Highlights
-
•
First frog (11 families, 6 genera, 29 spp.) haemoparasite survey in South Africa.
-
•
Showing a higher biodiversity as compared with similar research in Africa.
-
•
Intraerythrocytic and extracellular haemoparasites of five groups found.
-
•
Hepatozoon and Trypanosoma spp. were the most prevalent.
-
•
Protected areas boast higher parasite diversity compared with human impacted sites.
Keywords: Amphibian, Apicomplexan, Blood parasite survey, Frog haematozoans, Haemoflagellate, Microfilarid
Graphical Abstract
Abstract
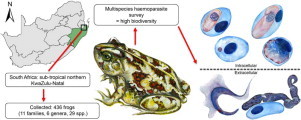
Since South Africa boasts a high biodiversity of frog species, a multispecies haemoparasite survey was conducted by screening the blood from 29 species and 436 individual frogs. Frogs were collected at three localities in sub-tropical KwaZulu-Natal, a hotspot for frog diversity. Twenty per cent of the frogs were infected with at least one of five groups of parasites recorded. Intraerythrocytic parasites comprising Hepatozoon, Dactylosoma, and viral or bacterial organisms, as well as extracellular parasites including trypanosomes and microfilarid nematodes were found. A significant difference (P < 0.01) in the prevalence of parasitaemia was found across species, those semi-aquatic species demonstrating the highest, followed by semi-terrestrial frog species. None of those species described as purely terrestrial and aquatic were infected. Hepatozoon and Trypanosoma species accounted for most of the infections, the former demonstrating significant differences in intensity of infection across species, families and habitat types (P = 0.028; P = 0.006; P = 0.007 respectively). Per locality, the first, the formally protected Ndumo Game Reserve, had the highest biodiversity of haemoparasite infections, with all five groups of parasites recorded. The other two sites, that is the area bordering the reserve and the Kwa Nyamazane Conservancy, had a lower diversity with no parasite infections recorded and only Hepatozoon species recorded respectively. Such findings could be ascribed to the anthropogenic impact on the latter two sites, the first by the rural village activities, and the second by the bordering commercial sugar cane agriculture. Future studies should include both morphological and molecular descriptions of the above parasites, as well as the identification of potential vectors, possibly clarifying the effects human activities may have on frog haemoparasite life cycles and as such their biodiversity.
1. Introduction
Amphibians are the most threatened vertebrate group, suffering large-scale declines in species diversity since at least, according to historical data, the 1970s (Stuart et al., 2004). A decade ago the IUCN's Global Amphibian Assessment indicated that a third of the estimated amphibian species had declined or become extinct (Stuart et al., 2004; Beebee and Griffiths, 2005). Such declines may be attributed to a number of factors ranging from habitat destruction, pollution and exploitation, to climate change and disease (Beebee and Griffiths, 2005). The disease known as chytridiomycosis (amphibian chytrid), caused by the fungal pathogen Batrachochytrium dendrobatidis, has been responsible for major global amphibian declines (Readel and Goldberg, 2010). Along with chytrid, amphibians are host to a wide variety of parasites (du Preez and Carruthers, 2009; Netherlands et al., 2014a), including intraerythrocytic and extracellular haemoparasites ranging from protozoans, comprising both intracellular apicomplexans (Davies and Johnston, 2000) and extracellular flagellates (Acosta et al., 2013), to extracellular nematode microfilariae (Baker, 2008) as well as those intracellular parasites of uncertain identity such as the viral and bacterial infections (Davies and Johnston, 2000; Davis et al., 2009). The most attention, however, has been given to those parasites of the first three groups mentioned, most likely due to the frequent findings and thus greater basis of knowledge of these organisms in anuran hosts. Furthermore, of these three groups, those of the Protozoa, particularly the apicomplexans, would appear to be the most studied of all (see Davies and Johnston, 2000; Netherlands et al., 2014a; Netherlands et al., 2014b).
However, since few parasite surveys on frogs have been carried out in sub-Saharan Africa, the degree of this haemoparasite diversity remains unknown (Readel and Goldberg, 2010; Netherlands et al., 2014a, 2014b). Yet, such diversity of knowledge regarding these parasites is necessary before further studies can be done on elucidating the effects that these parasites may have on their natural hosts, and the role these parasites may have in amphibian conservation.
Southern Africa currently boasts 159 known species of frogs in 33 genera and 13 families (du Preez and Carruthers, 2009; Channing and Baptista, 2013; Channing et al., 2013a, 2013b; Conradie, 2014). This study presents the results of a haemoparasite survey of frogs from three localities in KwaZulu-Natal, South Africa (see Fig. 1). Localities include the formally protected Ndumo Game Reserve (NGR) and the reserve's anthropogenically impacted surrounds, as well as Kwa Nyamazane Conservancy (KNC). All three sampling areas fall within a sub-tropical region known for its biological richness and as such the province, KZN, boasts the highest diversity of frog species in South Africa (see du Preez and Carruthers, 2009). The following study thus aimed to determine and record, through a multispecies haemoparasite survey on frogs, if this parasite diversity paralleled that of its rich frog diversity.
Fig. 1.
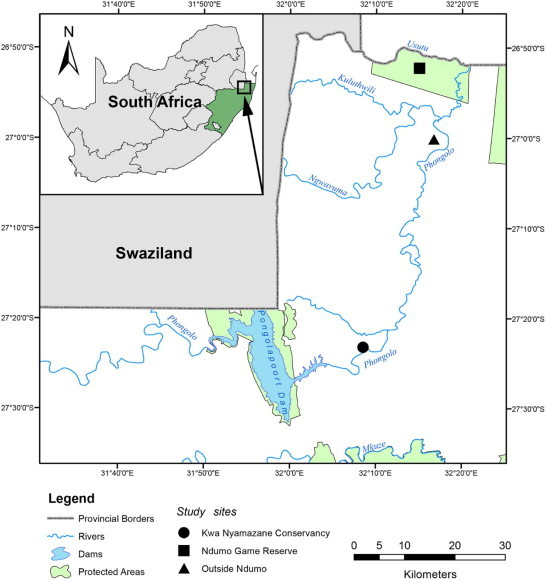
Map displaying the three sampling localities in northern KwaZulu-Natal, South Africa. Map displaying the three sampling localities at which frogs were surveyed for haemoparasite biodiversity, top to bottom: Ndumo Game Reserve (NGR), outside NGR and Kwa Nyamazane Conservancy, in northern KwaZulu-Natal, South Africa. All sampling sites were directly or indirectly linked to the Phongolo River.
2. Materials and methods
2.1. Study area and frog collection
Ndumo Game Reserve (NGR) (26°52′00.0″S 32°15′00.0″E) is situated in the West of the Maputaland bioregion, close to the borders of South Africa, Swaziland and Mozambique. The Maputaland bioregion, located in northern KwaZulu-Natal (KZN) and crossing into southern Mozambique, is one of the most biologically rich areas in southern Africa (Haddad, 2003) and has a sub-tropical climate. Ndumo is a large reserve, holding 10,117 ha of diverse habitats, including floodplains, sub-tropical bush, savannah and woodland, to riparian forest (Wesołowska and Haddad, 2009). The area directly surrounding the NGR (27°00′13.8″S 32°16′49.9″E) is not formally protected and thus is covered in rural tribal villages, causing the vegetation to be heavily impacted by the villagers' livestock and subsistence farming practices. Approximately 80 km to the south lies the Kwa Nyamazane Conservancy (KNC) (27°23′34.9″S 32°08′40.8″E), a small conservation area running along the Phongola River and surrounded by large sects of agricultural land, most of it utilised for sugar cane farming. These localities were specifically chosen as all three are located on, and are thus supplied by a permanent water source, the Phongola River (see Fig. 1).
Frogs were collected via active sampling at night in all three localities as mentioned above. All these sites were visited during the warmer and rainy months of February and November 2012, April and November 2013 and February 2014. During collection possible invertebrate vectors feeding on frogs were searched for; however none were observed. Captured frogs were held in disposable plastic bags and transported back to a field laboratory either at the NGR or KNC, where they were identified to species level using du Preez and Carruthers (2009).
2.2. Preparation and screening of frog blood
A drop of blood was collected from each frog via cardiac or femoral venipuncture using a sterile heparinised insulin syringe. A portion of this blood was used to prepare a thin blood smear, which once air-dried in a dust-proof container was fixed immediately using absolute methanol and stained thereafter using a modified solution of Giemsa stain (FLUKA, Sigma-Aldrich, Steinheim, Germany); the other portion was dropped into a microcentrifuge tube with an equal volume of 70% ethanol for future molecular analysis. All frogs were processed the morning after collection and were released within 24 h of capture.
Smears were screened using a 100 × immersion oil objective on a Nikon Eclipse E800 compound microscope (Nikon, Amsterdam, Netherlands), and images were captured with an attached Nikon digital camera. The average parasitaemia was calculated per 100 erythrocytes, with ~104 erythrocytes examined per blood smear following Cook et al. (2009). The estimated average parasitaemia for extracellular parasites were calculated as number of parasites/per-slide (ps) with an approximate field of 20,000 blood cells examined. This study received the relevant ethical approval (North-West University ethics approval no.: NWU-00005–14-S3), as well as approval to do research from the appropriate conservation authorities (Ezemvelo KZN Wildlife, permits: OP 674/2012, OP 5139/2012, OP 526/2014, and OP 839/2014.).
2.3. Statistical analysis
The Monte Carlo variant of the Fisher's exact test, set to 10,000 replicates with a confidence interval of 99%, was employed to investigate significance in variation of prevalence between species, families, habitat types and sampling periods. The habitat types were established based on those described by du Preez and Carruthers (2009). Frog species were classified as terrestrial (those species thriving and breeding away from a permanent water source for most of their lives), semi-terrestrial (species thriving away from a permanent water source, but needing such a source to breed), semi-aquatic (species requiring a position near a permanent water source for most of their lives in order to survive and breed) and aquatic (species permanently living and breeding in a water source, rarely leaving that source). The Kruskal–Wallis test was used since it is suitable for non-parametric data and does not assume normal data distribution and equal sample size. It was applied to determine significance levels (P < 0.05) of variation between infection intensity across species, families, habitat types, and sampling periods. It was further employed to determine significance of variation of the overall intensity of Hepatozoon and Trypanosoma across frog species, families, habitats and sampling periods. A non-parametric Levenes's test was used to verify the equality of variances in the samples (homogeneity of variance, P > 0.05) (Nordstokke and Zumbo, 2007, 2010). All statistical analyses were performed using IBM SPSS Statistics ver. 22 (SPSS, 2013).
3. Results
Blood smears were collected from 436 frogs of 29 species, 6 genera and 11 families (Table 1). Of these 15/29 (52%) of the frog species were infected with haemoparasites, making up 87/436 (20%) of the total number of frogs (Table 2). Five groups of haemoparasites were recorded including intraerythrocytic haemogregarine and haemogregarine-like species of the genus Hepatozoon and Dactylosoma respectively, and intraerythrocytic organisms of a viral or bacterial nature, the species of which could not be identified; furthermore, extracellular flagellate parasite species of the genus Trypanosoma and microfilarid nematode species were observed.
Table 1.
Frog species divided into associated habitat types and listed alphabetically with families, as well as numbers collected at Ndumo Game Reserve (NGR), the locality bordering NGR (BNGR) and the Kwa Nyamazane Conservancy (KNC).
| Habitat type | Frog species | Family | Locality | No. collected |
|---|---|---|---|---|
| Terrestrial (1) | Breviceps adspersus | Breviciptidae | NGR | 4 |
| Semi-terrestrial (9) | Amietophrynus garmani | Bufonidae | NGR | 23 |
| BNGR | 2 | |||
| KNC | 5 | |||
| Amietophrynus gutturalis | Bufonidae | NGR | 1 | |
| KNC | 3 | |||
| Amietophrynus maculatus | Bufonidae | NGR | 9 | |
| Chiromantis xerampelina | Rhacophoridae | NGR | 43 | |
| BNGR | 1 | |||
| Leptopelis mossambicus | Arthroleptidae | NGR | 2 | |
| Schismaderma carens | Bufonidae | NGR | 7 | |
| Tomopterna cryptotis | Pyxicephalidae | NGR | 6 | |
| Tomopterna krugerensis | Pyxicephalidae | NGR | 1 | |
| Tomopterna natalensis | Pyxicephalidae | NGR | 1 | |
| Semi-aquatic (17) | Afrixalus aureus | Hyperoliidae | NGR | 14 |
| Afrixalus delicatus | Hyperoliidae | NGR | 7 | |
| Afrixalus fornasinii | Hyperoliidae | NGR | 2 | |
| Cacosternum boettgeri | Pyxicephalidae | BNGR | 11 | |
| KNC | 1 | |||
| Hemisus marmoratus | Hemisotidae | NGR | 22 | |
| Hildebrandtia ornata | Ptychadenidae | NGR | 6 | |
| Hyperolius argus | Hyperoliidae | BNGR | 24 | |
| Hyperolius marmoratus | Hyperoliidae | NGR BNGR |
20 10 |
|
| KNC | 6 | |||
| Hyperolius pusillus | Hyperoliidae | NGR | 10 | |
| BNGR | 1 | |||
| KNC | 3 | |||
| Hyperolius tuberilinguis | Hyperoliidae | NGR | 12 | |
| Kassina maculata | Hyperoliidae | NGR | 8 | |
| Kassina senegalensis | Hyperoliidae | KNC | 3 | |
| Phrynobatrachus mababiensis | Phrynobatrachidae | NGR | 13 | |
| Phrynomantis bifasciatus | Microhylidae | NGR | 1 | |
| Ptychadena anchietae | Ptychadenidae | NGR | 77 | |
| KNC | 1 | |||
| Ptychadena mascareniensis | Ptychadenidae | NGR | 5 | |
| BNGR | 2 | |||
| Ptychadena mossambica | Ptychadenidae | NGR | 19 | |
| Aquatic (2) | Xenopus laevis | Pipidae | NGR | 1 |
| Xenopus muelleri | Pipidae | NGR | 46 | |
| BNGR | 3 | |||
| Total | 29 | 11 | 3 | 436 |
Table 2.
Frog species listed alphabetically and categorised according to their habitat type. Shown are the number of frogs examined and infected, prevalence of the five haemoparasite groups (P) and the intensity of the infections (I), along with the reference to the figures of these parasites (Fig.) in parentheses.
| Habitat type | Frog species | Examined | Infected | Hepatozoon spp., P; I (Fig.) | Dactylosoma spp., P; I (Fig.) | Viral or bacterial organisms, P; I (Fig.) | Trypanosoma spp., P; p/s (Fig.) | Microfilariae, P; p/s(Fig.) |
|---|---|---|---|---|---|---|---|---|
| Semi-terrestrial | Amietophrynus garmani | 30 | 7 | 5/7; 8.4% (Fig. 2A–B) | 2/7; 21 p/s (Fig. 2L–M) | |||
| Amietophrynus gutturalis | 4 | 3 | 3/8; 2.4% (Fig. 2A–B) | |||||
| Amietophrynus maculatus | 9 | 7 | 7/7; 19.5% (Fig. 2A–B) | 3/7; 3 p/s (Fig. 2O–P) | ||||
| Chiromantis xerampelina | 44 | 1 | 1/1;26 p/s (Fig. 2Q) | |||||
| Semi-aquatic | Hemisus marmoratus | 22 | 2 | 2/2; 0.8% (Fig. 2B) | ||||
| Hildebrandtia ornata | 6 | 1 | 1/1; 0.3% (Fig. 2E) | |||||
| Hyperolius marmoratus | 36 | 4 | 3/4; 20.5% (Fig. 2D) | 1/4; 1 p/s (Fig. 2N) | ||||
| Hyperolius tuberilinguis | 12 | 1 | 1/1; 20 p/s (Fig. 2R) | |||||
| Kassina maculata | 8 | 1 | 1/1; 4 p/s (Fig. 2M–S) | |||||
| Phrynobatrachus mababiensis | 13 | 1 | 1/1; 0.3% (Fig. 2B) | 1/1; 8 p/s (Fig. 2O) | ||||
| Phrynomantis bifasciatus | 1 | 1 | 1/1; 6 p/s (Fig. 2O–P) | |||||
| Ptychadena anchietae | 78 | 47 | 31/47; 2.4% (Fig. 2C) | 13/47; 1% (Fig. 2F–G) | 4/47; 75% (Fig. 2I) | 29/47; 13.3 p/s (Fig. 2L–P,R,T) | 1/47; 1 p/s | |
| Ptychadena mascareniensis | 5 | 2 | 1/2; 0.2% (Fig. 2B) | 2/2; 8.8 p/s (Fig. 2O) | ||||
| Ptychadena mossambica | 19 | 7 | 5/7; 3% (Fig. 2B) | 2/7; 99% (Fig. 2J) | 4/7; 6 p/s (Fig. 2O,N,R) | |||
| Schismaderma carens | 7 | 2 | 1/2; 0.8% (Fig. 2A–B) | 1/2; 28 p/s (Fig. 2K) | ||||
| Total | 15 | 294 | 87 | 59; 5.3% | 13; 1% | 6; 87% | 46; 10.6 p/s | 2; 14.5 p/s |
Prevalence = P; intensity = I; per slide = p/s (Figure reference = Fig.).
Hepatozoon species accounted for most of the infections at 59/436 (14%), followed by Trypanosoma species at 46/436 (11%); viral or bacterial infections, microfilarid infections and Dactylosoma species, accounted for 6/436 (1%), 2/436 (0.5%) and 13/436 (3%) of the overall prevalence respectively (Table 2). As for the intensity of the groups, Hepatozoon showed an overall (all infected frogs pooled) intensity of 5%, the Dactylosoma an overall intensity of 1%, the viral or bacterial infections an overall intensity of 87%, the Trypanosoma an overall intensity of 11 per blood slide, and the microfilarid nematode infections an overall intensity of 15 per blood slide (Table 2). The overall prevalence of haemoparasites (all parasite groups pooled) varied significantly by frog species (χ2 = 163.475, P < 0.01). Ptychadena anchietae demonstrated the highest prevalence at 47/78 (60%) and Chiromantis xerampelina the lowest at 1/44 (2%) (Table 2). Upon division of the frog species into groups including aquatic (two species), semi-aquatic (17 species), terrestrial (one species) and semi-terrestrial (nine species) (see Table 1), it was observed that only the semi-aquatic and semi-terrestrial groups contained infected species (Table 2). These two groups varied significantly in prevalence of infection (χ2 = 87.000, P < 0.01), with 79% of the infected individuals from the semi-aquatic group and only 21% from the semi-terrestrial group. Of the semi-aquatic group, the genus Ptychadena had the highest diversity of haemoparasites, infected with all types as recorded in Table 2. Furthermore, P. anchietae, of all the infected frog species, revealed the highest prevalence of parasites, making up 47/87 (54%) of the total with 47/78 (60%) of the P. anchietae themselves found to be infected.
Hepatozoon species accounted for most of the infections followed by Trypanosoma species, significance of intensity calculated via the use of the Kruskal–Wallis test. Hepatozoon intensity across frog species (χ2 = 17.683, P = 0.028), across families (χ2 = 11.717, P = 0.006), and across the different habitat types (χ2 = 7.227, P = 0.007) showed a significant difference. Hyperolius marmoratus, in the semi-aquatic group, and Amietophrynus maculatus, in the semi-terrestrial group, accounted for the highest intensities (Table 2). Hepatozoon intensity across the different sampling periods, however, showed no significant variance (χ2 = 4.177, P = 0.552). Trypanosoma intensity across frog species (χ2 = 11.919, P = 0.028) showed a significant difference; however, across families (χ2 = 3.802, P = 0.664), habitat types (χ2 = 0.330, P = 0.585) and sampling periods (χ2 = 6.675, P = 0.147), no significant difference was observed. In this case Hyperolius tuberilinguis, in the semi-aquatic group, and Chiromantis xerampelina, in the semi-terrestrial group, accounted for the highest intensities (Table 2).
Per locality, it was observed that the NGR, with 26 species examined, showed a prevalence of 77/360 (21%) as compared with outside the NGR, with eight species examined and a prevalence of 0/54 (0%), and the KNC, with seven species examined and a prevalence of 10/22 (45%). Furthermore, the NGR had a higher diversity of haemoparasites, including 50/360 (14%) infected with Hepatozoon species, 11/360 (3%) with Dactylosoma species, 5/360 (1%) with viral or bacterial organisms, 46/360 (13%) with Trypanosoma species and 2/360 (0.6%) with microfilaria as compared with the KNC frogs that were only infected with Hepatozoon.
4. Discussion
On the whole, 20% (87/436) of the frogs in this study were infected with at least one haemoparasite group, some infected up to five. This was similar to previous comparable studies such as that of Readel and Goldberg (2010) in western Uganda documenting a 17% (30/180) prevalence, even though this was found to be approximately half that of other studies in Africa (see Mohammed and Mansour, 1959; Ball, 1967; Readel and Goldberg, 2010). In this study, Hepatozoon species accounted for most of the infections at 14%, which was equally true for the survey done in Uganda by Readel and Goldberg (2010). On the contrary, Ball (1967), during a survey completed in Tanzania and Kenya, found a considerably higher prevalence of 29%. Readel and Goldberg (2010) suggested this may be attributable to availability of insect vectors. Trypanosome species were the second most common parasite infecting frogs in this study at 11%, just slightly higher than the 6% reported by Readel and Goldberg (2010). Both the results of the present and the Readel and Goldberg (2010) studies' conducted in Africa are in contrast to what has been recorded in other similar studies but on different continents (see Barta and Desser, 1984; Barta et al., 1989), in which the Trypanosoma demonstrate a higher prevalence to that of Hepatozoon or any other haemoparasite groups (see Werner, 1993). Microfilarid infections from the South African frogs studied here, were also seen to occur in low numbers, similar to Readel and Goldberg (2010). Infections not reported by Readel and Goldberg (2010) and Ball (1967), but reported in this study, were a Dactylosoma species and viral or bacterial infections. The only other study in Africa to report on parasite intensities was that by Readel and Goldberg (2010), in which Hepatozoon species contained an average intensity of 2.3%, Trypanosoma species had an average intensity of 7.2 parasites, and Microfilariae had an average intensity of 11.2 parasites. In comparison the total parasite intensity for the current study was 5.3% for Hepatozoon species, 10.6 for Trypanosoma and 14.5 for Microfilariae.
Similar to Readel and Goldberg (2010) significant differences (P < 0.01) in the prevalence of parasites among frog species were recorded during the current study, with P. anchietae showing the highest prevalence and C. xerampelina the lowest. Both frog species prefer habitats close to water (classified in this study as being semi-terrestrial) (du Preez and Carruthers, 2009). Ptychadena anchietae is a grass frog and is often found around the water's edge whilst C. xerampelina is an arboreal frog species. Since the abundance of possible vectors associated with water, such as mosquitoes and leeches, would be high in such habitats, it may explain the high prevalence of haemoparasites recorded from P. anchietae. The reason for such a low prevalence in C. xerampelina, particularly for Hepatozoon species, which may be mosquito transmitted in such an environment (Desser et al., 1995; Davies and Johnston, 2000), cannot be explained. This result is particularly peculiar since both frog species were infected with trypanosomes (only a single individual of C. xerampelina), which are mosquito and leech transmitted (Barta and Desser, 1984). One of the only possibilities could be, since Hepatozoon is transmitted via the ingestion of the infected invertebrate or vertebrate (Davies and Johnston, 2000), that C. xerampelina prefers a diet not inclusive of mosquitoes and other frogs. Future diet studies may help to clarify this finding.
Division of the frog species into groups showed that only the semi-aquatic and semi-terrestrial groups contained infected species, these two groups varying significantly in prevalence of infection (P < 0.01). The semi-aquatic group had the highest prevalence, likely attributable to the Ptychadena species, one of them P. anchietae. The Ptychadena species also showed the highest diversity of haemoparasites, infected with all five recorded groups. Furthermore, of all the frog species, P. anchietae was the only species to be parasitised with a species of Dactylosoma. These parasites are closely associated with water and thus are suggested to be transmitted by a leech vector (Barta, 1991). Reports of Dactylosoma parasitising frogs in Africa are numerous, accounts of these organisms from at least five countries and approximately eight species of frog (see Barta, 1991). One such report was from South Africa from the bufonid A. regularis (most likely Amietophrynus gutturalis) by Fantham et al. (1942). In all these reports the Dactylosoma species are referred to as a single species Dactylosoma ranarum (see Barta, 1991); however, only future molecular work will be able to clarify if the species here is one of the same. Furthermore, the Ptychadenidae were the only frogs found infected with viral or bacterial organisms. Viral or bacterial infections have been recorded from a cosmopolitan distribution of amphibians (see Desser, 1987). Unfortunately, very little is known about the identity, classification and effect of these organisms (see Desser, 1987; Davies and Johnston, 2000; Davis et al., 2009). Alves de Matos and Paperna (1993) presented the most recent study of uncertain erythrocyte virus infections from P. anchietae in South Africa. These virus or bacterial infections were found to be similar to several different viruses of the Frog Erythrocytic Virus (FEV) group such as Toddia, Pirhemocyton and other Rickettsiales. In contrast to the above findings in both the semi-aquatic and semi-terrestrial groups, the frog species from the terrestrial as well as aquatic groups were not observably parasitaemic. Since B. adspersus (terrestrial) spends most of its life underground (du Preez and Carruthers, 2009), contact with vectors would be rare. However, since the semi-aquatic group had the highest prevalence of parasites, most likely due to the frequent contact with vectors, it was expected that those of the aquatic group would be equally parasitised. Yet, as in Readel and Goldberg (2010), the species of Xenopus (aquatic) here were found to be uninfected. Such a finding is surprising as it would be expected that Xenopus should contain a rather high prevalence of parasites, particularly as it is well known that leeches feed on these frogs (see Badets and Du Preez, 2014; Kruger and Du Preez, 2015). Possible explanations for this could be that haemoparasites infecting Xenopus are extremely host specific or were simply not present in the area sampled.
Intensity of Hepatozoon species across frog species and families, as well as across habitat types were found to be significant (P = 0.007). Hyperolius marmoratus (Hyperolidae) of the semi-aquatic group, and A. maculatus (Bufonidae) from the semi-terrestrial group, had the highest intensity. Hyperolius marmoratus may be found permanently on the edges of water, where vector abundance and contact rates are likely to be high, thus accounting for this high intensity. Amietophrynus maculatus appears to favour more static, shallow water bodies, which are also favoured by mosquito species, the high contact rates with possibly Hepatozoon infected mosquitoes would thus be high. Hepatozoon species have been reported and described from a few Hyperolius species in Africa, though the majority have been reported from bufonid species such as A. maculatus (see Netherlands et al., 2014b). Trypanosoma species intensities varied significantly only across species (P = 0.028), being highest in the semi-aquatic H. tuberilinguis and the semi-terrestrial C. xerampelina. These two species are permanently associated with water, and thus always in close association with an abundance of possible vectors. A plethora of trypanosome species have been described and reported from numerous African frog species, unfortunately many reports contain inadequate taxonomic descriptions and in numerous cases they are simply referred to as a Trypanosoma sp. without any morphological data provided on the specific parasite (see Bardsley and Harmsen, 1973; Telford, 2009). Furthermore, since trypanosomes are known to be pleomorphic, the true diversity seen in this study cannot be realised, and thus future molecular work along with morphological description is intended in order to differentiate between species and life stages of these organisms. Intensity across sampling periods for both Hepatozoon and Trypanosoma species was insignificant (P = 0.552 and P = 0.147 respectively), likely due to sampling occurring only during the wet seasons.
Parasites have always been seen in a negative light, especially with regards to human and livestock health. However, within the natural environment parasites may be seen as a crucial part of a functional and healthy ecosystem. Parasites make a profound impact on the biodiversity of an ecosystem by influencing aspects such as host competition, migration, speciation and stability (Combes, 1996). Furthermore, parasites reflect their host species' environmental interactions, revealing feeding behaviour, geographical ranges and social systems (Dobson et al., 2008). In a stable and healthy natural ecosystem parasites and their hosts have had the opportunity to co-evolve, the parasite causing few pathogenic effects in a healthy host animal. If however, this well established co-existence is disturbed by, for example, habitat destruction or the indiscriminate movement of host animals between habitats; pathogenic effects may become apparent resulting in the destabilisation of the host population (see Combes, 1996). Additionally, in light of the above, the loss of parasite diversity would very likely have unforeseen, but grave consequences, especially with respect to regulation of host populations and their abundance within the communities (Dobson et al., 2008). As aptly put by Dobson et al. (2008), if the main aim of conservation biologists is to conserve fully functional food webs, it is imperative that parasites are thus also included within biodiversity conservation. Ndumo Game Reserve was found to harbour the highest diversity of both frog species and haemoparasites as compared with the other two sites (see Table 1), which are impacted by rural village settlements and peripheral commercial sugar cane agriculture respectively. Anthropogenic impacts, as found in Readel and Goldberg (2010), may account for the lack of diversity in these two sites, affecting vector distributions and contact rates between frog hosts and vectors.
This study represents the first multispecies haemoparasite survey done on frogs in South Africa. It is anticipated that through future work, including both morphological and molecular descriptions of the parasites reported in this study, that the biodiversity of this region will be elucidated. Furthermore, it is hoped that with this biodiversity knowledge and the identification of potential vectors, the effects human activities may have on frog haemoparasite life cycles and as such their biodiversity will be clarified.
Conflict of interest
The authors declared that there is no conflict of interest.
Fig. 2.
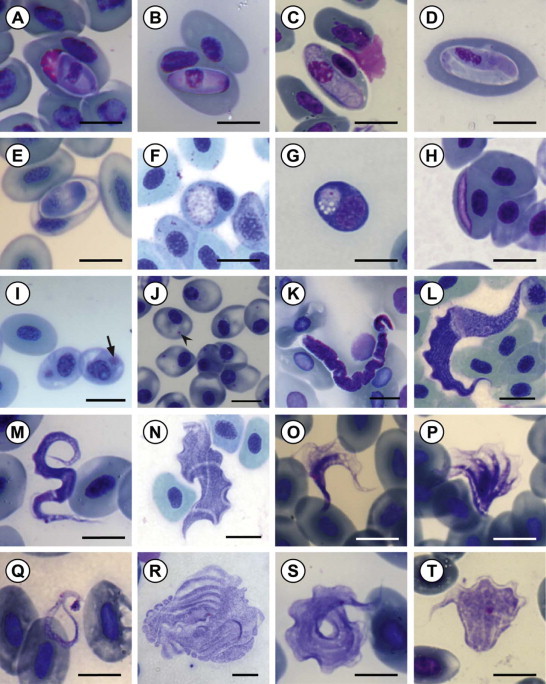
Micrographs of various frog haemoparasites encountered in the current study. Haemoparasites from the peripheral blood of 15 frog species collected from three localities in northern KwaZulu-Natal, stained with Giemsa stain (A–E) gamonts of Hepatozoon species; (F–G) primary and secondary stage gamonts of Dactylosoma species; (H–J) viral or bacterial inclusions; (K) microfilarid nematode species; (L–T) Trypanosoma species. Scale bar: 10 µm.
Acknowledgements
We are thankful to Ndumo Game Reserve and Kwa Nyamazane Conservancy for allowing us to collect samples, as well as to both Dr Leon Meyer and Mr Nico Wolmerans for their much appreciated help during collection. Prof. John R. Barta from the University of Guelph, Canada, for aiding in the identification of some of the haemogregarines as well as providing us with numerous sources of relevant literature. In addition, we are grateful for the financial assistance of the National Research Foundation (NRF) of South Africa to CAC (NRF Scarce Skills Postdoctoral Scholarship-Grant SFP13090332476) and to ECN (NRF Scarce Skills Masters Scholarship-Grant UID: 89924). Opinions expressed and conclusions arrived at, are those of the authors and are not necessarily to be attributed to the NRF. The fieldwork and running expenses of this research was funded by the Water Research Commission (WRC) of South Africa (Project K5-2185, NJ Smit, PI). Ezemvelo KZN Wildlife is thanked for research permits OP 674/2012, OP 5139/2012, OP 526/2014, and OP 839/2014.
References
- Acosta I.C.L., Costa A.P., Nunes P.H., Gondim M.F.N., Gatti A., Rossi J.L. Morphological and molecular characterization and phylogenetic relationships of a new species of trypanosome in Tapirus terrestris (lowland tapir), Trypanosoma terrestris sp. nov., from Atlantic Rainforest of southeastern Brazil. Parasit. Vectors. 2013;6:349. doi: 10.1186/1756-3305-6-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves de Matos A.P., Paperna I. Ultrastructure of erythrocytic virus of the South African anuran Ptychadena anchietae. Dis. Aquat. Org. 1993;16:105–109. [Google Scholar]
- Badets M., Du Preez L. Phoretic interaction between the kangaroo leech Marsupiobdella africana (Hirudinea: Glossiphoniidae) and the cape river crab Potamonautes perlatus (Decapoda: Potamonautidae) Int. J. Parasitol Parasites Wildl. 2014;3:6–11. doi: 10.1016/j.ijppaw.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D.G. John Wiley & Sons; 2008. Flynn's Parasites of Laboratory Animals; pp. 149–150. [Google Scholar]
- Ball G.H. Blood sporozoans from East African Amphibia. J. Eukaryot. Microbiol. 1967;14:521–527. doi: 10.1111/j.1550-7408.1967.tb02037.x. [DOI] [PubMed] [Google Scholar]
- Bardsley J.E., Harmsen R. The trypanosomes of anura. Adv. Parasitol. 1973;11:1–73. doi: 10.1016/s0065-308x(08)60184-0. [DOI] [PubMed] [Google Scholar]
- Barta J.R. The dactylosomatidae. Adv. Parasitol. 1991;30:1–37. doi: 10.1016/s0065-308x(08)60305-x. [DOI] [PubMed] [Google Scholar]
- Barta J.R., Desser S.S. Blood parasites of amphibians from Algonquin park, Ontario. J. Wildl. Dis. 1984;20:180–189. doi: 10.7589/0090-3558-20.3.180. [DOI] [PubMed] [Google Scholar]
- Barta J.R., Boulard Y., Desser S.S. Blood parasites of Rana esculenta from Corsica: comparison of its parasites with those of eastern North American ranids in the context of host phylogeny. Trans. Am. Microsc. Soc. 1989;108:6–20. [Google Scholar]
- Beebee T.J.C., Griffiths R.A. The amphibian decline crisis. A watershed for conservation biology? Biol. Conserv. 2005;125:271–285. [Google Scholar]
- Channing A., Baptista N. Amietia angolensis and A. fuscigula (Anura: Pyxicephalidae) in southern Africa: a cold case reheated. Zootaxa. 2013;3640:501–520. doi: 10.11646/zootaxa.3640.4.1. [DOI] [PubMed] [Google Scholar]
- Channing A., Hillers A., Lötters S., Rödel M.O., Schick S., Conradie W. Taxonomy of the super-cryptic Hyperolius nasutus group of long reed frogs of Africa (Anura: Hyperoliidae), with descriptions of six new species. Zootaxa. 2013;3620:301–350. doi: 10.11646/zootaxa.3620.3.1. [DOI] [PubMed] [Google Scholar]
- Channing A., Schmitz A., Burger M., Kielgast J. A molecular phylogeny of African Dainty Frogs, with the description of four new species (Anura: Pyxicephalidae: Cacosternum) Zootaxa. 2013;3701:518–550. doi: 10.11646/zootaxa.3701.5.2. [DOI] [PubMed] [Google Scholar]
- Combes C. Parasites, biodiversity and ecosystem stability. Biodivers. Conserv. 1996;5:953–962. [Google Scholar]
- Conradie W. The King of the Dwarves: a new cryptic species of Dainty Frog (Anura: Pyxicephalidae: Cacosternum) from the eastern Great Escarpment of South Africa. Zootaxa. 2014;3785:438–452. doi: 10.11646/zootaxa.3785.3.6. [DOI] [PubMed] [Google Scholar]
- Cook C.A., Smit N.J., Davies A.J. A redescription of Haemogregarina fitzsimonsi Dias, 1953 and some comments on Haemogregarina parvula Dias, 1953 (Adeleorina: Haemogregarinidae) from southern African tortoises (Cryptodira: Testudinidae), with new host data and distribution records. Folia Parasitol. 2009;56:173–179. doi: 10.14411/fp.2009.021. [DOI] [PubMed] [Google Scholar]
- du Preez L., Carruthers V. Struik Nature; Cape Town: 2009. A Complete Guide to the Frogs of Southern Africa. [Google Scholar]
- Davies A.J., Johnston M.R.L. The biology of some intraerythrocytic parasites of fishes, amphibians and reptiles. Adv. Parasitol. 2000;45:1–107. doi: 10.1016/s0065-308x(00)45003-7. [DOI] [PubMed] [Google Scholar]
- Davis A.K., Devore J.L., Milanovich J.R., Cecala K., Maerz J.C., Yabsley M.J. New findings from an old pathogen: intraerythrocytic bacteria (family Anaplasmatacea) in red-backed salamanders Plethodon cinereus. EcoHealth. 2009;6:219–228. doi: 10.1007/s10393-009-0250-0. [DOI] [PubMed] [Google Scholar]
- Desser S.S. Aegyptianella ranarum sp. n. (Rickettsiales, Anaplasmataceae): ultrastructure and prevalence in frogs from Ontario. J. Wildl. Dis. 1987;23:52–59. doi: 10.7589/0090-3558-23.1.52. [DOI] [PubMed] [Google Scholar]
- Desser S.S., Hong H., Martin D.S. The life history, ultrastructure, and experimental transmission of Hepatozoon catesbianae n. comb., an apicomplexan parasite of the bullfrog, Rana catesbeiana and the mosquito, Culex territans in Algonquin Park, Ontario. J. Parasitol. 1995;81:212–222. [PubMed] [Google Scholar]
- Dobson A., Lafferty K.D., Kuris A.M., Hechinger R.F., Jetz W. Homage to Linnaeus: how many parasites? How many hosts? Proc. Natl. Acad. Sci. U. S. A. 2008;105:11482–11489. doi: 10.1073/pnas.0803232105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantham H.B., Porter A., Richardson L.R. Some haematozoa observed in vertebrates in eastern Canada. Parasitology. 1942;34:199–226. [Google Scholar]
- Haddad C.R. Fruit chafers (Coleoptera: Scarabaeidae: Cetoniini) of the Ndumo Game Reserve and Tembe Elephant Park, KwaZulu-Natal. Afr. Entomol. 2003;11:130–133. [Google Scholar]
- Kruger N., Du Preez L.H. Reproductive strategies of the Kangaroo Leech, Marsupiobdella africana (Glossiphoniidae) Int. J. Parasitol Parasites Wildl. 2015 doi: 10.1016/j.ijppaw.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed A.H.H., Mansour N.S. A general survey of blood protozoa in some Egyptian amphibians and reptiles. Bull. Zool. Soc. Egypt. 1959;14:21–26. [Google Scholar]
- Netherlands E.C., Cook C.A., Smit N.J., du Preez L.H. Redescription and molecular diagnosis of Hepatozoon theileri (Laveran, 1905) (Apicomplexa: Adeleorina: Hepatozoidae), infecting Amietia quecketti (Anura: Pyxicephalidae) Folia Parasitol. 2014;61:293–300. [PubMed] [Google Scholar]
- Netherlands E.C., Cook C.A., Smit N.J. Hepatozoon species (Adeleorina: Hepatozoidae) of African bufonids, with morphological description and molecular diagnosis of Hepatozoon ixoxo sp. nov. parasitising three Amietophrynus species (Anura: Bufonidae) Parasit. Vectors. 2014;7:552. doi: 10.1186/s13071-014-0552-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordstokke D.W., Zumbo B.D. A cautionary tale about Levene's tests for equality of variances. J. Edu. Res. Policy Stud. 2007;7:1–14. [Google Scholar]
- Nordstokke D.W., Zumbo B.D. A new nonparametric Levene test for equal variances. Psicologica. 2010;31:401–430. [Google Scholar]
- Readel A.M., Goldberg T.L. Blood parasites of frogs from an equatorial African montane forest in western Uganda. J. Parasitol. 2010;96:448–450. doi: 10.1645/GE-2284.1. [DOI] [PubMed] [Google Scholar]
- SPSS . IBM Corp; Armonk, NY: 2013. IBM SPSS Statistics for Windows, Version 22.0. IBM Corp. Released. [Google Scholar]
- Stuart S.N., Chanson J.S., Cox N.A., Young B.E., Rodrigues A.S., Fischman D.L. Status and trends of amphibian declines and extinctions worldwide. Science. 2004;306:1783–1786. doi: 10.1126/science.1103538. [DOI] [PubMed] [Google Scholar]
- Telford S.R. CRC Press; New York: 2009. Hemoparasites of the Reptilia: Color Atlas and Text. [Google Scholar]
- Werner J.K. Blood parasites of amphibians from Sichuan Province, People's Republic of China. J. Parasitol. 1993;79:356–363. [PubMed] [Google Scholar]
- Wesołowska W., Haddad C.R. Jumping spiders (Araneae: Salticidae) of the Ndumo Game Reserve, Maputaland, South Africa. Afr. Invert. 2009;50:13–103. [Google Scholar]


