Abstract
AIM: To evaluate clinical response to initial corticosteroid (CS) treatment in Chinese ulcerative colitis patients (UC) and identify predictors of clinical response.
METHODS: Four hundred and twenty-three UC patients who were initially treated with oral or intravenous CS from 2007 to 2011 were retrospectively reviewed at eight inflammatory bowel disease centers in China, and 101 consecutive cases with one-year follow-up were analyzed further for clinical response and predictors. Short-term outcomes within one month were classified as primary response and primary non-response. Long-term outcomes within one year were classified as prolonged CS response, CS dependence and secondary non-response. CS refractoriness included primary and secondary non-response. Multivariate analyses were performed to identify predictors associated with clinical response.
RESULTS: Within one month, 95.0% and 5.0% of the cases were classified into primary response and non-response, respectively. Within one year, 41.6% of cases were assessed as prolonged CS response, while 49.5% as CS dependence and 4.0% as secondary non-response. The rate of CS refractoriness was 8.9%, while the cumulative rate of surgery was 6.9% within one year. After multivariate analysis of all the variables, tenesmus was found to be a negative predictor of CS dependence (OR = 0.336; 95%CI: 0.147-0.768; P = 0.013) and weight loss as a predictor of CS refractoriness (OR = 5.662; 95%CI: 1.111-28.857; P = 0.040). After one-month treatment, sustained high Sutherland score (≥ 6) also predicted CS dependence (OR = 2.347; 95%CI: 0.935-5.890; P = 0.014).
CONCLUSION: Tenesmus was a negative predictor of CS dependence, while weight loss and sustained high Sutherland score were strongly associated with poor CS response.
Keywords: Clinical response, Predictor, Corticosteroid, Ulcerative colitis
Core tip: This is the first large-scale study across mainland China to evaluate clinical response to initial corticosteroid (CS) treatment in ulcerative colitis (UC) and identify predictors of clinical response, although recent studies have focused on the treatment outcomes of new drugs for UC. CS therapy continues to one of the most effective treatments in active UC. In view of the great difference between yellow and white races in CS response, and the lack of large-scale, systematic CS studies in yellow-race UC patients, this study was meaningful in understanding the efficacy of and risk factors for initial CS treatment in yellow-race UC patients.
INTRODUCTION
Ulcerative colitis (UC) is a form of chronic inflammatory bowel disease (IBD) whose incidence is steadily on the rise in China during the last two decades[1-3]. The disease is clinically characterized by bloody diarrhea, abdominal pain, mucous stool and extra-intestinal manifestations[4]. As it relapses frequently, the current treatment strategies are aimed at controlling acute exacerbation and maintaining long-term remission. 5-aminosalicylates (5-ASA) or sulfasalazine (SASP) is effective for mild to moderate disease[5,6], while high-dose oral or intravenous corticosteroid (CS) is required for moderate to severe disease or when 5-ASA or SASP proves ineffective[7,8]. After inducing primary remission, CS should be tampered over several months. Actually, not all patients can reach primary remission or subsequent prolonged response. It has been reported that genetic factors may be involved in the clinical efficacy of CS[9-12]. As a result, CS dependence and CS refractoriness indicate the requirement of more aggressive therapy, such as immunosuppressors (e.g., azathioprine, 6-mercathioprine, methotrexate)[13,14], anti-tumor necrosis factor monoclonal antibody (infliximab and adalimumab)[15,16] and even total or partial colectomy[17]. Therefore, predicting individual CS response can help adjust the therapeutic schedule in time and prevent the disease from deterioration. In this study, we conducted a retrospective survey of all UC cases treated with systematic CS in eight IBD centers in China, aiming to identify the factors predicting clinical response to initial CS treatment among Chinese UC cases.
MATERIALS AND METHODS
Study population and data extraction
All hospitalized cases which had been diagnosed with UC according to the suggested guidelines for diagnosis of IBD in 2012[18] were reviewed in the eight IBD centers in China, which satisfied the following requirements: (1) the medical center was university hospital or grade A territory hospital; (2) diagnostic facilities for high-quality endoscopy, radiology and pathology were available; and (3) the center was part of a health system that offered universal cover for primary and specialist services with an established system for referral from primary to secondary care. This study was approved by the ethics committee of Zhongnan Hospital of Wuhan University.
After excluding the cases which had a history of CS use, 431 UC cases initially treated with oral or intravenous CS from 2007 to 2011 were included, among which 101 consecutively hospitalized cases were analyzed further. A UC case report form (CRF) was designed to collect primary data about the demographic, clinical, diagnostic and therapeutic characteristics before initial use of CS (M0) and 1 mo (M1), 3 mo (M3), 6 mo (M6), and 12 mo (M12) after the start of CS.
Clinical phenotype and severity
According to the Montreal classification, UC was categorized by disease extent into proctitis (E1), left-sided colitis (E2) and extensive colitis (E3). Disease severity was assessed by Sutherland score which was based on clinical and endoscopic characteristics. The range of 3-5 points was considered mild, while 6-10 points moderate and 11-12 points severe.
Assessment of clinical efficacy
Patients were initially treated with oral CS (prednisone 40-60 mg/d or equivalent) or intravenous CS (hydrocortisone 200-400 mg/d or equivalent). After clinical alleviation, CS tapered off within 3-4 mo. Short-term clinical efficacy was classified into two categories according to clinical symptoms within one month after the start of CS[19]: primary response (an obvious improvement in bowel movements, bloody stool and abdominal pain, without fever, weight loss or other systemic symptoms) and primary non-response (no improvement in clinical symptoms). After primary response, long-term clinical efficacy was reclassified into several categories according to subsequent follow-up within one year[19]: prolonged response (after the end of CS therapy, maintenance of remission still lasted at least one month), CS dependence (relapse at dose reduction or within one month after the end of CS therapy; or CS could not discontinue after administration for more than 3-4 mo), secondary non-response (primary remission was achieved, but then CS became ineffective), and CS refractoriness (including primary and secondary CS non-response).
Statistical analysis
Statistical analyses were performed with IBM SPSS Statistics V21.0. All the factors that might predict subsequent clinical efficacy of CS were subjected to univariate analysis. Variables were compared using χ2 test or Fisher’s exact test. Logistic regression analysis was carried out using the variables whose P-values were less than 0.1 in univariate analysis. Differences were considered significant when the P-value in multivariate analysis was less than 0.05. 95%CI was estimated for all parameters.
RESULTS
Patient characteristics
One hundred and one consecutive cases (47.5% female vs 52.5% male) were analyzed, of whom the majority had an age of more than 30, a disease duration of less than 5 years and a relapse frequency of less than 3 per year (Table 1). Eight (7.9%) cases had a history of intestinal surgery, including cholecystectomy in 1 case, appendectomy in 2, partial colectomy in 3, partial proctectomy in 1, sigmoid fistula operation in 1, and rectum fistula operation in 1. According to the Montreal classification, the disease was categorized by disease extent as proctitis (15.3%), left-sided colitis (15.3%) and extensive colitis (69.4%), while based on the Sutherland score, the disease were categorized by disease activity as mild (5.0%), moderate (47.5%) and severe (47.5%).
Table 1.
Demographic, clinical, laboratory characteristics and auxiliary medication in 101 ulcerative colitis cases before initial administration of corticosteroid n (%)
| Prolonged response | Dependence | Refractoriness | Total | |
| Cases | 42 | 50 | 9 | 101 |
| Female | 24 (57.1) | 22 (44.0) | 2 (22.8) | 48 (47.5) |
| Age (≥ 30 yr) | 39 (92.8) | 41 (82.0) | 6 (66.7) | 85 (84.2) |
| Disease duration (≥ 5 yr) | 12 (29.3) | 15 (31.9) | 2 (22.2) | 29 (33.3) |
| Relapse frequency (> 3/yr) | 2 (5.9) | 4 (9.3) | 0 | 6 (7.0) |
| Previous colectomy | 4 (9.5) | 3 (6.0) | 1 (11.1) | 8 (7.9) |
| UC location | ||||
| Proctitis | 7 (17.5) | 6 (12.2) | 2 (22.2) | 15 (15.3) |
| Left-side colitis | 5 (12.5) | 9 (18.4) | 1 (11.1) | 15 (15.3) |
| Extensive colitis | 28 (70.0) | 34 (69.4) | 6 (66.7) | 68 (69.4) |
| Sutherland score | ||||
| Mild (3-5) | 2 (4.8) | 2 (4.0) | 1 (11.1) | 5 (5.0) |
| Moderate (6-10) | 21 (50.0) | 25 (50.0) | 2 (22.2) | 48 (47.5) |
| Severe (11-12) | 19 (45.2) | 23 (46.0) | 6 (66.7) | 48 (47.5) |
| Heart rate (≥ 90/min) | 8 (19.5) | 18 (36.0) | 5 (55.6) | 31 (31.0) |
| Diarrhea (≤ 6/d) | 14 (33.3) | 25 (50.0) | 4 (44.4) | 43 (42.6) |
| Bloody stool | 34 (80.1) | 44 (88.0) | 8 (88.9) | 86 (85.1) |
| Mucus | 34 (81.0) | 39 (78.0) | 6 (66.7) | 79 (78.2) |
| Tenesmus | 25 (59.5) | 16 (32.0) | 4 (44.4) | 45 (44.6) |
| Abdominal pain | 34 (81.0) | 47 (94.0) | 9 (100.0) | 90 (89.1) |
| Abdominal distention | 6 (14.3) | 8 (16.0) | 4 (44.4) | 18 (17.8) |
| Weight loss | 17 (40.5) | 18 (36.0) | 7 (77.8) | 42 (41.6) |
| Fever | 13 (31.0) | 13 (26.0) | 5 (55.6) | 31 (30.7) |
| Extra-intestinal manifestation | 6 (14.3) | 7 (14.0) | 2 (22.2) | 15 (14.9) |
| Hemoglobin (< 12.0 mg/dL) | 32 (76.2) | 31 (62.0) | 9 (100.0) | 72 (71.3) |
| Leukocyte (4-10 × 109/L) | 21 (50.0) | 29 (58.0) | 9 (100.0) | 59 (58.4) |
| Neutrophil (50%-70%) | 20 (47.6) | 28 (56.0) | 6 (66.7) | 54 (53.5) |
| Platelet (> 300 × 1012/L) | 26 (61.9) | 26 (52.0) | 4 (44.4) | 56 (55.4) |
| Albumin (< 3.5 mg/dL) | 27 (64.3) | 27 (54.0) | 5 (55.6) | 59 (58.4) |
| K+ (< 3.5 mmol/L) | 16 (15.8) | 16 (32.0) | 3 (33.3) | 35 (34.7) |
| ESR (> 15 mm/h) | 27 (64.3) | 27 (54.0) | 7 (77.8) | 61 (60.4) |
| Auxiliary medication | ||||
| None | 2 (4.8) | 7 (14.0) | 1 (11.1) | 10 (9.9) |
| Only SASP or 5-ASA (A) | 32 (76.2) | 29 (58.0) | 5 (55.6) | 66 (65.3) |
| Only immunosuppressant (B) | 1 (2.4) | 4 (8.0) | 1 (11.1) | 6 (5.9) |
| Only infliximab (C) | 0 | 0 | 0 | 0 |
| A + B | 6 (14.3) | 9 (18.0) | 2 (22.2) | 17 (16.8) |
| A + C | 1 (2.4) | 1 (2.0) | 0 | 2 (2.0) |
The demographic, clinical and laboratory characteristic were displayed respectively according to the cohorts of different corticosteroid response. The auxiliary medication after the start of corticosteroid treatment was also displayed. UC: Ulcerative colitis; ESR: Erythrocyte sediment rate; 5-ASA: 5-aminosalicylates; SASP: Sulfasalazine.
The predominant manifestations were diarrhea (100%), abdominal pain (87.1%), bloody stool (85.1%), mucus (78.2%), tenesmus (44.6%), weight loss (41.6%), fever (30.7%), tachycardia (> 90/min) (31.0%) and abdominal distention (17.8%) (Table 1). Of all the cases, 15 (13.9%) had extra-intestinal manifestations, including arthritis or arthralgia in 8 cases, and oral ulcer in 6 cases. Table 1 shows the clinical characteristics of the patients before CS treatment.
Anemia (hemoglobin < 12.0 mg/dL) occurred in 71.3% of cases, low or high leukocyte count in 41.6%, and high erythrocyte sediment rate (ESR; > 15 mm/h) in 60.4% (Table 1). The albumin level was below 3.5 mg/dL in 58.4% of cases, and hypokalemia existed in 34.7% of cases.
Before initial administration of CS, the main drugs available for the 101 cases were 5-ASA or SASP and azathioprine (AZA) or 6-mercathioprine (6-MP). Subsequently, all the cases received CS orally or intravenously. After the start of CS treatment, 83.1% of cases chose 5-ASA/SASP as auxiliary medication, while 22.7% for AZA/6-MP, and 2.0% for infliximab. Approximately 18.8% of the cases received a combined therapy, mainly the combination of 5-ASA/SASP and AZA/6-MP (Table 1).
Short-term response (within one month)
Of the 101 cases, 96 (95.0%) reached primary response within one month, while 5 (5.0%) had primary non-response, among whom 3 underwent surgery later (Figure 1).
Figure 1.
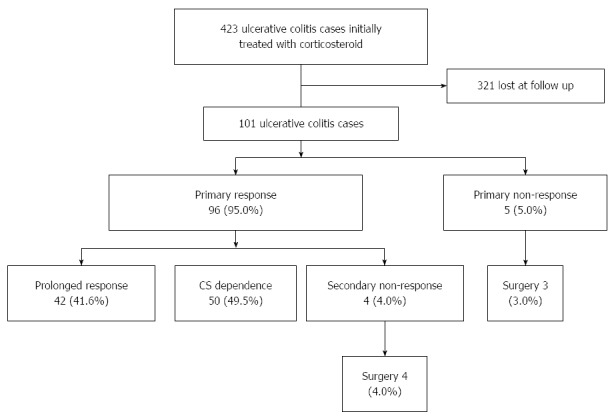
Clinical efficacy in 101 ulcerative colitis cases within one year after initial administration of corticosteroid.
Long-term response (within one year)
After primary response, 42 (41.6%) maintained prolonged CS response, but 50 (49.5%) became CS dependent and 4 (4.0%) lost response to CS. All the 4 cases of secondary non-response underwent surgery later. The rate of CS refractoriness (including primary and secondary CS non-response) was 8.9%, and the cumulative risk of surgery was 6.9% within one year.
Predictors of clinical response
After univariate and multivariate analyses of all the variables, tenesmus was found to be negatively related with CS dependence (OR = 0.336; 95%CI: 0.147-0.768; P = 0.013) (Table 2), and weight loss was positively associated with CS refractoriness (OR = 5.662; 95%CI: 1.111-28.857; P = 0.040) (Table 3). At one month after initial CS administration, sustained high Sutherland score (≥ 6) was a risk factor for CS dependence (OR = 2.347; 95%CI: 0.935-5.890; P = 0.014) (Table 4).
Table 2.
Logistic regression analysis of risk factors for corticosteroid dependence before start of corticosteroid
|
P value1 |
OR (95%CI) | ||
| Univariate | Multivariate | ||
| Tenesmus | 0.012 | 0.013 | 0.336 (0.147-0.768) |
| Anemia | 0.041 | 0.338 | 0.576 (0.233-1.422) |
| Hypokalemic (< 3.0 mmol/L) | 0.065 | 0.060 | 0.121 (0.014-1.028) |
| Diarrhea (> 6/d) | 0.135 | ||
| ESR (> 15 mm/h) | 0.193 | ||
| Weight loss | 0.260 | ||
| Tachycardia (≥ 90/min) | 0.280 | ||
| Hypoproteinemia (< 3.5 mg/dL) | 0.373 | ||
Data with univariate P < 0.5 are not displayed. All the variables were statistically analyzed. In univariate analysis, tenesmus and anemia before start of corticosteroid treatment were related with corticosteroid dependence (P = 0.012, 0.041). All the variables with P < 0.100 were further analyzed by logistic regression. After multivariate analysis, tenesmus at baseline was found to be associated with corticosteroid dependence (OR = 0.336, 95%CI: 0.147-0.768; P = 0.013). ESR: Erythrocyte sediment rate.
Table 3.
Logistic regression analysis of risk factors for corticosteroid refractoriness before start of corticosteroid
|
P value1 |
OR (95%CI) | ||
| Univariate | Multivariate | ||
| Weight loss | 0.051 | 0.040 | 5.662 (1.111-28.857) |
| Abdominal distention | 0.086 | 0.251 | 2.436 (0.601-9.882) |
| Fever | 0.188 | ||
| Tachycardia (≥ 90/min) | 0.196 | ||
| Age (< 30 yr) | 0.253 | ||
| ESR (> 15 mm/h) | 0.277 | ||
| Sutherland score (≥ 11) | 0.392 | ||
Data with univariate P < 0.5 are not displayed. All the variables were statistically analyzed. In univariate analysis, no variables were related with corticosteroid refractoriness. All the variables with P < 0.100 were further analyzed by logistic regression. After multivariate analysis, weight loss at baseline was found to be associated with corticosteroid refractoriness (OR = 5.662; 95%CI: 1.111-28.857; P = 0.040). ESR: Erythrocyte sediment rate.
Table 4.
Logistic regression analysis of risk factors for corticosteroid dependence at one month after start of corticosteroid
|
P value1 |
OR (95%CI) | ||
| Univariate | Multivariate | ||
| Sutherland score (≥ 6) | 0.037 | 0.014 | 2.347 (0.935-5.890) |
| Abnormal neutrophil% | 0.016 | 0.107 | 0.425 (0.168-1.075) |
| Weight loss | 0.021 | 0.336 | 2.400 (0.839-6.866) |
| Mucus | 0.096 | ||
| Diarrhea (> 6/d) | 0.136 | ||
| Hypokalemic (< 3.5 mmol/L) | 0.176 | ||
| ESR (> 15 mm/h) | 0.186 | ||
| Bloody stool | 0.343 | ||
Data with univariate P < 0.5 are not displayed. All the variables were statistically analyzed. In univariate analysis, Sutherland score (≥ 6), abnormal neutrophil% and weight loss after one month of corticosteroid treatment were related with corticosteroid dependence (P = 0.037, 0.016, 0.021). All the variables with P < 0.100 were further analyzed by logistic regression. After multivariate analysis, sustained high Sutherland score (≥ 6) after one-month treatment was found to be associated with corticosteroid dependence (OR = 2.347; 95%CI: 0.935-5.890; P = 0.014).
DISCUSSION
This was a large-scale study in which a retrospective and consecutive analysis of hospitalized UC patients with initial CS therapy across mainland China was performed. There have been several studies about predictors of clinical response to CS, but few reported the response to CS in Chinese cohorts, especially those who were treated with CS for the first time.
Considering the influences of potential selection bias, the findings of our study were still generally consistent with those of other studies, especially those by Chen et al[20] and Chow et al[21], which were based on all and partial UC cases who received the first course of CS therapy in Guangzhou and Hong Kong of China (Table 5). The short-term and long-term responses to CS in Chinese IBD patients might be concluded from the high similarity between our nationwide study and the regional studies by Chen et al[20] and Chow et al[21]. A study by Jeon et al[22] on Korea cases showed higher non-response and surgery rates, which may be due to the fact that the included cases were more severe (active moderate to severe UC patients who received intravenous CS after failure of oral CS therapy), since severity had been shown to be a predictor of poor outcome after CS therapy[23,24].
Table 5.
Different studies for ulcerative colitis cases with initial corticosteroid treatment
| Current study | Chen et al[20] | Chow et al[21] | Faubion et al[25] | Tung et al[26] | Jeon et al[22] | Ho et al[17] | |
| Area | Mainland China | Guangzhou | Hong Kong | Olmsted | Olmsted | Korea | UK |
| (China) | (China) | (USA) | (USA) | ||||
| Cases, n | 101 | 81 | 95 | 63 | 14 (≤ 19 yr) | 67.0 | 86 |
| Date | 2007-2011 | 2002-2010 | 1985-2007 | 1970-1993 | 1940-2001 | 1996-2010 | 1998-2003 |
| Study type | Multi-center | Single-center | Single-center | Population-based | Population-based | Single-center | Single-center |
| Within 1 mo, % | |||||||
| Response | 95 | 97.5 | 93.7 | 84 | 79 | 78.5 | 82 |
| Non-response | 5 | 2.5 | 6.3 | 16 | 21 | 19.7 | 25 |
| Within 1 yr, % | |||||||
| Pronged response | 41.6 | 67.1 | 46.3 | 49 | 57 | 46.3 | 55 |
| CS dependence | 49.5 | 32.9 | 47.4 | 22 | 14 | 42.6 | 17 |
| Surgery | 6.9 | 0 | 7.4 | 29 | 29 | 16.0 | 21 |
Part of the cases had previous use of corticosteroids. Different studies were conducted in different areas, dates and cohorts to evaluate corticosteroid response in ulcerative colitis according to the similar classification of corticosteroid response. CS: Corticosteroid.
There were obvious differences between our study and the studies by Faubion et al[25] and Ho et al[17], which reported much higher non-response and surgery rates in American cases from 1970 to 1993 and in UK patients from 1998 to 2003. This may be due to the lack of timely diagnosis and aggressive medication (immunomodulators and biological agents) in the early stages. The genetic susceptibility to CS between yellow and white races might also play a role. A study by Tung et al[26] on pediatric patients showed a higher rate of prolonged response and a lower rate of dependency, but the surgery rate was much higher, which indicates that the prognosis and response to CS between adults and pediatrics may be different. Further studies are needed to clarify this issue.
Compared with our study in which all the cases took initial CS treatment, not all the cases in the study by Chow et al[21] took systematic CS therapy for the first time. However, the two studies shared similar CS response rates, suggesting that initial requirement of CS played no significant role in clinical efficacy. Previous studies[27-29] have suggested that CS down-regulated the expression of CS receptor (CR), but its levels increased after finishing CS treatment. Therefore, within a long interval between two consecutive CS treatments, the density of CR could return to baseline, which would not affect the next CS therapy. This might explain the similarity between our study and that by Chow et al[21].
No previous studies reported the negative relationship between tenesmus and CS dependence (Table 2). As tenesmus was a typical symptom of inflammation and its existence reflected the predominance of distal inflammation, it was thought to be easily controlled by anti-inflammatory agents, especially daily enemas. After further analysis, it was found that compared with the cases without tenesmus, the ones with tenesmus had a much higher ESR before CS therapy (36.9 mm/h vs 27.1 mm/h) and a slightly lower ESR at one month (20.5 mm/h vs 22.0 mm/h). On the other hand, before therapy, 63.6% cases with tenesmus had a high Sutherland score of more than 10, while 50% cases without tenesmus reached that level. After 30 d of CS therapy, among the cases with tenesmus at baseline, only 22.9% had a Sutherland score of more than 6, while the score was more than 6 in 51.3% cases which had no tenesmus at baseline. In a study by Tong et al[30], composite sophora colon-soluble capsules in treating UC had a tendency to improve tenesmus (P = 0.056), but no changes were found in improving other symptoms. In a study by Patz et al[31], 9 of 10 patients with refractory distal UC had a clinical remission after short-chain fatty acid enemas, as reflected by a decrease in degree of tenesmus (1.6 vs 0.3, P < 0.05) and bleeding (2.2 vs 1.2, P < 0.05) and by global self-assessment. This also indicated that UC cases with tenesmus was relatively easy to reach symptom alleviation after medical therapy.
In our study, the cases with weight loss at diagnosis tended to respond less to the subsequent CS treatment (Table 3). Similarly, in the study by Chow et al[21], the univariate P-values were less than 0.05 both in predicting CS dependency of ulcerative colitis and CS refractoriness of Crohn’s disease, despite the multivariate P-value of more than 0.05. Moreover, Chow et al[21] reported anemia as a predictor of CS refractoriness, which was not verified by the study by Chen et al[20] and our study. In the study of Korean cohort by Jeon et al[22], anemia was predictive of CS non-response on 14 d, but it was not applicable at 1 mo, 3 mo and 1 year (P = 0.02, 0.247, 0.057). Other studies reported other variables, such as extensive colitis[32] and the score[33], as predictors of poor CS response including CS dependence, CS refractoriness and/or surgery. Given the tremendous discrepancy in different studies, large-scale prospective studies using the same evaluation criteria are needed to identify the variables in predicting CS refractoriness.
After one month of CS treatment, a higher proportion of CS dependent cases still suffered from a high Sutherland score (≥ 6), suggesting Sutherland score as another risk predictor for CS dependence (Table 4). In previous studies, few captured makers of disease activity such as CDAI and Sutherland score for correlation analysis. Before the start of CS, most cases suffered from moderate to severe disease, which narrowed the disparity in Sutherland score. After one month of CS therapy, we found that a sustained high Sutherland score (≥ 6) was predictive of CS dependence. Yoon et al[34] found that partial Mayo score was a predictive factor of CS dependence in CS-naïve UC cases, while Turner et al[35] reported the PUCAI which was calculated on day 3 and day 5 of steroid therapy could identify patients requiring salvage therapy. In all, as a comprehensive index, Sutherland score was a better predictor of CS dependence than single factors.
Besides, auxiliary meditation played no statistically influential role in CS response, which might be attributed to the low rates of immunosuppressants and infliximab (22.8% and 2.0%) and the irregular medication in this study. A large prospective study should be designed to assess the role of immunosuppressants and infliximab in Chinese UC patients after CS treatment.
Several limitations existed in our study. First, our study was a retrospective study, and the results needed to be confirmed by prospective studies. Second, it was not a population-based study, and the incidence of initial CS treatment in Chinese cohorts was unknown. Third, the majority of the study cases were diagnosed by endoscopy before CS administration, but few underwent a second endoscopy examination in the follow-up after the start of CS. The relationship between mucosal healing and CS efficacy was still unknown. Last but not least, the simple size was relatively small.
In conclusion, among moderate to severe Chinese UC cases with initial CS treatment, primary response and non-response occurred in 95.0% and 5.0% within one month. Prolonged CS response, CS dependence and secondary non-response existed in 41.6%, 49.5% and 4.0% of cases within one year. The cumulative rate of surgery was 6.9% within one year. Tenesmus negatively predicted CS dependence and weight loss predicted CS refractoriness. After one month of CS treatment, sustained high Sutherland score (≥ 6) was another risk factor for predicting CS dependence.
COMMENTS
Background
Despite the medication of new drugs (such as infliximab and adalimumab), corticosteroids (CS) are still widely used for moderate to severe ulcerative colitis (UC), but clinical response and its predictors have not been systematically evaluated to date in Chinese cohorts, especially among those with CS treatment for the first time.
Research frontiers
Tenesmus was found to be a negative predictor of CS dependence and weight loss as a predictor of CS refractoriness within one year. After one-month treatment, sustained high Sutherland score (≥ 6) also predicted CS dependence.
Innovations and breakthroughs
This was the first large-scale retrospective study of hospitalized UC patients with initial CS therapy across mainland China. There have been several studies about predictors of clinical response to CS, but few reported the response to CS in Chinese cohorts, especially among those who were treated with CS for the first time. After statistical analysis of the patients’ demographic, laboratory, clinical characteristics and auxiliary medication, tenesmus was found to be negatively associated with CS dependence. Previous studies ignored this symptom and the predictive role in clinical response.
Applications
This study identified the predictors of poor CS response, which could help digestive physicians adopt more aggressive treatment early among the patients with risk factors to avoid subsequent poor CS response.
Peer-review
This paper describes the results of a Chinese multicenter study evaluating the effectiveness of steroid therapy in patients with moderate to severe ulcerative colitis and investigating predictive factors for effectiveness and the development of steroid dependence after 1 year of treatment. It is therefore very interesting. Many studies have reported the treatment outcomes of new drugs for ulcerative colitis. Recently, however, few studies have examined outcomes of steroid therapy. Steroid preparations continue to be used for the treatment of active ulcerative colitis throughout the world. Because this paper focuses on the efficacy of steroid therapy and the development of steroid dependence, the most important problem associated with steroid therapy, it is considered very meaningful.
Footnotes
Supported by Grants from the Ministry of Public Health, No. 201002020; and Hubei Provincial Outstanding Medical Academic Leader Program (2013).
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: July 28, 2014
First decision: August 15, 2014
Article in press: November 11, 2014
P- Reviewer: Yokoyama K S- Editor: Yu J L- Editor: Wang TQ E- Editor: Liu XM
References
- 1.Ng SC, Tang W, Ching JY, Wong M, Chow CM, Hui AJ, Wong TC, Leung VK, Tsang SW, Yu HH, et al. Incidence and phenotype of inflammatory bowel disease based on results from the Asia-pacific Crohn’s and colitis epidemiology study. Gastroenterology. 2013;145:158–165.e2. doi: 10.1053/j.gastro.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y, Ouyang Q. Ulcerative colitis in China: retrospective analysis of 3100 hospitalized patients. J Gastroenterol Hepatol. 2007;22:1450–1455. doi: 10.1111/j.1440-1746.2007.04873.x. [DOI] [PubMed] [Google Scholar]
- 3.Wang YF, Ouyang Q, Hu RW. Progression of inflammatory bowel disease in China. J Dig Dis. 2010;11:76–82. doi: 10.1111/j.1751-2980.2010.00421.x. [DOI] [PubMed] [Google Scholar]
- 4.Wang YF, Zhang H, Ouyang Q. Clinical manifestations of inflammatory bowel disease: East and West differences. J Dig Dis. 2007;8:121–127. doi: 10.1111/j.1443-9573.2007.00296.x. [DOI] [PubMed] [Google Scholar]
- 5.Lichtenstein GR, Abreu MT, Cohen R, Tremaine W. American Gastroenterological Association Institute medical position statement on corticosteroids, immunomodulators, and infliximab in inflammatory bowel disease. Gastroenterology. 2006;130:935–939. doi: 10.1053/j.gastro.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 6.Farrell RJ, Kelleher D. Glucocorticoid resistance in inflammatory bowel disease. J Endocrinol. 2003;178:339–346. doi: 10.1677/joe.0.1780339. [DOI] [PubMed] [Google Scholar]
- 7.Travis SP. Review article: the management of mild to severe acute ulcerative colitis. Aliment Pharmacol Ther. 2004;20 Suppl 4:88–92. doi: 10.1111/j.1365-2036.2004.02056.x. [DOI] [PubMed] [Google Scholar]
- 8.Aspenvall L, Aadland E. [Severe ulcerative colitis] Tidsskr Nor Laegeforen. 1990;110:1520–1522. [PubMed] [Google Scholar]
- 9.Creed TJ, Probert CS. Review article: steroid resistance in inflammatory bowel disease - mechanisms and therapeutic strategies. Aliment Pharmacol Ther. 2007;25:111–122. doi: 10.1111/j.1365-2036.2006.03156.x. [DOI] [PubMed] [Google Scholar]
- 10.Cho JH. The genetics and immunopathogenesis of inflammatory bowel disease. Nat Rev Immunol. 2008;8:458–466. doi: 10.1038/nri2340. [DOI] [PubMed] [Google Scholar]
- 11.Van Limbergen J, Russell RK, Nimmo ER, Satsangi J. The genetics of inflammatory bowel disease. Am J Gastroenterol. 2007;102:2820–2831. doi: 10.1111/j.1572-0241.2007.01527.x. [DOI] [PubMed] [Google Scholar]
- 12.Li M, Gao X, Guo CC, Wu KC, Zhang X, Hu PJ. OCTN and CARD15 gene polymorphism in Chinese patients with inflammatory bowel disease. World J Gastroenterol. 2008;14:4923–4927. doi: 10.3748/wjg.14.4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jewell DP, Truelove SC. Azathioprine in ulcerative colitis: final report on controlled therapeutic trial. Br Med J. 1974;4:627–630. doi: 10.1136/bmj.4.5945.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fraser AG, Orchard TR, Jewell DP. The efficacy of azathioprine for the treatment of inflammatory bowel disease: a 30 year review. Gut. 2002;50:485–489. doi: 10.1136/gut.50.4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moss AC, Farrell RJ. Infliximab for induction and maintenance therapy for ulcerative colitis. Gastroenterology. 2006;131:1649–1651; discussion 1651. doi: 10.1053/j.gastro.2006.09.039. [DOI] [PubMed] [Google Scholar]
- 16.Mozaffari S, Nikfar S, Abdolghaffari AH, Abdollahi M. New biologic therapeutics for ulcerative colitis and Crohn’s disease. Expert Opin Biol Ther. 2014;14:583–600. doi: 10.1517/14712598.2014.885945. [DOI] [PubMed] [Google Scholar]
- 17.Ho GT, Mowat C, Goddard CJ, Fennell JM, Shah NB, Prescott RJ, Satsangi J. Predicting the outcome of severe ulcerative colitis: development of a novel risk score to aid early selection of patients for second-line medical therapy or surgery. Aliment Pharmacol Ther. 2004;19:1079–1087. doi: 10.1111/j.1365-2036.2004.01945.x. [DOI] [PubMed] [Google Scholar]
- 18.Chinese Medical Association Digestive Branch. Chinese consensus on diagnosis and treatment standard of inflammatory bowel disease. Chin J Intern Med. 2012;51:818–831. [Google Scholar]
- 19.Munkholm P, Langholz E, Davidsen M, Binder V. Frequency of glucocorticoid resistance and dependency in Crohn’s disease. Gut. 1994;35:360–362. doi: 10.1136/gut.35.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen BL, Chen YJ, Gao X, He Y, Xiao YL, Chen SH, Hu PJ. The natural history of first course of corcoticosteroid therapy for 235 patients with inflammatory bowel disease. Chin J Gastroenterol Hepatol. 2013;22:1006–5709. [Google Scholar]
- 21.Chow DK, Sung JJ, Tsoi KK, Wong VW, Wu JC, Leong RW, Chan FK. Predictors of corticosteroid-dependent and corticosteroid-refractory inflammatory bowel disease: analysis of a Chinese cohort study. Aliment Pharmacol Ther. 2009;29:843–854. doi: 10.1111/j.1365-2036.2009.03944.x. [DOI] [PubMed] [Google Scholar]
- 22.Jeon HH, Lee HJ, Jang HW, Yoon JY, Jung YS, Park SJ, Hong SP, Kim TI, Kim WH, Cheon JH. Clinical outcomes and predictive factors in oral corticosteroid-refractory active ulcerative colitis. World J Gastroenterol. 2013;19:265–273. doi: 10.3748/wjg.v19.i2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mack DR, Langton C, Markowitz J, LeLeiko N, Griffiths A, Bousvaros A, Evans J, Kugathasan S, Otley A, Pfefferkorn M, et al. Laboratory values for children with newly diagnosed inflammatory bowel disease. Pediatrics. 2007;119:1113–1119. doi: 10.1542/peds.2006-1865. [DOI] [PubMed] [Google Scholar]
- 24.Lindgren SC, Flood LM, Kilander AF, Löfberg R, Persson TB, Sjödahl RI. Early predictors of glucocorticosteroid treatment failure in severe and moderately severe attacks of ulcerative colitis. Eur J Gastroenterol Hepatol. 1998;10:831–835. doi: 10.1097/00042737-199810000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Faubion WA, Loftus EV, Harmsen WS, Zinsmeister AR, Sandborn WJ. The natural history of corticosteroid therapy for inflammatory bowel disease: a population-based study. Gastroenterology. 2001;121:255–260. doi: 10.1053/gast.2001.26279. [DOI] [PubMed] [Google Scholar]
- 26.Tung J, Loftus EV, Freese DK, El-Youssef M, Zinsmeister AR, Melton LJ, Harmsen WS, Sandborn WJ, Faubion WA. A population-based study of the frequency of corticosteroid resistance and dependence in pediatric patients with Crohn’s disease and ulcerative colitis. Inflamm Bowel Dis. 2006;12:1093–1100. doi: 10.1097/01.mib.0000235835.32176.85. [DOI] [PubMed] [Google Scholar]
- 27.Hearing SD, Norman M, Probert CS, Haslam N, Dayan CM. Predicting therapeutic outcome in severe ulcerative colitis by measuring in vitro steroid sensitivity of proliferating peripheral blood lymphocytes. Gut. 1999;45:382–388. doi: 10.1136/gut.45.3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matthews JG, Ito K, Barnes PJ, Adcock IM. Defective glucocorticoid receptor nuclear translocation and altered histone acetylation patterns in glucocorticoid-resistant patients. J Allergy Clin Immunol. 2004;113:1100–1108. doi: 10.1016/j.jaci.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 29.Kam JC, Szefler SJ, Surs W, Sher ER, Leung DY. Combination IL-2 and IL-4 reduces glucocorticoid receptor-binding affinity and T cell response to glucocorticoids. J Immunol. 1993;151:3460–3466. [PubMed] [Google Scholar]
- 30.Tong ZQ, Yang B, Chen BY, Zhao ML. A multi-center, randomized, single-blind, controlled clinical study on the efficacy of composite sophora colon-soluble capsules in treating ulcerative colitis. Chin J Integr Med. 2010;16:486–492. doi: 10.1007/s11655-010-0562-5. [DOI] [PubMed] [Google Scholar]
- 31.Patz J, Jacobsohn WZ, Gottschalk-Sabag S, Zeides S, Braverman DZ. Treatment of refractory distal ulcerative colitis with short chain fatty acid enemas. Am J Gastroenterol. 1996;91:731–734. [PubMed] [Google Scholar]
- 32.Choquet A, Yamamoto-Furusho JK, Reyes E, Takahashi-Monroy T, Vargas-Vorácková F, Uscanga L. [Predictors of colectomy in patients with ulcerative colitis. A cohort analysis of 184 cases] Rev Invest Clin. 2004;56:11–15. [PubMed] [Google Scholar]
- 33.Seo M, Okada M, Yao T, Matake H, Maeda K. Evaluation of the clinical course of acute attacks in patients with ulcerative colitis through the use of an activity index. J Gastroenterol. 2002;37:29–34. doi: 10.1007/s535-002-8129-2. [DOI] [PubMed] [Google Scholar]
- 34.Yoon JY, Cheon JH, Park JJ, Hong SP, Kim TI, Kim WH. Clinical outcomes and factors for response prediction after the first course of corticosteroid therapy in patients with active ulcerative colitis. J Gastroenterol Hepatol. 2011;26:1114–1122. doi: 10.1111/j.1440-1746.2011.06688.x. [DOI] [PubMed] [Google Scholar]
- 35.Turner D, Mack D, Leleiko N, Walters TD, Uusoue K, Leach ST, Day AS, Crandall W, Silverberg MS, Markowitz J, et al. Severe pediatric ulcerative colitis: a prospective multicenter study of outcomes and predictors of response. Gastroenterology. 2010;138:2282–2291. doi: 10.1053/j.gastro.2010.02.047. [DOI] [PubMed] [Google Scholar]


