Abstract
Background
With a minimum follow up of 2 years, the TAX324 study demonstrated a significant survival benefit of induction chemotherapy (IC) with docetaxel, cisplatin and 5FU (TPF) versus cisplatin and 5fluorouracil (PF) followed by chemoradiotherapy with carboplatin delivered as sequential therapy (ST) in locally advanced head and neck cancer (LAHNC). We report the long term results with 5 years minimum follow-up.
Methods
TAX324 was a randomized, open-label phase 3 trial comparing three cycles of TPF IC (docetaxel 75 mg/m2 of body-surface area, followed by intravenous cisplatin 100 mg/m2 and 5fluorouracil 1000 mg/m2 per day administered as a continuous 24-hour infusion for 4 days) with three cycles of PF (intravenous cisplatin 100 mg/m2, followed by fluorouracil 1000 mg/m2 per day as a continuous 24-hour infusion for 5 days). Both regimens were followed by 7 weeks of chemoradiotherapy with concomitant weekly carboplatin (AUC1.5). Randomization was performed centrally with the use of a biased-coin minimization technique. At study entry, patients were stratified according to the site of the primary tumor, nodal status (N0 or N1 vs. N2 or N3), and institution. For this long term analysis, data was gathered retrospectively. Overall survival (OS) and progression-free survival (PFS) were the primary endpoints. Data as of December 1, 2008 were analyzed. Tracheostomy and gastric feeding tube dependence were used as surrogates for treatment related long term toxicity. The median follow-up was 72.2 months (mo) (IQR for TPF =33 mo, PF =34 mo and for all pts =34 months). The analysis was based on data from all 501 patients. 61 patients were lost to follow-up and their data as of the initial analysis in 2005 was used.
Findings
OS was significantly better with TPF versus PF (HR=0.74, 95%CI: 0.58–0.94), with an estimated 5-yr survival rate of 0.52 and 0.42 in the TPF and PF arms, respectively. Median survival time was 70.6 mo (95%CI: 49.0–89.0 mo) with TPF versus 34.8 mo (the 95%CI: 22.6–48.0 mo) in the PF group (p=0.014). PFS was also significantly better with TPF (38.1 mo; 95%CI 19.3–66.1 mo vs. 13.2 mo, 95%CI 10.6–20.7 mo; HR= 0.75, 95%CI: 0.60–0.94). Subjects with hypopharyngeal and laryngeal cancer had significantly superior PFS with TPF (HR=0.68, the 95%CI: 0.47–0.98). No significant difference for dependence on gastric feeding tubes and tracheotomies was detected between the treatment groups. In the TPF arm 3 out of 91 patients (3%) remained feeding tube dependent (no information in 40 cases) while 8 out of 71 (10%) patients required feeding tubes in the PF arm (no information in 30 cases). 6 out of 92 (7%) patients had tracheostomies (no information in 39) versus 8/71 (13%) (no information in 30) in the TPF and PF groups, respectively.
Interpretation
IC with TPF provides long term survival benefit compared to PF in LAHNC. Patients who are candidates for IC should be treated with TPF.
Introduction
Squamous-cell carcinoma of the head and neck (HNC) accounts for more than 40,000 newly diagnosed cancer cases per year in the United States and 8% of cancers annually worldwide (1, 2). The majority of patients present with potentially curable, locally advanced disease. Historically, only approximately 50% of these patients live for 3 years following standard therapy and 40 to 60% eventually develop locoregional recurrences, distant metastases or a second primary tumor (3–5).
A variety of strategies combining chemotherapy with surgery and radiotherapy (RT) have been explored to improve outcomes. Concomitant chemoradiotherapy (CRT) and induction chemotherapy/sequential therapy (IC/ST) are current treatment standards for patients with LAHNC to improve survival and for organ preservation (4–9). Currently, several randomized trials are ongoing comparing IC with CRT which should help to define the role of IC. Historically, IC with cisplatin and fluorouracil PF) has demonstrated benefit in LAHNC by reducing tumor size and micrometastases prior to definitive radiotherapy (10, 11). A comprehensive meta-analysis showed that IC with PF significantly improved the rate of survival at 5 years, as compared with standard radiotherapy plus surgery in patients with locally advanced disease (12, 13).
The initial results from randomized trials demonstrate that the addition of docetaxel to IC with PF results in a significant survival benefit in LAHNC. The TAX323 trial in unresectable disease, TAX324 in both resectable and unresectable disease and the GORTEC 2000-1 trial in larynx and hypopharynx cancer all found that TPF IC resulted in improved survival and/or laryngectomy free survival rates compared with treatment with PF (9, 14, 15). The TAX 324 trial compared a sequential plan of IC followed by chemoradiotherapy and demonstrated a significant 30% improvement in survival in patients with resectable and unresectable LAHNC with a median follow up of 42 months when TPF IC was used (9). It is important to determine whether the benefit in survival is durable and the results are sustained for a longer duration. To assess the long term outcomes of IC with TPF, we evaluated patients enrolled on the TAX324 trial with a minimum follow-up of 5 years.
Methods
Patients and data collection
Details of the TAX 324 Phase III trial protocol eligibility have been previously published (9). Between May 21, 1999, and December 3, 2003, patients from 55 centers in the United States, Canada, Argentina, and Europe were analyzed in the initial analysis of TAX 324 and had been randomized to treatment with TPF or PF. For this analysis, data as of December 1, 2008, with a median follow-up time of 72.2 months (mo) (95% CI: 68.8–75.5) was obtained retrospectively. All 55 centers were contacted to provide information on subjects based on review of their medical records. 501 patients were evaluated in the initial and in the updated analysis on an intent-to-treat basis. Among those 234 were dead, and 267 were alive at the time of the initial analysis based on data as of December, 2005. As of December 1, 2008, 171 patients were still alive, 35 died, and 61(12%) were lost to follow-up. No sufficient evidence was found to believe that loss to follow-up was informative or related to any factors associated with lifetime. Table A in the web appendix shows the characteristics of the 61 patients who were lost to follow-up and demonstrates no significant differences between the treatment arms. Table 1 provides on-study (demographic and cancer) characteristics for 501 subjects randomized and treated on the TAX 324 study (255 in the TPF group and 246 in the PF group). More than 80% of the patients were male, and the predominant primary site of disease was the oropharynx. The TPF group had more patients with T4 lesions than did the PF group (49% vs. 37%); subjects’ on-study characteristics were otherwise well balanced The questionnaire to gather clinical information was approved by the Institutional Review Board (IRB) at Dana Farber Cancer Institute and most participating centers obtained IRB approval at their sites for this study as well.
Table 1.
On-study characteristics
| Variable | TPF (N=255) | PF (N=246) |
|---|---|---|
| Age – yr | ||
| Median | 55 | 56 |
| Range | 38–82 | 33–80 |
| Sex – no. (%) | ||
| Male | 215 (84) | 204 (83) |
| Female | 40 (16) | 42 (17) |
| WHO performance status – no. (%) | ||
| 0 | 142 (56) | 126 (51) |
| 1 | 113 (44) | 117 (48) |
| Unknown | 0 | 3 (1) |
| Site of primary tumor – no. (%) | ||
| Hypopharynx | 42 (16) | 34 (13) |
| Larynx | 48 (19) | 42 (17) |
| Oral cavity | 33 (13) | 38 (15) |
| Oropharynx | 132 (52) | 132 (53) |
| Other | 0 | 0 |
| Stage of primary tumor – no. (%) | ||
| T1 | 13 (5) | 9 (4) |
| T2 | 43 (17) | 56 (23) |
| T3 | 74 (29) | 88 (35) |
| T4 | 124 (48) | 90 (37) |
| TX§ | 1 (<1) | 3 (1) |
| Nodal state – no. (%) | ||
| N0 | 42 (16) | 35 (14) |
| N1 | 53 (21) | 49 (20) |
| N2 | 128 (50) | 123 (50) |
| N3 | 32 (13) | 38 (15) |
| NX¶ | 0 | 1 (<1) |
| Overall stage of disease – no. (%) | ||
| III | 41 (16) | 46 (19) |
| IV | 214 (84) | 199 (81) |
| Unknown | 0 | 1 (<1) |
| HPV status – no. (%) | ||
| negative | 81 (32) | 79 (32) |
| positive | 35 (14) | 30 (12) |
| unknown | 139 (55) | 137 (56) |
| Reason for inoperability – no. (%) | ||
| Technical unresectability | 92 (36) | 84 (34) |
| Low surgical curability | 78 (31) | 75 (30) |
| Organ preservation | 85 (33) | 87 (35) |
| Status & | ||
| Alive | 131(51) | 101(41) |
| Dead | 124(49) | 145(59) |
| Progression status | ||
| PD | 142(56) | 164(67) |
| No PD | 113(44) | 82(33) |
PF denotes cisplatin and fluorouracil, TPF docetaxel plus cisplatin and fluorouracil
The proportion of survived is higher in TPF arm compared to PF, p=0.025
Randomization and Masking
In this open label trial, 1:1 randomization was performed centrally with the use of a biased-coin minimization technique at an independent contract research organization (CRO). At study entry, patients were stratified according to the site of the primary tumor, nodal status (N0 or N1 vs. N2 or N3) and institution as described in the initial report (9). For the update of this study, lead investigators from each center collected the data and ensured its accuracy and completeness. Data was entered into the database without knowledge of the treatment assignment and the treatment key was applied at the time of statistical analysis.
Treatment
Induction Chemotherapy
The treatment regimen has been previously described (9). Briefly, patients who were randomized to receive TPF received docetaxel 75 mg/m2 of body-surface area), followed by intravenous cisplatin (100 mg/m2). 5-Fluorouracil (1000 mg/m2 per day) was administered as a continuous 24-hour infusion for 4 days. Patients in the PF arm received intravenous cisplatin (100 mg/m2), followed by fluorouracil (1000 mg/m2 per day as a continuous 24-hour infusion for 5 days. Induction chemotherapy (IC was given every 3 weeks for three cycles. Patients were taken off study for disease progression, unacceptable toxic effects, withdrawal of consent by the patient, or a reduction of less than 25% in tumor size after cycle 2.
Chemoradiotherapy
All patients remaining on protocol were assigned to receive chemoradiotherapy (CRT) beginning 3 to 8 weeks after the start of the third cycle of IC (day 22 to day 56 of cycle 3). Weekly carboplatin at an area under the curve (AUC) of 1.5 was given for a maximum of 7 weekly doses during the course of radiotherapy. The radiation dose administered to the primary tumor was between 70 and 74 Gy (2 Gy per day 5 days per week). Uninvolved lymph nodes were treated with at least 50 Gy and involved lymph nodes received 60 to 74 Gy, depending on whether an elective neck dissection was indicated after completion of treatment. All patients were treated using a 3-field technique; no intensity modulated radiation therapy (IMRT) was used. Of the patients who did not remain on protocol therapy in either arm, the majority received definitive RT or CRT, as previously reported.
Surgery
In patients with an initial nodal stage of N2 and a partial response to IC, N3 disease, or residual disease after CRT, surgery was performed 6 to 12 weeks after completion of CRT. Surgery was also allowed for patients who did not complete CRT and had resectable residual disease at the primary site or in the neck.
Assessments and Outcomes
The primary objective for the current analysis was to assess survival. Overall survival (OS) was calculated from date of randomization until date of death or date last known alive. Progression free survival (PFS) was defined as the length of time from the date of randomization to any recurrent or progressive disease or death in remission for those subjects scored as experiencing events, and until date last known alive and free of disease for those who were censored. The long-term toxicity of the regimen was measured by the presence of a tracheotomy or enteral feeding tube at the time of data collection. A questionnaire was approved by the institutional review board at the Dana Farber Cancer Institute and distributed to all sub-sites. For patients who were lost to follow-up in the US, social security numbers were used to inquire about deaths at the national death registry and for those alive, contact information was retrieved through public records and the patients were contacted. Tracheotomy and PEG dependence were recorded based on all available data sources.
Statistical Analysis
TAX324 had a power of 91% to detect a hazard ratio for death of 0.65 on the basis of an assumed median survival of 43 months in the TPF group and 28 months in the PF group, with use of a two-sided log-rank test at a level of significance of 0.05. A minimum follow-up of 24 months and a total of 227 events were required. A maximum of 250 patients per group were to be recruited on the assumption that 15% would drop out early or be lost to follow-up as described in the initial TAX324 report (9).
The analyses of this update are based on all eligible patients randomized and treated on the TAX 324 protocol (n=501). The Fisher’s exact test and general linear model approach were used to compare patients’ clinical and demographic characteristics between two treatment arms. The method of Kaplan and Meier was used to estimate OS and PFS functions. The log-rank test was used to compare time-to-event (OS, PFS) functions. Patients were evaluated in groups as having received IC with TPF versus PF. Stepwise Cox regression was chosen to model OS and PFS. We used a condition that a variable had to be significant at the 0.25 level before it can be entered into regression model, and that a variable in the model has to be significant at the 0.15 level to remain in the model. All statistical tests were done at the 0.05 level of significance. Estimated parameters are reported with the corresponding 95% confidence intervals (CI). The results are reported at a median follow-up time of 72.2 months. The software used was SAS version 9.2 (SAS Institute, Inc.), and S-plus version 8.1 (TIBCO). TAX324 was registered with clinicaltrials.gov number NCT00273546.
Role of the funding source
This study was initiated by the investigators at the Dana Farber Cancer Institute and was supported by Sanofi-Aventis. The design, collection, analysis and interpretation as well as the writing of the manuscript was conducted independently of Sanofi-Aventis. JL, MP and OG had full access to all of the data and JL had the final responsibility to submit for publication.
Results
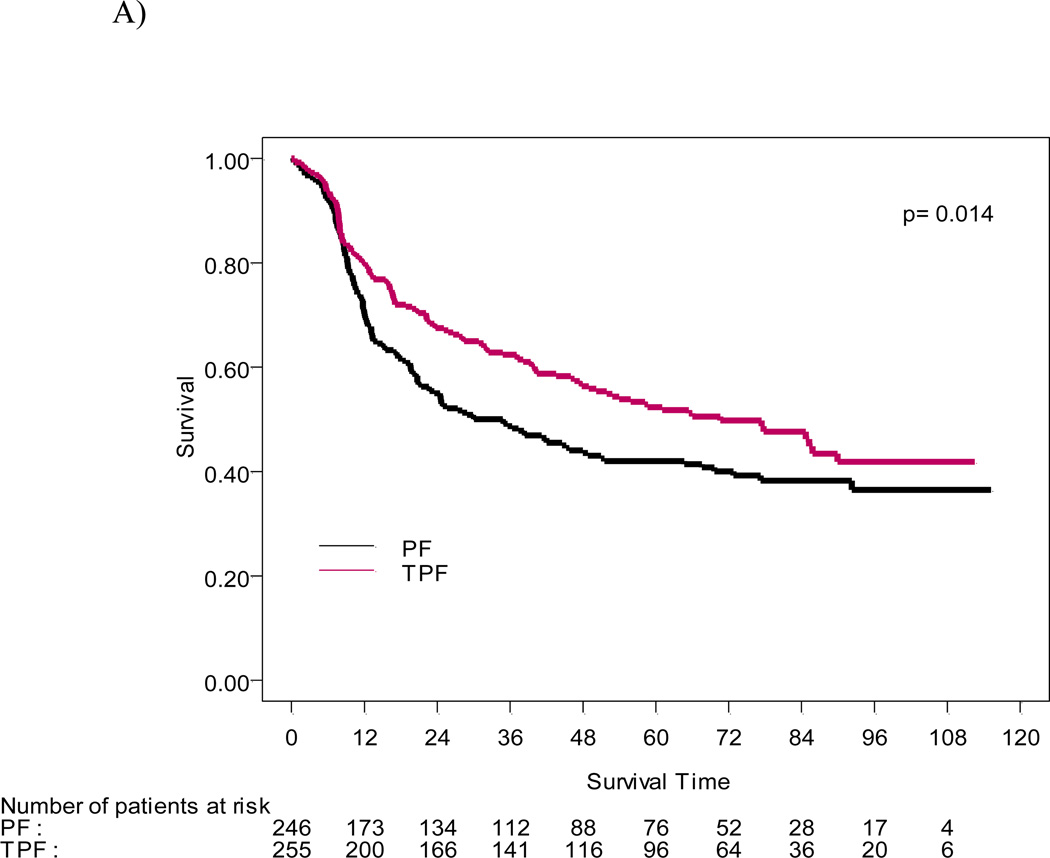
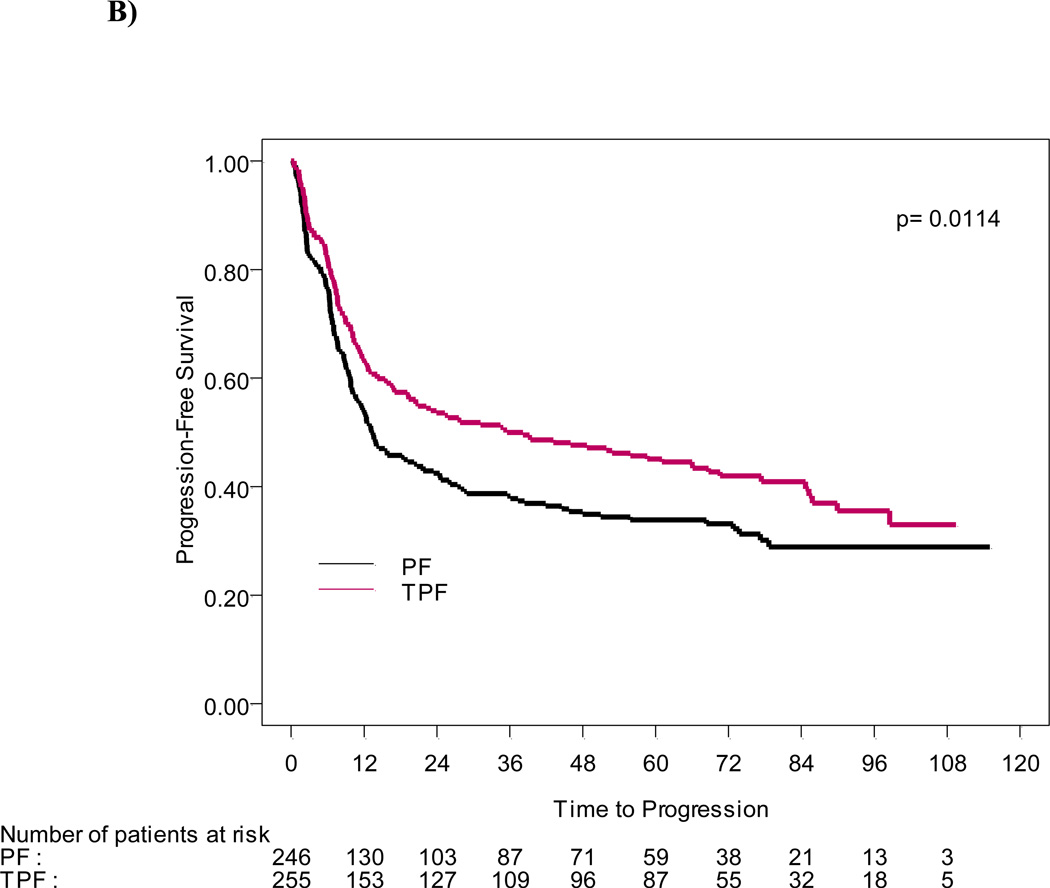
With a median follow-up of 6.0 years (72.2 months), the survival advantage seen in the original report was sustained. Median survival time was 70.6 mo (the 95%CI: 49.0–89.0 mo) in the patient group treated with TPF versus 34.8 mo (the 95%CI: 22.6–48.0 mo) in the PF group (p=0.014), and an estimated 5-yr OS rate of 52% and 42% in TPF and PF arms, respectively. The risk of death was significantly reduced for patients in the TPF arm compared to PF (HR= 0.74, the 95%CI: 0.58–0.94) (Figure 1A). Progression-free survival was also significantly longer in the TPF group (38.1mo; 19.3–66.1 mo) than in patients who received PF the (13.2mo, 95%CI 10.6–20.7mo; HR= 0.75, 95% CI: 0.60–0.94) (Figure 1B).
Figure 1.
A) OS and B) PFS functions estimated by the Kaplan-Meier method for 501 patients treated on TPF and PF arms.
In subgroup analysis by site at the minimum 2-year time point, patients with hypopharyngeal and laryngeal tumors had significantly longer OS and PFS. In the current analysis, patients treated with TPF continued to demonstrate a benefit. Among the 166 patients with hypopharyngeal and laryngeal primary tumors, PFS was significantly longer in the TPF arm (p=0.037, figure 2A). The risk of disease progression for patients treated with TPF was significantly lower than for those in the PF treatment arm; (HR=0.68, 95%CI: 0.47–0.98). The difference in survival for larynx and hypopharynx patients between the two treatment arms was not statistically significant (p=0.11), likely reflecting the effect of salvage surgery after progression for this subset (not shown). Patients treated with TPF had median OS of 51.9 mo, (95%CI: 31.6–85.3 mo) while the group treated with PF had a median OS of 23.5 mo (95%CI: 13.4–42.0 mo; HR=0.73, 95%CI: 0.49–1.08).
Figure 2.
A: PFS functions estimated by the Kaplan-Meier method for 166 patients with hypopharyngeal and laryngeal primary tumor sites
The median PFS for subjects treated on TPF and PF arms are 20.9 mo, (95%CI 12.4–58.7) months and 10.1 mo, (the 95%CI 7.7–13.6) months, respectively; p=0.037, the log-rank test . The HR is 0.68 (the 95%CI 0.47–0.98), corresponding to a 32% reduction in risk for disease progression or death for patients who received TPF.
B: OS for patients with oropharyngeal cancer. OS functions estimated by the Kaplan-Meier method for 264 patients with oropharyngeal cancer. OS was significantly superior in patients with oropharyngeal primary tumors who received induction chemotherapy with TPF (p=0.045, the log rank test).
Although no significant difference between treatment arms could be detected for patients with oropharyngeal primary tumors in the initial analysis, the current analysis revealed that the comparative survival benefit of TPF versus PF became more pronounced over time. The estimated overall survival with TPF by the Kaplan-Meier method was now significantly superior compared to PF (p=0.045, Figure 2B). Median OS has not been reached (NR) in the TPF arm (the 95%CI: 77.4-NR), but it was 64.7 mo in PF arm (HR= 0.69, the 95%CI: 0.48–0.99). There was no statistically discernible difference in OS for patients with tumors of the oral cavity at the minimum of 2 years as well as at the minimum of 5 years follow-up time-point (Table 2).
Table 2.
Overall survival (OS) and progression free survival (PFS) summary
| Variable | TPF (N=255) |
PF (N=246) |
Hazard Ratio (95% CI)† |
P Value‡ |
|---|---|---|---|---|
| Overall survival§ | ||||
| Alive&& | 131 | 101 | ||
| Dead | 124 | 145 | ||
| Median duration of survival (mo) | 71 | 35 | 0.74 (0.58–0.94) | 0.014 |
| Rate – % | ||||
| At 2 yr | 67 | 55 | ||
| At 3 yr | 62 | 49 | ||
| At 5 yr | 52 | 42 | ||
| Median duration of survival according to site of primary tumor (mo) | ||||
| Oropharynx | NR | 65 | 0.69 (0.48–0.99) | 0.045 |
| Hypopharynx | 32 | 20 | 0.74 (0.42–1.30) | 0.29 |
| Larynx | 58 | 25 | 0.72 (0.41–1.24) | 0.23 |
| Oral Cavity | 37 | 14 | 0.89 (0.50–1.59) | 0.7 |
| Hypopharynx&Larynx | 52 | 24 | 0.73(0.49–1.08) | 0.12 |
| Median duration of Survival according to reason for inoperability (mo) | ||||
| Technical unresectability | 48 | 21 | 0.77 (0.52–1.13) | 0.17 |
| Low surgical curability | 86 | 48 | 0.80 (0.51–1.25) | 0.33 |
| Organ preservation | 85 | 42 | 0.61 (0.40–0.94) | 0.03 |
| Median duration according to disease stage (mo) | ||||
| III | 90 | 65 | 0.69 (0.36–1.31) | 0.26 |
| IV | 59 | 25 | 0.74 (0.57–0.97) | 0.03 |
| Median duration according to primary tumor stage ( mo) | ||||
| T1&T2 | NR | 49 | 0.743(0.24–0.78) | 0.005 |
| T3&T4 | 49 | 27 | 0.85 (0.65–1.11) | 0.23 |
| Median duration according to nodal stage ( mo) | ||||
| N0&N1 | 65 | 35 | 0.75 (0.50–1.11) | 0.16 |
| N2&N3 | 78 | 29 | 0.74 (0.55–1.00) | 0.049 |
| Progression-free survival | ||||
| Median duration (mo) | 38 | 13 | 0.75 (0.60–0.94) | 0.011 |
| Rate – % | ||||
| At 2 yr | 54 | 42 | ||
| At 3 yr | 50 | 38 | ||
| At 5 yr | 45 | 34 |
PF denotes cisplatin and fluorouracil, TPF docetaxel plus cisplatin and fluorouracil, and NR not reached.
Hazard ratios are for death in the TPF group as compared with the PF group. Outcomes were as follows: death (in the analysis of overall survival), progression or death (in the analysis of progression-free survival
out of 501 pts 232 (46%) are alive
P values were calculated by the log-rank test.
The median follow-up was 71 months in the TPF group and 72 months in the PF group.
Patients with higher tumor stages (Stage IV) benefited from treatment with TPF and had better overall survival compared with the PF group at the earlier analysis (p=0.02). This difference between treatment arms was sustained at 5 years minimum (p=0.03), while no statistically significant difference was found in patients with stage III disease (p=0.26, table 2). No significant differences between local, loco-regional and distant failures were detected between treatment arms.
A resulting Cox regression survival model included the following risk factors, gender, treatment arm, tumor stage, site of primary tumor, WHO status as well as reason for inoperability. There was no effect of age, or age category (split at 65 years), ethnicity, and nodal status on OS or PFS. Long-term toxicity was also assessed in the current data using the presence of a tracheostomy or gastric feeding tube as a surrogate (Table 3). To ensure reliability of the data, only cases with confirmed tracheotomy and gastric feeding tubes were evaluated and in 140 cases no information about feeding tracheotomy and feeding tube status could be obtained. Three of 91 (3%) patients treated with TPF compared with 8 of 71 (10%) patients with data in the PF arm were dependent on a gastric feeding tube. There was no difference between the two arms in dependence on a gastric feeding tube or the need of tracheostomy. Among patients who had received TPF, 6 out of 92 (7%) with data had a tracheostomy while 8 of 71 (11%) patients with data in the PF arm had a tracheostomy at the time of the last follow-up.
Table 3.
| Toxicity | TPF treated | PF treated | Results of the Fisher’s exact test, the 2-sided p-value |
|---|---|---|---|
| Gastric Feeding Tube | P=0.14 | ||
| No | 88 | 63 | |
| Yes | 3 | 8 | |
| No information | 40 | 30 | |
| Tracheostomy | P=0.60 | ||
| No | 86 | 63 | |
| Yes | 6 | 8 | |
| No information | 39 | 30 | |
Discussion
In this long term analysis of TAX324 with a median follow-up of 6 years, OS and PFS was significantly better in patients treated with TPF versus PF. Hypopharyngeal and laryngeal cancer patients also had significantly superior PFS with TPF and those with oropharyngeal primary tumors in the TPF arm had a OS advantage. Toxicities appeared similar in both treatment arms measured by similar gastric feeding tube dependence rates and number of patients requiring tracheostomies, although no information could be obtained in a relatively large number of cases.
Patients with head and neck cancer most frequently present as locally advanced disease. Despite progress through the use of multimodality treatment involving surgery, radiotherapy and chemotherapy which has become the standard of care in recent years, the survival remains poor and treatment related morbidity is a major problem in long term survivors. Data from early trials and encouraging results from meta-analyses have revived interest in the use of neoadjuvant or induction chemotherapy prior to definitive local treatment. Based on the initial reports of the GORTEC2000-1, TAX323 and TAX324 trials, IC followed by either RT or CRT is frequently used for the treatment of curable LAHNC (9, 14, 15) and TPF has been approved by the Federal Drug Administration (FDA) for the treatment of LAHNC. Nonetheless, the role of IC is still poorly defined and randomized trials are under way to determine whether induction chemotherapy is superior to concomitant chemo/RT.
In this context, the long term analysis of IC trials such as this study is important to determine the consequences of therapy and the durability of the results since 5-year analyses have frequently revealed reduced survival and altered outcomes in head and neck cancer trials (4, 7, 16). Long-term studies in head and neck cancer are challenging: First, the high risk of second primary tumors related to field cancerization as well as morbidity and mortality due to alcohol and tobacco tend to diminish treatment benefits over time(18, 19). Second, late mortality from therapy may also effect survival (20, 21). Nonetheless, long term analysis provides critical data that help us understand the durability and consequences of treatment. For example, in the initial analysis of RTOG 91-11, a larynx preservation trial, upfront bolus cisplatin-based CRT resulted in superior larynx preservation compared to IC with PF and radiotherapy alone while there was no significant difference in laryngectomy free survival and OS. However in the later analysis at 5 years follow-up, laryngectomy free survival was superior for both CRT and IC compared to RT alone. Overall survival continued to be similar in all treatment arms (7). The updated results of our study suggest that the survival benefit of ST with TPF continues well beyond 2 years of the original analysis and was sustained at the same high level, a testimony to the efficacy of TPF as an induction regimen.
Tumor response to therapy and prognosis are influenced by the location of the primary tumor. In the initial analysis of TAX324, patients with oropharyngeal tumors constituted the largest cohort and also enjoyed by far the best outcomes. At 2 years, the difference in overall survival between oropharyngeal patients treated with TPF versus PF did not reach statistical significance. With longer follow up, however, the difference between the treatment groups was sustained and reached statistical significance at a minimum follow-up of 5 years. Similar findings were observed for patients with oropharyngeal primary tumors in the updated analysis of the randomized Phase III trial of RT with or without cetuximab (22). This may be related to the high rate of oropharyngeal cancer associated with human papillomavirus (HPVOPC). HPVOPC responds well to chemotherapy and radiation and these patients generally have a good prognosis(23, 24). Therefore, because of the small numbers of deaths in either treatment arm, a longer follow-up was necessary to allow for a sufficient number of events to detect differences between two already very effective regimens. Furthermore, the lower rate of second primary tumors in HPVOPC contributes to the difference of outcomes over time and reduced mortality as de novo cancer of the aerodigestive tract is less frequent and the efficacy of the regimen is not impacted by second primary tumors.
Age has not been generally recognized as a determinant of outcomes in HNC. Data from a large meta-analysis suggest that the benefit of chemotherapy diminishes with age and younger patients may benefit more from the addition of chemotherapy in the treatment of LAHNC(13). . Our study is not powered sufficiently to determine whether these findings are also true in patients over age 65 or over age 70, where prior studies have not been able to demonstrate a benefit of chemotherapy. Further studies are needed to answer these questions.
Our study has several limitations: TAX324 was a prospective, randomized trial, however, the data for this follow-up analysis were gathered retrospectively. While survival information for 88% of patients could be collected, reliable data on tracheotomy and gastric feeding tubes were difficult to obtain on all patients and therefore limit interpretation. We were also unable to gather reliable quality of life data in this study. It was not possible to determine whether laryngectomies were performed because of late toxicity or as salvage treatment because of disease recurrence, limiting the interpretation of tracheotomy as a surrogate marker for toxicity While the inclusion criteria were designed to reflect common presentations of head and neck cancer, patients with performance status of less than 2 were excluded. Hence this population represents results in a relatively healthy cohort of patients. Nonetheless, this long term analysis confirms the efficacy of TPF IC in patients with LAHNC and provides evidence that subsets of patients with larynx and hypopharynx as well as oropharynx cancer benefit from sequential therapy with the three drug IC regimen. These data confirm TPF as the treatment regimen of choice for induction chemotherapy and that TPF has a lasting impact on survival in LAHNC.
Supplementary Material
Panel: Research in context.
Systematic review
We aimed to identify all prospective therapeutic clinical trials undertaken in locally advanced head and neck cancer with emphasis on induction chemotherapy, published in peer-reviewed journals. We used general search strategies to identify articles in online medical literature databases, primarily in PubMed, including the search terms “clinical trial AND head and neck cancer AND induction chemotherapy”. Articles were individually reviewed. The data were not combined or subjected to meta-analysis.
Interpretation
The observed sustained superiority of TPF over PF further solidifies the role of TPF as induction chemotherapy. Based on these data, patients with a good performance status who are candidates for induction chemotherapy should receive TPF.
Acknowledgement
This study was supported by Sanofi-Aventis. We thank Ana Martinez- Bouteleux and Rosemary Costello R.N. as well as all study staff at the study sites for their help collecting the data.
Footnotes
Author contributions
JL and MP made a substantial contribution to conception and design, acquisition of data and writing of the article and had full access to the raw data. OG and MT made substantial contribution to the statistical analysis and writing of this article. RH made significant contributions to the study concept and writing of this article. JF made significant contributions to the acquisition of data and writing of this article. KC, NS, RT, DS made significant contributions to writing of this article.
Conflict of interest
JL, OG, JF and DS report no conflict of interest.
MT’s academic institution has received research support from Sanofi Aventis.
KC has received travel support from Sanofi-Aventis and his academic institution has received research support from Sanofi Aventis.
RT has received consultancy honoraria and speaker’s fees from Sanofi-Aventis.
RH has received consultant’s fees from Sanofi-Aventis and Bristol-Meyer-Squibb.
NS has been an employee of Sanofi-Aventis until September, 2010 and holds stock of Sanofi-Aventis.
MP has received consultancy fees for participating in Data Safety and Monitoring Boards (DSMB) for Glaxo-Smith-Kline, Amgen, Merck-EMD, Novartis. He has participated in advisory boards for Pfitzer and has received consultancy fees from Biovex. He is holding patents with Allopexx and has received royalties. His academic institution has received research funding from Sanofi-Aventis. He has received honoraria for lectures at ASCO.
Contributor Information
Jochen H. Lorch, Instructor of Medicine, Dana Farber Cancer Instute, 44 Binney Street, Boston MA 02115, Phone: (617) 632 3090, Jochen_Lorch@dfci.harvard.edu.
Olga Goloubeva, Division of Biostatistics, Marlene and Stewart Greenebaum Cancer Center, University of Maryland, Medical School Teaching Facility, Suite 261, 10 South Pine Street, Baltimore, Maryland 21201, Phone: (410) 706 8502, OGoloubeva@som.umaryland.edu.
Robert I. Haddad, Associate Professor, Dana Farber Cancer Institute, 44 Binney Street, Boston MA 02115, Phone: (617) 632 3090, Robert_Haddad@dfci.harvard.edu
Kevin Cullen, Professor of Medicine, University of Maryland School of Medicine, 22 S. Greene Street, Baltimore, MD 21201, Phone: (410) 328 5506, kcullen@umm.edu.
Nicholas Sarlis, Incyte Corporation, Experimental Station, Route 141 & Henry Clay Road, Bldg. E400; Rm. 6220F, Wilmington, DE 19880, Phone: (302)-498-7047, nsarlis@incyte.com.
Roy Tishler, Associate Professor, Dana Farber Cancer Instute, 44 Binney Street, Boston MA 02115, Phone: (617) 632 3090, Roy_Tishler@dfci.harvard.edu.
Ming Tan, Professor of Medicine, Division of Biostatistics, Marlene and Stewart Greenebaum Cancer Center, University of Maryland, Medical School Teaching Facility, Suite 261, 10 South Pine Street, Baltimore, Maryland 21201, Phone: (410) 706 8502, mttan@som.umaryland.edu.
John Fasciano, 28 State Street 25th floor, Boston, MA 02109, Phone : (617) 570 9216, John.Fasciano@mssb.com.
Daniel E. Sammartino, 535 E 14th St. , Apt. 8B, New York, NY 10009, Phone: (401) 316 1674, Daniel_Sammartino@NYMC.edu
Marshall R. Posner, Professor of Medicine, and Professor of Gene and Cell Medicine, The Tisch Cancer Institute, Division of Hematology/Medical Oncology, Mount Sinai School of Medicine, 1 Gustave L. Levy Place, Box 1128, New York, NY 10029, Phone: (212) 659 5461, marshall.posner@mssm.edu
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005 Mar-Apr;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Horner M, Ries L, Krapcho M, Neyman N, Aminou R, Howlader N, et al. SEER Cancer Statistics Review, 1975–2006. National Cancer Institute; Bethesda, MD: 2009. http://seer.cancer.gov/csr/1975_2006/, based on November 2008 SEER data submission. [Google Scholar]
- 3.Adelstein DJ, Li Y, Adams GL, Wagner H, Jr, Kish JA, Ensley JF, et al. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol. 2003 Jan 1;21(1):92–98. doi: 10.1200/JCO.2003.01.008. [DOI] [PubMed] [Google Scholar]
- 4.Denis F, Garaud P, Bardet E, Alfonsi M, Sire C, Germain T, et al. Final results of the 94-01 French Head and Neck Oncology and Radiotherapy Group randomized trial comparing radiotherapy alone with concomitant radiochemotherapy in advanced-stage oropharynx carcinoma. J Clin Oncol. 2004 Jan 1;22(1):69–76. doi: 10.1200/JCO.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 5.Haddad RI, Shin DM. Recent advances in head and neck cancer. N Engl J Med. 2008 Sep 11;359(11):1143–1154. doi: 10.1056/NEJMra0707975. [DOI] [PubMed] [Google Scholar]
- 6.Lefebvre JL, Rolland F, Tesselaar M, Bardet E, Leemans CR, Geoffrois L, et al. Phase 3 randomized trial on larynx preservation comparing sequential vs alternating chemotherapy and radiotherapy. J Natl Cancer Inst. 2009 Feb 4;101(3):142–152. doi: 10.1093/jnci/djn460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forastiere A, Maor M, Weber R, Pajak TF, et al. Long term results of Intergroup RTOG 91-11: A phase III trial to preserve the larynx- Induction cisplatin/5FU and radiation therapy versus concurrent cisplatin and radiatio therapy versus radiation therapy. Proceedings of the American Society of Clinical Oncology. 2006:5517. [Google Scholar]
- 8.Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006 Feb 9;354(6):567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 9.Posner MR, Hershock DM, Blajman CR, Mickiewicz E, Winquist E, Gorbounova V, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007 Oct 25;357(17):1705–1715. doi: 10.1056/NEJMoa070956. [DOI] [PubMed] [Google Scholar]
- 10.Domenge C, Hill C, Lefebvre JL, De Raucourt D, Rhein B, Wibault P, et al. Randomized trial of neoadjuvant chemotherapy in oropharyngeal carcinoma. French Groupe d'Etude des Tumeurs de la Tete et du Cou (GETTEC) Br J Cancer. 2000 Dec;83(12):1594–1598. doi: 10.1054/bjoc.2000.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paccagnella A, Orlando A, Marchiori C, Zorat PL, Cavaniglia G, Sileni VC, et al. Phase III trial of initial chemotherapy in stage III or IV head and neck cancers: a study by the Gruppo di Studio sui Tumori della Testa e del Collo. J Natl Cancer Inst. 1994 Feb 16;86(4):265–272. doi: 10.1093/jnci/86.4.265. [DOI] [PubMed] [Google Scholar]
- 12.Pignon JP, Bourhis J, Domenge C, Designe L. Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: three meta-analyses of updated individual data. MACH-NC Collaborative Group. Meta-Analysis of Chemotherapy on Head and Neck Cancer. Lancet. 2000 Mar 18;355(9208):949–955. [PubMed] [Google Scholar]
- 13.Pignon JP, le Maitre A, Maillard E, Bourhis J. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009 Jul;92(1):4–14. doi: 10.1016/j.radonc.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 14.Vermorken JB, Remenar E, van Herpen C, Gorlia T, Mesia R, Degardin M, et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. 2007 Oct 25;357(17):1695–1704. doi: 10.1056/NEJMoa071028. [DOI] [PubMed] [Google Scholar]
- 15.Calais G, Pointreau Y, Alfonsi M, Sire C, Tuchais C, Tortochaux J, et al. Randomized phase III trial comparing induction chemotherapy using cisplatin (P) fluorouracil (F) with or without docetaxel (T) for organ preservation in hypopharynx and larynx cancer. Preliminary results of GORTEC 2000-01. Proceedings of the American Society of Clinical Oncology. 2006;24:5506. [Google Scholar]
- 16.Taylor SGt, Murthy AK, Vannetzel JM, Colin P, Dray M, Caldarelli DD, et al. Randomized comparison of neoadjuvant cisplatin and fluorouracil infusion followed by radiation versus concomitant treatment in advanced head and neck cancer. J Clin Oncol. 1994 Feb;12(2):385–395. doi: 10.1200/JCO.1994.12.2.385. [DOI] [PubMed] [Google Scholar]
- 17.Pointreau Y, Garaud P, Chapet S, Sire C, Tuchais C, Tortochaux J, et al. Randomized Trial of Induction Chemotherapy With Cisplatin and 5-Fluorouracil With or Without Docetaxel for Larynx Preservation. J Natl Cancer Inst. 2009 Apr 1;101(7):498–506. doi: 10.1093/jnci/djp007. 2009. [DOI] [PubMed] [Google Scholar]
- 18.Cooper JS, Pajak TF, Rubin P, Tupchong L, Brady LW, Leibel SA, et al. Second malignancies in patients who have head and neck cancer: incidence, effect on survival and implications based on the RTOG experience. Int J Radiat Oncol Biol Phys. 1989 Sep;17(3):449–456. doi: 10.1016/0360-3016(89)90094-1. [DOI] [PubMed] [Google Scholar]
- 19.Jones AS, Morar P, Phillips DE, Field JK, Husband D, Helliwell TR. Second primary tumors in patients with head and neck squamous cell carcinoma. Cancer. 1995 Mar 15;75(6):1343–1353. doi: 10.1002/1097-0142(19950315)75:6<1343::aid-cncr2820750617>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 20.Smith GL, Smith BD, Buchholz TA, Giordano SH, Garden AS, Woodward WA, et al. Cerebrovascular Disease Risk in Older Head and Neck Cancer Patients After Radiotherapy. J Clin Oncol. 2008 Nov 1;26(31):5119–5125. doi: 10.1200/JCO.2008.16.6546. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Avraham E, Teresa L, Carol RB, Laura AD, Marc JH, Amy EM, et al. Objective assessment of swallowing dysfunction and aspiration after radiation concurrent with chemotherapy for head-and-neck cancer. International journal of radiation oncology, biology, physics. 2002;53(1):23–28. doi: 10.1016/s0360-3016(02)02712-8. [DOI] [PubMed] [Google Scholar]
- 22.Bonner JA, Harari PM, Giralt J, Cohen RB, Jones CU, Sur RK, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer, 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2009 Nov;6(11):21–28. doi: 10.1016/S1470-2045(09)70311-0. [DOI] [PubMed] [Google Scholar]
- 23.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010 Jul 1;363(1):24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rischin D, Young RJ, Fisher R, Fox SB, Le QT, Peters LJ, et al. Prognostic significance of p16INK4A and human papillomavirus in patients with oropharyngeal cancer treated on TROG 02.02 phase III trial. J Clin Oncol. 2010 Sep 20;28(27):4142–4148. doi: 10.1200/JCO.2010.29.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.