Abstract
AIM: To investigate the activity of vesicular glutamate transporter-3 (VGLUT3) in a visceral hyperalgesia rat model of irritable bowel syndrome, and the role of mast cells (MCs).
METHODS: Transient intestinal infection was induced by oral administration of Trichinella spiralis larvae in rats. On the 100th day post-infection (PI), the rats were divided into an acute cold restraint stress (ACRS) group and a non-stressed group. Age-matched untreated rats served as controls. The abdominal withdrawal reflex was used to measure the visceromotor response to colorectal distension (CRD). The expression levels of VGLUT3 in peripheral and central neurons were analyzed by immunofluorescence and western blotting.
RESULTS: VGLUT3 expression in the L6S1 dorsal root ganglion cells was significantly higher in the PI group than in the control group (0.32 ± 0.009 vs 0.22 ± 0.008, P < 0.01), and there was no significant difference in the expression of VGLUT3 between MC-deficient rats and their normal wild-type littermates. Immunofluorescence showed that the expression levels of VGLUT3 in PI + ACRS rats were enhanced in the prefrontal cortex of the brain compared with the control group.
CONCLUSION: VGLUT3 is involved in the pathogenesis of visceral hyperalgesia. Coexpression of c-fos, 5-hydroxytryptamine and VGLUT3 after CRD was observed in associated neuronal pathways. Increased VGLUT3 induced by transient intestinal infection was found in peripheral nerves, and was independent of MCs. Moreover, the expression of VGLUT3 was enhanced in the prefrontal cortex in rats with induced infection and stress.
Keywords: Vesicular glutamate transporter-3, Irritable bowel syndrome, Mast cell, Infection, Stress, Neuron
Core tip: It has been shown that visceral hyperalgesia in response to various stimuli from the gut of irritable bowel syndrome (IBS) patients is an important factor in abdominal pain. Our research indicated that the vesicular glutamate transporter 3 (VGLUT3), which concentrates the excitatory neurotransmitter glutamate into synaptic vesicles in peripheral and central neurons, is involved in the pathogenesis of visceral hyperalgesia. An infection-induced increase in VGLUT3 may be one of the main reasons for visceral hyperalgesia in IBS patients.
INTRODUCTION
Irritable bowel syndrome (IBS) is a common gastrointestinal disorder. Patients commonly present with abdominal pain associated with altered bowel habits. However, these symptoms are very difficult to manage effectively. Underlying visceral hyperalgesia due to various stimuli has been implicated in the abdominal pain of IBS patients. The modulatory function of the nervous system, including the brain-gut axis and mediators, has been thought to be important in visceral hyperalgesia associated with the gut[1].
The excitatory neurotransmitter glutamate has been shown to mediate the pain transmission process in the visceral afferent neuronal pathways, and vesicular glutamate transporters (VGLUTs) have been shown to be the most important molecules in concentrating glutamate into synaptic vesicles[2,3]. VGLUT1 and VGLUT2 are the most reliable markers for glutamatergic neurons in the adult rodent brain. VGLUT1 is expressed mainly in the cerebral and cerebellar cortices and hippocampus, as well as in certain thalamic nuclei, whereas VGLUT2 is expressed mainly in deeper brain regions including the thalamus, hypothalamus and brainstem[2,4].
VGLUT3 differs from VGLUT1 and VGLUT2 in its expression and intracellular location; for example, it is expressed in neurons classically defined by the presence of another transmitter, such as serotonin, dopamine, gamma-aminobutyric acid, and acetylcholine[3,5,6]. Thus, it is presumed that the function of VGLUT3 is more complicated than the functions of VGLUT1 and VGLUT2.
Seal et al[7] used Vglut3 gene knockout mice (Vglut3-/-), for the first time to evaluate the function of VGLUT3 primary afferent neurons in somatic sensory input. This study yielded the following results: (1) in the absence of injury or inflammation, no significant differences were observed in the response to testing with Von Frey hairs between Vglut3-/- mice and wild type littermates; and (2) in a model of inflammation induced by the injection of carrageenan into the hindpaw, the threshold for withdrawal from stimulation was significantly decreased in wild type mice, but only small changes occurred in Vglut3-/- mice. This indicated that the loss of Vglut3 specifically impairs mechanical hyperalgesia in response to normally innocuous stimuli that accompany inflammation. The activation of spinal neurons has been observed when the pudendal sensory nerve or pelvic nerve receive stimulation, which also indicates that VGLUT3 plays a role in convergent somatic and visceral sensory pathways[8].
A previous study has confirmed that VGLUT3 is involved in conduction of visceral pain sensations and visceral hyperalgesia in rats[9]. Moreover, we observed that visceral hyperalgesia induced by acute cold restraint stress (ACRS), but not infection, was dependent on mast cells (MCs)[10]. The present study was therefore carried out to investigate the expression of VGLUT3 and the relationship between VGLUT3 and MCs in peripheral nerves in visceral hyperalgesia in rats, as well as the changes in VGLUT3 in the brains of rats.
MATERIALS AND METHODS
Animals
Specific pathogen male MC-deficient rats (Ws/Ws) and their normal wild-type littermates (+/+) (TGC Inc., Kanagawa, Japan) were housed in standard polypropylene cages containing 2.5 cm wood chip bedding material. Cages were maintained at 22 °C with an automatic 12 h light/dark cycle. Rats received a standard laboratory diet and tap water ad libitum. Experiments were carried out when the rats reached 12 wk of age. All procedures were preformed in such a manner as to minimize the number of animals used and to minimize suffering. All procedures were approved by the Animal Care Committee of Peking University.
Visceral hyperalgesia induced by transient T. spiralis
Rats were infected by administration of 1.0 mL of 0.9% saline solution containing 1500 T. spiralis larvae by gavage. An equivalent volume of vehicle (saline) was administered to the control rats. These larvae were isolated from the skeletal muscle of infected Kunming mice after digestion with standard 2.5% pepsin-0.5% HCl solution, as described by some researchers. The rats then had a recovery period of 100 d following T. spiralis administration (post-infection, PI).
Visceral hyperalgesia induced by ACRS
Briefly, 100 d after recovery, one half of the controls and one half of the PI rats were restrained in individual polymethyl methacrylate restraint cages, and these animals were designated as ACRS and PI + ACRS groups and placed in a cold room at 4 °C for 2 h. ACRS was routinely performed from 8:00 am to 10:00 am.
Visceromotor response to colorectal distension
After an overnight fast, the animals were lightly anesthetized with diethyl ether, and a disposable silicon balloon urethral catheter (6 Fr, Sewoon, South Korea) was lubricated and placed into the rat’s distal colon so that the tip of the balloon was 1 cm proximal to the anus. The catheter was taped to the base of the tail to prevent displacement. Rats were restrained in a plastic containment device and were allowed to acclimate for 10 min before testing. Sensitivity to colorectal distension (CRD) was determined using the abdominal withdrawal reflex (AWR) as widely described previously. The rats received a standard CRD procedure, in which the first balloon dilation was 1 mL, followed by increasing phases of distension (in 0.2 mL increments) applied for 20 s every 5 min until the AWR score reached 3. This evaluation was performed by three independent observers, and the AWR score was assigned as follows: 0 = no behavioral response to distension; 1 = brief head movements followed by cessation of movement; 2 = contraction of abdominal muscle without lifting of abdomen; 3 = lifting of abdomen; and 4 = body arching and lifting of the pelvic structure.
Immunofluorescence
L6S1 dorsal root ganglions (DRGs) and brains were removed and frozen tissue sections (10 μm) were post-fixed with acetone (-20 °C, 10 min) and permeabilized with 1% Triton X-100 for 2 h, and then blocked with 10% normal goat serum in PBS with 0.3% Triton X-100 for 30 min at room temperature. Sections were incubated overnight at 4 °C with guinea pig anti-VGLUT3 antibodies (1:5000, Millipore, Bedford, MA, United States), rabbit anti-c-fos antibodies (1:200, Santa Cruz Biotechnology, Santa Cruz, CA, United States) and rat anti-5-hydroxytryptamine (5-HT) (1:50, Abcam, Cambridge, MA, United States), respectively. After washing, sections were then incubated for 30 min at 37 °C with rhodamine-labeled goat anti-guinea pig (1:100, KPL, Gaithersburg, MD, United States) antibodies, FITC-conjugated goat anti-rat (1:100) antibodies and FITC-conjugated goat anti-rabbit (1:100) antibodies (Sigma). Sections were counterstained with DAPI-Fluoromount (SouthernBiotech, Birmingham, AL, United States). Digital images of five slices per individual sample per animal were captured under identical parameters for each with fluorescence microscopy (Leica DM3000, Leica Microsystems, Wetzlar, Germany). The mean gray level intensity for a region of interest in these images was determined using the Image Pro Plus 6.0 image analysis software system (Media Cybernetics, Silver Spring, MD, United States).
Western blot analysis
The L6S1 DRGs and the brain were quickly removed, and the prefrontal cortex (PFC), Raphé nuclei, striatum and hippocampal regions were dissected in a cold atmosphere (0-4 °C) and stored at -80 °C before use. Frozen tissues were homogenized in ice-cold RIPA lysis buffer containing 50 mmol/L Tris, pH 7.4, 150 mmol/L NaCl, 1% v/v Triton X-100, 1% sodium deoxycholate, 1% SDS, and ‘‘Complete’’, mini, EDTA-free protease inhibitor cocktail (Roche Diagnostics, Mannheim, Germany). Proteins were separated with 10% SDS-polyacrylamide gel electrophoresis and then transferred to PVDF membranes (BioRad) at 200 mA for 2 h. Nonspecific binding sites were blocked at room temperature with 0.1% (PBS-T) containing 5% nonfat milk for 1 h. Blots were then incubated overnight at 4 °C with a primary antibody in PBS-T containing 2% non-fat milk. The two primary antibodies used were guinea pig anti-VGLUT3 antibodies (1:500, Millipore), and rabbit anti-β-actin antibodies (1:2000, CWBIO, Shanghai, China). The membranes were then incubated with a secondary antibody (IRDye 800CW conjugated donkey anti-guinea pig, 1:10000; IRDye 800CW conjugated goat anti-rabbit, 1:10000, LI-COR Biotechnology, Lincoln, NE, United States) for 1 h at room temperature in darkness. Images of the bands in the membranes were captured and analyzed with a LI-COR odyssey scanner (LI-COR Biotechnology). The relative expression of each protein was calculated as the ratio of signal density to β-actin density.
Statistical analysis
Data are presented as the mean ± SE. The statistical significance of data was determined using one-way analysis of variance (ANOVA) followed by a least significant difference test or Student-Newman-Keuls test. The differences in immunoreactive neurons after CRD between groups were analyzed with the Student t test. Statistical calculations were performed using SPSS version 13.0 (SPSS Inc., Chicago, IL, United States). A P-value < 0.05 was considered significant.
RESULTS
Visceral hyperalgesia induced by ACRS dependent on MCs
In response to CRD, the distension volume to reach an AWR score of 3 was significantly lower in +/+ rats after transient T. spiralis intestinal infection (P < 0.05) and ACRS (P < 0.05). Although T. spiralis intestinal infection decreased the visceral threshold of pain sensitivity to CRD in Ws/Ws rats (P < 0.01), it seemed to have no effect on pain triggered by ACRS in these rats (Figure 1).
Figure 1.
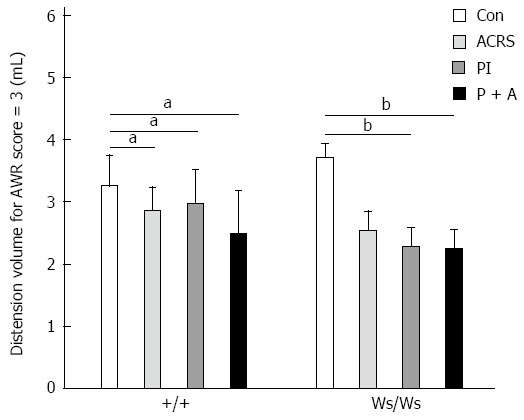
Distension volumes needed to reach an abdominal withdrawal reflex score of 3. Mean ± SE, n = 6; aP < 0.05, bP < 0.01 vs control group. Con: Control group; PI: Post-infection; ACRS: Acute cold restraint stress; P + A: PI + ACRS group; AWR: Abdominal withdrawal reflex.
Coexpression of VGLUT3 with c-fos and 5-HT in rats with CRD
In this study, we examined the co-localization of VGLUT 3 immunoreactivity (ir) with serotonin-ir in L6S1 DRGs in rats with CRD. L6S1 DRG sections from rats were fluorescently labeled for serotonin-ir and VGLUT3-ir. The results indicated that some serotonergic cells of the L6S1 DRGs also expressed VGLUT3 (Figure 2). Co-distribution of c-fos-ir and VGLUT3-ir was also found in the L6S1 spinal dorsal horn in rats with CRD (Figure 3).
Figure 2.
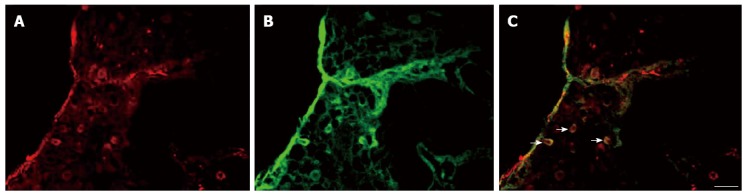
Coexpression of vesicular glutamate transporter-3 and 5-hydroxytryptamine in L6S1 dorsal root ganglions. A: Vesicular glutamate transporter-3 (VGLUT3) positive cells; B: 5-hydroxytryptamine positive cells; C: Merged images of A and B. Scale bar: 100 μm.
Figure 3.
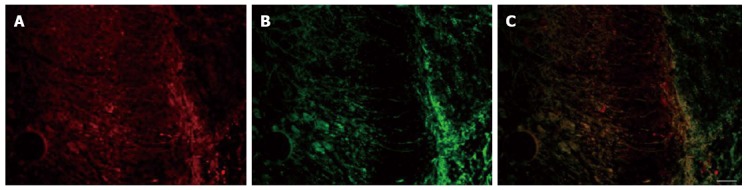
Coexpression of vesicular glutamate transporter-3 and c-fos in the L6S1 spinal dorsal horn. A: Vesicular glutamate transporter-3 (VGLUT3)-positive cells; B: C-fos-positive cells; C: Merged images of A and B. Scale bar: 100 μm.
Increased VGLUT3 expression induced by T. spiralis infection in peripheral neurons was not dependent on MCs
VGLUT3-ir signals were observed in L6S1 DRGs, as shown in Figure 4A-C. It is clear that VGLUT3-ir was mainly found in the perikaryon (but not the nucleus) of ganglion cells of DRG neurons. Western immunoblot analysis was used to further confirm the changes in VGLUT3 expression in the L6S1 DRGs. Compared with the control group from +/+ rats, a significant increase in the level of VGLUT3 protein was observed in the L6S1 DRGs in post T. spiralis-infected rats (PI vs control: 0.32 ± 0.009 vs 0.22 ± 0.008, P < 0.01; PI + ACRS vs control: 0.32 ± 0.015 vs 0.22 ± 0.008, P < 0.01), but an increase was not found in ACRS rats (0.22 ± 0.011). Compared with the control group of Ws/Ws rats, a significant increase in the level of VGLUT3 protein was also observed in the L6S1 DRGs in post T. spiralis-infected rats (PI vs control: 0.30 ± 0.011 vs 0.22 ± 0.009, P < 0.01; PI + ACRS vs control: 0.31 ± 0.011 vs 0.22 ± 0.009, P < 0.01), and an increase was not found in ACRS rats (0.21 ± 0.010), as shown by quantitative densitometry evaluation of these immunoblots (Figure 5).
Figure 4.
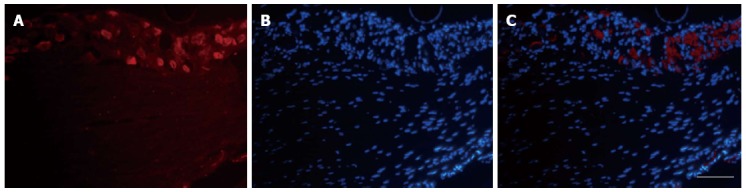
Expression of vesicular glutamate transporter-3 in L6S1 dorsal root ganglions neurons in rats with colorectal distension. A: Representative immunofluorescence images of vesicular glutamate transporter-3 (VGLUT3) immunoreactivity (ir)-positive neurons in L6S1 dorsal root ganglions (DRGs); B: Cell nuclei staining in DRGs; C: Merged images of A and B. Scare bar: 100 μm.
Figure 5.
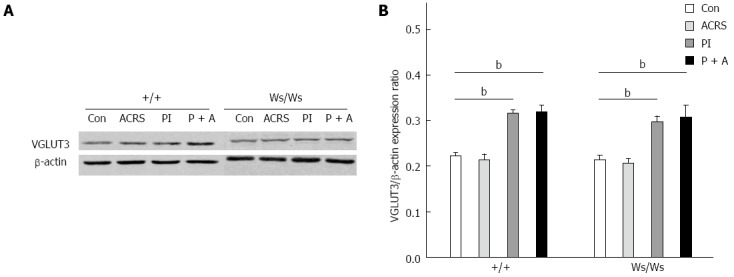
Expression of vesicular glutamate transporter-3 protein in L6S1 dorsal root ganglions neurons in +/+ and Ws/Ws rats. A: Representative western blotting for VGLUT3 in L6S1 DRG extracts; B: Quantitative analysis of vesicular glutamate transporter-3 (VGLUT3) protein. Data are expressed as normalized density to β-actin. bP < 0.01 vs control group.
Changes in VGLUT3 expression in four regions of the brain
Immunofluorescence and immunoblot methods were used to examine the expression of VGLUT3 in control and model rats. As illustrated in Figure 6A-D, VGLUT3-immunolabeled cells were identical in these regions of the brain. With quantification of VGLUT3-ir labeling intensity in different regions of the brain (Figure 6E), it was evident that the labeling intensity of VGLUT3-ir positive neurons was significantly higher in the PFC of PI + ACRS rats compared with control rats (219 ± 14.5 vs 115 ± 10.1, F = 6.4; P = 0.005). No significant differences were found in the VGLUT3-ir signals in the Raphé nuclei, striatum and hippocampus regions of the brain in the rats.
Figure 6.
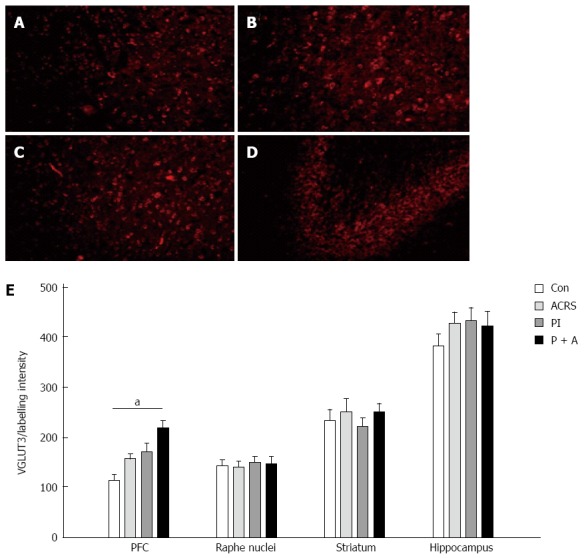
Representative immunofluorescence images of vesicular glutamate transporter-3-ir positive neurons in different brain regions. A: Prefrontal cortex; B: Raphé nuclei; C: Striatum; D: Hippocampus; E: Quantification of vesicular glutamate transporter-3 (VGLUT3)-3-ir labeling intensity in the different regions of the brain. aP < 0.05. Original magnification, A-D: × 200.
DISCUSSION
The present study confirmed that VGLUT3 was co-localized with 5-HT and c-fos in rats with CRD. Increased expression of VGLUT3 in the peripheral primary afferent neurons was observed in rats with intestinal T. spiralis infection but did not occur after acute stress, and was not dependent on MCs. Increased expression of VGLUT3 in the brain was also observed in rats with infection and stress.
IBS likely involves a complex set of interactions between the central and peripheral nervous systems that affect a patient’s tolerance to pain signals from the viscera. It has been demonstrated that intestinal infections and stressful events are the most common causes of IBS. T. spiralis infection has been used as an animal model of intestinal infections in some studies[11]. The larvae of the parasites invade the duodenal and jejunal mucosa causing severe inflammation accompanied by histological damage, with subsequent spontaneous recovery in approximately 6 to 8 wk. However, visceral hyperalgesia lasts for a prolonged time. Some studies have also confirmed that stressful life experiences, particularly responses to stressors, are significantly associated with the development of IBS[12]. Therefore, we used these animal models to further verify the role of VGLUT3 in peripheral and central neurons in the process of visceral hyperalgesia. Indeed, glutamate is one of the important excitatory neurotransmitters in the central nervous system. In the dorsal horn, glutamate is released by primary afferent fibers and local excitatory interneurons and participates in sensory transmission[13]. Central branches of primary afferent fibers arise from the DRG and end in the dorsal horn of the spinal cord.
Earlier studies have shown that VGLUT3 has more complex functions than VGLUT1 and T2. In previous investigations, VGLUT1 and T2 were not modulated in the striatum and the substantia nigra of the 6-hydroxydopamine-lesioned rat, and only VGLUT3 played a role in the pathogenesis of Parkinson’s disease[14]. In addition, one study found increased expression of VGLUT in the cortex and caudate-putamen of rats subjected to transient middle cerebral artery occlusion[15]. Western blot and immunohistochemistry revealed an increase in the VGLUT1 signal in the cortex and caudate-putamen until 3 d of reperfusion followed by a reduction 7 d after the ischemic insult. In contrast, VGLUT2 and VGLUT3 were drastically reduced. Confocal microscopy revealed an increase in VGLUT2 and VGLUT3 immunolabeling in the reactive astrocytes of the ischemic corpus callosum and cortex. Other data have shown that VGLUT3, but not VGLUT1 and T2, can significantly increase the Ca2+-dependent release of glutamate from astrocytes[16]. Seal et al[17] determined that VGLUT3 is encoded by the SLC17A8 gene and transports the neurotransmitter into synaptic vesicles before it is released into the synaptic cleft. The genetic deletion of Slc17a8 in mice results in profound deafness as a result of a loss of transmission between the sensory hair cells in the inner ear and the afferent sensory neurons. In addition, they found that VGLUT3-deficient mice exhibited nonconvulsive seizures, suggesting VGLUT3 plays a modulatory role in the network function of interneurons[18]. Numerous studies have demonstrated a variable distribution of VGLUT3 in the mammalian brain. In the present study, we focused on regions of the brain that are responsive to proximal colon distension or were previously determined to contain large populations of glutamatergic neurons such as the PFC, Raphé nuclei, striatum and hippocampus[3,4,19,20]. Our study demonstrated that transient intestinal infection causes an increase in VGLUT3-ir signals in the PFC region of the brain, but not in the Raphé nuclei, striatum and hippocampus regions. Although VGLUT3 protein in the PFC and Raphé nuclei was not measured, no significant differences were found in the expression of VGLUT3 protein in the striatum and hippocampus region. This result may be similar to that in which expression of the VGLUT2 was increased in the cortex of genetic-absence epileptic rats[21]. Some results also demonstrated augmented colorectal distension-induced PFC activity in rats similar to that observed in IBS patients[22]. Therefore, our results suggest that visceral hyperalgesia may be associated with the expression of VGLUT3 in the DRGs and PFC region of the brain. In addition, it has been reported that acute stress significantly increased extracellular glutamate levels, and VGLUT1 immunoreactivity was increased in the hippocampus after 10 d of chronic unpredictable stress[23]. Thus, VGLUT3 may play a different role on a functional level compared with VGLUT1 and VGLUT2.
The influence of VGLUT3 on the peripheral nervous system has also been discussed in some studies. Linke et al[24] applied immunocytochemistry to examine the distribution of immunoreactivity of all three VGLUTs during prenatal development of the myenteric plexus in the human small intestine. They found that VGLUTs were predominantly located in the ganglionic neuropil, interganglionic varicose fibers and perisomatic puncta, but cytoplasmic labeling of differing intensities was also found. VGLUT3-ir was less abundant in the developing myenteric plexus than VGLUT1 and VGLUT2-ir. It was mainly expressed in the ganglionic neuropil and in the perisomatic puncta throughout the gestational period under evaluation. The aortic depressor nerve (ADN) primarily transmits baroreceptor signals from the aortic arch to the nucleus tractus solitarii. Cell bodies of neurons that send peripheral fibers to form the ADN are located in the nodose ganglion. VGLUT3-ir containing neurons transmit cardiovascular signals via the ADN to the brain stem[25]. DRGs are the primary afferent neuron in the information transmission of visceral sensation. Our study indicates that intestinal infection rather than ACRS caused the upregulation of VGLUT3 expression in DRG neurons.
Some studies have demonstrated that MCs have a close relationship with visceral hyperalgesia[26,27]. An increased number of MCs can be observed in the colonic mucosa of a subset of IBS patients[28]. In our previous study, we demonstrated that the upregulation of mediators (PAR2 and NGF) and signal proteins (pERK1/2 and TRPV1) has a close relationship with the presence of MCs. MCs also play an important role in visceral hyperalgesia induced by infection in rats[10,12]. Our studies have indicated that the increased expression of VGLUT3 in rats with visceral hyperalgesia induced by infection is not dependent on MCs. Thus, MCs are not the main cause of increased VGLUT3 in rats.
In summary, the present study confirmed that increased expression of VGLUT3 occurred in peripheral neurons in rats with visceral hyperalgesia, and there was no relationship between the changes in VGLUT3 and MCs. Enhanced VGLUT3-ir was observed in rats with visceral hyperalgesia induced by infection and stress. When the rats received stimulation from the gut, coexpression of VGLUT3 with c-fos and 5-HT was observed in peripheral and central neurons.
COMMENTS
Background
Irritable bowel syndrome (IBS) is a highly prevalent gastrointestinal disorder that is often accompanied by visceral hyperalgesia. Primary sensory neurons convey the sensation of pain from the gut to the spinal cord using the excitatory transmitter glutamate, and vesicular glutamate transporters-3 (VGLUT3) is the most important molecule in concentrating glutamate into synaptic vesicles. The activity of VGLUT3 in central and peripheral nerves in visceral hyperalgesia rats and the role of mast cells have not been determined.
Research frontiers
VGLUT3 may be specifically involved in mechanical pain sensation, and in particular in mechanical hyperalgesia resulting from normally innocuous stimuli that accompanies inflammation injury and trauma.
Innovations and breakthroughs
The authors first observed the role of VGLUT3 in the visceral nociceptive transmission process induced by colorectal distension in rats. Intestinal infection could enhance the VGLUT3 immune activity in peripheral and central nervous, and this was not dependent on mast cells. VGLUT3 may be an important molecule involved in the brain-gut interaction in IBS patients.
Applications
Rose bengal (RB) is the most potent known VGLUT inhibitor, which could provide new clues to develop drugs to regulate visceral sensitivity.
Terminology
Visceral hyperalgesia occurs when stimuli that are normally perceived as innocuous can evoke persistent pain following inflammation.
Peer-review
This manuscript presents novel findings.
Footnotes
Supported by National Natural Science Foundation of China, No. 30940033; and the Doctoral Program of Higher Education of China.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: September 5, 2014
First decision: September 27, 2014
Article in press: December 8, 2014
P- Reviewer: Puljak L S- Editor: Ma YJ L- Editor: Cant MR E- Editor: Liu XM
References
- 1.Elsenbruch S. Abdominal pain in Irritable Bowel Syndrome: a review of putative psychological, neural and neuro-immune mechanisms. Brain Behav Immun. 2011;25:386–394. doi: 10.1016/j.bbi.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 2.Fremeau RT, Troyer MD, Pahner I, Nygaard GO, Tran CH, Reimer RJ, Bellocchio EE, Fortin D, Storm-Mathisen J, Edwards RH. The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron. 2001;31:247–260. doi: 10.1016/s0896-6273(01)00344-0. [DOI] [PubMed] [Google Scholar]
- 3.Fremeau RT, Burman J, Qureshi T, Tran CH, Proctor J, Johnson J, Zhang H, Sulzer D, Copenhagen DR, Storm-Mathisen J, et al. The identification of vesicular glutamate transporter 3 suggests novel modes of signaling by glutamate. Proc Natl Acad Sci USA. 2002;99:14488–14493. doi: 10.1073/pnas.222546799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herzog E, Bellenchi GC, Gras C, Bernard V, Ravassard P, Bedet C, Gasnier B, Giros B, El Mestikawy S. The existence of a second vesicular glutamate transporter specifies subpopulations of glutamatergic neurons. J Neurosci. 2001;21:RC181. doi: 10.1523/JNEUROSCI.21-22-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El Mestikawy S, Wallén-Mackenzie A, Fortin GM, Descarries L, Trudeau LE. From glutamate co-release to vesicular synergy: vesicular glutamate transporters. Nat Rev Neurosci. 2011;12:204–216. doi: 10.1038/nrn2969. [DOI] [PubMed] [Google Scholar]
- 6.Fremeau RT, Voglmaier S, Seal RP, Edwards RH. VGLUTs define subsets of excitatory neurons and suggest novel roles for glutamate. Trends Neurosci. 2004;27:98–103. doi: 10.1016/j.tins.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Seal RP, Wang X, Guan Y, Raja SN, Woodbury CJ, Basbaum AI, Edwards RH. Injury-induced mechanical hypersensitivity requires C-low threshold mechanoreceptors. Nature. 2009;462:651–655. doi: 10.1038/nature08505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wiedey J, Alexander MS, Marson L. Spinal neurons activated in response to pudendal or pelvic nerve stimulation in female rats. Brain Res. 2008;1197:106–114. doi: 10.1016/j.brainres.2007.12.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang CQ, Wei YY, Leng YX, Zhong CJ, Zhang YS, Wan Y, Duan LP. Vesicular glutamate transporter-3 contributes to visceral hyperalgesia induced by Trichinella spiralis infection in rats. Dig Dis Sci. 2012;57:865–872. doi: 10.1007/s10620-011-1970-x. [DOI] [PubMed] [Google Scholar]
- 10.Yang CQ, Wei YY, Zhong CJ, Duan LP. Essential role of mast cells in the visceral hyperalgesia induced by T. spiralis infection and stress in rats. Mediators Inflamm. 2012;2012:294070. doi: 10.1155/2012/294070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bercík P, Wang L, Verdú EF, Mao YK, Blennerhassett P, Khan WI, Kean I, Tougas G, Collins SM. Visceral hyperalgesia and intestinal dysmotility in a mouse model of postinfective gut dysfunction. Gastroenterology. 2004;127:179–187. doi: 10.1053/j.gastro.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 12.Leng YX, Wei YY, Chen H, Zhou SP, Yang YL, Duan LP. Alteration of cholinergic and peptidergic neurotransmitters in rat ileum induced by acute stress following transient intestinal infection is mast cell dependent. Chin Med J (Engl) 2010;123:227–233. [PubMed] [Google Scholar]
- 13.Millan MJ. The induction of pain: an integrative review. Prog Neurobiol. 1999;57:1–164. doi: 10.1016/s0301-0082(98)00048-3. [DOI] [PubMed] [Google Scholar]
- 14.Chung EK, Chen LW, Chan YS, Yung KK. Up-regulation in expression of vesicular glutamate transporter 3 in substantia nigra but not in striatum of 6-hydroxydopamine-lesioned rats. Neurosignals 2006- 2007;15:238–248. doi: 10.1159/000101704. [DOI] [PubMed] [Google Scholar]
- 15.Sánchez-Mendoza E, Burguete MC, Castelló-Ruiz M, González MP, Roncero C, Salom JB, Arce C, Cañadas S, Torregrosa G, Alborch E, et al. Transient focal cerebral ischemia significantly alters not only EAATs but also VGLUTs expression in rats: relevance of changes in reactive astroglia. J Neurochem. 2010;113:1343–1355. doi: 10.1111/j.1471-4159.2010.06707.x. [DOI] [PubMed] [Google Scholar]
- 16.Ni Y, Parpura V. Dual regulation of Ca2+-dependent glutamate release from astrocytes: vesicular glutamate transporters and cytosolic glutamate levels. Glia. 2009;57:1296–1305. doi: 10.1002/glia.20849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seal RP, Akil O, Yi E, Weber CM, Grant L, Yoo J, Clause A, Kandler K, Noebels JL, Glowatzki E, et al. Sensorineural deafness and seizures in mice lacking vesicular glutamate transporter 3. Neuron. 2008;57:263–275. doi: 10.1016/j.neuron.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahnert-Hilger G, Jahn R. Into great silence without VGLUT3. Neuron. 2008;57:173–174. doi: 10.1016/j.neuron.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 19.Higley MJ, Gittis AH, Oldenburg IA, Balthasar N, Seal RP, Edwards RH, Lowell BB, Kreitzer AC, Sabatini BL. Cholinergic interneurons mediate fast VGluT3-dependent glutamatergic transmission in the striatum. PLoS One. 2011;6:e19155. doi: 10.1371/journal.pone.0019155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schäfer MK, Varoqui H, Defamie N, Weihe E, Erickson JD. Molecular cloning and functional identification of mouse vesicular glutamate transporter 3 and its expression in subsets of novel excitatory neurons. J Biol Chem. 2002;277:50734–50748. doi: 10.1074/jbc.M206738200. [DOI] [PubMed] [Google Scholar]
- 21.Touret M, Parrot S, Denoroy L, Belin MF, Didier-Bazes M. Glutamatergic alterations in the cortex of genetic absence epilepsy rats. BMC Neurosci. 2007;8:69. doi: 10.1186/1471-2202-8-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gibney SM, Gosselin RD, Dinan TG, Cryan JF. Colorectal distension-induced prefrontal cortex activation in the Wistar-Kyoto rat: implications for irritable bowel syndrome. Neuroscience. 2010;165:675–683. doi: 10.1016/j.neuroscience.2009.08.076. [DOI] [PubMed] [Google Scholar]
- 23.Raudensky J, Yamamoto BK. Effects of chronic unpredictable stress and methamphetamine on hippocampal glutamate function. Brain Res. 2007;1135:129–135. doi: 10.1016/j.brainres.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Linke N, Bódi N, Resch BE, Fekete E, Bagyánszki M. Developmental pattern of three vesicular glutamate transporters in the myenteric plexus of the human fetal small intestine. Histol Histopathol. 2008;23:979–986. doi: 10.14670/HH-23.979. [DOI] [PubMed] [Google Scholar]
- 25.Lin LH, Talman WT. Vesicular glutamate transporters and neuronal nitric oxide synthase colocalize in aortic depressor afferent neurons. J Chem Neuroanat. 2006;32:54–64. doi: 10.1016/j.jchemneu.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 26.Guilarte M, Santos J, de Torres I, Alonso C, Vicario M, Ramos L, Martínez C, Casellas F, Saperas E, Malagelada JR. Diarrhoea-predominant IBS patients show mast cell activation and hyperplasia in the jejunum. Gut. 2007;56:203–209. doi: 10.1136/gut.2006.100594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klooker TK, Braak B, Koopman KE, Welting O, Wouters MM, van der Heide S, Schemann M, Bischoff SC, van den Wijngaard RM, Boeckxstaens GE. The mast cell stabiliser ketotifen decreases visceral hypersensitivity and improves intestinal symptoms in patients with irritable bowel syndrome. Gut. 2010;59:1213–1221. doi: 10.1136/gut.2010.213108. [DOI] [PubMed] [Google Scholar]
- 28.Chadwick VS, Chen W, Shu D, Paulus B, Bethwaite P, Tie A, Wilson I. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology. 2002;122:1778–1783. doi: 10.1053/gast.2002.33579. [DOI] [PubMed] [Google Scholar]


