Abstract
AIM: To investigate the efficacy of probiotics in irritable bowel syndrome (IBS) patients.
METHODS: PubMed, Cochrane library, Scopus, Google Scholar, and Clinicaltrial.gov databases were searched for literature published between September 2007 and December 2013. The applied Mesh terms were “probiotics,” “irritable bowel syndrome,” and “irritable bowel syndrome treatment.” The collected data contained24 clinical trials, of which 15 were eligible for meta-analysis and nine were reviewed systematically. All studies were randomized placebo-controlled trials in patients with IBS that investigated the efficacy of probiotics in IBS improvement. The Jadad score was used to assess the methodological quality of trials. The quality scale ranges from 0 to 5 points, with a score ≤ 2 indicating a low quality report, and a score of ≥ 3 indicating a high quality report. Relative risk (RR), standardized effect size, and 95%CI were calculated using the DerSimonian-Laird method. The Cochran Q test was used to test heterogeneity with P < 0.05. Funnel plots were constructed and Egger’s and Begg-Mazumdar tests were performed to assess publication bias.
RESULTS: A total of 1793 patients were included in the meta-analysis. The RR of responders to therapies based on abdominal pain score in IBS patients for two included trials comparing probiotics to placebo was 1.96 (95%CI: 1.14-3.36; P = 0.01). RR of responders to therapies based on a global symptom score in IBS patients for two included trials comparing probiotics with placebo was 2.43 (95%CI: 1.13-5.21; P = 0.02). For adequate improvement of general symptoms in IBS patients, the RR of seven included trials (six studies) comparing probiotics with placebo was 2.14 (95%CI: 1.08-4.26; P = 0.03). Distension, bloating, and flatulence were evaluated using an IBS severity scoring system in three trials (two studies) to compare the effect of probiotic therapy in IBS patients with placebo, the standardized effect size of mean differences for probiotics therapy was -2.57 (95%CI: -13.05--7.92).
CONCLUSION: Probiotics reduce pain and symptom severity scores. The results demonstrate the beneficial effects of probiotics in IBS patients in comparison with placebo.
Keywords: Evidence-based medicine, Irritable bowel syndrome, Meta-analysis, Probiotics, Systematic review, Clinical trial
Core tip: Irritable bowel syndrome (IBS) is a gastrointestinal tract dysfunction with a complicated etiology. Probiotics may influence IBS symptoms. The present meta-analysis included 1793 patients with all subtypes of IBS from 15 randomized, double-blind clinical trials conducted during 2007-2013. The current and previous meta-analyses are mainly limited by the use of different scales to analyze the mean differences of symptoms among various studies. Thus, further clinical trials are still needed to conclude the effectiveness of probiotics on specific major IBS symptoms of patients. Probiotics may have a beneficial therapeutic role in IBS patients.
INTRODUCTION
Irritable bowel syndrome (IBS) is a gastrointestinal tract dysfunction with a complicated etiology[1]. Prevalence of IBS varies between Asian and North American societies, but the total range in the general population is estimated at 5%-11%[2-4]. Besides the interference with daily life of patients and caregivers, socioeconomic costs of IBS have increased, as the majority of IBS patients are young (20-39 years)[2].
Abdominal pain, stool pattern alteration, distention, bloating, straining, abdominal discomfort, and urgency are major symptoms observed in IBS[5,6]. Genetic background, environmental factors, history of inflammatory bowel disease in a family member, and psychological factors, such as stressful social activities, are involved in the pathogenesis of IBS[7]. The level of severity of IBS depends on various factors, such as chronic immunity reactions after intestinal microbiome alteration, visceral hypersensitivity associated with gut-brain pathways, and impaired bowel permeability. It is believed that initiation of IBS in some people is associated with a post-microbial infection[8,9]. However, the precise cause of IBS is currently unknown[10-14].
Pharmacologic, psychologic, and complementary approaches are considered as therapeutic options in IBS patients[15]. Pharmacologic medications include antispasmodics, selective serotonin reuptake inhibitors[16], tricyclic antidepressants[17], and 5-hydroxytryptamine type-3 antagonists such as ramosetron and alosetron[18], and lubiprostone and linaclotide[19]. However, due to lack of favorable efficacy and associated adverse events with pharmacologic treatments, some IBS patients look for alternative treatments such as herbal medications and Chinese acupuncture[2,20-22]. Probiotics are live microorganisms which have been demonstrated to exhibit potential effects on human health[23]. Probiotics may influence the IBS symptoms including abdominal pain, bloating, distension, flatulence, altered bowel movements, and gut microbiota[24].
The nature of probiotics explains their beneficial role in intestinal function as they can protect against pathogenic bacteria via their antimicrobial properties[25]. Probiotics also amplify the intestinal tight junctions and stabilize the permeability. Moreover, probiotics stimulate goblet cells to produce mucus to enhance the intestinal barrier function, normalize bowel movements, and reduce visceral hypersensitivity[25] in pediatric and adult patients[26,27].Several probiotic strains showed beneficial outcomes in IBS patients[28,29]. The present study was performed to update the previous meta-analysis[30] with consideration of further clinical trials. A systematic review has been also conducted to assess the efficacy of probiotics in IBS patients in clinical trials that were not eligible for inclusion in the meta-analysis.
MATERIALS AND METHODS
Data sources
PubMed, Cochrane library, Scopus, Google Scholar, and Clinicaltrial.gov databases were searched for articles published between September 2007 and December 2013. The applied Mesh terms were “probiotics,” “irritable bowel syndrome,” and “irritable bowel syndrome treatment.”
Study selection
Three reviewers inspected the topic and abstracts of all articles to eliminate identical studies, review articles, systematic reviews, and meta-analysis investigations. All relevant characteristics of included trials, such as IBS type, probiotic strain, dose of probiotics, trial and follow-up duration, and patient characteristics and outcomes, were collected and summarized. All randomized controlled trials that considered IBS symptom improvement as outcome of interest were included. The reference lists of searched articles were reviewed to identify any additional eligible articles.
Assessment of trial quality
The Jadad score, which indicates the quality of studies based on their description of randomization, blinding, and dropouts (withdrawals), was used to assess the methodological quality of trials[31]. The quality scale ranges from 0 to 5 points, with a score of ≤ 2 indicating a low quality report, and a score of ≥ 3 indicating a high quality report.
Statistical analysis
Data from selected studies were extracted in the form of 2 × 2 tables by study characteristics. Included studies were weighted by effect size and pooled. Data were analyzed using StatsDirect software version 3.0.107 (StatsDirect Ltd., Cheshire, United Kingdom). Relative risk (RR), standardized effect size, and 95%CI were calculated using a DerSimonian-Laird (for random effects) method. The Cochran Q test was used to test heterogeneity and P < 0.05 was considered as significant. In case of heterogeneity or few included studies, the random effects model was used. Egger’s and Begg-Mazumdar tests were used to evaluate publication bias indicators in a funnel plot.
RESULTS
Based on the electronic search, 11748 publications were identified. A total of 8719 studies were found to be inappropriate because they did not clearly meet our inclusion criteria. Of the remaining studies, those without a control group, using probiotics in combination with herbal medication or prebiotics, and with unsuitable details about inclusion criteria were excluded. Furthermore, one trial that had applied the Likert score, two trials that were performed in pediatric patients, and two crossover trials were excluded (Figure 1). Fifteen trials met our criteria for meta-analysis, which included the use of Rome II (n = 7), Rome III (n = 6), and International Classification of Health Problems in Primary Care and World Organization of Family Doctors (n = 2) criteria. The quality score of included trials were assessed and reported according to Jadad quality score; all trials had high quality with scores ranging from 3 to 5 (Table 1). A total of 1793 patients with diarrhea-predominant IBS (D-IBS), constipation-predominant IBS (C-IBS), and alternative IBS (A-IBS) were included.
Figure 1.
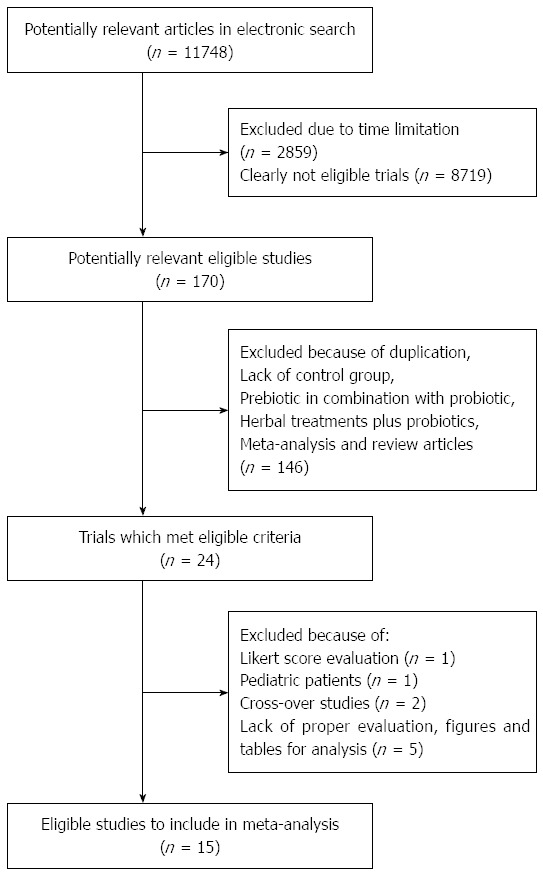
Flow diagram for study selection.
Table 1.
Jadad quality score of randomized controlled trial included in the meta-analysis
| Study | Randomization | Blinding | Withdrawals and dropouts | Total score |
| Kajander et al[33] | 2 | 2 | 1 | 5 |
| Williams et al[32] | 1 | 2 | 1 | 4 |
| Zeng et al[39] | 1 | 2 | 1 | 4 |
| Enck et al[35] | 1 | 1 | 1 | 3 |
| Drouault-Holowacz et al[37] | 2 | 2 | 1 | 5 |
| Sinn et al[42] | 2 | 2 | 1 | 5 |
| Enck et al[36] | 1 | 2 | 1 | 4 |
| Simrén et al[40] | 2 | 2 | 1 | 5 |
| Sondergaard et al[43] | 2 | 2 | 1 | 5 |
| Guglielmetti et al[44] | 2 | 2 | 1 | 5 |
| Ducrotté et al[45] | 1 | 2 | 1 | 4 |
| Kruis et al[34] | 2 | 2 | 1 | 5 |
| Ki cha et al[38] | 2 | 2 | 1 | 5 |
| Dapoigny et al[46] | 1 | 2 | 1 | 4 |
| Roberts et al[41] | 2 | 2 | 1 | 5 |
The characteristics of studies are scrutinized in Table 2. Nine other clinical trials that were not applicable for our meta-analysis were reviewed systematically. The characteristics and outcomes of these trials are summarized in Table 3.
Table 2.
Characteristics of studies included in the meta-analysis
| Trial | Type of IBS | Criteria |
Age (yr) |
Sex (Male/Female) |
Probiotic | Probiotic dosage | Duration of treatment | Follow-up | Outcome | ||
| Probiotic | Placebo | Probiotic | Placebo | ||||||||
| Kajander et al[33] | All types | Rome II | 50 | 46 | 2/41 | 4/39 | Lactobacillusrhamnosus GG | 1 × 107 CFU | 20 wk | 3 wk | ↑ Stabilization of intestinal microbiota |
| L. rhamnosus Lc705 | ↓ Distension and abdominal pain in probiotic group | ||||||||||
| Propionibacteriumfreudenreichiissp. shermanii JS | ↓ IBS symptoms | ||||||||||
| Bifidobacteriumanimalisssp. lactis Bb-12 | |||||||||||
| Williams et al[32] | All types | Rome II | 40 | 38 | 3/25 | 8/20 | L. acidophilus CUL60 | 2.5 × 1010 CFU | 8 wk | 2 wk | ↑ QoL |
| L. acidophilus CUL21 | ↓ Symptom severity, bloating not improved | ||||||||||
| B. lactis CUL34, | |||||||||||
| B. bifidum CUL20 | |||||||||||
| Zeng et al[39] | D-IBS | Rome II | 44.6 | 45.8 | 10/4 | 9/6 | Streptococcusthermophilus | 1 × 108 CFU | 4 wk | - | Mucosal barrier function and bowel symptoms improved |
| L. bulgaricus | 1 × 107 CFU | ↓ Small bowel permeability | |||||||||
| L. acidophilus | |||||||||||
| B. longum | |||||||||||
| Enck et al[35] | All types | ICHPPC and WONCA | 49.8 | 49.4 | 76/72 | 75/75 | Escherichia coli (Symbioflor 2) | 1.5-4.5 ×107 CFU | 8 wk | ND | ↓ Typical symptoms of IBS patients |
| Drouault-Holowacz et al[37] | All types | Rome II | 47 | 44 | 8/40 | 16/36 | B. longum LA101 | 1 × 1010 CFU | 4 wk | - | ↑ QoL |
| L. acidophilus LA102 | ↓ Flatulence | ||||||||||
| Lactococcusl actis LA103 | ↓ Abdominal pain and bloating | ||||||||||
| S. thermophilus LA104 | |||||||||||
| Sinn et al[42] | All types | Rome III | 41.9 | 47.5 | 6/14 | 8/12 | L. acidophilus SDC 2012, 2013 | 2 × 109 CFU | 4 wk | - | ↓ IBS symptoms, abdominal pain and discomfort |
| Enck et al[36] | All types | ICHPPC and WONCA | 49.8 | 49.4 | 77/72 | 73/75 | E. coli and Enterococcusfaecalis (Pro Symbioflor) | 3-9 × 107 CFU | 8 wk | - | ↓ 50% global symptom score and abdominal pain score |
| Simrén et al[40] | All types | Rome II | 42 | 44 | 11/26 | 11/26 | L. paracasei F19 | 5 × 107 CFU | 8 wk | 8 wk | Improvement in both groups in pain frequency, pain and bloating severity, satisfaction with bowel habits, and interference with daily life |
| L. acidophilus La5 | |||||||||||
| B. lactis Bb-12 | |||||||||||
| Sondergaard et al[43] | ND | Rome II | 53.9 | 48.5 | 7/20 | 6/19 | L. paracasei F19 | 5 × 107 CFU | 8 wk | 8 wk | Symptom relief in both groups;no difference between probiotics and placebo |
| L. acidophilus La5 | (500 mL) | ||||||||||
| B. lactis Bb-12 | |||||||||||
| Guglielmetti et al[44] | All types | Rome III | 36.65 | 40.98 | 21/41 | 19/41 | B. bifidum MIMBb75 | 1 × 109 CFU | 4 wk | 4 wk | ↓ IBS symptoms like: pain, discomfort distension, bloating, digestive disorders |
| ↑ QoL | |||||||||||
| Ducrotté et al[45] | D-IBS (in majority of patients) | Rome III | 36.53 | 38.4 | 70/38 | 81/25 | L. plantarum 299v | 1 × 1010 CFU | 4 wk | 3 wk | ↓ Abdominal pain and bloating |
| Kruis et al[34] | D-IBS | Rome II | 46.3 | 45.1 | 12/48 | 16/44 | E. coli (Nissle 1917) | 2.5-25 × 109 CFU | 12 wk | - | No significant effects of probiotics in general symptoms, but enteric flora altered due to gastroenterocolitis or administration of antibiotics before IBS initiation |
| Ki Cha et al[38] | D-IBS | Rome III | 37.9 | 40.3 | 12/13 | 14/11 | L. acidophilus | 1 × 1010 CFU | 8 wk | 2 wk | ↑ QoL |
| L. plantarum | |||||||||||
| L. rhamnosus | |||||||||||
| B. breve | |||||||||||
| B. lactis | |||||||||||
| B. longum | |||||||||||
| S. thermophilus | |||||||||||
| Dapoigny et al[46] | All types | Rome III | 46.1 | 48.8 | 5/20 | 10/15 | L. caseirhamnosus (LCR 35) | 6 × 108 CFU | 4 wk | 2 wk | ↓ IBS patients complaining of diarrhea |
| (250 mg) | ↓ 50% reduction in IBS severity score in probiotic arm | ||||||||||
| Roberts et al[41] | C-IBS, A-IBS | Rome III | 44.66 | 43.71 | 14/74 | 14/77 | B. lactis CNCMI-2494 | 1.25 × 1010 CFU | 12 wk | - | Significant improvement in IBS symptoms in both groups |
A-IBS: Alternating irritable bowel syndrome; CFU: Colony forming unit; IBS: Irritable bowel syndrome; C-IBS: Constipation-predominant irritable bowel syndrome; D-IBS: Diarrhea-predominant irritable bowel syndrome; ICHPPC: International classification of health problems in primary care; ND: Not determined; QOL: Quality of life; WONCA: World organization of family doctors.
Table 3.
Characteristics of studies included in the systematic review
| Trial | Type of IBS | Criteria |
Age (yr) |
Sex (Male/Female) |
Probiotic | Probiotic dosage | Duration of treatment | Follow-up | Outcome | ||
| Probiotic | Placebo | Probiotic | Placebo | ||||||||
| Agrawal et al[51] | C-IBS | Rome III | 39.6 | 0/19 | 0/19 | Bifidobacteriumlactis DN-173010 | 1.25 × 1010 CFU | 4 wk | 1 wk | ↓ Abdominal distension and bloating | |
| Hun[48] | D-IBS | Rome II | 48.36 | 9/41 | Bacillusc oagulans GBI-306086 | 8× 108 CFU | 8 wk | - | ↓ Bloating and abdominal pain | ||
| Dolin[67] | D-IBS | Rome III | 52.3 | 44 | 7/19 | 6/23 | B. coagulans GBI-306086 | 2 × 109 CFU | 8wk | 2 wk | ↓ Number of daily bowel movements |
| Guandalini et al[47] | Alltypes | Rome II | 4-18 | 31/28 | VSL#3 | 4.5 × 1011 bacteria | 6 wk | 6 wk after 2-wk wash-out | ↓ Percentage of symptoms, severity and frequency of abdominal pain and bloating | ||
| ↑ QoL | |||||||||||
| Ligaarden et al[53] | All types | Rome II | 46.5 (18-75) | 5/11 | Lactobacillusplantarum MF1298 | 1 × 1010 CFU | 3 wk | - | Daily symptom scores not different between probiotic and placebo groups | ||
| Francavilla et al[50] | ND | Rome II | 6.5 | 6.3 | 43/24 | 35/23 | L. rhamnosus GG | 3 × 109 CFU | 12wk | 8 wk | ↓ Frequency and severity of pain, and improved intestinal permeability |
| Hong et al[49] | All types | Rome III | 33 | 33 | 12/25 | 10/26 | Lactobacillus sp. HY7801 | 4 × 109 CFU | 8 wk | - | ↑ Intestinal barrier function in females |
| B. longum HY804 | ↓ Pain and flatulence defection | ||||||||||
| L. brevis HY7401 | |||||||||||
| Choi et al[54] | D-IBS, A-IBS | Rome II | 40.2 | 40.6 | 18/17 | 19/20 | Saccharomycesboulardii | 2 × 1011 CFU | 4 wk | - | ↑ QoL |
| Michail et al[52] | D-IBS | Rome III | 21.8±17 | 5/10 | 3/6 | VSL#3 | 9 × 1011 bacteria | 8 wk | - | ↑ QoL | |
| No change in gut microbiota | |||||||||||
| ↑ Specific GSRS-IBS scores | |||||||||||
A-IBS: Alternating irritable bowel syndrome; CFU: Colony Forming Unit; C-IBS: Constipation-predominant irritable bowel syndrome; D-IBS: Diarrhea-predominant irritable bowel syndrome; GSRS: Gastrointestinal Symptom Rating Scale; IBS: Irritable bowel syndrome; ND: Not determined; VSL#3: A probiotic combination of L. casei, L.plantarum, L. acidophilus, L. delbrueckii ssp. bulgaricus, B. longum, B. breve, B. infantis, and S. thermophiles; QoL: Quality of life.
Results of meta-analysis
Pain assessments in IBS patients for comparison of probiotics to placebo therapy: Abdominal pain severity was evaluated by IBS severity scoring system in one study for 8-10 wk to compare the effect of probiotics therapy with placebo in IBS patients[32]. The reduction of abdominal pain severity from baseline was -23.42 ± 30.05 and -17.19 ± 27.73 after 8 and 10 wk, respectively, in the probiotics therapy group,and -12.29 ± 30.08 and -8.06 ± 27.98, respectively, in the placebo group.
In another study that assessed abdominal pain severity using a 6 mo symptom diary with a 0-4 scale, the mean of severity of reduction was -3.00 ± 9.95 in the probiotics group in comparison with 0.00 ± 10.27 in the placebo group[33].
Abdominal pain improvement: The RR for abdominal pain improvement in one trial was 1.10 (95%CI: 0.77-1.60), which was not statistically significant[34].
Responders to therapies based on abdominal pain score: The summary for RR of responders to therapies based on abdominal pain score in IBS patients for two included trials comparing probiotics to placebo was 1.96 (95%CI: 1.14-3.36; P = 0.01) (Figure 2A)[35,36]. The Cochrane Q test for heterogeneity indicated that the studies were not heterogeneous (P = 0.11) (Figure 2B) and could be combined. However, because too few studies were included, the random effects model for individual and summary RR was applied.
Figure 2.
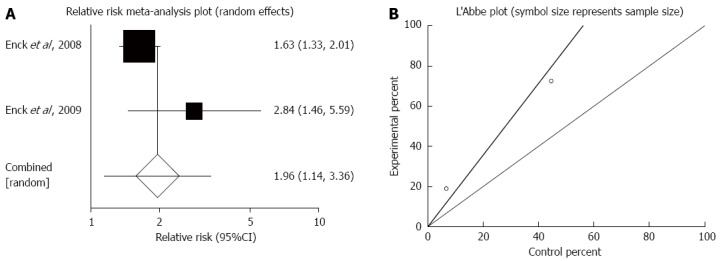
Responders to therapies based on abdominal pain score. A: Individual and pooled relative risk in studies comparing probiotics and placebo in irritable bowel syndrome patients; B: Heterogeneity indicators for the studies.
Abdominal pain score by visual analogue scale (VAS): The summary for standardized effect size of mean differences of abdominal pain score by VAS ranging from 0 to 10 in IBS patients for probiotics therapy for two included trials comparing with placebo was -0.56 (95%CI: -1.29-0.16; P = 0.13) (Figure 3)[37,38]. The Cochrane Q test for heterogeneity indicated that the studies were not heterogeneous (P = 0.73) and could be combined. However, because too few studies were included, the random effects model for individual and summary effect size for standardized mean was applied.
Figure 3.
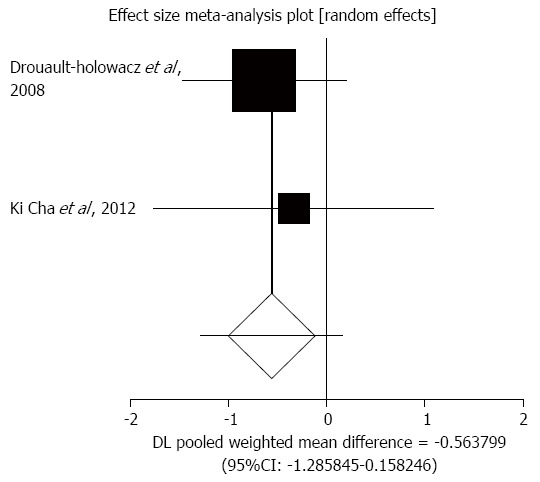
Individual and pooled effect size for standardized mean for abdominal pain in studies comparing probiotics and placebo in irritable bowel syndrome patients.
In one study that scored abdominal pain score on VAS ranging from 0 to 100, the mean score reduction was -7.65 ± 9.69 in the probiotics group vs -0.98±7.67 in the placebo group[39].
Distension/bloating/flatulence assessments in IBS patients for comparison of probiotics to placebo therapy
Distension/bloating/flatulence (DBF): DBF was evaluated by an IBS severity scoring system in three trials from two studies to compare the effect of probiotics therapy with placebo in IBS patients[32,40]. The summary of standardized effect size of mean differences of DBF for probiotics therapy was -2.57 (95%CI: -13.05-7.92; P = 0.63) (Figure 4).The Cochrane Q test for heterogeneity indicated that the studies were not heterogeneous (P = 0.64) and could be combined. However, the random effects model for individual and summary effect size for standardized mean was applied because of the small number of included studies.
Figure 4.
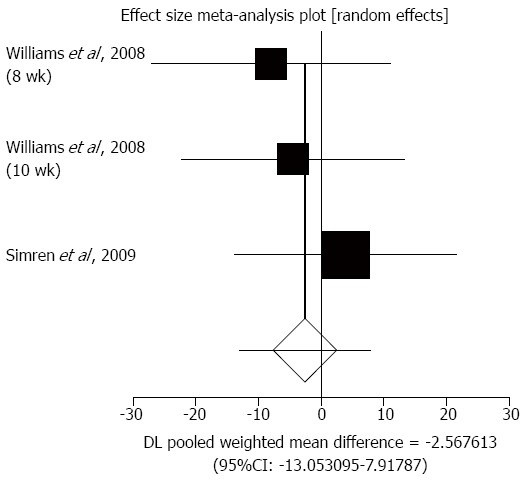
Individual and pooled effect size for standardized mean for distension, bloating, and flatulence in studies comparing probiotics and placebo in irritable bowel syndrome patients.
In another study that assessed DBF using 6-mo symptom diary with a 0-4 scale, the mean severity reduction was -4.00 ± 11.36 in probiotics group vs -2.00 ± 11.2 in the placebo group[33].
Flatulence: The RR for flatulence improvement in one trial was 1.20 (95%CI: 0.85-1.77), which was not statistically significant[34].
Bloating: In one study that evaluated bloating improvement using a 0-100 VAS, the mean score reduction was 0.34 ± 6.49 in probiotics group in comparison to 0.44 ± 5.25 in the placebo group[39].
Bowel habit dissatisfaction: Three trials in two studies evaluated bowel habit dissatisfaction using an IBS severity scoring system to compare the effect of probiotics therapy with placebo in IBS patients[32,40]. The summary for standardized effect size of mean differences of bowel habit dissatisfaction for probiotics therapy was -5.31 (95%CI: -13.87-3.25; P = 0.22) (Figure 5). The Cochrane Q test for heterogeneity indicated that the studies were not heterogeneous (P = 0.66) and could be combined. However, the random effects model for individual and summary of effect size for standardized mean was applied because of the small number of included studies.
Figure 5.
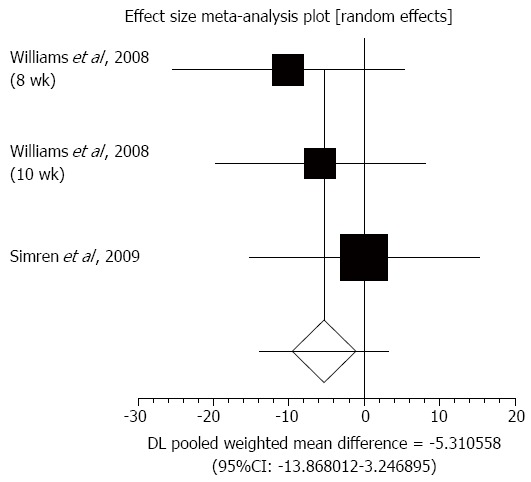
Individual and pooled effect size for standardized mean for bowel habit dissatisfaction in studies comparing probiotics and placebo in irritable bowel syndrome patients.
Assessment of IBS improvement in IBS patients for comparison of probiotics to placebo therapy
Global score improvement: IBS improvement was evaluated by subjective global assessment in one study for 4, 8, and 12 wk to compare the effect of probiotics therapy with placebo in IBS patients[41]. RR was 1.20 (95%CI: 0.89-1.60), 0.90 (95%CI: 0.63-1.20), and 0.90 (95%CI: 0.61-1.20) after4, 8, and 12 wk, respectively.
Sum score: A sum score was evaluated using an IBS symptom score (abdominal pain + distension + flatulence + rumbling) in one study for 4-5, 13-14, and 20 wk to compare the effect of probiotics therapy to placebo in IBS patients[33]. The reduction of sum score from baseline was -13.00 ± 24.40, -15.00 ± 26.84, and -14.00 ± 26.83 after 4-5, 13-14, and 20 wk, respectively, for patients receiving probiotics therapy, and -6.00 ± 22.55, -6.00 ± 22.55, and -3.00 ± 21.97, respectively, with placebo.
Responders to therapies based on global symptom score
The summary for RR of responders to therapies based on a global symptom score in IBS patients for two included trials comparing probiotics with placebo was 2.43 (95%CI: 1.13-5.21; P = 0.02) (Figure 6A)[35,36]. The Cochrane Q test for heterogeneity indicated that the studies were not heterogeneous (P = 0.06) (Figure 6B) and could be combined. Because of the small number of included studies, the random effects model for individual and summary for RR was applied.
Figure 6.
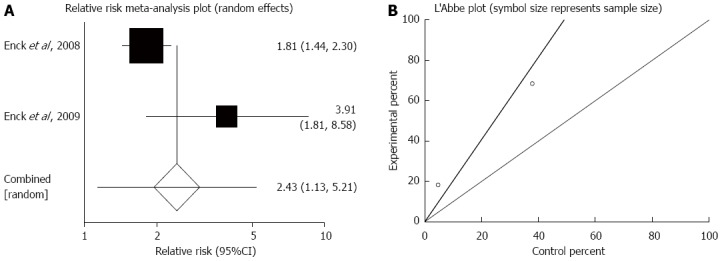
Responders to therapies based on global symptom score. A: Individual and pooled relative risk in studies comparing probiotics and placebo in irritable bowel syndrome patients; B: Heterogeneity indicators for the studies.
Adequate general symptoms improvement
The summary for RR of adequate general symptoms improvement in IBS patients for seven included trials from six studies comparing probiotics with placebo was 2.14 (95%CI: 1.08-4.26; P = 0.03) (Figure 7A)[34,37,42-45]. The Cochrane Q test for heterogeneity indicated that the studies were heterogeneous non-combinable (P < 0.01) (Figure 7B), and thus the random effects model for individual and summary for RR was applied. For evaluation of publication bias, Egger’s regression of normalized effect vs precision for all included studies for “adequate general symptoms improvement” in IBS patients among probiotics vs placebo therapy was 4.34 (95%CI: -3.13-11.81; P = 0.20), and the Begg-Mazumdar test on standardized effect vs variance indicated τ = -0.33 (P = 0.38) (Figure 7C).
Figure 7.
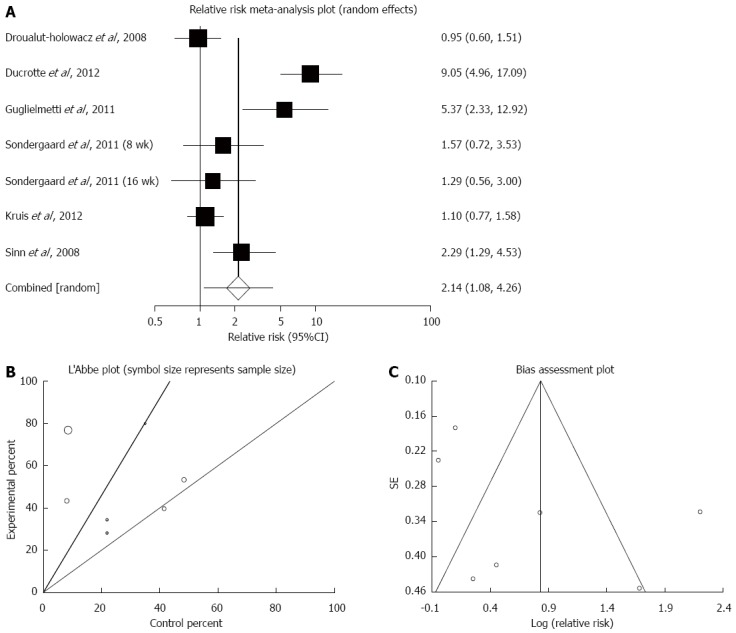
Adequate general symptoms improvement. A: Individual and pooled relative risk in studies comparing probiotics and placebo irritable bowel syndrome patients; B: Heterogeneity indicators for the studies; C: Publication bias indicators.
Symptom severity score: Symptom severity score was evaluated by an IBS severity scoring system in one study for 8 and 10 wk to compare the effect of probiotics therapy with placebo in IBS patients[32]. The reduction of symptom severity score from baseline was -132.45 ± 118.30 and -93.49 ± 103.45 for probiotics therapy group in 8 and 10 wk, respectively, and -80.08 ± 116.16 and -58.67 ± 96.38, respectively for patients in the placebo group.
Severity score improvement: RR for severity score improvement in C-IBS and A-IBS types in one trial was 1.29 (95%CI: 0.72-2.16), which was not statistically significant[46]. The RR for severity score improvement was significant in D-IBS types in one trial, at 1.74 (95%CI: 1.06-2.66)[46].
Results of systematic review
A systematic review was performed to summarize non-eligible clinical trials for the meta-analysis that were excluded because of heterogeneity or different measurement scales for IBS symptoms. All the nine studies were classified as randomized placebo-controlled trials. Four, six, and eight weeks of treatment with probiotics were reported.
Abdominal pain: Four weeks administration of probiotics ameliorated the abdominal pain in three clinical trials in comparison to placebo[37,42,45]. In one multi-center crossover study in children, probiotics alleviated the intensity and frequency of abdominal pain after 6 wk; the mean reduction of abdominal pain score from baseline was 1.0 ± 0.2 and 0.5 ± 0.2 in probiotics and placebo groups, respectively[47]. Hun[48] reported the improvement of abdominal pain in a probiotics arm. However, this symptom was also improved in the placebo group after 6 and 8 wk. Another study showed that the abdominal pain score was significantly decreased in the probiotic group; the mean abdominal pain score was decreased from baseline (53 ± 21.4) -26.0 and -29.5 after 4 and 8 wk of probiotics administration, respectively[49]. An evaluation of pain relief in a crossover trial amongst children reported improvement with probiotics in 36/67 children at 12 wk and 49/67 at 20 wk[50]. The placebo group in that study showed improvement among 23/69 and 38/69 patients after 12 and 20 wk, respectively. Simrén et al[40] demonstrated the positive effect of probiotics in abdominal pain after 1 wk in comparison to placebo. However, there was no significant different between probiotics and placebo groups after 8 wk of treatment.
DBF: Flatulence and bloating were improved in probiotics-receiving adult patients after 4 wk[37,44,45]. Moreover, probiotics alleviated distension and bloating in adult female patients with C-IBS[51]. Another investigation demonstrated that the mean abdominal distension/bloating in the placebo group declined from baseline after 8 and 10 wk (-14.74 and -7.52, respectively) compared to -22.80 and -12.04, respectively, in the probiotics group[32]. A crossover clinical trial showed that bloating/gassiness in 42/59 IBS children was improved, including 16 in the placebo group[47]. The overall assessment showed the effectiveness of probiotics on abdominal bloating compared to placebo in the 6-wk trial. Simrén et al[40] reported a significant reduction of bloating severity after 2 wk of treatment with probiotics compared to placebo, but no significant difference was observed between the two compared groups at the end of trial.
Global IBS score: The specific gastrointestinal symptom rating scale-IBS score was improved after 8 wk of probiotics digestion in D-IBS patients[52].However, the daily symptom score did not change among probiotics- and placebo-receiving patients in a crossover trial[53]. Probiotics were effective in reducing the global IBS score from baseline after 4 wk[39]. In comparison to placebo, 50% reduction was observed in global symptom score after probiotics ingestion[36]. In another clinical trial, IBS severity score was decreased by 40% in probiotics-receiving patients, whereas the reduction was reported as 28% in placebo-receiving patients[46].
IBS symptoms relief: Treatment with probiotics for 4, 6, and 8 wk resulted insignificant beneficial effects on IBS symptoms in comparison to placebo[35,41-44,47].
Quality of life (QoL): Probiotics administration decreased disease-associated complications in IBS patients after 4 wk[37,44,54]. In a crossover trial, the elevated QoL in children after 6 wk of probiotics digestion was identified[47]. In 2008, Williams et al[32] reported that probiotics improved the QoL from baseline rather than placebo. Another study performed by Ki Cha et al[38] reported that the changes of QoL from baseline in the follow-up period were statistically similar in probiotics- and placebo-receiving patients. Michail et al[52] showed a significant positive effect on the overall average QoL score in both probiotics and placebo groups after 8 wk of treatment; QoL was improved from 3.0 ± 1.3 to 2.1 ± 0.8 in the active treatment group and from 2.4 ± 1.0 to 1.8 ± 0.6 in the control group. Twelve-week treatment similarly resulted in QoL improvement in placebo and probiotics groups in D-IBS patients[34].
Intestinal barrier function and gut microbiota
In IBS patients, bowel function and gut microbiota are changed. Probiotics modify the impaired intestinal permeability in pediatric and D-IBS patients after 4 and 12 wk of treatment, respectively[39,50]. Furthermore, probiotics caused significant improvement in intestinal barrier function among IBS female patients in an 8-wk trial[49]. Probiotics digestion alleviated the increased small bowel permeability in 28.6% of patients in comparison to 53.3% in a placebo group[39]. It was therefore concluded that probiotics improved mucosal barrier function in D-IBS adult patients. Steady state of gut microbiota in the probiotics-treated arm was increased, but it was decreased in the placebo arm[33]. However, the balanced gut microbiota was not observed in probiotics-receiving patients in comparison to placebo after 8 and 12 wk of treatment[34,52].
DISCUSSION
IBS is manifested with gut-brain axis interactions, changes in serologic biomarkers, enhanced inflammatory indicators such as myeloperoxidase, tumor necrosis factor α, and lipid peroxides, and gut microbiome disruption, and is also associated with genetic background and environmental factors[55-58]. Among all factors, change in intestinal microbial flora is important in the initiation of IBS. Furthermore, diet as an environmental factor influences the human microflora[59]. Despite pharmacologic approaches and novel drugs for IBS management, the use of probiotics in IBS has been confirmed by recovery and gradual healing[60-62]. Additionally, probiotics stabilize immune dysregulation in IBS, thus enhancing cellular integrity to protect the colon[63,64]. Probiotics also modify the intestinal microbiota, altering the fermentation pattern inside the colon and reducing flatulence[65].
Although probiotic organisms exert beneficial effects to the host, they can act as a double-edged sword with both negative and positive effects. Therefore, precaution is necessary before they are administered[66]. Pain assessment analysis showed that probiotics significantly reduce pain severity after eight and ten weeks of administration[32,33]. However, the reduction rate was rather higher at week eight than week ten, suggesting reduced effectiveness with long-term use. The responder rate based on abdominal pain was significantly more than placebo[35,36]. Probiotics did not improve abdominal pain significantly vs placebo in two trials[34,38], and did not significantly affect the severity of DBF[32-34,39,40]. Global IBS symptoms were not improved[41], but IBS sum score was decreased after use of probiotics[33]. The responder rate was significantly higher in probiotics-treated groups when global symptom improvement was considered[35,36]. Probiotics were effective in inducing an adequate improvement of general IBS symptoms[34,37,42-45]. The severity of symptoms was decreased[32], but was not improved with probiotics compared with placebo[46]. The same results of clinical improvement in a previous meta-analysis demonstrated the effectiveness of probiotics on IBS symptoms[30].
The numbers of reported withdrawals are shown in Table 4. The majority of withdrawals were due to adverse events in probiotics and lack of efficacy in placebo groups. Four and seven patients in placebo and probiotics groups, respectively, discontinued treatment due to adverse events[33,34,36]. Lack of efficacy was reported as the reason of withdrawal in three patients in two trials[34,35]. Symptom worsening was reported in five patients who received placebo in two trials[38,46]. The results of the systematic review demonstrated the beneficial effect of probiotics on QoL[32,34,37,38,44,47,54], abdominal pain[37,42,45,47-50], DBF[32,37,44,45,51,54], IBS diagnostic scores[36,39,46,52,53] , and IBS total symptoms[33,35,42,44,47,67].
Table 4.
Numbers and causes of reported withdrawals in the included clinical trials in the meta-analysis
| Study | Group (n) |
Cause of withdrawal |
|||
| Adverse effect | Non-compliance | Lack of efficacy | Symptom worsening | ||
| Drouault-Holowacz et al[37] | Placebo (53) | NR | 1 | NR | NR |
| Probiotic (53) | NR | 5 | NR | NR | |
| Kajander et al[33] | Placebo (43) | 2 | NR | NR | NR |
| Probiotic (43) | 2 | NR | NR | NR | |
| Kruis et al[34] | Placebo (60) | NR | NR | 2 | NR |
| Probiotic (60) | 2 | NR | NR | NR | |
| Enck et al[35] | Placebo (148) | NR | 1 | 1 | NR |
| Probiotic (149) | 2 | NR | NR | NR | |
| Enck et al[36] | Placebo (150) | 2 | NR | NR | NR |
| Probiotic (148) | 3 | NR | NR | NR | |
| Dapoigny et al[46] | Placebo (26) | NR | NR | NR | 3 |
| Probiotic (26) | NR | NR | NR | NR | |
| Ki Cha et al[38] | Placebo (25) | NR | NR | NR | 2 |
| Probiotic (25) | NR | NR | NR | NR | |
NR: Not reported.
Generally, use of different scales to analyze the mean differences of symptoms in various studies has been the main limitation of all existing meta-analyses concerning IBS, and the present one is no an exception. Thus, further clinical trials are still needed to confirm the effectiveness of probiotics on specific major IBS symptoms and patient QoL. Collectively, probiotics may have a beneficial therapeutic role in IBS patients.
COMMENTS
Background
Irritable bowel syndrome (IBS) is a gastrointestinal tract dysfunction, affecting the general population, and young people in particular. This syndrome is manifested with abdominal pain, stool pattern alteration, distention, bloating, straining, abdominal discomfort, and urgency. Genetic background and environmental factors are important in the pathogenesis of IBS, but the precise cause of IBS is still unknown. Because of adverse effects of pharmacologic drugs, some physicians and IBS patients tend to use probiotics.
Research frontiers
Probiotics are live microorganisms that confer positive effects into the host after oral administration. Several probiotics strains have shown beneficial outcomes in IBS patients.
Related publications
The authors’ study was performed to update the previous meta-analysis with the inclusion of additional clinical trials. Moreover, a systematic review was conducted to assess the efficacy of probiotics in IBS patients in clinical trials that were not eligible for meta-analysis section.
Innovations and breakthroughs
A total of 11748 articles published between 2007 and 2013 regarding the efficacy of probiotics in IBS were identified and studied. Of these, 15 trials met inclusion criteria for this meta-analysis and were analyzed. Rome, International Classification of Health Problems in Primary Care, and World Organization of Family Doctors criteria were applied to include IBS patients. Totally, 1793 patients with diarrhea- and constipation-predominant IBS, and alternative IBS were included. Nine other clinical trials that were not applicable for our meta-analysis were reviewed systematically.
Applications
Pain assessment analysis showed that probiotics significantly reduce pain severity. The responder rate based on abdominal pain was significantly greater than with placebo. The responder rate was significantly higher in probiotics treated groups when global symptom improvement was considered. Probiotics were effective for improving general IBS symptoms. The majority of withdrawals were due to adverse events with probiotics and lack of efficacy in placebo groups. The results of the systematic review demonstrated the beneficial effect of probiotics on quality of life, abdominal pain, distension, bloating and flatulence, IBS diagnostic scores and IBS total symptoms. Generally, use of different scales to analyze the mean differences of symptoms in various studies has been the main limitation of all existing meta-analyses in IBS. Thus, well-designed clinical trials are still needed to reach a consensus on the effectiveness of probiotics on IBS symptoms and patient quality of life. Collectively, probiotics seem to have a beneficial therapeutic role in IBS patients if administered accurately.
Peer-review
In this study, we concluded that probiotics confer beneficial effects for alleviation of IBS symptoms. Generally, use of different scales to analyze the mean differences of symptoms in various studies is the main limitation of all existing meta-analyses in IBS. Further well-designed clinical trials are still needed to confirm the effectiveness of probiotics on major IBS symptoms and patient quality of life.
Footnotes
Conflict-of-interest: Authors declare no conflict of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: June 4, 2014
First decision: July 9, 2014
Article in press: September 30, 2014
P- Reviewer: Hauser G S- Editor: Qi Y L- Editor: AmEditor E- Editor: Ma S
References
- 1.Gwee KA, Lu CL, Ghoshal UC. Epidemiology of irritable bowel syndrome in Asia: something old, something new, something borrowed. J Gastroenterol Hepatol. 2009;24:1601–1607. doi: 10.1111/j.1440-1746.2009.05984.x. [DOI] [PubMed] [Google Scholar]
- 2.Wilkins T, Pepitone C, Alex B, Schade RR. Diagnosis and management of IBS in adults. Am Fam Physician. 2012;86:419–426. [PubMed] [Google Scholar]
- 3.Spiller R, Aziz Q, Creed F, Emmanuel A, Houghton L, Hungin P, Jones R, Kumar D, Rubin G, Trudgill N, et al. Guidelines on the irritable bowel syndrome: mechanisms and practical management. Gut. 2007;56:1770–1798. doi: 10.1136/gut.2007.119446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hulisz D. The burden of illness of irritable bowel syndrome: current challenges and hope for the future. J Manag Care Pharm. 2004;10:299–309. doi: 10.18553/jmcp.2004.10.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malagelada JR. A symptom-based approach to making a positive diagnosis of irritable bowel syndrome with constipation. Int J Clin Pract. 2006;60:57–63. doi: 10.1111/j.1368-5031.2005.00744.x. [DOI] [PubMed] [Google Scholar]
- 6.Surdea-Blaga T, Băban A, Dumitrascu DL. Psychosocial determinants of irritable bowel syndrome. World J Gastroenterol. 2012;18:616–626. doi: 10.3748/wjg.v18.i7.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ringel Y, Maharshak N. Intestinal microbiota and immune function in the pathogenesis of irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2013;305:G529–G541. doi: 10.1152/ajpgi.00207.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barbara G, Cremon C, Pallotti F, De Giorgio R, Stanghellini V, Corinaldesi R. Postinfectious irritable bowel syndrome. J Pediatr Gastroenterol Nutr. 2009;48 Suppl 2:S95–S97. doi: 10.1097/MPG.0b013e3181a15e2e. [DOI] [PubMed] [Google Scholar]
- 9.Ghoshal UC, Ranjan P. Post-infectious irritable bowel syndrome: the past, the present and the future. J Gastroenterol Hepatol. 2011;26 Suppl 3:94–101. doi: 10.1111/j.1440-1746.2011.06643.x. [DOI] [PubMed] [Google Scholar]
- 10.Keszthelyi D, Troost FJ, Masclee AA. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. Methods to assess visceral hypersensitivity in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2012;303:G141–G154. doi: 10.1152/ajpgi.00060.2012. [DOI] [PubMed] [Google Scholar]
- 11.Spiller RC. Irritable bowel syndrome. Br Med Bull. 2004;72:15–29. doi: 10.1093/bmb/ldh039. [DOI] [PubMed] [Google Scholar]
- 12.Camilleri M. Current and future pharmacological treatments for diarrhea-predominant irritable bowel syndrome. Expert Opin Pharmacother. 2013;14:1151–1160. doi: 10.1517/14656566.2013.794223. [DOI] [PubMed] [Google Scholar]
- 13.Jeffery IB, Quigley EM, Öhman L, Simrén M, O’Toole PW. The microbiota link to irritable bowel syndrome: an emerging story. Gut Microbes. 2012;3:572–576. doi: 10.4161/gmic.21772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hammerle CW, Surawicz CM. Updates on treatment of irritable bowel syndrome. World J Gastroenterol. 2008;14:2639–2649. doi: 10.3748/wjg.14.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quigley EM, Craig OF. Irritable bowel syndrome; update on pathophysiology and management. Turk J Gastroenterol. 2012;23:313–322. doi: 10.4318/tjg.2012.0551. [DOI] [PubMed] [Google Scholar]
- 16.Suares NC, Ford AC. Diagnosis and treatment of irritable bowel syndrome. Discov Med. 2011;11:425–433. [PubMed] [Google Scholar]
- 17.Rahimi R, Nikfar S, Rezaie A, Abdollahi M. Efficacy of tricyclic antidepressants in irritable bowel syndrome: a meta-analysis. World J Gastroenterol. 2009;15:1548–1553. doi: 10.3748/wjg.15.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rahimi R, Nikfar S, Abdollahi M. Efficacy and tolerability of alosetron for the treatment of irritable bowel syndrome in women and men: a meta-analysis of eight randomized, placebo-controlled, 12-week trials. Clin Ther. 2008;30:884–901. doi: 10.1016/j.clinthera.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Mozaffari S, Nikfar S, Abdollahi M. Metabolic and toxicological considerations for the latest drugs used to treat irritable bowel syndrome. Expert Opin Drug Metab Toxicol. 2013;9:403–421. doi: 10.1517/17425255.2013.759558. [DOI] [PubMed] [Google Scholar]
- 20.Rahimi R, Abdollahi M. Herbal medicines for the management of irritable bowel syndrome: a comprehensive review. World J Gastroenterol. 2012;18:589–600. doi: 10.3748/wjg.v18.i7.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manheimer E, Cheng K, Wieland LS, Min LS, Shen X, Berman BM, Lao L. Acupuncture for treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2012;5:CD005111. doi: 10.1002/14651858.CD005111.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ojcius DM, Jiang SB, Persechini PM, Detmers PA, Young JD. Cytoplasts from cytotoxic T lymphocytes are resistant to perforin-mediated lysis. Mol Immunol. 1991;28:1011–1018. doi: 10.1016/0161-5890(91)90187-o. [DOI] [PubMed] [Google Scholar]
- 23.Jones ML, Martoni CJ, Tamber S, Parent M, Prakash S. Evaluation of safety and tolerance of microencapsulated Lactobacillus reuteri NCIMB 30242 in a yogurt formulation: a randomized, placebo-controlled, double-blind study. Food Chem Toxicol. 2012;50:2216–2223. doi: 10.1016/j.fct.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 24.Dai C, Zheng CQ, Jiang M, Ma XY, Jiang LJ. Probiotics and irritable bowel syndrome. World J Gastroenterol. 2013;19:5973–5980. doi: 10.3748/wjg.v19.i36.5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gareau MG, Sherman PM, Walker WA. Probiotics and the gut microbiota in intestinal health and disease. Nat Rev Gastroenterol Hepatol. 2010;7:503–514. doi: 10.1038/nrgastro.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Korterink JJ, Ockeloen L, Benninga MA, Tabbers MM, Hilbink M, Deckers-Kocken JM. Probiotics for childhood functional gastrointestinal disorders: a systematic review and meta-analysis. Acta Paediatr. 2014;103:365–372. doi: 10.1111/apa.12513. [DOI] [PubMed] [Google Scholar]
- 27.Enck P, Klosterhalfen S, Martens U. [Probiotic therapy of the irritable bowel syndrome] Dtsch Med Wochenschr. 2011;136:371–375. doi: 10.1055/s-0031-1272538. [DOI] [PubMed] [Google Scholar]
- 28.Ortiz-Lucas M, Tobías A, Saz P, Sebastián JJ. Effect of probiotic species on irritable bowel syndrome symptoms: A bring up to date meta-analysis. Rev Esp Enferm Dig. 2013;105:19–36. doi: 10.4321/s1130-01082013000100005. [DOI] [PubMed] [Google Scholar]
- 29.Whelan K. Probiotics and prebiotics in the management of irritable bowel syndrome: a review of recent clinical trials and systematic reviews. Curr Opin Clin Nutr Metab Care. 2011;14:581–587. doi: 10.1097/MCO.0b013e32834b8082. [DOI] [PubMed] [Google Scholar]
- 30.Nikfar S, Rahimi R, Rahimi F, Derakhshani S, Abdollahi M. Efficacy of probiotics in irritable bowel syndrome: a meta-analysis of randomized, controlled trials. Dis Colon Rectum. 2008;51:1775–1780. doi: 10.1007/s10350-008-9335-z. [DOI] [PubMed] [Google Scholar]
- 31.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 32.Williams EA, Stimpson J, Wang D, Plummer S, Garaiova I, Barker ME, Corfe BM. Clinical trial: a multistrain probiotic preparation significantly reduces symptoms of irritable bowel syndrome in a double-blind placebo-controlled study. Aliment Pharmacol Ther. 2009;29:97–103. doi: 10.1111/j.1365-2036.2008.03848.x. [DOI] [PubMed] [Google Scholar]
- 33.Kajander K, Myllyluoma E, Rajilić-Stojanović M, Kyrönpalo S, Rasmussen M, Järvenpää S, Zoetendal EG, de Vos WM, Vapaatalo H, Korpela R. Clinical trial: multispecies probiotic supplementation alleviates the symptoms of irritable bowel syndrome and stabilizes intestinal microbiota. Aliment Pharmacol Ther. 2008;27:48–57. doi: 10.1111/j.1365-2036.2007.03542.x. [DOI] [PubMed] [Google Scholar]
- 34.Kruis W, Chrubasik S, Boehm S, Stange C, Schulze J. A double-blind placebo-controlled trial to study therapeutic effects of probiotic Escherichia coli Nissle 1917 in subgroups of patients with irritable bowel syndrome. Int J Colorectal Dis. 2012;27:467–474. doi: 10.1007/s00384-011-1363-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Enck P, Zimmermann K, Menke G, Müller-Lissner S, Martens U, Klosterhalfen S. A mixture of Escherichia coli (DSM 17252) and Enterococcus faecalis (DSM 16440) for treatment of the irritable bowel syndrome--a randomized controlled trial with primary care physicians. Neurogastroenterol Motil. 2008;20:1103–1109. doi: 10.1111/j.1365-2982.2008.01156.x. [DOI] [PubMed] [Google Scholar]
- 36.Enck P, Zimmermann K, Menke G, Klosterhalfen S. Randomized controlled treatment trial of irritable bowel syndrome with a probiotic E.-coli preparation (DSM17252) compared to placebo. Z Gastroenterol. 2009;47:209–214. doi: 10.1055/s-2008-1027702. [DOI] [PubMed] [Google Scholar]
- 37.Drouault-Holowacz S, Bieuvelet S, Burckel A, Cazaubiel M, Dray X, Marteau P. A double blind randomized controlled trial of a probiotic combination in 100 patients with irritable bowel syndrome. Gastroenterol Clin Biol. 2008;32:147–152. doi: 10.1016/j.gcb.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 38.Ki Cha B, Mun Jung S, Hwan Choi C, Song ID, Woong Lee H, Joon Kim H, Hyuk J, Kyung Chang S, Kim K, Chung WS, et al. The effect of a multispecies probiotic mixture on the symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J Clin Gastroenterol. 2012;46:220–227. doi: 10.1097/MCG.0b013e31823712b1. [DOI] [PubMed] [Google Scholar]
- 39.Zeng J, Li YQ, Zuo XL, Zhen YB, Yang J, Liu CH. Clinical trial: effect of active lactic acid bacteria on mucosal barrier function in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2008;28:994–1002. doi: 10.1111/j.1365-2036.2008.03818.x. [DOI] [PubMed] [Google Scholar]
- 40.Simrén M, Ohman L, Olsson J, Svensson U, Ohlson K, Posserud I, Strid H. Clinical trial: the effects of a fermented milk containing three probiotic bacteria in patients with irritable bowel syndrome - a randomized, double-blind, controlled study. Aliment Pharmacol Ther. 2010;31:218–227. doi: 10.1111/j.1365-2036.2009.04183.x. [DOI] [PubMed] [Google Scholar]
- 41.Roberts LM, McCahon D, Holder R, Wilson S, Hobbs FD. A randomised controlled trial of a probiotic ‘functional food’ in the management of irritable bowel syndrome. BMC Gastroenterol. 2013;13:45. doi: 10.1186/1471-230X-13-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sinn DH, Song JH, Kim HJ, Lee JH, Son HJ, Chang DK, Kim YH, Kim JJ, Rhee JC, Rhee PL. Therapeutic effect of Lactobacillus acidophilus-SDC 2012, 2013 in patients with irritable bowel syndrome. Dig Dis Sci. 2008;53:2714–2718. doi: 10.1007/s10620-007-0196-4. [DOI] [PubMed] [Google Scholar]
- 43.Søndergaard B, Olsson J, Ohlson K, Svensson U, Bytzer P, Ekesbo R. Effects of probiotic fermented milk on symptoms and intestinal flora in patients with irritable bowel syndrome: a randomized, placebo-controlled trial. Scand J Gastroenterol. 2011;46:663–672. doi: 10.3109/00365521.2011.565066. [DOI] [PubMed] [Google Scholar]
- 44.Guglielmetti S, Mora D, Gschwender M, Popp K. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life--a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2011;33:1123–1132. doi: 10.1111/j.1365-2036.2011.04633.x. [DOI] [PubMed] [Google Scholar]
- 45.Ducrotté P, Sawant P, Jayanthi V. Clinical trial: Lactobacillus plantarum 299v (DSM 9843) improves symptoms of irritable bowel syndrome. World J Gastroenterol. 2012;18:4012–4018. doi: 10.3748/wjg.v18.i30.4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dapoigny M, Piche T, Ducrotte P, Lunaud B, Cardot JM, Bernalier-Donadille A. Efficacy and safety profile of LCR35 complete freeze-dried culture in irritable bowel syndrome: a randomized, double-blind study. World J Gastroenterol. 2012;18:2067–2075. doi: 10.3748/wjg.v18.i17.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guandalini S, Magazzù G, Chiaro A, La Balestra V, Di Nardo G, Gopalan S, Sibal A, Romano C, Canani RB, Lionetti P, et al. VSL#3 improves symptoms in children with irritable bowel syndrome: a multicenter, randomized, placebo-controlled, double-blind, crossover study. J Pediatr Gastroenterol Nutr. 2010;51:24–30. doi: 10.1097/MPG.0b013e3181ca4d95. [DOI] [PubMed] [Google Scholar]
- 48.Hun L. Bacillus coagulans significantly improved abdominal pain and bloating in patients with IBS. Postgrad Med. 2009;121:119–124. doi: 10.3810/pgm.2009.03.1984. [DOI] [PubMed] [Google Scholar]
- 49.Hong YS, Hong KS, Park MH, Ahn YT, Lee JH, Huh CS, Lee J, Kim IK, Hwang GS, Kim JS. Metabonomic understanding of probiotic effects in humans with irritable bowel syndrome. J Clin Gastroenterol. 2011;45:415–425. doi: 10.1097/MCG.0b013e318207f76c. [DOI] [PubMed] [Google Scholar]
- 50.Francavilla R, Miniello V, Magistà AM, De Canio A, Bucci N, Gagliardi F, Lionetti E, Castellaneta S, Polimeno L, Peccarisi L, et al. A randomized controlled trial of Lactobacillus GG in children with functional abdominal pain. Pediatrics. 2010;126:e1445–e1452. doi: 10.1542/peds.2010-0467. [DOI] [PubMed] [Google Scholar]
- 51.Agrawal A, Houghton LA, Morris J, Reilly B, Guyonnet D, Goupil Feuillerat N, Schlumberger A, Jakob S, Whorwell PJ. Clinical trial: the effects of a fermented milk product containing Bifidobacterium lactis DN-173 010 on abdominal distension and gastrointestinal transit in irritable bowel syndrome with constipation. Aliment Pharmacol Ther. 2009;29:104–114. doi: 10.1111/j.1365-2036.2008.03853.x. [DOI] [PubMed] [Google Scholar]
- 52.Michail S, Kenche H. Gut microbiota is not modified by Randomized, Double-blind, Placebo-controlled Trial of VSL#3 in Diarrhea-predominant Irritable Bowel Syndrome. Probiotics Antimicrob Proteins. 2011;3:1–7. doi: 10.1007/s12602-010-9059-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ligaarden SC, Axelsson L, Naterstad K, Lydersen S, Farup PG. A candidate probiotic with unfavourable effects in subjects with irritable bowel syndrome: a randomised controlled trial. BMC Gastroenterol. 2010;10:16. doi: 10.1186/1471-230X-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Choi CH, Jo SY, Park HJ, Chang SK, Byeon JS, Myung SJ. A randomized, double-blind, placebo-controlled multicenter trial of saccharomyces boulardii in irritable bowel syndrome: effect on quality of life. J Clin Gastroenterol. 2011;45:679–683. doi: 10.1097/MCG.0b013e318204593e. [DOI] [PubMed] [Google Scholar]
- 55.Mearin F, Perelló A, Balboa A. [Irritable bowel syndrome and inflammatory bowel disease: Is there a connection?] Gastroenterol Hepatol. 2009;32:364–372. doi: 10.1016/j.gastrohep.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 56.de Kivit S, Tobin MC, Forsyth CB, Keshavarzian A, Landay AL. Regulation of Intestinal Immune Responses through TLR Activation: Implications for Pro- and Prebiotics. Front Immunol. 2014;5:60. doi: 10.3389/fimmu.2014.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grover M. Role of gut pathogens in development of irritable bowel syndrome. Indian J Med Res. 2014;139:11–18. [PMC free article] [PubMed] [Google Scholar]
- 58.Mozaffari S, Esmaily H, Rahimi R, Baeeri M, Sanei Y, Asadi-Shahmirzadi A, Salehi-Surmaghi MH, Abdollahi M. Effects of Hypericum perforatum extract on rat irritable bowel syndrome. Pharmacogn Mag. 2011;7:213–223. doi: 10.4103/0973-1296.84235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jeffery IB, O’Toole PW. Diet-microbiota interactions and their implications for healthy living. Nutrients. 2013;5:234–252. doi: 10.3390/nu5010234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Simrén M, Barbara G, Flint HJ, Spiegel BM, Spiller RC, Vanner S, Verdu EF, Whorwell PJ, Zoetendal EG. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut. 2013;62:159–176. doi: 10.1136/gutjnl-2012-302167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mozaffari S, Nikfar S, Abdollahi M. The safety of novel drugs used to treat irritable bowel syndrome. Expert Opin Drug Saf. 2014;13:625–638. doi: 10.1517/14740338.2014.902932. [DOI] [PubMed] [Google Scholar]
- 62.Mozaffari S, Rahimi R, Abdollahi M. Implications of melatonin therapy in irritable bowel syndrome: a systematic review. Curr Pharm Des. 2010;16:3646–3655. doi: 10.2174/138161210794079254. [DOI] [PubMed] [Google Scholar]
- 63.Martínez C, González-Castro A, Vicario M, Santos J. Cellular and molecular basis of intestinal barrier dysfunction in the irritable bowel syndrome. Gut Liver. 2012;6:305–315. doi: 10.5009/gnl.2012.6.3.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Major G, Spiller R. Irritable bowel syndrome, inflammatory bowel disease and the microbiome. Curr Opin Endocrinol Diabetes Obes. 2014;21:15–21. doi: 10.1097/MED.0000000000000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jiang T, Savaiano DA. Modification of colonic fermentation by bifidobacteria and pH in vitro. Impact on lactose metabolism, short-chain fatty acid, and lactate production. Dig Dis Sci. 1997;42:2370–2377. doi: 10.1023/a:1018895524114. [DOI] [PubMed] [Google Scholar]
- 66.Didari T, Solki S, Mozaffari S, Nikfar S, Abdollahi M. A systematic review of the safety of probiotics. Expert Opin Drug Saf. 2014;13:227–239. doi: 10.1517/14740338.2014.872627. [DOI] [PubMed] [Google Scholar]
- 67.Dolin BJ. Effects of a proprietary Bacillus coagulans preparation on symptoms of diarrhea-predominant irritable bowel syndrome. Methods Find Exp Clin Pharmacol. 2009;31:655–659. doi: 10.1358/mf.2009.31.10.1441078. [DOI] [PubMed] [Google Scholar]


