deductive reasoning, and the fusiform may mediate the integration of information with a working premise (Fangmeier et al. 2006). The postcentral gyrus has been shown to be connected with mental preparation for successful problem solving (Tian et al. 2011). We expected the precuneus to show greater activation in experts, but identified similar activations in both groups, suggesting that even novices were employing some pattern recognition, or nonanalytic reasoning in answering. This is not entirely surprising, as our “novices” have completed both college and medical school, and are currently engaged in postgraduate medical education, so that they are not entirely new to the field. This may represent an intermediate step in the development of expertise, as experts also demonstrate distinctive features during nonanalytical reasoning (see next section).
Expert patterns
Among the regions in which experts evince greater activation is the VLPFC, which mediates working memory retrieval (Wolf et al. 2006). The left VLPFC has been implicated in cognitive control of memory including task switching, knowledge-based retrieval, integration of past events, and resolution of task interference (Badre and Wagner 2006), whereas the right facilitates the update of action plans which may be part of the automaticity of expertise. (Levy and Wagner 2011). Experts also differentially activate the lateral OFC, which may prepare for outcome changes (Windmann et al. 2006) that contribute to decision making (Kringelbach 2005). This region may thus facilitate connections between prestored knowledge and the new clinical scenario.
Experts demonstrate greater activation in several regions that may orchestrate the “chunking” or pattern recognition believed to be integral to nonanalytic reasoning, including the inferior occipital gyrus, middle occipital gyrus (Ruff et al. 2003) and parahippocampal gyrus (Aguirre et al. 1996). On the other hand, we believe that differential expert activation of the insula, a region involved in the integration of sensory information (Medford and Critchley 2010) as well as empathy, emotion, and the processing of uncertainty (Singer et al. 2009), may be a manifestation of the “gut instinct” that comes from experience. Our experts also showed greater recruitment of the dorsal anterior cingulate cortex, a region reportedly involved in conflict resolution during error detection (Braver et al. 2001). The ACC processes cognitive and affective representations, in addition to sensory and motor information, in order to evaluate error (Bush et al. 2000). Activation of ACC in tandem with the insula supports a recent model of multimodal integration in response selection (Medford and Critchley 2010). Cultivation of this functional network may therefore spawn better-informed choices and minimize diagnostic error.
Another region of unique activation in experts is the bilateral cerebellum. Recent studies have suggested that the cerebellum may process not only motor control but also mediate cognitive control in the form of rule retrieval (Crescentini et al. 2011; Balsters et al. 2012), which may contribute to the automaticity of nonanalytic clinical reasoning. Although we did not find activation in the caudate as we had predicted with experts, the lentiform nucleus of the basal ganglia was more active. Lesion studies suggest this region is involved in drive and initiative (Brown et al. 1997). Lastly thalamic activation suggests an elevated intensity, alertness, and arousal (Sturm et al., 1999) unique to experts.
Novice patterns
The ventral ACC was the only region where novices showed greater activation than experts. The ventral ACC is involved in the emotional response to error (Braver et al. 2001), so this may represent an emotional response (Etkin et al. 2011).to their greater uncertainty when challenged with MCQs.
Neural efficiency and expertise
Direct comparisons between the expert and novice group revealed significantly less activation in frontal and selective posterior regions for the experts. The relative reduction of activity in these areas may mean that experts are more efficiently able to incorporate these areas into their diagnostic decisions, whereas novices require more cognitive effort to accomplish this. Thus, our findings suggest that experts may make better “first moves” because they can more efficiently activate relevant areas of the brain for the task of clinical reasoning.
Our results reveal relative deactivation of the DLPFC of the experts, supporting our hypothesis. As previously discussed, the DLPFC is essential to attention shifting, working memory and inhibitory control (e.g., Glascher et al. 2012) and such neural efficiency suggests that experts may require fewer neural resources to accomplish the task demands. In addition, the relative reduced activation in the DMPFC suggests experts are more efficient with referential processing (Yaoi et al. 2009), and the evaluation of the self's qualities within the goal of the moment (Beer et al. 2010). The relative LOFC deactivation in experts suggests neural efficiency when weighing outcome uncertainty and probabilistic choices (Windmann et al. 2006). In addition, the efficiency in the dorsal anterior cingulate of the experts suggests that error evaluation requires less effort to accomplish a high level of performance (Braver et al. 2001). The relative reduction in the lOFC and ACC suggests that although these regions are uniquely recruited by experts during nonanalytical reasoning (see Table 1), they are more efficient in processing compared to novices.
Significant differences in several frontal regions were only revealed during direct comparisons between the groups. The experts demonstrated significantly less activation in the ventromedial prefrontal cortex (VMPFC), a region in processing of metacognitive representations such as outcome selection (Amodio and Frith 2006). Our results also revealed a relative deactivation in the ventral ACC of experts, suggesting that they require less neural resources when affectively evaluating possible errors (Etkin et al. 2011). Neural efficiency is also present in the medial OFC (Windmann et al. 2006) of the experts. Notably, the medial OFC is also reportedly involved in empathy and compassion (Klimecki et al. 2012), consistent with the efficiency demonstrated in the postcentral gyrus, a region that mediates advanced mentalizing about emotion and its relationship to empathy, which lead to a greater ability to empathize (Hooker et al. 2008). Collectively, these regions are sensitive to the development of social cognition and perhaps serve as a locus for professionalism. This could, in other words, indicate that experts are activating examples of actual patients with answering MCQ vignettes and thus professionalism issues are being considered and/or incorporated into their answers.
Not only do our results support the notion that expertise is mediated by neural efficiency in terms of deactivation, but we demonstrated that such refinement also requires selective heightened activation relative to novices. Although we had predicted that the precuneus and caudate would demonstrate greater activation in experts compared novices, our results revealed instead the rostrolateral prefrontal cortex (RLPFC) and the cuneus as regions significantly greater in the experts. The RLPFC is a region involved in cognitive processing of abstract, stimulus-independent information, in addition to planning and prospective memory (Gilbert et al. 2006; Wagner et al. 2006; Rubens and Zanto 2011). The right lateralization is related to processing demands (Bunge et al. 2009) and acts in concert with the hippocampus during relational encoding (Wendelken and Bunge 2010). The cuneus has been associated with reasoning (Ruff et al. 2003), specifically deductive reasoning (Barbey and Barsalou 2010) which may utilize visuospatial information.
Limitations of this investigation include our relatively small sample and the lack of a period of formal rest or inactivity; however, as we sought to capture the construct of reasoning, and in particular the construct of nonanalytic reasoning, we believe that comparing answering and reflecting phases would result in more meaningful, task-specific findings.
Conclusions
Implications of our work include the idea that there may be a functional neuroimaging pattern or “locus” of clinical reasoning expertise during educational evaluation. We believe that the activation of multiple areas of the brain is likely due to the complexity of the task (clinical reasoning). Thus, we may also have identified a multiregion expertise network for clinical reasoning as both novices and experts activated the same areas during educational evaluation, with rare exception. Due to the complexity of clinical reasoning, it may be that such a network is needed for seemingly effortless (or at least more efficient) processing of complex data from patients to arrive at a diagnosis. In addition experts had less activation in several areas of the frontal lobe when answering MCQs supporting the notion of neural efficiency.
The differences and similarities between experts and novices suggest that there is a core network of regions that play a role in moving from novice to expert in clinical reasoning. Indeed our results support a recent review in which expertise was characterized within a two-stage framework with decreased activity and cerebral functional reorganization relating to chunks and knowledge structure (Guida et al. 2012). This is encouraging as it suggests that, if reproducible, future work may be able to plot the trajectory of expert performance and provide more specific feedback to individuals based on the pattern of functional neuroactivation. Such development of single subject analysis was recently discussed in the context of clinical diagnosis (Bullmore 2012), and although such approaches are not yet available, it has potential to contribute to the mitigation of diagnostic errors. In summary, our study utilized established educational theory, two separate participant groups (experts and novices), as well as task items (MCQs) that have been well-validated for assessing clinical reasoning, to provide evidence that expertise involves a distributed and refined brain network during nonanalytical reasoning.
Acknowledgments
Grant funding from American Board of Internal Medicine Foundation supported this study. The Henry M. Jackson Foundation for the Advancement of Military Medicine supported MEC during her involvement in this research. All individuals listed as authors are qualified for authorship. There are no financial contributions, dual commitments, or other potential conflicts of interest.
Disclaimer
The views expressed herein are those of the authors and do not necessarily reflect the Department of Defense or other federal agencies.
Conflict of Interest
None declared.
References
- Aguirre GK, Detre JA, Alsop DC, D'Esposito M. The parahippocampus subserves topographical learning in man. Cereb. Cortex. 1996;6:823–829. doi: 10.1093/cercor/6.6.823. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat. Rev. Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Computational and neurobiological mechanisms underlying cognitive flexibility. Proc. Natl. Acad. Sci. USA. 2006;103:7186–7191. doi: 10.1073/pnas.0509550103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsters JH, Whelan CD, Robertson IH, Ramnani N. Cerebellum and cognition: evidence for the encoding of higher order rules. Cereb. Cortex. 2012;23:1433–1443. doi: 10.1093/cercor/bhs127. [DOI] [PubMed] [Google Scholar]
- Barbey AK, Barsalou LW. Reasoning and problem solving: Models. Encyclopedia of neuroscience Oxford: Academic Press; 2010. [Google Scholar]
- Beer JS, Lombardo MV, Bhanji JP. Roles of medial prefrontal cortex and orbitofrontal cortex in self-evaluation. J. Cogn. Neurosci. 2010;22:2108–2119. doi: 10.1162/jocn.2009.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boreham NC. The dangerous practice of thinking. Med. Educ. 1994;28:172–179. doi: 10.1111/j.1365-2923.1994.tb02695.x. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Gray JR, Molfese DL, Snyder A. Anterior cingulate cortex and response conflict: effects of frequency, inhibition and errors. Cereb. Cortex. 2001;11:825–836. doi: 10.1093/cercor/11.9.825. [DOI] [PubMed] [Google Scholar]
- Braver TS, Paxton JL, Locke HS, Barch DM. Flexible neural mechanisms of cognitive control within human prefrontal cortex. Proc. Natl. Acad. Sci. USA. 2009;106:7351–7356. doi: 10.1073/pnas.0808187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LL, Schneider JS, Lidsky TI. Sensory and cognitive functions of the basal ganglia. Curr. Opin. Neurobiol. 1997;7:157–163. doi: 10.1016/s0959-4388(97)80003-7. [DOI] [PubMed] [Google Scholar]
- Bullmore E. The future of functional MRI in clinical medicine. NeuroImage. 2012;62:1267–1271. doi: 10.1016/j.neuroimage.2012.01.026. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Helskog EH, Wendelken C. Left, but not right, rostrolateral prefrontal cortex meets a stringent test of the relational integration hypothesis. NeuroImage. 2009;46:338–342. doi: 10.1016/j.neuroimage.2009.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Chen G, Saad ZS, Nath AR, Beauchamp MS, Cox RW. FMRI group analysis combining effect estimates and their variances. NeuroImage. 2012;60:747–765. doi: 10.1016/j.neuroimage.2011.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Crescentini C, Seyed-Allaei S, de Pisapia N, Jovicich J, Amati D, Shallice T. Mechanisms of rule acquisition and rule following in inductive reasoning. J. Neurosci. 2011;31:7763–7774. doi: 10.1523/JNEUROSCI.4579-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durning SJ, Graner J, Artino AR, Pangaro LN, Beckman T, Holmboe E, Jr., et al. Using functional neuroimaging combined with a think-aloud protocol to explore clinical reasoning expertise in internal medicine. Mil. Med. 2012;177:72–78. doi: 10.7205/milmed-d-12-00242. [DOI] [PubMed] [Google Scholar]
- Elstein AS, Shulman LS, Sprafka SA. Medical problem solving: a ten-year retrospective. Eval. Health Prof. 1990;13:5–36. [Google Scholar]
- Ericsson KA. The Cambridge handbook of expertise and expert performance. Cambridge; New York: Cambridge University Press; 2006. [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn. Sci. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fangmeier T, Knauff M, Ruff CC, Sloutsky V. FMRI evidence for a three-stage model of deductive reasoning. J. Cogn. Neurosci. 2006;18:320–334. doi: 10.1162/089892906775990651. [DOI] [PubMed] [Google Scholar]
- Figner B, Knoch D, Johnson EJ, Krosch AR, Lisanby SH, Fehr E, et al. Lateral prefrontal cortex and self-control in intertemporal choice. Nat. Neurosci. 2010;13:538–539. doi: 10.1038/nn.2516. [DOI] [PubMed] [Google Scholar]
- Fletcher L, Carruthers P. Metacognition and reasoning. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012;367:1366–1378. doi: 10.1098/rstb.2011.0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert SJ, Spengler S, Simons JS, Steele JD, Lawrie SM, Frith CD, et al. Functional specialization within rostral prefrontal cortex (area 10): a meta-analysis. J. Cogn. Neurosci. 2006;18:932–948. doi: 10.1162/jocn.2006.18.6.932. [DOI] [PubMed] [Google Scholar]
- Glascher J, Adolphs R, Damasio H, Bechara A, Rudrauf D, Calamia M, et al. Lesion mapping of cognitive control and value-based decision making in the prefrontal cortex. Proc. Natl. Acad. Sci. USA. 2012;109:14681–14686. doi: 10.1073/pnas.1206608109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot AD. Thought and choice in chess. The Hague, the Netherlands: Mouton; 1965. [Google Scholar]
- Guida A, Gobet F, Tardieu H, Nicolas S. How chunks, long-term working memory and templates offer a cognitive explanation for neuroimaging data on expertise acquisition: a two-stage framework. Brain Cogn. 2012;79:221–244. doi: 10.1016/j.bandc.2012.01.010. [DOI] [PubMed] [Google Scholar]
- Higgs J, Jones MA, Loftus S, Christensen N. Clinical reasoning in the health professions. Butterworth-Heinemann, UK: Elsevier; 2008. ISBN 978-0-7506-888-57. [Google Scholar]
- Hooker CI, Verosky SC, Germine LT, Knight RT, D'Esposito M. Mentalizing about emotion and its relationship to empathy. Soc. Cogn. Affect Neurosci. 2008;3:204–217. doi: 10.1093/scan/nsn019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimecki OM, Leiberg S, Lamm C, Singer T. Functional neural plasticity and associated changes in positive affect after compassion training. Cereb. Cortex. 2012;23:1552–15561. doi: 10.1093/cercor/bhs142. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat. Rev. Neurosci. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Levy BJ, Wagner AD. Cognitive control and right ventrolateral prefrontal cortex: reflexive reorienting, motor inhibition, and action updating. Ann. N. Y. Acad. Sci. 2011;1224:40–62. doi: 10.1111/j.1749-6632.2011.05958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medford N, Critchley HD. Conjoint activity of anterior insular and anterior cingulate cortex: awareness and response. Brain Struct. Funct. 2010;214:535–549. doi: 10.1007/s00429-010-0265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubauer AC, Fink A. Intelligence and neural efficiency. Neurosci. Biobehav. Rev. 2009;33:1004–1023. doi: 10.1016/j.neubiorev.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Norman GR, Eva KW. Diagnostic error and clinical reasoning. Med. Educ. 2010;44:94–100. doi: 10.1111/j.1365-2923.2009.03507.x. [DOI] [PubMed] [Google Scholar]
- Northoff G, Bermpohl F. Cortical midline structures and the self. Trends Cogn. Sci. 2004;8:102–107. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Price CJ, Friston KJ. Cognitive conjunction: a new approach to brain activation experiments. NeuroImage. 1997;5:261–270. doi: 10.1006/nimg.1997.0269. [DOI] [PubMed] [Google Scholar]
- Rubens MT, Zanto TP. Characterizing the involvement of rostrolateral prefrontal cortex in prospective memory. J. Neurosci. 2011;31:9067–9069. doi: 10.1523/JNEUROSCI.1891-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff CC, Knauff M, Fangmeier T, Spreer J. Reasoning and working memory: common and distinct neuronal processes. Neuropsychologia. 2003;41:1241–1253. doi: 10.1016/s0028-3932(03)00016-2. [DOI] [PubMed] [Google Scholar]
- Russo JE, Johnson EJ, Stephens DL. The validity of verbal protocols. Mem. Cognit. 1989;17:759–769. doi: 10.3758/bf03202637. [DOI] [PubMed] [Google Scholar]
- Schmidt HG, Boshuizen HP. On acquiring expertise in medicine. Special Issue: European educational psychology. Educat. Psychol. Rev. 1993;5:205–221. [Google Scholar]
- Schuwirth L. Is assessment of clinical reasoning still the Holy Grail? Med. Educ. 2009;43:298–300. doi: 10.1111/j.1365-2923.2009.03290.x. [DOI] [PubMed] [Google Scholar]
- Schuwirth LWT, Verheggen MM, van der Vleuten CPM, Boshuizen HPA, Dinant GJ. Do short cases elicit different thinking processes than factual knowledge questions do? Med. Educ. 2001;5:348–356. doi: 10.1046/j.1365-2923.2001.00771.x. [DOI] [PubMed] [Google Scholar]
- Simon HA. Invariants of human behavior. Annu. Rev. Psychol. 1990;41:1–19. doi: 10.1146/annurev.ps.41.020190.000245. [DOI] [PubMed] [Google Scholar]
- Singer T, Critchley HD, Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends Cogn. Sci. 2009;13:334–340. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Stolper E, van de Wiel M, van Royen P, van Bokhoven M, van der Weijden T, Dinant GJ. Gut feelings as a third track in general practitioners’ diagnostic reasoning. J. Gen. Intern. Med. 2011;26:197–203. doi: 10.1007/s11606-010-1524-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm W, de Simone A, Krause BJ, Specht K, Hesselmann V, Radermacher I, et al. Functional anatomy of intrinsic alertness: evidence for a fronto-pariental-thalamic-brainstem network in the right hemisphere. Neuropsychologia. 1999;37:797–805. doi: 10.1016/s0028-3932(98)00141-9. [DOI] [PubMed] [Google Scholar]
- Sylvester CY, Wager TD, Lacey SC, Hernandez L, Nichols TE, Smith EE, et al. Switching attention and resolving interference: fMRI measures of executive functions. Neuropsychologia. 2003;41:357–370. doi: 10.1016/s0028-3932(02)00167-7. [DOI] [PubMed] [Google Scholar]
- Tian F, Tu S, Qiu J, Lv JY, Wei DT, Su YH, et al. Neural correlates of mental preparation for successful insight problem solving. Behav. Brain Res. 2011;216:626–630. doi: 10.1016/j.bbr.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Wagner G, Koch K, Reichenbach JR, Sauer H, Schlosser RG. The special involvement of the rostrolateral prefrontal cortex in planning abilities: an event-related fMRI study with the Tower of London paradigm. Neuropsychologia. 2006;44:2337–2347. doi: 10.1016/j.neuropsychologia.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Wan X, Nakatani H, Ueno K, Asamizuya T, Cheng K, Tanaka K. The neural basis of intuitive best next-move generation in board game experts. Science. 2011;331:341–346. doi: 10.1126/science.1194732. [DOI] [PubMed] [Google Scholar]
- Wendelken C, Bunge SA. Transitive inference: distinct contributions of rostrolateral prefrontal cortex and the hippocampus. J. Cogn. Neurosci. 2010;22:837–847. doi: 10.1162/jocn.2009.21226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windmann S, Kirsch P, Mier D, Stark R, Walter B, Gunturkun O, et al. On framing effects in decision making: linking lateral versus medial orbitofrontal cortex activation to choice outcome processing. J. Cogn. Neurosci. 2006;18:1198–1211. doi: 10.1162/jocn.2006.18.7.1198. [DOI] [PubMed] [Google Scholar]
- Wolf RC, Vasic N, Walter H. Differential activation of ventrolateral prefrontal cortex during working memory retrieval. Neuropsychologia. 2006;44:2558–2563. doi: 10.1016/j.neuropsychologia.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Yaoi K, Osaka N, Osaka M. Is the self special in the dorsomedial prefrontal cortex? An fMRI study. Soc. Neurosci. 2009;4:455–463. doi: 10.1080/17470910903027808. [DOI] [PubMed] [Google Scholar]


