Abstract
AIM: To investigate the clinical characteristics and prognostic factors of cutaneous metastasis of cholangiocarcinoma by a retrospective analysis of published cases.
METHODS: An extensive search was conducted in the English literature within the PubMed database using the following keywords: cutaneous metastasis or skin metastasis and cholangiocarcinoma or bile duct. The data of 30 patients from 21 articles from 1978 to 2014 were analyzed. Patient data retrieved from the articles included the following: age, gender, time cutaneous metastasis occurred, number of cutaneous metastases throughout life, sites of initial cutaneous metastasis, anatomic site, pathology and differentiation of cholangiocarcinoma, and immunohistochemical results of the cutaneous metastasis. The assessment of overall survival after cutaneous metastasis (OSCM) was the primary endpoint.
RESULTS: The median age at diagnosis of cutaneous metastasis of cholangiocarcinoma was 60.0 years (range: 35-77). This metastasis showed a predilection towards males, with a male to female ratio of 3.29. In 8 cases (27.6%), skin metastasis was the first sign of cholangiocarcinoma. Additionally, 18 cases (60.0%) manifested single cutaneous metastasis, while 12 cases (40.0%) demonstrated multiple skin metastases. In 50.0% of patients, the metastasis occurred in the drainage region, while 50.0% of patients had distant cutaneous metastases. The scalp was the most frequently involved region of distant skin metastasis, occurring in 36.7% of patients. The median OSCM of cholangiocarcinoma was 4.0 mo. Patient age and cutaneous metastatic sites showed no significant relation with OSCM, while male gender and single metastasis of the skin were associated with a poorer OSCM (hazard ratio: 0.168; P = 0.005, and hazard ratio: 0.296; P = 0.011, respectively).
CONCLUSION: The prognosis of cutaneous metastasis of cholangiocarcinoma is dismal. Both male gender and single skin metastasis are associated with a poorer OSCM.
Keywords: Cutaneous metastasis, Cholangiocarcinoma, Clinical characteristics, Pathological features, Prognosis
Core tip: Skin metastasis from internal malignancy is rare, especially from cholangiocarcinoma. The clinical characteristics and prognosis of the metastases are unclear and need to be further defined. We performed a systemic analysis of 30 cases in the English literature from 1978 to 2014 to expand our understanding of this condition. To the best of our knowledge, this is the first comprehensive analysis of cutaneous metastasis of cholangiocarcinoma.
INTRODUCTION
Skin metastasis is rare, involving 0.7%-9% of all cancer patients[1]. Lung and breast cancer are the leading causes of cutaneous metastasis in males and females, respectively[1]. The early recognition of skin metastasis of previously undiagnosed cancers is critical for timely intervention[1,2].
Cutaneous metastases originating from cholangiocarcinoma are rarely encountered in clinical practice. To date, little is known about the clinicopathological features and prognosis of this condition, as only sporadic case reports or series are available. To obtain a full understanding of the disease, systemic reviews and analyses are required. To the best of our knowledge, our research is the first comprehensive analysis of cutaneous metastasis of cholangiocarcinoma.
MATERIALS AND METHODS
An extensive search of the English literature was performed in PubMed using the keywords “cutaneous metastasis” or “skin metastasis” and “cholangiocarcinoma” or “bile duct”. Only cases with a definite pathologic diagnosis and disease course description were included. In total, 30 cases have been described in case reports or series between 1978 and 2014[3-23].
The data that were retrieved included the following: age, gender, time cutaneous metastasis occurred, number of cutaneous metastases throughout life, sites of initial cutaneous metastasis, anatomic site, pathology and differentiation of cholangiocarcinoma, and immunohistochemical results of the cutaneous metastasis. The primary aim was to determine overall survival after cutaneous metastasis (OSCM), defined as the period from diagnosis of cutaneous metastasis to death or latest follow-up. The evaluation of OSCM will help to clarify the value of cutaneous metastasis in predicting the survival outcome in cholangiocarcinoma patients.
The Kaplan-Meier method was used to generate the cumulative survival curve, and the log-rank (Mantel-Cox) test was used to compare survival differences. We used SPSS version 17.10 (SPSS, Inc., Chicago, IL, United States) for data analysis, and P < 0.05 was considered statistically significant.
RESULTS
Clinical and pathological features
As shown in Table 1, the median age at diagnosis of cutaneous metastasis of cholangiocarcinoma was 60.0 years (range: 35-77). This metastasis affected more males, with a male to female ratio of 3.29. Skin metastasis was the first sign of cholangiocarcinoma in 8 cases (27.6%). For those with a definite diagnosis of cholangiocarcinoma, the mean time for cutaneous metastasis development was 14.51 mo.
Table 1.
Clinical features of patients with cutaneous metastasis of cholangiocarcinoma
| Age (n = 30) | n (%) |
| Median at diagnosis (yr) 60.0 | |
| Range (yr) 35-77 | |
| 30-40 | 2 (6.7) |
| 41-50 | 6 (20.0) |
| 51-59 | 6 (20.0) |
| 60-70 | 9 (30.0) |
| > 70 | 7 (23.3) |
| Sex (n = 30) | |
| Male | 23 (76.7) |
| Female | 7 (23.3) |
| Cutaneous metastasis occurrence (n = 30) | |
| Before diagnosis of cholangiocarcinoma | 8 (26.7) |
| After diagnosis of cholangiocarcinoma | 21 (70.0) |
| Not available | 1 (3.3) |
| Number of cutaneous metastasis throughout life (n = 30) | |
| Single | 18 (60.0) |
| Multiple | 12 (40.0) |
| Sites of initial cutaneous metastasis (n = 30) | |
| Drainage site | 15 (50.0) |
| Distant site | 15 (50.0) |
| Scalp1 | 11 (36.7) |
| Head2 | 2 (6.7) |
| Shoulder | 1 (3.3) |
| Trunk | 1 (3.3) |
Including a case with initial skin metastasis of scalp and head and two cases with initial skin metastasis of scalp and trunk;
Including a case with the initial skin metastasis on the head, face, and trunk.
In total, 18 cases (60.0%) manifested single cutaneous metastasis, while 12 cases (40.0%) demonstrated multiple skin metastases. In 50.0% of patients, the metastasis occurred in the drainage region, namely the percutaneous biliary drainage or catheterization site, while 50.0% of patients had distant cutaneous metastasis. The scalp was the most frequently involved region of distant skin metastasis, occurring in 36.7% of all patients.
The cutaneous metastases presented as nodules, papules, erythema and lesions with or without ulcers, which were sized between 0.3 cm and 4 cm. Figure 1 shows a metastatic nodule and erythema in the scalp and along the drainage tube, respectively. The size of skin metastases had no association with OSCM.
Figure 1.
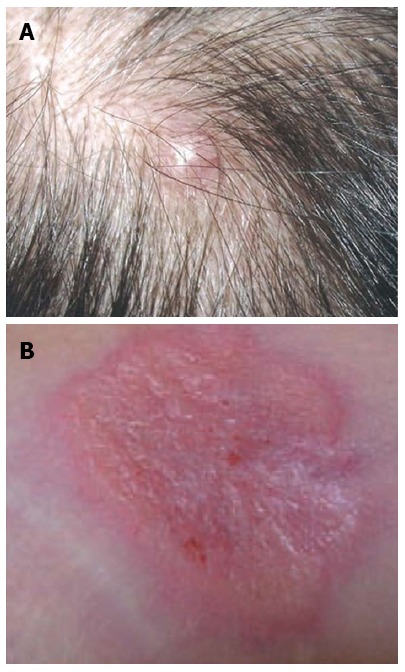
Typical manifestations of skin metastasis of cholangiocarcinoma. A: A metastatic nodule in the scalp[11]; B: Erythema along the catheter tract[8].
In total, 24 cases had explicit descriptions of the anatomic site of cholangiocarcinoma, of which 4 cases were extrahepatic, 10 cases were hilar, and 10 cases were intrahepatic. With respect to the pathology of cholangiocarcinoma, 26 cases had clear documentation, of which 25 cases were adenocarcinoma and one case was small cell cholangiocarcinoma. Additionally, 17 cases had information on the differentiation of the primary tumor. The well, well-to-moderate, moderate-to-poor, poor, and undifferentiated types comprised 6, 2, 1, 7, and 1 cases, respectively. Only 9 cases had detailed immunohistochemical analysis of the cutaneous metastasis (Table 2).
Table 2.
Immunohistochemical analysis of cutaneous metastasis in 9 cases
| Case number | Immunohistochemistry results |
| 1 | CK7+, CEA-, CK20- |
| 2 | CK7+, CK19+ |
| 3 | CK19+, CEA+ |
| 4 | CK7+, CK8/18/19+, CEA+, CK20- |
| 5 | CK7+, CAM5.2+, AE1/3+, CK20- |
| 6 | CK7+, CK20+, CA199 (focally+), TTF1- |
| 7 | CK7+, CK19+ |
| 8 | CK7 (strongly+), CK20 (weakly+) |
| 9 | CK7+, CK20+, p53+ |
When cutaneous metastasis (or metastases) occurred, 8/24 patients underwent surgery, including to the skin lesion, while 16/24 did not. Chemotherapy was performed in 8/23 patients, while 15/23 patients did not undergo chemotherapy. Only 4/23 patients received local radiotherapy to the skin metastasis, while 19/23 did not. However, the response to chemotherapy or radiotherapy was poor.
Prognostic factors of overall survival
As shown in Figure 2, the median OSCM of cholangiocarcinoma was only 4.0 mo (n = 24). When cutaneous metastasis occurred, 12/13 patients had concurrent metastasis of other sites, such as the bone, lung, brain, and lymph nodes. In 17 cases, there was no description of concurrent metastasis in other sites.
Figure 2.
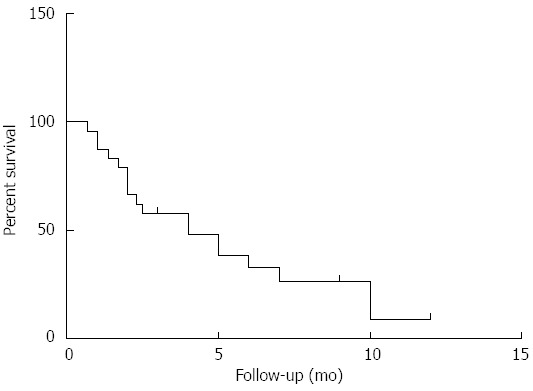
Overall survival after cutaneous metastasis of cholangiocarcinoma (n = 24). The median OSCM of cholangiocarcinoma was only 4.0 mo. OSCM: Overall survival after cutaneous metastasis.
As shown in Figure 3A and B, male gender and single metastasis were associated with a poorer OSCM (male vs female, 2.4 mo vs 10 mo; hazard ratio (HR) = 0.168; 95% confidence interval (CI): 0.058-0.490; P = 0.005; single metastasis vs multiple metastases: 2.3 mo vs 7 mo; HR = 0.296; 95%CI: 0.104-0.842; P = 0.011.
Figure 3.
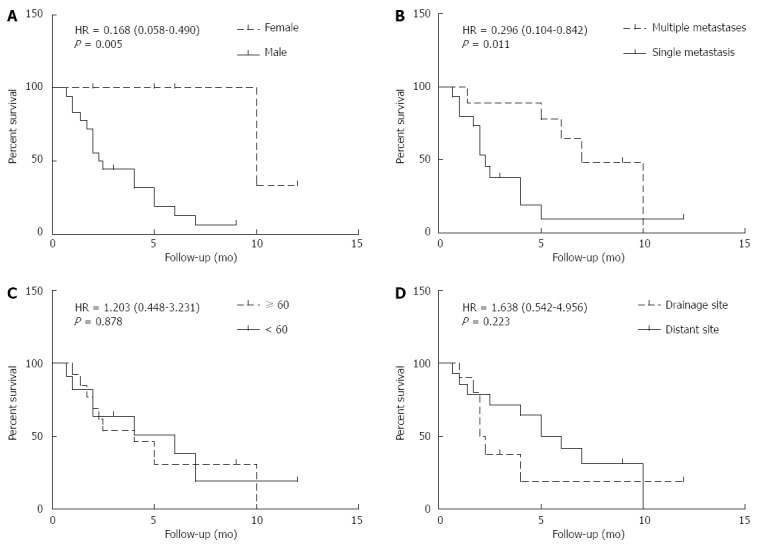
Prognostic factors of overall survival after cutaneous metastasis. A: Male gender was associated with a poorer OSCM (HR = 0.168; 95%CI: 0.058-0.490; P = 0.005); B: Single metastasis was associated with a poorer OSCM (HR = 0.296; 95%CI: 0.104-0.842; P = 0.011); C: Age showed no significant association with OSCM; D: Cutaneous metastatic site had no significant association with OSCM. HR: Hazard ratio; CI: Confidence interval.
As shown in Figure 3C and D, age and cutaneous metastatic sites showed no significant relationship with OSCM (≥ 60 years vs < 60 years, 4 mo vs 6 mo: HR = 1.203; 95%CI: 0.448-3.231; P = 0.878; drainage vs distant, 2.15 mo vs 5.5 mo: HR = 1.638; 95%CI: 0.542-4.956; P = 0.223).
DISCUSSION
Cholangiocarcinoma is a rare neoplasm originating from the epithelial cells of the bile duct, and it accounts for less than 2%[23] of all cancers. Cholangiocarcinoma is typically defined according to the anatomic site, as intrahepatic, hilar, or extrahepatic cholangiocarcinoma[24]. Unfortunately, cholangiocarcinoma has a very poor prognosis, with the majority of patients losing the opportunity for radical surgery. Cholangiocarcinoma is prone to metastasize to the lung, liver, peritoneum, and retroperitoneal lymph nodes, while cutaneous metastasis is uncommon[23]. Currently, cutaneous metastasis has only been described in sporadic case reports or series. No systematic research has been initiated to elucidate the clinicopathological features, prognosis and overall survival related to cutaneous metastasis, and we are the first group to analyze and summarize the characteristics of skin metastasis of cholangiocarcinoma.
Interestingly, in 27.6% (8/29) of cases, skin metastasis was the first sign of cholangiocarcinoma, suggesting that bile duct examination should be performed for cutaneous metastasis of unknown origin. The confirmation of primary malignancy was the prerequisite of rational treatment. In our research, skin metastasis of cholangiocarcinoma could be further divided into two subtypes, involving the site of percutaneous biliary drainage (PTBD) or distant regions, which have different tumor behavior. The former showed seeding of the tumor along the catheter tract, while the latter was associated with distant dissemination. However, skin metastasis of the drainage site composed half of all the cutaneous metastasis in cholangiocarcinoma, suggesting that more attention should be paid to this complication of PTBD. Hepatocellular carcinoma has also been related to cutaneous metastasis in the biopsy site. When addressing scalp metastasis of unknown origin, we should keep the possibility of bile duct-derived carcinoma in mind, as our data showed that the scalp was the most frequently involved site of distant skin metastasis in cholangiocarcinoma. This hematogenous dissemination likely resulted from the intracranial venous sinuses, which communicated with valveless vertebral venous plexus. Additionally, the abdominal and pelvic venous blood enters into the vertebral venous plexus[11].
Skin metastasis of cholangiocarcinoma usually indicates a poor prognosis, with a median OSCM of 4 mo. In our analysis, the longest OSCM was 12 mo, while the shortest was only 21 d. In univariate analysis, female gender showed an OSCM advantage over male gender (10 mo vs 2.4 mo). However, the number of female cases with OSCM data was small (only 6), and more cases should be recruited in the future to confirm this trend. Surprisingly, the group with multiple skin metastases was superior to the group with a single metastasis with respect to OSCM. Among the 18 patients with a single cutaneous metastasis, 12 cases (66.7%) had a lesion at the drainage site. Patients who required PTBD usually had more advanced primary tumors and, thus, were more likely to have a single skin metastasis. As more advanced primary tumors were usually associated with a shorter overall survival, this phenomenon partly accounts for the unexpected observation that patients with a single skin metastasis had a shorter survival than those with multiple metastases.
Skin metastasis of cholangiocarcinoma is rather rare compared with lung cancer or breast cancer. Over a span of nearly 50 years (from 1978 to 2014), only 30 cases could be found in the English language literature. Only published cases with a description of disease course could be included, and the characteristics of unpublished cases may not be representative. Despite the limitation in case numbers, we have helped to expand the understanding of this unique entity. With new reports of such cases in the future, more consensus will be achieved.
In conclusion, the prognosis of cholangiocarcinoma with cutaneous metastasis is dismal. The scalp is the most frequently involved site of distant skin metastasis. Male gender and single metastasis are associated with a relatively poorer OSCM.
COMMENTS
Case characteristics
We investigated the clinical characteristics and prognostic factors of cutaneous metastasis of cholangiocarcinoma by retrospective analysis of 30 cases from 21 published articles from 1978 to 2014.
Clinical diagnosis
Cutaneous metastasis of cholangiocarcinoma.
Differential diagnosis
Cutaneous metastasis of other origins.
Imaging diagnosis
Computed tomography or magnetic resonance imaging of related sites demonstrated the lesions of cutaneous metastasis.
Pathological diagnosis
Pathologic examination showed metastatic carcinoma consistent with cholangiocarcinoma.
Treatment
Patients underwent surgery or radiotherapy to the local cutaneous metastasis or chemotherapy or none of these strategies.
Related reports
To the best of our knowledge, this is the first comprehensive analysis of cutaneous metastasis of cholangiocarcinoma.
Term explanation
The overall survival after cutaneous metastasis, defined as the period from diagnosis of cutaneous metastasis to death or latest follow-up, will help to clarify the value of cutaneous metastasis for predicting survival outcome in cholangiocarcinoma patients.
Experiences and lessons
The prognosis of cutaneous metastasis of cholangiocarcinoma is very poor, and both male gender and single metastasis are associated with worse overall survival after cutaneous metastasis.
Peer-review
This is a comprehensive, detailed analysis with interesting findings, as it is the first combined analysis of clinical data and survival of many cases of cutaneous metastasis of cholangiocarcinoma, which is an extremely rare manifestation of this disease.
Footnotes
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: September 30, 2014
First decision: October 14, 2014
Article in press: December 8, 2014
P- Reviewer: Kawakami H, Mott JL, Rerknimitr R, Tanaka T, Vasilieva LE S- Editor: Ma YJ L- Editor: Cant MR E- Editor: Zhang DN
References
- 1.Hussein MR. Skin metastasis: a pathologist’s perspective. J Cutan Pathol. 2010;37:e1–20. doi: 10.1111/j.1600-0560.2009.01469.x. [DOI] [PubMed] [Google Scholar]
- 2.Brownstein MH, Helwig EB. Patterns of cutaneous metastasis. Arch Dermatol. 1972;105:862–868. [PubMed] [Google Scholar]
- 3.Geramizadeh B, Giti R, Malekhosseini SA. Cutaneous metastasis of cholangiocarcinoma: report of two cases. Int J Organ Transplant Med. 2013;4:172–174. [PMC free article] [PubMed] [Google Scholar]
- 4.Noro T, Ohdaira H, Takizawa R, Kawasaki N, Kitajima M, Suzuki Y. Metastasis to the skin at the drain site after complete resection of the lower bile duct cancer: report of a case. Surg Today. 2012;42:1248–1252. doi: 10.1007/s00595-012-0340-4. [DOI] [PubMed] [Google Scholar]
- 5.Kondo NI, Shirabe K, Mano Y, Taketomi A, Yoshizumi T, Ikegami T, Masuda T, Kayashima H, Hashimoto N, Morita K, et al. Late recurrence after resection of mass-forming intrahepatic cholangiocarcinoma: report of a case. Surg Today. 2012;42:1210–1214. doi: 10.1007/s00595-012-0332-4. [DOI] [PubMed] [Google Scholar]
- 6.Hyun SY, Lee JH, Shin HS, Lee SW, Park YN, Park JY. Cutaneous metastasis from cholangiocarcinoma as the first clinical sign: a report of two cases. Gut Liver. 2011;5:100–104. doi: 10.5009/gnl.2011.5.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.West KL, Selim MA, Puri PK. Cutaneous metastatic cholangiocarcinoma: a report of three cases and review of the literature. J Cutan Pathol. 2010;37:1230–1236. doi: 10.1111/j.1600-0560.2010.01619.x. [DOI] [PubMed] [Google Scholar]
- 8.Balzani A, Clerico R, Schwartz RA, Panetta S, Panetta C, Skroza N, Innocenzi D, Calvieri S. Cutaneous implantation metastasis of cholangiocarcinoma after percutaneous transhepatic biliary drainage. Acta Dermatovenerol Croat. 2005;13:118–121. [PubMed] [Google Scholar]
- 9.Bloom RA, Gordon RL, Manny Y, Engelberg M. Seeding of cholangiocarcinoma along T-tube tracts. Gastrointest Radiol. 1984;9:167–169. doi: 10.1007/BF01887827. [DOI] [PubMed] [Google Scholar]
- 10.Stergiopoulos C, Kountouras J, Kapetanakis N, Katsinelos P, Kokkali S, Tsapournas G, Zavos C, Zaramboukas T. Distant cutaneous metastasis preceding the diagnosis of ductal cholangiocarcinoma. J Eur Acad Dermatol Venereol. 2009;23:242–243. doi: 10.1111/j.1468-3083.2008.02845.x. [DOI] [PubMed] [Google Scholar]
- 11.Lu CI, Wong WR, Hong HS. Distant cutaneous metastases of cholangiocarcinoma: report of two cases of a previously unreported condition. J Am Acad Dermatol. 2004;51:S108–S111. doi: 10.1016/j.jaad.2004.01.042. [DOI] [PubMed] [Google Scholar]
- 12.Dogan G, Karincaoglu Y, Karincaoglu M, Aydin NE. Scalp ulcer as first sign of cholangiocarcinoma. Am J Clin Dermatol. 2006;7:387–389. doi: 10.2165/00128071-200607060-00008. [DOI] [PubMed] [Google Scholar]
- 13.Kwon E, Jeon SY. P378: Multiple cutaneous metastases from cholangiocarcinoma Konyang University. Show North Area (Green) 2014;66:432–432. [Google Scholar]
- 14.Kwak JY, Kim KM, Jeong CU, Yu KJ. A case of cholangiocarcinoma with distant cutaneous metastasis on the scalp. Korean Medical Institute Estimates Published Academic. 2012:129. [Google Scholar]
- 15.Pasquali P, Fortuño A, Casañas C, Fonoll M, Landeyro J, Gonzalez J. Scalp and chest cutaneous metastases of cholangiocarcinoma. Int J Dermatol. 2013;52:1414–1417. doi: 10.1111/j.1365-4632.2011.05186.x. [DOI] [PubMed] [Google Scholar]
- 16.Shorvon PJ, Leung JW, Corcoran M, Mason RR, Cotton PB. Cutaneous seeding of malignant tumours after insertion of percutaneous prosthesis for obstructive jaundice. Br J Surg. 1984;71:694–695. doi: 10.1002/bjs.1800710916. [DOI] [PubMed] [Google Scholar]
- 17.Oleaga JA, Ring EJ, Freiman DB, McLean GK, Rosen RJ. Extension of neoplasm along the tract of a transhepatic tube. AJR Am J Roentgenol. 1980;135:841–842. doi: 10.2214/ajr.135.4.841. [DOI] [PubMed] [Google Scholar]
- 18.Verbeek PC, Van der Heyde MN, Ramsoekh T, Bosma A. Clinical significance of implantation metastases after surgical treatment of cholangiocarcinoma. Semin Liver Dis. 1990;10:142–144. doi: 10.1055/s-2008-1040467. [DOI] [PubMed] [Google Scholar]
- 19.Loew R, Dueber C, Schwarting A, Thelen M. Subcutaneous implantation metastasis of a cholangiocarcinoma of the bile duct after percutaneous transhepatic biliary drainage (PTBD) Eur Radiol. 1997;7:259–261. doi: 10.1007/s003300050147. [DOI] [PubMed] [Google Scholar]
- 20.Yanagi T, Matsumura T, Yoshizaki N. Cholangiocarcinoma with skin metastases. J Am Acad Dermatol. 2007;56:S58–S60. doi: 10.1016/j.jaad.2006.02.057. [DOI] [PubMed] [Google Scholar]
- 21.Ueda K, Okada N, Yoshikawa K. A case of cutaneous metastasis of bile duct carcinoma. J Am Acad Dermatol. 1991;25:848–849. doi: 10.1016/s0190-9622(08)80981-4. [DOI] [PubMed] [Google Scholar]
- 22.Reingold IM, Smith BR. Cutaneous metastases from hepatomas. Arch Dermatol. 1978;114:1045–1046. [PubMed] [Google Scholar]
- 23.Lee WJ, Kim MS, Chang SE, Lee MW, Choi JH, Moon KC, Koh JK. Multiple cutaneous metastases from hilar cholangiocarcinoma. Clin Exp Dermatol. 2009;34:e174–e176. doi: 10.1111/j.1365-2230.2008.03022.x. [DOI] [PubMed] [Google Scholar]
- 24.Choi EK, Yoo IeR, Kim SH, O JH, Choi WH, Na SJ, Park SY. The clinical value of dual-time point 18F-FDG PET/CT for differentiating extrahepatic cholangiocarcinoma from benign disease. Clin Nucl Med. 2013;38:e106–e111. doi: 10.1097/RLU.0b013e318266f402. [DOI] [PubMed] [Google Scholar]


