Abstract
AIM: To evaluate the outcomes of patients with medium-sized hepatocellular carcinoma (HCC) who underwent percutaneous microwave ablation (MWA).
METHODS: We retrospectively reviewed all patients with a single medium-sized HCC who underwent percutaneous MWA from January 2010 to January 2013. Technical success, technical effectiveness and complications were subsequently observed. Survival curves were constructed using the Kaplan-Meier method. The Cox proportional hazards model was fitted to each variable. The relative prognostic significance of the variables for predicting overall survival rate, recurrence-free survival rate and local tumor recurrence(s) was assessed using univariate analysis. All variables with a P value < 0.20 were subjected to multivariate analysis.
RESULTS: The study included 182 patients (mean age, 58 years; age range: 22-86 years) with a single HCC (mean size, 3.72 ± 0.54 cm; range: 3.02-5.00 cm). The estimated technical effectiveness rate was 93% in 182 patients. The major complication rate was 2.7% (5/182), including liver abscess in 4 cases, and abdominal bleeding at the puncture site in 1 case. Thirty-day mortality rate was 0.5% (1/182). One patient died due to liver abscess-related septicemia. Cumulative recurrence-free survival and overall survival (OS) rates were 51%, 36%, 27% and 89%, 74%, 60% at 1, 2, and 3 years, respectively. Age (P = 0.017) and tumor diameter (P = 0.029) were independent factors associated with local tumor recurrence. None of the factors had a statistically significant impact on recurrence-free survival. Serum albumin level (P = 0.009) and new lesion(s) (P = 0.029) were independently associated with OS.
CONCLUSION: Percutaneous MWA is a relatively safe and effective treatment for patients with medium-sized HCC.
Keywords: Hepatocellular carcinoma, Medium-sized tumor, Microwave ablation, Outcomes, Cox analysis
Core tip: This is the first study on the clinical outcome of a large population with medium-sized hepatocellular carcinoma (HCC) treated with microwave ablation (MWA). Our results confirmed that percutaneous MWA is a relatively safe and effective treatment for patients with a single medium-sized HCC.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the third leading cause of cancer mortality worldwide[1]. Surgical resection and radiofrequency ablation (RFA) are the main treatments for early-stage HCC patients, who are not candidates for liver transplantation[2-4]. If these therapies are contraindicated, other minimally invasive options are used[5-8]. One option is microwave ablation (MWA), which produces a larger ablation zone than RFA, and has similar efficacy to RFA[9,10].
There have been many reports regarding the clinical outcome of small HCC (≤ 3 cm or ≤ 5 cm) treated with RFA or surgical resection[11-13]. Tumor size is significantly associated with overall survival (OS)[12,14,15]. However, there have been few reports on the clinical outcomes of patients with a single medium-sized (> 3 cm and ≤ 5 cm) HCC treated with percutaneous MWA.
Therefore, the aim of this study was to evaluate the outcomes of patients with a single medium-sized HCC who underwent percutaneous MWA.
MATERIALS AND METHODS
Ethics
This study was approved by the Institutional Review Board of the Eastern Hepatobiliary Surgery Hospital Ethics Committee of the Second Military Medical University, Shanghai, China. Informed consent from patients was waived as this was a retrospective study. However, written informed consent for percutaneous MWA procedures was obtained from patients prior to each treatment.
Study population
Between January 2010 and January 2013, our Minimally Invasive Therapy Center admitted 4998 patients with HCC. The EASL non-invasive diagnostic criteria[2] were used in the diagnosis of cirrhosis and HCC. We reviewed consecutive patients with a single medium-sized HCC without prior therapy who had undergone percutaneous MWA. We recorded tumor maximum diameter in accordance with the measurements of dynamic computer tomography (CT), magnetic resonance imaging (MRI) scans, or ultrasonography (US). Of these measurements, the largest diameter was selected.
The inclusion criteria for ablation were as follows: (1) liver function classed as Child-Pugh class A or B; (2) no evidence of extra-hepatic metastases or vascular invasion; (3) normal pro-thrombin time value ± 4 s; and (4) platelet count ≥ 45000 cells/mm3, otherwise, platelet transfusion was performed. The exclusion criteria for ablation were as follows: (1) the above-mentioned indications were unmet; and (2) the distance from the tumor to important structures, for example, pericardium, gallbladder, bowel loops and hepatic hilum was < 0.5 cm. If the tumor satisfied these criteria, patients with tumors ≤ 2 cm underwent RFA, and those with tumors > 2 cm underwent MWA at our institution.
Percutaneous MWA procedure
Percutaneous MWA procedures were performed under real-time ultrasonic guidance. Local anesthesia was administered without conscious-sedation as breath control of the patient was required for the insertion of two antennas into the tumors. These two antennas were applied based on cost-effectiveness; the delivery power was set at 100 W. Multi-site ablation was performed until the entire tumor with a 0.5-1 cm margin was covered by the ablation zone. Exposure time was normally within 8 min, as the volume [(4.8 ± 0.9) × (4.0 ± 0.9) × (4.0 ± 0.9 cm)] of MWA was always achieved within 8 min when a single antenna was applied[9]. Before March 2011, the FORSEATM MW delivery system, and 14 gauge cooled-shaft antennas with a 1.0 cm active tip (both from Qinghai Electronic Institute, Nanjing, China) were used. After March 2011, the KY-2000 MW delivery system, and 18 gauge cooled-shaft antennas with a 1.1 cm active tip (both from Kang-You Electronic Institute, Nanjing, China) were used.
Definition of terminology
Definition of terminology was based on the standardization by the International Working Group on Image-Guided Tumor Ablation[16]. Technical success was indicated when the tumor was treated according to protocol, and was covered completely by an ablation area on a contrast enhanced (CE)-CT scan within 24 h. Technical effectiveness was defined as ablation of lesions initially showing contrast enhancement during the arterial phase and washout in the venous phase not demonstrated by CE-MRI and/or CE-CT scan 1 mo after ablation. Major complications were defined according to current guidelines[16]. All other complications were regarded as minor. Intra-hepatic recurrence included local tumor recurrence(s) and new lesion(s). Local tumor recurrences were defined as ablation lesions and areas in contact with the ablation lesion which showed contrast enhancement during the arterial phase and washout in the venous phase. Portal hypertension was diagnosed by the presence of esophageal varices or splenomegaly with platelet counts < 100 × 109[2].
Follow-up after percutaneous MWA
For early evaluation of therapeutic response or possible complications, a CE-CT scan was performed within 24 h. Follow-up with CE-MRI and/or CE-CT scan, liver function tests and alpha-fetoprotein (AFP) were performed in all patients monthly for the first 2 mo. Follow-up with liver enzyme and AFP tests, CE-MRI and/or CE-CT scans were performed every 2 mo up to two years, and then every 4-6 mo according to the risk of recurrence.
Treatment strategy after initial percutaneous MWA
When technical success was not achieved at the immediate follow-up, and intra-hepatic recurrence was observed during subsequent follow-up, additional percutaneous MWA was primarily selected. When percutaneous MWA was not feasible, other therapeutic modalities were carried out for the tumor according to the guidelines for the management of HCC[2].
Analysis of therapeutic efficacy and survival
Local therapeutic efficacy in terms of technical success and effectiveness was assessed on a tumor basis. Recurrence-free survival and OS were evaluated on a per patient basis. The OS time was defined as the interval between the operation time and either death or end of the study (April 1, 2013). The time of recurrence-free survival was defined as the time from the operation to either recurrence including death, or end of the study (April 1, 2013).
Statistical analysis
Survival and recurrence curves were calculated using the Kaplan-Meier method. Prognostic factors for recurrence-free survival, local tumor recurrence(s), and OS were assessed using univariate analysis. Potential prognostic factors for recurrence-free survival and local tumor recurrence(s) included all the factors shown in Table 1. In addition to the factors in Table 1, potential prognostic factors of OS included local tumor recurrence(s) and new lesion(s). A univariate Cox proportional hazards model was fitted to each variable, and all variables with a P value < 0.20 were subjected to multivariate analysis to assess their value as independent predictors of recurrence-free survival and OS.
Table 1.
Baseline characteristics of the study population (n = 182)
| Clinical features | Number of patients |
| HCC size range 3.02-5.0 cm mean size 3.72 ± 0.54 cm | |
| Sex | |
| Males | 155 |
| Females | 27 |
| Age (yr) | |
| ≤ 60 | 105 |
| > 60 | 77 |
| Child-Pugh classification | |
| A | 170 |
| B | 12 |
| ALB (g/L) | |
| ≤ 35 | 37 |
| > 35 | 145 |
| ALT (U/L) | |
| ≤ 41 | 107 |
| > 41 | 75 |
| Portal hypertension | |
| Yes | 82 |
| No | 100 |
| Total bilirubin (μmol/L) | |
| ≤ 18.8 | 115 |
| 18.8-34.2 | 50 |
| > 34.2 | 17 |
| AFP (μg/L) | |
| ≤ 20 | 98 |
| 20-400 | 52 |
| > 400 | 32 |
| Etiology of HCC | |
| HBV | 140 |
| HCV | 3 |
| HBV + HCV | 3 |
| Other reason(s) | 36 |
| PLT (× 109/L) | |
| < 45 | 14 |
| 45-100 | 66 |
| ≥ 100 | 102 |
PLT: Platelet; ALB: Albumin; ALT: Alanine aminotransferase; AFP: Alpha-fetoprotein; HBV: Hepatic B virus; HCV: Hepatic C virus; HCC: Hepatocellular carcinoma.
A P value < 0.05 was considered statistically significant. Data analyses were performed using the commercially available software, IBM SPSS 20.0 (Chicago, IL, United States).
RESULTS
Patients
One hundred and eighty-two consecutive patients (male:female = 155:27, mean age, 58 years, range: 22-86 years) with a single medium-sized HCC (mean size, 3.72 ± 0.54 cm; range: 3.02-5.00 cm) were initially diagnosed and enrolled in this study. Eighty-seven patients were diagnosed with cirrhosis. The Child-Pugh scores in 12 patients with Child-Pugh classification B were ≤ 8. None of the patients had ascites. Maximum tumor diameters were recorded according to CT/MRI scans in 94 patients, and US scans in 88 patients. The clinical characteristics of the patients and tumors are summarized in Table 1.
Twenty-eight patients were lost to follow-up. One patient died within 30 d due to septicemia secondary to a liver abscess in the ablation area. The mean duration of follow-up was 17.8 mo (range: 3.2-37.0 mo) and 153 patients had clinical follow-up.
Technical success and technical effectiveness of initial treatment
At the follow-up visit, technical success and effectiveness rates were 100% (182/182), and 100% (153/153), respectively. The estimated technical effectiveness rate was 93% in 182 patients, as 7% (13) of patients with local tumor recurrence at 1 month were classified as technical effectiveness failure. Thirteen of 28 patients were lost to follow-up. Figure 1 shows MRI scans of the liver of one patient before and after treatment with MWA.
Figure 1.
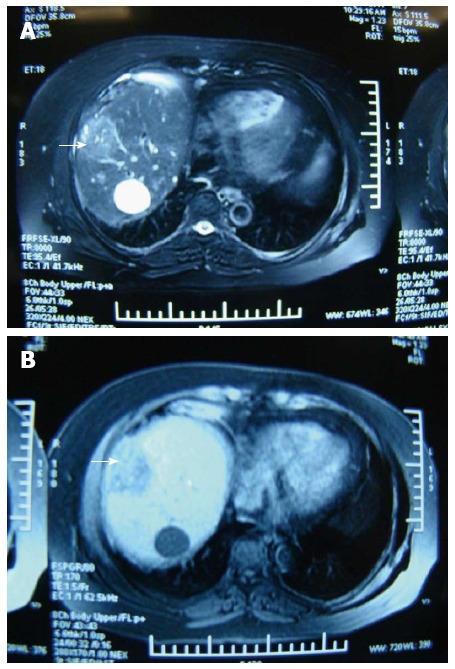
Magnetic resonance imaging of the liver in a patient with medium-sized hepatocellular carcinoma before and after treatment with microwave ablation. A: Magnetic resonance imaging of liver before treatment with microwave ablation (MWA). Medium-sized hepatocellular carcinoma (HCC) is labeled (arrow); B: MRI of liver at 1 mo after treatment with MWA. Medium-sized HCC is labeled (arrow). The tumor diameter was 4 cm × 4 cm. The power was set to 100 W. The ablation time was 3 min.
Intra-hepatic recurrences and management
Eighty-three (54.2%, 83/153) patients developed intra-hepatic recurrence, while 66 (43.1%, 66/153) patients showed no recurrence, and 4 patients died without recurrence. Of these 4 cases, 1 patient died following a car accident, 1 patient died due to hypovolemic shock caused by esophageal variceal bleeding, and 2 patients died due to liver failure. Local tumor recurrence(s), new lesion(s) or both occurred in 29, 52 and 2 patients, respectively. The local tumor recurrence rate was 20.3% (31/153). Twenty-nine patients developed local tumor recurrence(s), and 2 patients developed both new tumors and recurrences managed with MWA, hepatic resection, TACE (transcatheter arterial chemoembolization), percutaneous ethanol injection, or radiation therapy. A summary of patient management is shown in Figure 2.
Figure 2.
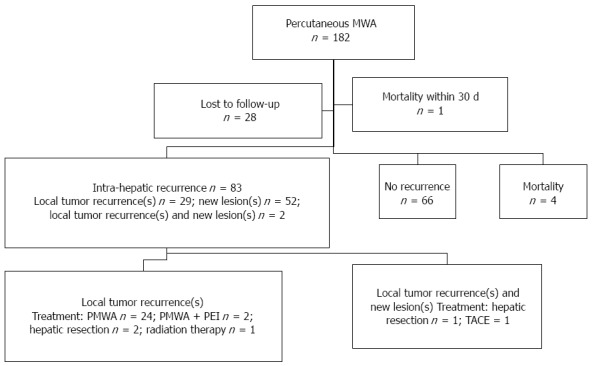
Intra-hepatic recurrences and management. Local tumor recurrence(s) and intra-hepatic recurrence(s) occurred in 31 patients, which were managed with various therapeutic modalities. PMWA: Percutaneous microwave ablation; TACE: Transcatheter arterial chemoembolization; PEI: Percutaneous ethanol injection.
Cumulative local tumor recurrence rates were estimated to be 23%, 25%, and 25%, and intra-hepatic recurrence rates were estimated to be 48%, 61%, and 73% at 1, 2, and 3 years in 182 patients, respectively (Figure 3). In univariate and multivariate analysis, age (P = 0.010 and P = 0.017) and tumor diameter (P = 0.016 and P = 0.029) were independent factors significantly associated with local tumor recurrence(s). Of the 83 patients who suffered a first intra-hepatic recurrence, 33 (38.4%, 33/83) experienced a second recurrence. Forty-five patients (54.2%, 45/83) underwent a second MWA, and 13 patients (39.4%, 13/33) underwent a third MWA.
Figure 3.
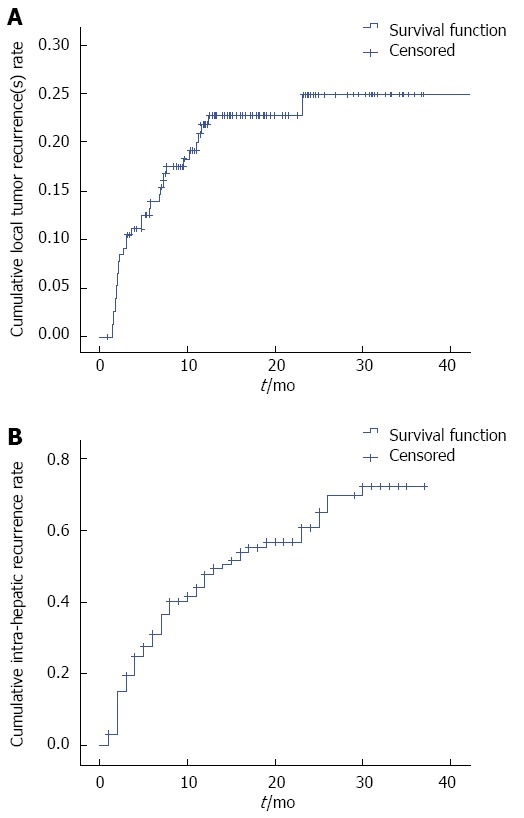
Local tumor recurrence(s) and intra-hepatic recurrence curves for 182 patients with medium-sized hepatocellular carcinoma who underwent microwave ablation.
Recurrence-free survival and OS
Except for the 5 patients (4 + 1) mentioned above who died without recurrence, 26 patients developed recurrence and died due to HCC progression during follow-up.
Cumulative recurrence-free survival rates were estimated to be 51%, 36%, and 27% at 1, 2, and 3 years, respectively (Figure 4). In univariate and multivariate analysis, recurrence-free survival rates were not affected by the variables shown in Table 2.
Figure 4.
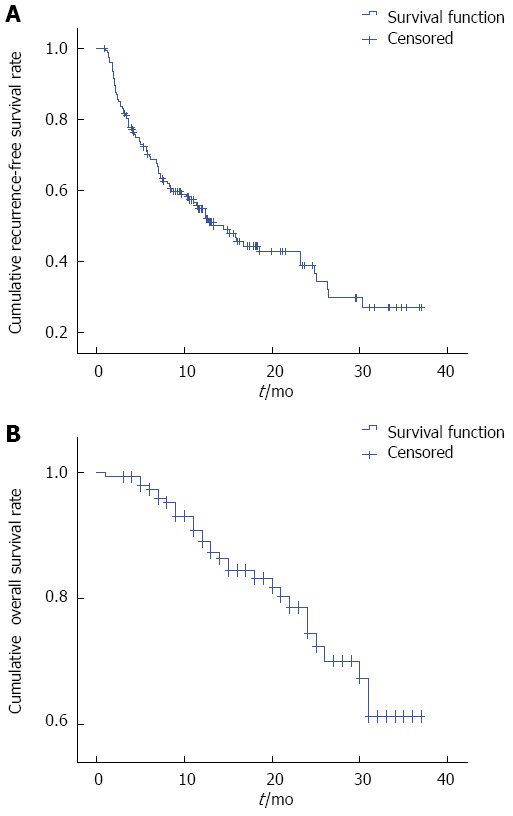
Recurrence-free survival and overall survival curves for 182 patients with medium-sized hepatocellular carcinoma who underwent microwave ablation.
Table 2.
Cox survival analysis of predictors for recurrence-free survival in 182 patients with hepatocellular carcinoma following microwave ablation
|
Univariate |
Multivariate |
|||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Tumor diameter | 1.173 | 0.784-1.755 | 0.439 | |||
| Sex (male/female) | 1.223 | 0.662-2.258 | 0.521 | |||
| Age (> 60 yr/≤ 60 yr) | 0.825 | 0.535-1.272 | 0.384 | |||
| Child-Pugh classification (A/B) | 0.507 | 0.232-1.106 | 0.088 | 0.665 | 0.271-1.635 | 0.372 |
| ALB (> 35 g/L/≤ 35 g/L) | 1.367 | 0.799-2.336 | 0.254 | |||
| ALT (> 41 U/L, ≤ 41 U/L) | 0.956 | 0.619-1.475 | 0.837 | |||
| Total bilirubin (> 34.2 mmol/L/18.8-34.2 mmol/L/≤ 18.8 mmol/L) | 0.749 | 0.547-1.026 | 0.072 | 0.805 | 0.563-1.151 | 0.234 |
| AFP (> 400 mg/L/20-400 mg/L/≤ 20 mg/L) | 0.861 | 0.660-1.124 | 0.271 | |||
| Etiology of HCC [HBV/HCV/HBV+HCV/Other reason(s)] | 0.901 | 0.747-1.086 | 0.274 | |||
| PLT (≥ 100 × 109/L/45 × 109-100 × 109/L/< 45 × 109/L) | 1.079 | 0.781-1.490 | 0.647 | |||
| Portal hypertension (Yes/No) | 0.936 | 0.607-1.443 | 0.764 | |||
PLT: Platelet; ALB: Albumin; ALT: Alanine aminotransferase; AFP: Alpha-fetoprotein; HBV: Hepatic B virus; HCV: Hepatic C virus; HCC: Hepatocellular carcinoma.
Cumulative OS rates were estimated to be 89%, 74% and 60% at 1, 2, and 3 years, respectively (Figure 4). In univariate analysis, portal hypertension (P = 0.004), serum albumin (P = 0.000), total bilirubin (P = 0.003), AFP (P = 0.049), platelet count (P = 0.003), and new lesion(s) (P = 0.001) all had a statistically significant impact on OS. In multivariate analysis, serum albumin (P = 0.009), and new lesion(s) (P = 0.029) were significantly associated with OS (Table 3).
Table 3.
Cox survival analysis of predictors for overall survival in 182 patients with hepatocellular carcinoma following microwave ablation
|
Univariate |
Multivariate |
|||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Tumor diameter | 1.314 | 0.692-2.439 | 0.404 | |||
| Sex (male/female) | 1.403 | 0.537-3.667 | 0.490 | |||
| Age (> 60 yr/≤ 60 yr) | 0.912 | 0.444-1.871 | 0.801 | |||
| Child-Pugh classification (A/B) | 0.492 | 0.149-1.622 | 0.244 | |||
| ALB (> 35 g/L/≤ 35 g/L) | 5.969 | 2.938-12.129 | 0.000 | 3.296 | 1.340-8.111 | 0.009 |
| ALT (> 41 U/L, ≤ 41 U/L) | 0.921 | 0.454-1.868 | 0.819 | |||
| Total bilirubin (> 34.2 mmol/L/18.8-34.2 mmol/L/≤ 18.8 mmol/L) | 0.488 | 0.306-0.778 | 0.003 | 0.881 | 0.528-1.469 | 0.626 |
| AFP(> 400 mg/L/20-400 mg/L/≤ 20 mg/L) | 0.657 | 0.433-0.998 | 0.049 | 0.834 | 0.533-1.305 | 0.427 |
| Etiology of HCC [HBV/HCV/HBV + HCV/Other reason(s)] | 0.910 | 0.658-1.258 | 0.566 | |||
| PLT (≥ 100 × 109/L/45 × 109-100 × 109/L/< 45 × 109/L) | 2.040 | 1.277-3.259 | 0.003 | 1.148 | 0.451-2.924 | 0.772 |
| Portal hypertension (Yes/No) | 0.327 | 0.154-0.695 | 0.004 | 0.802 | 0.188-3.413 | 0.765 |
| Local tumor recurrence(s) (Yes/No) | 0.543 | 0.190-1.553 | 0.255 | |||
| New lesion(s) (Yes/No) | 3.678 | 1.688-8.017 | 0.001 | 2.517 | 1.098-5.771 | 0.029 |
PLT: Platelet; ALB: Albumin; ALT: Alanine aminotransferase; AFP: Alpha-fetoprotein; HBV: Hepatic B virus; HCV: Hepatic C virus; HCC: Hepatocellular carcinoma.
Serum albumin level and new lesion(s) were independently associated with OS. When albumin was stratified as > or ≤ 35 g/L, cumulative OS rates were estimated to be 95.5%, 81.4%, 75.0% and 59.5%, 45.4%, 36.4% at 1, 2, and 3 years, respectively. When the presence of new lesions was stratified according to “no” or “yes”, cumulative OS rates were estimated to be 94%, 84.8%, 84.8% and 80.7%, 69%, 38.4% at 1, 2, and 3 years, respectively.
Complications
Complications were experienced by 15 patients (8.2%, 15/182) during follow-up. Major complications were identified in 5 cases (2.7%, 5/182) including liver abscess in 4 cases, and abdominal bleeding at the puncture site in 1 case. Liver abscess occurred within the ablation zone of coagulation necrosis in 3 patients, and was due to bile duct injury in 1 patient. One patient died of liver abscess-related septicemia. The 30-day mortality rate was 0.5% (1/182). The other complications observed were minor. All patients with complications received supportive care with antibiotics and hemostatics. With the exception of one patient who died, all patients recovered after treatment.
DISCUSSION
When liver transplantation is not possible, surgical resection is recommended for early-stage HCC[2,3]. In addition, RFA is considered to be curative for early-stage HCC[2]. Some authors consider RFA as the treatment of choice for very early stage HCC, even when surgical resection is possible[4]. However, surgical resection is considered the main treatment for medium-sized HCC[17]. When surgical resection is not appropriate treatment for medium-sized HCC, the combination of RFA and TACE has been recommended by some authors for the following reasons[17-22]: (1) the complete necrosis rate of medium-sized HCCs in patients who underwent RFA decreased; (2) TACE enhanced the efficacy of RFA; and (3) the effectiveness of the combination therapy was similar to that of hepatectomy.
Because the majority of MWAs is not or is only minimally affected by the heat sink effect[23], the ablation zone of MWA is larger than that of RFA[9], and patients treated with combination RFA and TACE suffer more than patients treated with MWA. Medium-sized HCCs have been treated with percutaneous MWA when liver transplantation and surgical resection were contraindicated and/or patients preferred minimally invasive therapy.
In the current study, the technical success and effectiveness rates were similar to those seen with combination RFA and TACE[18,19]. Our experience indicated that, in addition to the above-mentioned reasons for this success, three additional reasons were: (1) a higher delivery power was set for a large ablation area[24]; (2) MWA operators had significant clinical experience; and (3) patients who underwent ablation were selected.
In a previous study, the 3-year recurrence rate exceeded 50%[25,26] after hepatic resection. In the present study, a similar result (71%) was obtained. MWA and hepatic resection of HCC are not necessarily associated with recurrences[27]. Tumor pathology and angiogenesis may be the main factors in recurrence[28]. In a previous study, the OS rate of patients with medium-sized HCC and small HCC (< 5 cm) ranged from 66.67%-95.45% and 69.57%-92.17%, respectively[11-14]. Tumor size was found to be significantly associated with OS[12,14,15]. Although the OS rate in patients with a solitary medium-sized HCC who underwent MWA was 60%, this result was inferior to that in a previous study. When patients preferred MWA, or were not candidates for surgery or liver transplantation, MWA was an alternative therapy.
Frequently reported prognostic factors of HCC included tumor size, Child-Pugh classification, and recurrence in multivariate analysis in some studies[4,14,15,19,29,30]. However, in the current study, serum albumin level and the appearance of new lesion(s) were prognostic factors. The reason for the difference between previous studies and the present study may be related to the following: (1) tumor size ranged from 3 cm to 5 cm and may have been at the same stage; and (2) Child-Pugh classification and recurrence were ambiguous factors. In addition, platelet count was a prognostic factor in univariate analysis. This result is consistent with that of a previous study in patients with small HCCs (≤ 2 cm) who underwent resection, where a platelet count of ≤ 150 × 109 was found to be an independent prognostic factor regarding OS[30]. The reason for this phenomenon is unknown.
The major complication rate (2.7%) was similar for RFA with or without TACE for medium-sized HCCs (0%-3%)[18,19] and ablation for large HCCs (5-10 cm) (2.6%-3.29%)[31-34]. Therefore, the treatment schedule for patients with a single medium-sized HCC who underwent percutaneous MWA was relatively safe.
Our study had several limitations. Firstly, these data were obtained from a single center. A multicenter study with a larger number of patients and a longer observation period would be helpful. Secondly, not all HCC cases were confirmed by histopathology. Only clinical criteria were used[2]. Thirdly, this study was not a prospective trial and lacked a control group. Large randomized controlled studies are needed to confirm these results.
In conclusion, when liver transplantation and surgical resection are contraindicated and/or patients prefer minimally invasive therapy, percutaneous MWA is a relatively safe and efficacious treatment for patients with a single medium-sized HCC.
COMMENTS
Background
There have been few reports on the clinical outcomes of patients with a single medium-sized hepatocellular carcinoma (HCC) who have undergone percutaneous microwave ablation (MWA), although there have been many reports regarding the outcomes of patients with small HCC (≤ 3 cm or ≤ 5 cm) treated with radiofrequency ablation (RFA) or surgical resection.
Research frontiers
This is the first study on patients with a medium-sized HCC who underwent MWA.
Innovations and breakthroughs
When liver transplantation and surgical resection are contraindicated and/or patients prefer minimally invasive therapy, percutaneous MWA is a relatively safe and efficacious treatment for patients with medium-sized HCC.
Applications
This study involved 182 patients with medium-sized HCC treated with MWA. The major limitation in the study was the absence of a control group.
Peer-review
This research is very useful because it is still controversial whether locoregional methods such as MWA or RFA are beneficial for patients with medium-sized HCC (> 3 cm).
Footnotes
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: August 21, 2014
First decision: September 15, 2014
Article in press: December 22, 2014
P- Reviewer: Gao Y, Hayano K, Khatib M, Pompili M S- Editor: Ma YJ L- Editor: Logan S E- Editor: Zhang DN
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.European Association For The Study Of The Liver1; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Makuuchi M, Kokudo N, Arii S, Futagawa S, Kaneko S, Kawasaki S, Matsuyama Y, Okazaki M, Okita K, Omata M, et al. Development of evidence-based clinical guidelines for the diagnosis and treatment of hepatocellular carcinoma in Japan. Hepatol Res. 2008;38:37–51. doi: 10.1111/j.1872-034X.2007.00216.x. [DOI] [PubMed] [Google Scholar]
- 4.Livraghi T, Meloni F, Di Stasi M, Rolle E, Solbiati L, Tinelli C, Rossi S. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology. 2008;47:82–89. doi: 10.1002/hep.21933. [DOI] [PubMed] [Google Scholar]
- 5.Shiina S, Tagawa K, Unuma T, Terano A. Percutaneous ethanol injection therapy for the treatment of hepatocellular carcinoma. AJR Am J Roentgenol. 1990;154:947–951. doi: 10.2214/ajr.154.5.2157329. [DOI] [PubMed] [Google Scholar]
- 6.Chan AC, Cheung TT, Fan ST, Chok KS, Chan SC, Poon RT, Lo CM. Survival analysis of high-intensity focused ultrasound therapy versus radiofrequency ablation in the treatment of recurrent hepatocellular carcinoma. Ann Surg. 2013;257:686–692. doi: 10.1097/SLA.0b013e3182822c02. [DOI] [PubMed] [Google Scholar]
- 7.Seki T, Wakabayashi M, Nakagawa T, Itho T, Shiro T, Kunieda K, Sato M, Uchiyama S, Inoue K. Ultrasonically guided percutaneous microwave coagulation therapy for small hepatocellular carcinoma. Cancer. 1994;74:817–825. doi: 10.1002/1097-0142(19940801)74:3<817::aid-cncr2820740306>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 8.Vogl TJ, Müller PK, Hammerstingl R, Weinhold N, Mack MG, Philipp C, Deimling M, Beuthan J, Pegios W, Riess H. Malignant liver tumors treated with MR imaging-guided laser-induced thermotherapy: technique and prospective results. Radiology. 1995;196:257–265. doi: 10.1148/radiology.196.1.7540310. [DOI] [PubMed] [Google Scholar]
- 9.Qian GJ, Wang N, Shen Q, Sheng YH, Zhao JQ, Kuang M, Liu GJ, Wu MC. Efficacy of microwave versus radiofrequency ablation for treatment of small hepatocellular carcinoma: experimental and clinical studies. Eur Radiol. 2012;22:1983–1990. doi: 10.1007/s00330-012-2442-1. [DOI] [PubMed] [Google Scholar]
- 10.Zhang L, Wang N, Shen Q, Cheng W, Qian GJ. Therapeutic efficacy of percutaneous radiofrequency ablation versus microwave ablation for hepatocellular carcinoma. PLoS One. 2013;8:e76119. doi: 10.1371/journal.pone.0076119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, Lin XJ, Lau WY. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321–328. doi: 10.1097/01.sla.0000201480.65519.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang J, Yan L, Cheng Z, Wu H, Du L, Wang J, Xu Y, Zeng Y. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg. 2010;252:903–912. doi: 10.1097/SLA.0b013e3181efc656. [DOI] [PubMed] [Google Scholar]
- 13.Wang JH, Wang CC, Hung CH, Chen CL, Lu SN. Survival comparison between surgical resection and radiofrequency ablation for patients in BCLC very early/early stage hepatocellular carcinoma. J Hepatol. 2012;56:412–418. doi: 10.1016/j.jhep.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 14.Liang P, Dong B, Yu X, Yu D, Wang Y, Feng L, Xiao Q. Prognostic factors for survival in patients with hepatocellular carcinoma after percutaneous microwave ablation. Radiology. 2005;235:299–307. doi: 10.1148/radiol.2351031944. [DOI] [PubMed] [Google Scholar]
- 15.Rossi S, Ravetta V, Rosa L, Ghittoni G, Viera FT, Garbagnati F, Silini EM, Dionigi P, Calliada F, Quaretti P, et al. Repeated radiofrequency ablation for management of patients with cirrhosis with small hepatocellular carcinomas: a long-term cohort study. Hepatology. 2011;53:136–147. doi: 10.1002/hep.23965. [DOI] [PubMed] [Google Scholar]
- 16.Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD, Dupuy DE, Gervais D, Gillams AR, Kane RA, Lee FT, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2005;16:765–778. doi: 10.1097/01.RVI.0000170858.46668.65. [DOI] [PubMed] [Google Scholar]
- 17.Torzilli G, Belghiti J, Kokudo N, Takayama T, Capussotti L, Nuzzo G, Vauthey JN, Choti MA, De Santibanes E, Donadon M, et al. A snapshot of the effective indications and results of surgery for hepatocellular carcinoma in tertiary referral centers: is it adherent to the EASL/AASLD recommendations?: an observational study of the HCC East-West study group. Ann Surg. 2013;257:929–937. doi: 10.1097/SLA.0b013e31828329b8. [DOI] [PubMed] [Google Scholar]
- 18.Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Ierace T, Solbiati L, Gazelle GS. Hepatocellular carcinoma: radio-frequency ablation of medium and large lesions. Radiology. 2000;214:761–768. doi: 10.1148/radiology.214.3.r00mr02761. [DOI] [PubMed] [Google Scholar]
- 19.Morimoto M, Numata K, Kondou M, Nozaki A, Morita S, Tanaka K. Midterm outcomes in patients with intermediate-sized hepatocellular carcinoma: a randomized controlled trial for determining the efficacy of radiofrequency ablation combined with transcatheter arterial chemoembolization. Cancer. 2010;116:5452–5460. doi: 10.1002/cncr.25314. [DOI] [PubMed] [Google Scholar]
- 20.Kim JH, Won HJ, Shin YM, Kim SH, Yoon HK, Sung KB, Kim PN. Medium-sized (3.1-5.0 cm) hepatocellular carcinoma: transarterial chemoembolization plus radiofrequency ablation versus radiofrequency ablation alone. Ann Surg Oncol. 2011;18:1624–1629. doi: 10.1245/s10434-011-1673-8. [DOI] [PubMed] [Google Scholar]
- 21.Ni JY, Liu SS, Xu LF, Sun HL, Chen YT. Transarterial chemoembolization combined with percutaneous radiofrequency ablation versus TACE and PRFA monotherapy in the treatment for hepatocellular carcinoma: a meta-analysis. J Cancer Res Clin Oncol. 2013;139:653–659. doi: 10.1007/s00432-012-1369-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamakado K, Nakatsuka A, Takaki H, Yokoi H, Usui M, Sakurai H, Isaji S, Shiraki K, Fuke H, Uemoto S, et al. Early-stage hepatocellular carcinoma: radiofrequency ablation combined with chemoembolization versus hepatectomy. Radiology. 2008;247:260–266. doi: 10.1148/radiol.2471070818. [DOI] [PubMed] [Google Scholar]
- 23.Cheng BQ, Jia CQ, Liu CT, Fan W, Wang QL, Zhang ZL, Yi CH. Chemoembolization combined with radiofrequency ablation for patients with hepatocellular carcinoma larger than 3 cm: a randomized controlled trial. JAMA. 2008;299:1669–1677. doi: 10.1001/jama.299.14.1669. [DOI] [PubMed] [Google Scholar]
- 24.Yu NC, Raman SS, Kim YJ, Lassman C, Chang X, Lu DS. Microwave liver ablation: influence of hepatic vein size on heat-sink effect in a porcine model. J Vasc Interv Radiol. 2008;19:1087–1092. doi: 10.1016/j.jvir.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 25.Hines-Peralta AU, Pirani N, Clegg P, Cronin N, Ryan TP, Liu Z, Goldberg SN. Microwave ablation: results with a 2.45-GHz applicator in ex vivo bovine and in vivo porcine liver. Radiology. 2006;239:94–102. doi: 10.1148/radiol.2383050262. [DOI] [PubMed] [Google Scholar]
- 26.Muto Y, Moriwaki H, Ninomiya M, Adachi S, Saito A, Takasaki KT, Tanaka T, Tsurumi K, Okuno M, Tomita E, et al. Prevention of second primary tumors by an acyclic retinoid, polyprenoic acid, in patients with hepatocellular carcinoma. Hepatoma Prevention Study Group. N Engl J Med. 1996;334:1561–1567. doi: 10.1056/NEJM199606133342402. [DOI] [PubMed] [Google Scholar]
- 27.Takayama T, Sekine T, Makuuchi M, Yamasaki S, Kosuge T, Yamamoto J, Shimada K, Sakamoto M, Hirohashi S, Ohashi Y, et al. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet. 2000;356:802–807. doi: 10.1016/S0140-6736(00)02654-4. [DOI] [PubMed] [Google Scholar]
- 28.Wang ZL, Liang P, Dong BW, Yu XL, Yu de J. Prognostic factors and recurrence of small hepatocellular carcinoma after hepatic resection or microwave ablation: a retrospective study. J Gastrointest Surg. 2008;12:327–337. doi: 10.1007/s11605-007-0310-0. [DOI] [PubMed] [Google Scholar]
- 29.Hernandez-Gea V, Toffanin S, Friedman SL, Llovet JM. Role of the microenvironment in the pathogenesis and treatment of hepatocellular carcinoma. Gastroenterology. 2013;144:512–527. doi: 10.1053/j.gastro.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim YS, Lim HK, Rhim H, Lee MW, Choi D, Lee WJ, Paik SW, Koh KC, Lee JH, Choi MS, et al. Ten-year outcomes of percutaneous radiofrequency ablation as first-line therapy of early hepatocellular carcinoma: analysis of prognostic factors. J Hepatol. 2013;58:89–97. doi: 10.1016/j.jhep.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 31.Roayaie S, Obeidat K, Sposito C, Mariani L, Bhoori S, Pellegrinelli A, Labow D, Llovet JM, Schwartz M, Mazzaferro V. Resection of hepatocellular cancer ≤2 cm: results from two Western centers. Hepatology. 2013;57:1426–1435. doi: 10.1002/hep.25832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liang P, Wang Y, Yu X, Dong B. Malignant liver tumors: treatment with percutaneous microwave ablation--complications among cohort of 1136 patients. Radiology. 2009;251:933–940. doi: 10.1148/radiol.2513081740. [DOI] [PubMed] [Google Scholar]
- 33.Bertot LC, Sato M, Tateishi R, Yoshida H, Koike K. Mortality and complication rates of percutaneous ablative techniques for the treatment of liver tumors: a systematic review. Eur Radiol. 2011;21:2584–2596. doi: 10.1007/s00330-011-2222-3. [DOI] [PubMed] [Google Scholar]
- 34.Livraghi T, Meloni F, Solbiati L, Zanus G. Complications of microwave ablation for liver tumors: results of a multicenter study. Cardiovasc Intervent Radiol. 2012;35:868–874. doi: 10.1007/s00270-011-0241-8. [DOI] [PubMed] [Google Scholar]


