Abstract
There is increasing evidence that exposure to air pollutants are associated with human disease and may act through epigenetic modification of the nuclear genome, but there have been few publications describing their impact upon the mitochondrial genome. Mitochondrial DNA may be more susceptible to pollutant-induced changes via increased oxidative stress in the cell, and therefore this field of research is of growing interest. Many techniques employed to study DNA methylation of the nuclear genome are also applicable to mitochondrial epigenetic studies. In this chapter, we describe a protocol for the isolation of mitochondrial DNA from peripheral blood samples and the analysis of 5-methlycytosine content by bisulfite-pyrosequencing.
Keywords: Mitochondrial DNA, DNA methylation, Air pollution, Pollutant, Bisulfite-Pyrosequencing
1. Introduction
Environmental stressors, such as airborne particulate matters and metals, have been reported to cause increased cellular oxidative stress and are subsequently implicated in aging (1,2) and disease, including diabetes (3,4) and neurodegenerative disease (5). Such associations with disease may arise through the increased oxidative stress directly or indirectly leading to epigenetic changes in the cell (6). While there have been numerous epigenetic studies over the past decade investigating the association of environmental exposures with nuclear DNA (7–9), mitochondrial epigenetics has not been studied well (10).
Mitochondria are considered to be both a source and a target of cellular oxidative stress (11). The close proximity of mitochondrial DNA (mtDNA) to the source of reactive oxygen species (ROS) makes mtDNA more vulnerable to oxidative DNA damage. As mitochondria do not contain histone complexes, the field of mitochondrial epigenetics refers only to measuring 5-methylcytosine (5-mC) and 5-hydroxymethycytosine (5-hmC) at CpG or non-CpG sites in mtDNA. The presence of mitochondrial DNA 5-mC and 5-hmC has been increasingly well established recently and has led to several studies investigating its biological function and implication in disease (12), such as those into neurodegenerative disease (13,14), aging (15), and cervical cancer (16). Recent work has also demonstrated an effect of air pollutants upon mtDNA 5-mC methylation, which was hypothesized to occur through oxidative stress (17). However, this field is still in its infancy, and much further work is required to elucidate the impact of exposure to pollutants upon mitochondrial epigenetics.
Most techniques available for the purpose of studying nuclear DNA (nDNA) methylation have been adapted for use with mtDNA, such as immunoprecipitation (18), mass spectrometry (19,20), pyrosequencing (17), and bisulfite genomic sequencing (21). Current Infinium Methylation arrays by Illumina are solely designated for detecting nuclear DNA methylation and therefore do not enable the study of mtDNA methylation. Here in this chapter, we will discuss the most sensitive, robust and easy-to-use method, pyrosequencing, that is also the most widely used technique in epidemiological studies into the effect of environmental exposures upon DNA methylation (22,23).
2. Materials
2.1. MtDNA isolation (see Note 1)
XIT™ Mitochondrial DNA kit (G-Biosciences, St Louis, MO) (see Note 2).
RBC Lysis Buffer (G-Biosciences, St Louis, MO).
2.2. Bisulfite conversion (see Note 3)
EZ DNA Methylation™ Kit (ZYMO RESEARCH, Irvine, CA) (see Note 4).
Thermal cycler (Bio-Rad, Hercules, CA).
2.3. PCR amplification
2.4. Pyrosequencing
All PyroMark reagents and equipment are available from Qiagen (Venlo, Limburg).
PyroMark Q96 MD (Qiagen, Venlo, Limburg).
PyroMark Q96 HS Capillary Tips.
PyroMark Q96 HS Reagent Tips.
PyroMark Q96 HS Capillary Tip Holder.
PyroMark Annealing Buffer.
PyroMark Binding Buffer.
PyroMark Denaturation Solution.
PyroMark Wash Buffer.
PyroMark Gold Q96 CDT Reagents.
PyroMark Q96 HS Plate.
PyroMark Q96 HS Sample Prep Thermoplate.
PyroMark Q96 Vacuum Prep Troughs.
Streptavidin Sepharose High Performance beads (GE Healthcare, Little Chalfont, UK).
Shaker.
Heat block.
Sequencing primer (see Note 7).
Millex-FG Syringe Filter, 0.20 μm, hydrophobic PTFE, 50mm (Millipore, Amsterdam Zuidoost, Netherlands).
3. Methods
3.1. MtDNA isolation
3.1.1. Lysis of Erythrocytes
3.1.2. Isolation of mtDNA from the total nucleic acid fraction
Add 500 μl of ice-cold 1X SubCell Buffer-II to the pellet. Gently vortex to suspend the cells and incubate on ice for 10 minutes.
Centrifuge at 700 × g for 10 minutes to pellet the nuclei. Transfer the supernatant to a new tube.
Centrifuge the supernatant at 12,000 × g for 15 minutes. The resulting pellet contains mitochondria.
Discard the supernatant and resuspend the pellet in 500 μl of 1X SubCell Buffer-II without BSA.
Centrifuge at 12,000 × g for 10 minutes and remove the supernatant.
Resuspend the mitochondrial pellet in 500 μl Mitochondrial Lysis Buffer and incubate at room temperature for 10–15 minutes.
Add 5 μl LongLife™ Proteinase K to the tube and mix by inverting the tube 10–20 times.
Incubate at 55°C for at least 2 hours (see Note 10).
Following this, incubate the sample on ice for 1 minute to quickly cool (see Note 11).
Add 100 μl XIT™ Protein Precipitation Buffer to the sample and mix by inverting the tube 10–20 times.
Centrifuge at 14,000 × g for 2 minutes. Carefully transfer the supernatant to a fresh tube (see Note 12).
Add 500 μl isopropanol to the supernatant and mix by gently inverting the sample 30–50 times.
Centrifuge at 14,000 × g for 5 minutes.
Discard the supernatant and use a P10 pipette to carefully remove all liquid.
Add 200 μl 70% ethanol and invert the tube twice to wash the pellet.
Centrifuge at 14,000 × g for 2 minutes.
Discard the supernatant and drain the tube on a piece of clean absorbent paper.
Allow the tube to air dry for 15 minutes.
Add 50 μl of pre-warmed (room temperature) TE buffer and resuspend the mtDNA (see Note 13).
Store the DNA at 4°C if it is to be used immediately. For long-term storage, store at −20°C or −80°C.
Assess the purity of isolated mtDNA (optional) (see Note 14) (Fig. 1).
Figure 1. An example of q-PCR using the ‘chrM:3313-3322’ and HBB assays.
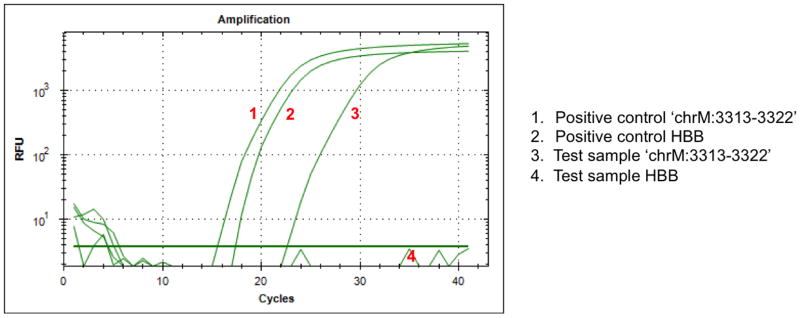
The q-PCR graph shows that isolated mtDNA (‘Test’) contains no nuclear DNA but does contain mtDNA, as demonstrated by the amplification of the mitochondrial-encoded chrM:3313-3322 (#3) but not the nuclear-encoded HBB (#4). Positive controls for each of the assays are shown by the #1 and 2 amplification curves.
3.2. Bisulfite conversion
Add 750 μl water and 210 μl of M-Dilution Buffer to a tube of CT Conversion Reagent (see Note 15).
Mix at room temperature with frequent vortexing or shaking for 10 minutes (see Note 16).
Add 5 μl of M-Dilution Buffer to the DNA sample and adjust the total volume to 50 μl with water. Mix the sample by pipetting up and down (see Note 17).
Incubate the mtDNA and M-Dilution Buffer sample at 37°C for 15 minutes.
After the incubation, add 100 μl of the prepared CT Conversion Reagent to each mtDNA sample mix.
Incubate the sample in a thermal cycler at 50°C for 12–16 hours followed by 4°C for 10 minutes.
Add 400 μl of M-Binding Buffer to a Zymo-Spin™ IC Column and place the column into a Collection Tube.
Transfer the sample from Step 6 into the Zymo-Spin™ IC Column containing the M-Binding Buffer. Close the cap and mix by inverting the column several times.
Centrifuge at full speed (>10,000 × g) for 30 seconds. Discard the flow-through in the Collection Tube.
Add 100 μl of M-Wash Buffer to the column. Centrifuge at full speed for 30 seconds and discard the flow-through.
Add 200 μl of M-Desulphonation Buffer to the column and let stand at room temperature (20–30°C) for 15–20 minutes. After the incubation, centrifuge at full speed for 30 seconds and discard the flow-through.
Add 200 μl of M-Wash Buffer to the column. Centrifuge at full speed for 30 seconds. Add another 200 μl of M-Wash Buffer and centrifuge for an additional 30 seconds.
Discard the Collection Tube and place the column into a 1.5 ml microcentrifuge tube. Add 10 μl of M-Elution Buffer (pre-warmed to 37°C) directly to the column matrix. Incubate for 1 minute at room temperature and centrifuge for 30 seconds at full speed to elute the DNA.
The bisulfite-converted DNA should be stored at −20°C or −80°C for longer-term storage (see Note 18).
3.3. PCR preparation
Add 12 μl of high purity DNase-free water into clean PCR tube.
Transfer 10 μl of PCR Taq mixture to the tube.
Add each 1 μl each of the forward and reverse primers, from working solutions at 10 μM. One of the two primers should be biotin-labeled (see Note 5).
Add 1 μl of bisulfite-converted mtDNA solution into the PCR mixture to make a final volume of 25μl (see Note 19).
Preform the PCR using standard cycling conditions, e.g. 95°C for 5 minutes, 40 cycles of (95°C for 30 seconds, the primer-specific annealing temperature for 30 seconds, 72°C for 1 minute), 72°C for 10 minutes, and 4°C forever.
3.4. Pyrosequencing
Pyrosequencing enables the relative quantification of nucleotides in a known sequence, and it is therefore commonly used to analyze DNA methylation and to determine single nucleotide polymorphism (SNP) haplotypes. By a chain of enzymatic reactions, the incorporation of nucleotides into the growing DNA strand releases light that is detected and quantified by a camera. This technique offers a highly sensitive and cost-effective approach to study DNA sequences of <100 nucleotides in length.
The methylation of mitochondrial genes can be analyzed with a standard pyrosequencing approach, using either the Q96 or Q24 platform. All solutions (e.g. the PCR products, the pyrosequencing sequencing primer, and sample preparation solutions) should be allowed to reach room temperature prior to starting, the PyroMark PSQ machine should be turned on one hour prior to use, and the heat block set to 80°C.
3.4.1. Immobilization of PCR product to beads
A bead master mix should be made, consisting of 40 μl Binding buffer, 2 μl of Streptavidin Sepharose beads and 28 μl of high-purity water per sample (see Note 20).
Transfer 70 μl of the mix to each well of a 96-well plate that is to be used.
Add 10 μl of PCR products from Section 3.3 into the relevant wells of a 96-well plate to make a final volume of 80 μl (see Note 21).
After sealing the plate with a plastic or foil sheet, incubate the mixture for 10 minutes with vigorous agitation on a shaker to mix the beads and biotin-labeled PCR products at room temperature.
3.4.2. Strand separation
Place five of the troughs delivered with the Vacuum Prep Tool/Worktable on the lab bench or on the Vacuum Prep Worktable.
Fill the troughs with approximately 180 ml of 70% ethanol, Wash buffer, and two with high purity water (e.g. MilliQ-grade). Fill the fifth trough with approximately 120 ml of Denaturation solution (see Note 22).
Ensure the vacuum switch is off, and start the vacuum pump.
Apply vacuum to the Vacuum Prep Tool by opening the vacuum via the switch (‘On’).
Wash the empty probes of the Vacuum Prep Tool by lowering the tool into high purity water for approximately 20 seconds. While doing this, ensure that the proper vacuum has been attained through all 96 probes.
Capture the bead/DNA-template complexes on the filter probes by slowly lowering the Vacuum Prep Tool into the PCR plate from Section 3.4.4. (see Note 23).
Make sure that the liquid has been aspirated evenly from all the wells in the plate and that all the beads have been captured on the probe tips. The surface of the remaining solution should be level with or lower than the filter probe tips in the wells.
Move the Vacuum Prep Tool to the trough containing 70% ethanol and let the solution flush through the filters for 5 seconds.
Move the Vacuum Prep Tool to the trough containing the Denaturation solution and flush through the filters for 5 seconds.
Move to the tool to the trough containing Wash buffer and flush through the filters for 5–10 seconds.
To allow the liquid to completely drain from the probes, hold the tool vertically at beyond 90°. Hold this position for a few seconds and then return the tool to the horizontal position.
Close the vacuum on the tool by moving the switch to the ‘Off’ position.
Release the beads in a PSQ HS 96 Plate, prefilled with 0.3 μM sequencing primer in 12 μl Annealing buffer. Shake the Vacuum Prep Tool while allowing the filter probes to rest on the bottom of the wells (see Note 24).
3.4.3. Primer annealing
After sealing the plate with a plastic or foil sheet, heat the PSQ HS 96 Plate with the samples at 80°C for 2 minutes using the PSQ HS 96 Sample Prep Thermoplate Kit.
Remove the plate from the heating block and let the samples cool to room temperature for 10 minutes.
3.4.4. Sequencing reaction
Create the assay run file using the PyroMark Q96 software.
When creating the file, choose instrument parameters appropriate for the capillary tips to be used (see Note 25).
Fill the PyroMark Q96 HS Capillary Tips with the relevant volumes of nucleotides, enzyme and substrate, as described within the assay run file, and place the HS Capillary Tips Holder in the PyroMark Q96 MD instrument. Check the function of the dispensing tips by running a dispensing test using the test plate.
Following the completion of the 10 minute incubation at room temperature, insert the PSQ HS 96 Plate from Step 19 in the PyroMark Q96 MD instrument and start the run.
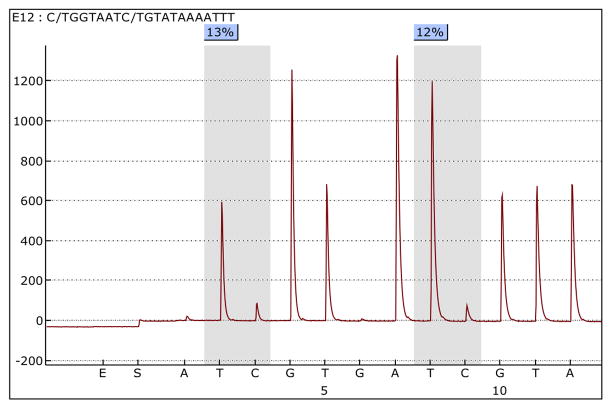
Once the run has been completed, pyrograms for each sample can be analyzed and the percentage methylation at each interrogated CpG site established (see Note 26) (Fig. 2).
Run pyrosequencing control oligos to establish the consistency and sensitivity of the assay (optional) (see Note 27) (Fig. 3).
Figure 2. An example pyrosequencing pyrogram using the tRNA-Leu assay.
The first CpG analyzed shows 13% methylation and the second CpG shows 12% methylation.
Figure 3. An example pyrosequencing pyrogram of control oligos.
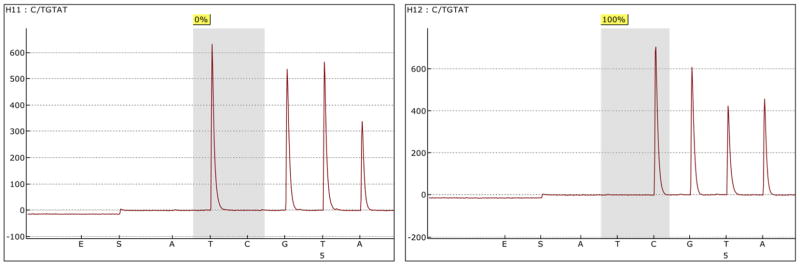
CpG sites representing the sensitivity of measuring 0% (left) and 100% (right) methylation by pyrosequencing reaction.
Footnotes
If DNA has already been isolated using a conventional approach, the sample will contain a mixture of nuclear DNA and mtDNA. In these circumstances, extra care will be required with the design of the mtDNA pyrosequencing assays or other methylation assays. There are a significant number of homologous sequences between nDNA and mtDNA, resulting from the evolutionary origins of mitochondria (24). These nuclear mitochondrial DNA (numt) sequences will subsequently affect assays designed to study the methylation of mtDNA. To overcome this potential for confounding of results, care should be taken to avoid the consensus sequences between nDNA and mtDNA in designing mtDNA methylation detection assays.
There are several commercially available kits for the isolation of mtDNA from tissues and blood. The protocol has to be modified according to the type of tissue isolated, and it is therefore important to receive advice from the technical support from each company. As peripheral blood is the most readily available specimen in epidemiological studies, we will describe a protocol for use with this sample type.
The bisulfite conversion process converts unmethylated cytosines to uracil and ultimately to thymine following PCR, while methylated-cytosines remain unmodified. The percentage of methylated residues at any given site in a known sequence can therefore be determined by employing a quantitative approach, such as pyrosequencing, to measure the cytosine:thymine (methylated:unmethylated) ratio. It should be noted that this approach cannot distinguish between 5-mC and 5-hmC. 5-hmC levels in mtDNA can be measured using commercially available kits (e.g. from Epigentek, Zymo Research, and Cambridge Epigenetix). Here, we will describe a standard protocol for bisulfite-converting DNA using the EZ DNA Methylation™ Kit (Zymo Research). The starting amount of DNA should be optimized for each experiment, with between 20 ng and100 ng of isolated mtDNA commonly used for bisulfite-conversion. It should be noted that the quantity of mtDNA that is isolated is lower than with extractions of nuclear genomic DNA. The integrity of the isolated mtDNA will impact upon the yield from this column-based approach, and therefore also performance of the samples with downstream applications.
96-well plate formats are available from Zymo Research.
Regions of interest require PCR-amplification prior to pyrosequencing. One of the primers must be 5′-biotin-labeled in order for the DNA to be immobilized on Streptavidin Sepharose beads as part of the sample preparation for pyrosequencing. The primer that requires biotinylation is established as part of the assay design process, as it is dependent upon the sequencing primer and subsequent direction of sequencing. The assay design should also take into account that the starting template is bisulfite-converted DNA and will not therefore contain cytosine residues. We use the MethPrimer online tool (http://www.urogene.org/cgi-bin/methprimer/methprimer.cgi) to design PCR primers for use with pyrosequencing and bisulfite-converted DNA, which gives primers with a very successful rate of PCR amplification (25).
In this protocol, we will use an example of an assay for the mitochondrial-encoded tRNA-Leu gene. The primer sequences are as follows: tRNA-Leu-forward, TAGGGTTTGTTAAGATGGTAGAGTT; tRNA-Leu-reverse (biotinylated), ACAATAAAAAATAAAAAATTAACCATAAAT. The Human mitochondrial DNA sequence can be obtained from NCBI GenBank J01415.2.
Sequencing primer tRNA-Leu-sp: TAGGGTTTGTTAAGATGGTAGAGTT. Sequencing primers for pyrosequencing should be selected in the range between 10 and 15 nucleotides in length, and should be located close to the target CpG(s). The primer sequence should not include CpG sites. The sequencing entry for the pyroassay is: C/tGGTAATC/tGTATAAAATTT.
Do not vortex. Ensure the RBC Lysis Buffer is at room temperature.
If pellet is not white, repeat steps 1 and 2 with a further 1ml RBC Lysis Buffer.
Invert the tube periodically. The incubation can be extended overnight to ensure maximal yield.
Do not store the samples on ice for longer than this.
The precipitated protein should have formed a tight white pellet (when this is not observed, incubate the sample on ice for 5 minutes and repeat the centrifugation).
Optional: incubate at 55–65°C for one hour, followed by an overnight incubation at room temperature to ensure complete resuspension of the genomic DNA
In order to confirm that there is no contamination with nuclear DNA, q-PCR can be performed to detect one gene from the nuclear genome (e.g. hemoglobin, beta) and one from the mitochondrial genome (e.g. chrM:3313-3322). Example q-PCR primer sequences for hemoglobin, beta (HBB) are as follows: Forward primer GCTTCTGACACAACTGTGTTCACTAGC; reverse primer CACCAACTTCATCCACGTTCACC. For the mitochondrial-encoded chrM:3313-3322, the following primer sequences can be used: forward primer, CACCCAAGAACAGGGTTTGT; reverse primer, TGGCCATGGGTATGTTGTTA. Standard q-PCR reaction mixes and cycling conditions can be used (e.g. using iQ SYBR Green Supermix, Bio-Rad), along with standard positive (e.g. nDNA) and negative (e.g. no template) controls. Samples of pure mtDNA should display amplification of the mitochondrial-encoded gene and no amplification of the nuclear gene (Fig. 1).
Each 2 ml tube of CT Conversion Reagent is designed for use with 10 separate bisulfite conversion reactions. CT Conversion Reagent is light-sensitive and should be handled accordingly.
It is normal to see traces of white undissolved crystals of sodium bisulfite or metabisulfite, and this does not result in incomplete bisulfite conversion later.
The quantity of isolated mtDNA is lower than with extractions of nuclear genomic DNA, and therefore the starting DNA input should be optimized for the experiment. We commonly use between 20 ng and 100 ng of mtDNA for bisulfite conversion.
Frequent freeze-thaw cycles may harm bisulfite-converted mtDNA and so this should be minimized, such as through the aliquoting of the sample.
If the yield of PCR products is not sufficient for satisfactory pyrosequencing performance, the input of bisulfite-converted mtDNA can be increased to 2 μl or more.
The bottle containing the Streptavidin Sepharose beads should be gently shaken prior to use until a homogenous solution is obtained.
The green color of the mix does not interfere with the pyrosequencing process later. Take note of the well position of the samples as this order will be the same as pyrosequencing running order.
The Wash buffer is supplied as a 10x solution and should be diluted to a 1x working solution using ultra-pure water.
The Streptavidin Sepharose beads sediment quickly and therefore the capturing of the beads must take place within three minutes of the agitation being terminated.
The probes can be cleaned by flushing through with high purity water and agitating the tool for 10 seconds with the vacuum on. Ensure to release the vacuum once this is completed, and return the tool to the ‘Park’ station.
For capillary dispensing tips, the instrument parameter file is ‘Code 005 CDT’. Details of the instrument parameters can be obtained through Qiagen’s technical support service.
Pyrosequencing data can be extracted and converted to an. EXL file by going to the ‘Analysis Result’ tab and selecting ‘Save’, then ‘All’ to save all the data from the plate. The resulting. TXT file can be exported to an. EXL file by selecting ‘Import Data from a txt file’ in Microsoft Excel, selecting ‘Delimited’ and then the delimiters ‘Tab’ and ‘Semicolon’.
In order to ensure that pyrosequencing performance is consistent and sensitive, custom-designed control oligos may be used. These oligos can be run prior to samples to establish the validity of the assay, or can be run with the samples as controls. Our custom designed control oligo consists of two templates (PSQcon-C: 5′-TATTAGATCGACGGGAACAAACGTTGAATTC -3′ and PSQcon-T: 5′-TATTAGATCAACGGGAACAAACGTTGAATTC -3′) and a common sequencing oligo (PSQcon-SP: 5′-CAACGTTTGTTCCCGT -3′). The mixture for the 100% methylation control can be prepared using 1 μl of PSQcon-C (10 pmol/μl), 0.5 μl of PSQcon-SP (pmol/μl) and 10.5 μl of Annealing Buffer per well of the PSQ HS 96 Plate. The mixture for 0% methylation control can be prepared using 1 μl of PSQcon-T (pmol/μl), 0.5 μl of PSQcon-SP (pmol/μl) and 10.5 μl of Annealing Buffer per well of the PSQ HS 96 Plate. Precede Sequencing Reaction Step 21 with the Sequencing entry C/TGTAT. An example pyrogram is shown as Fig. 3.
Contributor Information
Hyang-Min Byun, Email: hmbyun@hsph.harvard.edu.
Timothy M. Barrow, Email: timothy.barrow@uniklinik-freiburg.de.
References
- 1.Zhang X, Lin S, Funk WE, Hou L. Environmental and occupational exposure to chemicals and telomere length in human studies. Occup Environ Med. 2013;70:743–749. doi: 10.1136/oemed-2012-101350. [DOI] [PubMed] [Google Scholar]
- 2.Dosunmu R, Alashwal H, Zawia NH. Genome-wide expression and methylation profiling in the aged rodent brain due to early-life Pb exposure and its relevance to aging. Mech Ageing Dev. 2012;133:435–443. doi: 10.1016/j.mad.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bodin J, Bolling AK, Becher R, Kuper F, Lovik M, Nygaard UC. Transmaternal Bisphenol A Exposure Accelerates Diabetes Type 1 Development in NOD Mice. Toxicol Sci. 2013 doi: 10.1093/toxsci/kft242. [DOI] [PubMed] [Google Scholar]
- 4.Pan WC, Seow WJ, Kile ML, Hoffman EB, Quamruzzaman Q, Rahman M, et al. Association of low to moderate levels of arsenic exposure with risk of type 2 diabetes in Bangladesh. Am J Epidemiol. 2013;178:1563–1570. doi: 10.1093/aje/kwt195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loane C, Pilinis C, Lekkas TD, Politis M. Ambient particulate matter and its potential neurological consequences. Rev Neurosci. 2013;24:323–335. doi: 10.1515/revneuro-2013-0001. [DOI] [PubMed] [Google Scholar]
- 6.Silins I, Hogberg J. Combined toxic exposures and human health: biomarkers of exposure and effect. Int J Environ Res Public Health. 2011;8:629–647. doi: 10.3390/ijerph8030629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byun HM, Motta V, Panni T, Bertazzi PA, Apostoli P, Hou L, et al. Evolutionary age of repetitive element subfamilies and sensitivity of DNA methylation to airborne pollutants. Part Fibre Toxicol. 2013a;10:28. doi: 10.1186/1743-8977-10-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tarantini L, Bonzini M, Apostoli P, Pegoraro V, Bollati V, Marinelli B, et al. Effects of particulate matter on genomic DNA methylation content and iNOS promoter methylation. Environ Health Perspect. 2009;117:217–222. doi: 10.1289/ehp.11898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bollati V, Baccarelli A, Hou L, Bonzini M, Fustinoni S, Cavallo D, et al. Changes in DNA methylation patterns in subjects exposed to low-dose benzene. Cancer Res. 2007;67:876–880. doi: 10.1158/0008-5472.CAN-06-2995. [DOI] [PubMed] [Google Scholar]
- 10.Byun HM, Baccarelli AA. Environmental exposure and mitochondrial epigenetics: study design and analytical challenges. Hum Genet. 2014 doi: 10.1007/s00439-013-1417-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davidson SM, Duchen MR. Endothelial mitochondria: contributing to vascular function and disease. Circ Res. 2007;100:1128–1141. doi: 10.1161/01.RES.0000261970.18328.1d. [DOI] [PubMed] [Google Scholar]
- 12.Iacobazzi V, Castegna A, Infantino V, Andria G. Mitochondrial DNA methylation as a next-generation biomarker and diagnostic tool. Mol Genet Metab. 2013;110:25–34. doi: 10.1016/j.ymgme.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 13.Chestnut BA, Chang Q, Price A, Lesuisse C, Wong M, Martin LJ. Epigenetic regulation of motor neuron cell death through DNA methylation. J Neurosci. 2011;31:16619–16636. doi: 10.1523/JNEUROSCI.1639-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manev H, Dzitoyeva S, Chen H. Mitochondrial DNA: A Blind Spot in Neuroepigenetics. Biomol Concepts. 2012;3:107–115. doi: 10.1515/bmc-2011-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dzitoyeva S, Chen H, Manev H. Effect of aging on 5-hydroxymethylcytosine in brain mitochondria. Neurobiol Aging. 2012;33:2881–2891. doi: 10.1016/j.neurobiolaging.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun C, Reimers LL, Burk RD. Methylation of HPV16 genome CpG sites is associated with cervix precancer and cancer. Gynecol Oncol. 2011;121:59–63. doi: 10.1016/j.ygyno.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Byun HM, Panni T, Motta V, Hou L, Nordio F, Apostoli P, et al. Effects of airborne pollutants on mitochondrial DNA methylation. Part Fibre Toxicol. 2013b;10:18. doi: 10.1186/1743-8977-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shock LS, Thakkar PV, Peterson EJ, Moran RG, Taylor SM. DNA methyltransferase 1, cytosine methylation, and cytosine hydroxymethylation in mammalian mitochondria. Proc Natl Acad Sci U S A. 2011;108:3630–3635. doi: 10.1073/pnas.1012311108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Infantino V, Castegna A, Iacobazzi F, Spera I, Scala I, Andria G, et al. Impairment of methyl cycle affects mitochondrial methyl availability and glutathione level in Down’s syndrome. Mol Genet Metab. 2011;102:378–382. doi: 10.1016/j.ymgme.2010.11.166. [DOI] [PubMed] [Google Scholar]
- 20.Song L, James SR, Kazim L, Karpf AR. Specific method for the determination of genomic DNA methylation by liquid chromatography-electrospray ionization tandem mass spectrometry. Anal Chem. 2005;77:504–510. doi: 10.1021/ac0489420. [DOI] [PubMed] [Google Scholar]
- 21.Hong EE, Okitsu CY, Smith AD, Hsieh CL. Regionally specific and genome-wide analyses conclusively demonstrate the absence of CpG methylation in human mitochondrial DNA. Mol Cell Biol. 2013;33:2683–2690. doi: 10.1128/MCB.00220-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mikeska T, Bock C, El-Maarri O, Hubner A, Ehrentraut D, Schramm J, et al. Optimization of quantitative MGMT promoter methylation analysis using pyrosequencing and combined bisulfite restriction analysis. J Mol Diagn. 2007;9:368–381. doi: 10.2353/jmoldx.2007.060167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aparicio A, North B, Barske L, Wang X, Bollati V, Weisenberger D, et al. LINE-1 methylation in plasma DNA as a biomarker of activity of DNA methylation inhibitors in patients with solid tumors. Epigenetics. 2009;4:176–184. doi: 10.4161/epi.4.3.8694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hazkani-Covo E, Zeller RM, Martin W. Molecular poltergeists: mitochondrial DNA copies (numts) in sequenced nuclear genomes. PLoS Genet. 2010;6:e1000834. doi: 10.1371/journal.pgen.1000834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li LC, Dahiya R. MethPrimer: designing primers for methylation PCRs. Bioinformatics. 2002;18:1427–1431. doi: 10.1093/bioinformatics/18.11.1427. [DOI] [PubMed] [Google Scholar]