Graphical Abstract

Keywords: Chewing lice, Gyropidae, Insular syndrome, Island biogeography, Trimenoponidae, Trombiculidae
Highlights
-
•
The parasitological fauna associated at Cavia intermedia was previously unknown.
-
•
We sampled ectoparasites from 65% of the total host population.
-
•
The louse species Gliricola lindolphoi and Trimenopon hispidum were collected.
-
•
The trombiculid mites Arisocerus hertigi and Eutrombicula sp. were also collected.
-
•
We compared the results to ectoparasite records for other Cavia species.
Abstract
Cavia intermedia is a rodent species critically endangered and is found only on a 10 hectare island off the southern Brazilian coast. To identify the ectoparasites of C. intermedia, 27 specimens (14 males and 13 females), representing approximately 65% of the estimated total population, were captured and examined. A total of 1336 chewing lice of two species were collected: Gliricola lindolphoi (Amblycera: Gyropidae) and Trimenopon hispidum (Amblycera: Trimenoponidae). In addition, chiggers Arisocerus hertigi (Acari: Trombiculidae) and Eutrombicula sp. (Acari: Trombiculidae) were collected from the ears of all captured animals. This low species richness compared to those for other Cavia species is expected for island mammals. Although the results presented here are not conclusive about the relationship between C. intermedia and ectoparasites, this low species richness found might be reflected in a low level of investment by the hosts in the basal immune defense, since investments in white blood cell production by mammals are influenced by the diversity of parasites in the environment. Additionally, considering that it might result in host vulnerability to other parasites that might be introduced through exotic or migratory host species, the monitoring of C. intermedia, including parasitological and immunological assessments, is recommended as a key component of conservation efforts.
1. Introduction
Species richness on islands results from a dynamic balance between migration and extinction processes, which depend on island size and its distance from the adjacent continent (MacArthur and Wilson, 1967). Thus, insular environments are characterized primarily by a decrease in species richness. As a consequence, many biological processes are affected and differentiated from those on the mainland, including host–parasite interactions, mainly through differences in the number of parasite species, their biological characteristics and host specificity (Magnanou and Morand, 2006). Parasites play an important role in host ecology, immune investment, population dynamics, and behavior, so it seems relevant to identify and quantify the parasite assemblages associated with archipelago mammals, specially the insular endemic species (Linardi and Guimarães, 2000; Berglund et al., 2009; Bordes and Morand, 2009).
Cavia intermedia is an endemic species from the largest island (9.86 ha) of Moleques do Sul Archipelago, in Santa Catarina State, Southern Brazil (Cherem et al., 1999). The species probably diverged from a common ancestral population of C. magna as the result of vicariance associated with archipelago formation, approximately 8000 years ago (Gava et al., 1998; Cherem et al., 1999; Furnari, 2013). It is categorized as “critically endangered” at the global level according to the International Union for Conservation of Nature (IUCN) criteria (Chapman, 2008). It is probably the mammal with the smallest geographic distribution in the world (Alcover et al., 1998) and its average population size was estimated as just 42 individuals (Salvador and Fernandez, 2008). The ectoparasites of this species have not previously been described.
The aim of this study was to document the ectoparasites of C. intermedia, record their prevalence and abundance, examine the influence of host sex on these parameters and describe the host–parasite interactions based on comparisons to other species of the genus Cavia.
2. Methods
The Moleques do Sul Archipelago (27°51′S; 48°26′W) consists of three oceanic islands. It is located 8 km from Santa Catarina Island and 14 km from the coast of Brazil, and is part of the Serra do Tabuleiro State Park in Santa Catarina State, Southern Brazil (Fig. 1). A detailed description of the area was provided by Salvador and Fernandez (2008).
Fig. 1.
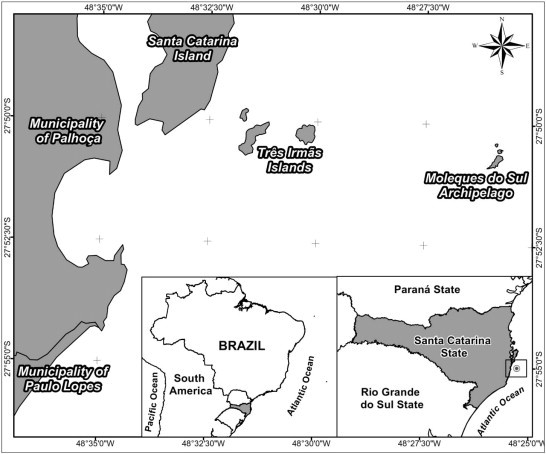
Location map of Moleques do Sul Archipelago.
Cavia intermedia were captured over 9 days in December, 2009, and for 4 days in February, 2010, using 34 traps baited with maize and placed in areas of high host density. Animals captured were numbered with ear-tags (Fish and small animal tag size 1, National Band and Tag Co., Newport, Kentucky, USA) and released at the place of capture. Techniques were approved by the Brazilian Federal Wildlife Agency (IBAMA) (license number # 033/07, process number # 02026.000394/2007-18) and are in accordance with guidelines published by the American Society of Mammalogists for use of wild mammals in research (Gannon and Sikes, 2007).
Lice were collected by brushing the hair coat on to a white tray, after rubbing cotton with ethyl ether on the host's body, and were then preserved in 70% ethanol. The sorting and counting of the lice was performed using a stereomicroscope. At least one sample containing several lice from each individual host was stored on permanent slides and identified according to Werneck (1942, 1948) and Emerson and Price (1975). Both the validity of specific names of lice and host were confirmed based on Price et al. (2003).
Chiggers were collected from the ears of the hosts by using forceps and they were stored in 70% alcohol. They were mounted in Hoyer's medium and examined on a light microscope with phase-contrast optics, according to Krantz and Walter (2009). They were identified according to Brennan and Goff (1977) and Brennan and Jones (1964), following the terminology of Goff et al. (1982). All mites and lice have been deposited at the Acari Collection of Instituto Butantan (IBSP130, IBSP11989, IBSP11990, IBSP11991).
Prevalence and abundance of lice were calculated according to Bush et al. (1997). Differences in abundance and prevalence among louse species were evaluated using a t-test and chi-square, respectively. Possible differences in parasite abundance (all species together or separately) between male and female hosts were evaluated with a t-test. Before performing the t-test, Levene's test was used to evaluate the homoscedasticity of the data. The parasitological parameters were not calculated for chiggers, because the collection of these parasites was not standardized.
3. Results and discussion
Twenty-seven C. intermedia (14 males and 13 females) were captured, which corresponds to approximately 65% of the total population as estimated by Salvador and Fernandez (2008). 1336 Mallophaga of two species were collected, Gliricola lindolphoi (Amblycera: Gyropidae) (Fig. 2) and Trimenopon hispidum (Amblycera: Trimenoponidae) (Fig. 3; Table 1). The morphological diagnosis of G. lindolphoi was based on: meso and methatorax fused into pterothorax; maxillary palpi 2-segmented; male genitalia with elongate and wide basal plate; straight parameres; genital sac with many sclerites; females presenting the longest terminal seta of the posterior margin shorter than the length of the last tergite. The morphological diagnosis of T. hispidum was based on: subtriangular head with straight lateral and posterior margins; pigmented eyes; presence of two claws on each of tarsi II–III and five pairs of abdominal spiracles.
Fig. 2.

Gliricola lindoiphoi male collected on Cavia intermedia. The bar corresponds to 100 µm.
Fig. 3.
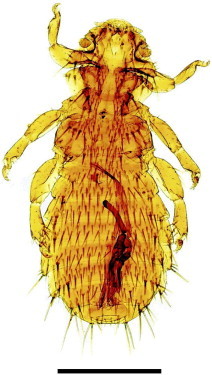
Trimenopon hispidum male collected on Cavia intermedia. The bar corresponds to 100 µm.
Table 1.
Abundance and prevalence of louse species associated with Cavia intermedia at Moleques do Sul Island, Santa Catarina, Brazil. Legend: ♂ = male adults; ♀ = female adults and Ny = nymphs.
| Louse species | Prevalence (%) | Abundance (parasites/host) | Sex and developmental stage | Total | ||
|---|---|---|---|---|---|---|
| ♂ | ♀ | Ny | ||||
| Trimenopon hispidum | 100 | 32.0 | 299 | 243 | 323 | 865 |
| Gliricola lindolphoi | 89 | 17.4 | 96 | 113 | 262 | 471 |
| Total | _ | 49.5 | 395 | 356 | 585 | 1336 |
Chiggers of two species, Arisocerus hertigi (Acari: Trombiculidae) (Fig. 4) and Eutrombicula sp. (Acari: Trombiculidae), were collected from the ears of all captured cavies. The morphological diagnosis of A. hertigi were based on: palpal tarsus with 7 branched setae; galeal seta nude; tibial claw trifurcate; 3 genualae I; a genuala II and III; a tibiala III and a mastitarsala III; palpal setae B/B/NNN; coxal setae 1.1.1; 2 pairs of sternal setae; PL>AL>AM, 20–22 dorsal setae; arranged 2H-6-6-(2–4)-2; 12–15 ventral setae; arranged 2st-2st-8-2-2; the sensilla are unilaterally expanded only one side and PL setae are long. The morphological diagnosis of Eutrombicula sp. were based on: palpal tibial claw bifurcate; cheliceral blade with tricuspid cap; palpal tarsus with 7 branched setae; a subterminala; and a tarsala; scutum roughly rectangular; wider than long; sensillae branched flageliform; five scutal setae; eyes 2/2, in a plate; leg segmentation 7-7-7; two or 3 genualae I; one genuala II and III; one tibiala III; 0–2 mastitibialae III; 1–3 mastitarsalae III.
Fig. 4.

Arisocerus hertigi collected on Cavia intermedia. The bar corresponds to 200 µm.
Prevalence did not differ significantly among the two louse species and was high for both (χ 2 = 0.18, df = 2, p = 0.915). The mean abundance was 49.5 (±39.1) parasites/host. Abundance of T. hispidum was greater than that of G. lindolphoi (Table 1) (t = 3.54, df = 26, p = 0.001). Host sex did not affect mean abundance (t = −0.5, df = 25, p = 0.62) or the abundance of either louse species individually (T. hispidum: t = −1.033, df = 25, p = 0.311; G. lindolphoi: t = −0.049, df = 25, p = 0.961).
This work shows, for the first time, the occurrence of two chewing louse species, T. hispidum and G. lindolphoi, and two trombiculid mites, A. hertigi and Eutrombicula sp., associated with C. intermedia, a rodent species highly endangered and endemic to an island in southern Brazil. Approximately 50 species of ectoparasites have been reported for the genus Cavia (Table 2), but in the current study only a few were found on C. intermedia. This low number of parasite species is expected for island mammals, as reported for C. fulgida (Guitton et al., 1986) and C. porcelllus (Linardi et al., 1991). The structure of parasites assembly on islands is strongly related to the low richness of free-living species typical of these environments (MacArthur and Wilson, 1967). This occurs because the parasites depend on the hosts to disperse to these sites and also to establish, especially for those who require more than one species of host to complete its life cycle (Magnanou and Morand, 2006).
Table 2.
Checklist of arthropods parasites from Cavia spp.
Until now, C. aperea, C. fulgida, C. a. pamparum and C. porcellus were reported as hosts of T. hispidum. This louse species is common in these hosts, but G. lindolphi is known only from C. aperea (Table 2), and is rare, being reported here for only the sixth time (Emerson and Price, 1975; Cardozo-de-Almeida et al., 2003; Krüger, 2006).
Arisocerus hertigi was originally described in rodents (Dasyproctidae) and marsupials from Sommerfield, Paraguay (Didelphidae) (Brennan and Jones, 1964). Subsequently, this species was also found parasitizing marsupials (Didelphidae) in the Federal District, Brazil (Goff and Gettinger, 1989). Therefore, this work reports the first record of this species in Caviidae.
The genus Eutrombicula, the most important in terms of human and animal health in the Neotropical Region, is composed of about 80 species (Brennan and Reed, 1974; Stekol′Nikov and González-Acuña, 2010). According to Daniel and Stekol′Nikov (2004) many species of this genus were identified “by default”. Thus, the correct identification of the material from C. intermedia will be possible only after a taxonomic revision of the genus.
We observed 100% prevalence and an abundance of 33 parasites/host for T. hispidum on C. intermedia. These values are slightly higher than those found in two other studies of Cavia spp., except for a location where the estimated abundance was much lower. Valim et al. (2004) estimated a prevalence of 100% and an abundance of 4.8 parasite/host and a prevalence of 90% and abundance of 29.1 parasites/host for T. hispidum on C. porcellus in Duque de Caxias and Silva Jardim in Rio de Janeiro State, Brazil, respectively. Krüger (2006) reported a prevalence of 97% and abundance of 23 parasites/host for T. hispidum on C. aperea from Pelotas, Rio Grande do Sul State, Brazil. While the prevalence of G. lindolphoi in this study (89%) is much higher than previously reported by Krüger (2006) (48%), the abundance (17.4 parasites/host) is similar to that reported by this author on C. aperea (16 parasites/host).
The differences between these studies and the results reported here are possibly related to high population densities of the C. intermedia, as stated by Salvador and Fernandez (2008). Since the transmission of lice occurs through direct contact between hosts, it is expected that parasite prevalence and abundance are positively related to population density of the host (Stanko et al., 2002; Magnanou and Morand, 2006). Although some studies reported differences in abundance between host genders perhaps linked to host behavior (Soliman et al., 2001; Moore and Wilson, 2002) or hormonal (Dlugosz et al., 2014) differences, there were no significant differences for C. intermedia.
4. Conclusion
The results presented here do not provide a complete picture of the relationship between C. intermedia and its ectoparasites, because this type of interaction is dynamic and changes with time (Linardi and Guimarães, 2000). However, we found some evidence for the effects of insularity on parasitism: a parasite fauna of low richness; parasitological parameters different from those found in studies on other Cavia spp. on the mainland of Brazil; the presence of generalist species (trombiculid mites); and direct life cycle species (lice) (Magnanou and Morand, 2006). Parasitological studies of Cavia spp., especially of C. magna, may help to clarify the interaction of C. intemedia and its ectoparasites.
Although the results presented here are not conclusive about the relationship between C. intermedia and ectoparasites, this low species richness found might be reflected in a low level of investment by the hosts in the basal immune defense, since investments in white blood cell production by mammals are influenced by the diversity of parasites in the environment (Bordes and Morand, 2009). Additionally, considering that it might result in host vulnerability to other parasites that might be introduced through exotic or migratory host species, the monitoring of C. intermedia, including parasitological and immunological assessments, is recommended as a key component of conservation efforts.
Acknowledgments
We wish to thank José Ramiro Botelho for laboratory support, Gabriela Ferreira de Souza and Caroline Oswald for the helping in capturing the rodents, Jonas Sponchiado and Jean Susan Al-Qureshi for manuscript review, and to Juliana Scotton and Luciano Candisani for the figures. Thanks are also extended to Instituto Brasileiro do Meio Ambiente (IBAMA) and Fundação do Meio Ambiente FATMA for the license to capture the animals. This research was sponsored by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and the Coordenação de Aperfeiçoamento de Pessoal do Ensino Superior (CAPES).
References
- Alcover J.A., Campillo X., Macias M., Sans A. A mammal species of the world: additional data on insular mammals. Am. Mus. Novit. 1998;3248:1–29. [Google Scholar]
- Bastos, F.A.N., 2008. Revisão taxonômica das espécies do gênero Ornithonyssus (Acari: Macronyssidae) parasitos de pequenos mamíferos terrestres no Brasil e avaliação da infecção desses ácaros por Rickettsia spp. Master dissertation. Universidade de São Paulo, São Paulo, Brazil.
- Berglund H., Järemo J., Bengtsson G. Endemism predicts intrinsic vulnerability to nonindigenous species on islands. Am. Nat. 2009;174(1):94–101. doi: 10.1086/598501. [DOI] [PubMed] [Google Scholar]
- Bordes F., Morand S. Coevolution between multiple helminth infestations and basal immune investment in mammals: cumulative effects of polyparasitism? Parasitol. Res. 2009;106:33–37. doi: 10.1007/s00436-009-1623-6. [DOI] [PubMed] [Google Scholar]
- Brennan J.M., Goff M.L. Keys to the genera of chiggers of the western hemisphere (Acarina: Trombiculidae) J. Parasitol. 1977;63(3):554–566. [PubMed] [Google Scholar]
- Brennan J.M., Jones E.K. Five new species of chiggers from South America (Acarina: Trombiculidae) J. Med. Entomol. 1964;1:307–310. doi: 10.1093/jmedent/1.3.307. [DOI] [PubMed] [Google Scholar]
- Brennan J.M., Reed J.T. The genus Eutrombicula in Venezuela (Acarina: Trombiculidae) J. Parasitol. 1974;60(4):699–711. [PubMed] [Google Scholar]
- Bush A.O., Lafferty K.D., Lotz J.M., Shostak A.W. Parasitology meets Ecology on its own terms: Margolis et al. revisited. J. Parasitol. 1997;83(4):575–583. [PubMed] [Google Scholar]
- Cardozo-de-Almeida M., Linardi P.M., Costa J. The type specimens of chewing lice (Isecta, Mallophaga) deposited in the entomological collection of Instituto Oswaldo Cruz, Rio de Janeiro, RJ, Brazil. Mem. Inst. Oswaldo Cruz. 2003;98(2):233–240. doi: 10.1590/s0074-02762003000200012. [DOI] [PubMed] [Google Scholar]
- Castro D.D.C., Mauri R., Cicchino A.C., Mosquera S. Ectoparasitos de roedores de La província de Buenos Aires, Argentina (Acarina, Anoplura, Mallophaga y Suctoria) Rev. Soc. Entomol. Argent. 1987;44:317–327. [Google Scholar]
- Chapman R.E. Cavia intermedia. 2008. http://www.iucnredlist.org IUCN Red List of Threatened Species.Version 2013.1. Available from:
- Cherem J.J., Olimpio J., Ximenez A. Descrição de nova espécie do gênero Cavia Pallas, 1766 (Mammalia – Caviidae) das Ilhas dos Moleques do Sul, Santa Catarina, Sul do Brasil. Biotemas. 1999;12:95–117. [Google Scholar]
- Cicchino A.C., Castro D.C. Contribuicion al conocimento de los malofagos argentinos XV. Una nueva espécie del gênero Philandesia Kellogg y Nakayama, 1914 (Mallophaga – Trimenoponidae) Hist. Nat. 1984;4:25–32. [Google Scholar]
- Cruz K.D., Ribbeck R., Daugschies A. Vorkommen und verbreitung von ektoparasiten bei meerrschweinchen (Cavia spp.) in Peru, Südamerika. Berl. Munch. Tierarztl. Wochenschr. 2003;116:102–107. [PubMed] [Google Scholar]
- Daniel M., Stekol′Nikov A.A. Chigger mites of the genus Eutrombicula ewing, 1938 (Acari: Trombiculidae) from Cuba, with the description of three new species. Folia Parasitol. 2004;51:359–366. doi: 10.14411/fp.2004.045. [DOI] [PubMed] [Google Scholar]
- Dittmar K. Evaluation of ectoparasites on the guinea pig mummies of el Yaral and Moquegua Valley, in Southern Peru. Chungara. 2000;32(1):123–125. [Google Scholar]
- Dittmar K. Arthropod and helminth parasites of the wild Guinea pig, Cavia aperea, from the Andes and the Cordillera in Peru, South America. J. Parasitol. 2002;88:409–411. doi: 10.1645/0022-3395(2002)088[0409:AAHPOT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Dlugosz E.M., Downs C.J., Khokhlova I.S., Degen A.A., Krasnov B.R. Ectoparasite performance when feeding on reproducing mammalian females: an unexpected decrease when on pregnant hosts. J. Exp. Biol. 2014;217:1058–1064. doi: 10.1242/jeb.098376. [DOI] [PubMed] [Google Scholar]
- Emerson K.C., Price R.D. Brigham Young University Science Bulletin; 1975. Mallophaga of Venezuelan mammals. (Biological Series 20). 76 pp. [Google Scholar]
- Evans D.E., Martins J.R., Guglielmone A.A. A review of the ticks (Acari, Ixodida) of Brazil, their host and geographic distribution – 1. The State of Rio Grande do Sul, Southern Brazil. Mem. Inst. Oswaldo Cruz. 2000;95(4):453–470. doi: 10.1590/s0074-02762000000400003. [DOI] [PubMed] [Google Scholar]
- Furnari N. New findings on the origin of Cavia intermedia, one of the world′s rarest mammals. Mammal Rev. 2013;43(4):323–326. [Google Scholar]
- Gannon W.L., Sikes R.S. Guidelines of the American Society of Mammalogists for use of wild mammals in research. J. Mammal. 2007;88(3):809–823. doi: 10.1093/jmammal/gyw078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gava A., Freitas T.R.O., Olimpio J. A new karyotype for the genus Cavia from a southern island of Brazil (Rodentia – Caviidae) Genet. Mol. Biol. 1998;21(1):77–80. [Google Scholar]
- Goff M.L., Gettinger D. Two new species of schoengastiine chiggers (Acari: Trombiculidae) from Brazil and rediagnosis of Arisocerus Brennan, 1970. J. Med. Entomol. 1989;26(6):554–558. doi: 10.1093/jmedent/26.6.554. [DOI] [PubMed] [Google Scholar]
- Goff M.L., Loomis R.B., Welbourn W.C., Wrenn W.J. A glossary of chigger terminology (Acari: Trombiculidae) J. Med. Entomol. 1982;19(3):221–238. doi: 10.1093/jmedent/19.3.221. [DOI] [PubMed] [Google Scholar]
- Guitton N., Araújo-Filho N.A., Sherlock Í.A. Ectoparasitos de roedores e marsupiais nos ambiente Silvestre de Ilha Grande, Estado do Rio de Janeiro, Brasil. Mem. Inst. Oswaldo Cruz. 1986;81:233–234. doi: 10.1590/s0074-02761986000200014. [DOI] [PubMed] [Google Scholar]
- Krantz G.W., Walter D.E. Texas Tech University Press; Lubbock, Texas: 2009. A Manual of Acarology. 807 pp. [Google Scholar]
- Krüger C., Mascarenhas C.S., Müller G., Brum J.G.W. Ocorrência de Siphonaptera em preá Cavia aperea Exerleben, 1777 (Rodentia: Caviidae) no Rio Grande do Sul, Brasil. Arq. Bras. Med. Vet. Zootec. 2010;62(5):1288–1290. [Google Scholar]
- Krüger, C.P., 2006. Artrópodes e helmintos parasitos de Cavia aperea Exerleben, 1777 (Rodentia: Caviidae) no sul do Brasil (Master dissertation). Universidade Federal de Pelotas, Rio Grande do Sul, Brazil.
- Linardi P.M., Guimarães L.R. Museu de Zoologia USP/FAPESP Press; São Paulo: 2000. Sifonápteros do Brasil. [Google Scholar]
- Linardi P.M., Botelho J.R., Rafael J.A., Valle C.M.C., Cunha A., Machado P.A.R. Ectoparasitos de pequenos mamíferos da Ilha de Maracá, Roraima, Brasil. I. Ectoparasitofauna, registros geográficos e de hospedeiros. Acta Amaz. 1991;21:131–140. [Google Scholar]
- MacArthur R., Wilson E. Princeton University Press; New Jersey: 1967. The Theory of Biogeography. [Google Scholar]
- Magnanou E., Morand S. Insularity and micromammal-macroparasite relationships. In: Monrad S., Krasnov B.R., Poulin R., editors. Micromammals and Macroparasites: From Evolutionary Ecology to Management. Editora Springer Press; 2006. [Google Scholar]
- Moore S.L., Wilson K. Parasites as viability cost of sexual selection in natural population of mammals. Science. 2002;297:2015–2018. doi: 10.1126/science.1074196. [DOI] [PubMed] [Google Scholar]
- Price R.D., Hellenthal R.A., Palma R.L. World checklist of chewing lice with host associations and keys to families and genera. In: Price R.D., Hellenthal R.A., Palma R.L., Johnson K.P., Clayton D.H., editors. The Chewing Lice: World Checklist and Biological Overview. Illinois Natural History Survey Special Publication; 2003. 448pp. [Google Scholar]
- Salvador C.H., Fernandez F.A.S. Population dynamics and conservation status of the insular cavy Cavia intermedia (Rodentia: Caviidae) J. Mammal. 2008;89:721–729. [Google Scholar]
- Soliman S., Main A.J., Marzouk A.S., Motasser A.A. Seasonal studies on commensal rats and their ectoparasites in a rural area of Egypt: the relationship of ectoparasites to the species, locality and relative abundance of the host. J. Parasitol. 2001;87:545–553. doi: 10.1645/0022-3395(2001)087[0545:SSOCRA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Stanko M., Miklisová D., Bellocq J.G., Morand S. Mammal density and patterns of ectoparasite species richness and abundance. Oecologia. 2002;131:289–295. doi: 10.1007/s00442-002-0889-5. [DOI] [PubMed] [Google Scholar]
- Stekol′Nikov A.A., González-Acuña D. Four new species of chigger mites (Acari: Trombiculidae) of the genus Eutrombicula from Chile. Int. J. Acarol. 2010;36:313–325. [Google Scholar]
- Valim M.P., Amorim M., Serra-freire N.M. Parasitismo por Acari e Phthiraptera em cobaios [Cavia porcellus (Linnaeus, 1758)] de ambientes rural e urbano nos municípios de Silva Jardim e Duque de Caxias, Rio de Janeiro, Brasil. Braz. J. Vet. Res. Anim. Sci. 2004;41:240–446. [Google Scholar]
- Werneck F.L. Sobre algumas espécies do gênero Gliricola (Mallophaga) Mem. Inst. Oswaldo Cruz. 1942;37:297–319. [Google Scholar]
- Werneck F.L. Os Malófagos de Mamíferos. Parte I: Amblycera e Ischnocera (Philopteridae e parte de Trichodectidae) Rev. Bras. Biol. 1948 [Google Scholar]


