Abstract
During a major solar particle event (SPE), astronauts in space are at risk of exposure to an increased dose of proton radiation. The whole body distribution of the absorbed SPE proton dose is inhomogeneous, and such an inhomogeneous SPE proton dose can be simulated by electron radiation. Using Yucatan minipigs as an animal model, we compared the time courses of leukocyte count changes after exposure to proton simulated SPE (pSPE) radiation or electron simulated SPE (eSPE) radiation. The results demonstrated that the time required after irradiation to reach the lowest leukocyte counts was generally comparable between the pSPE and eSPE radiation exposures. However, the leukocyte count often recovered faster after electron irradiation compared to proton irradiation at the corresponding doses. In addition, the radiation dose required to achieve comparable magnitudes of leukocyte count decrease was higher in the eSPE animals than for the pSPE animals. In conclusion, based on the magnitude of the decrease and the time required to reach the lowest leukocyte counts after irradiation, the pSPE radiation was more effective than the eSPE radiation in reducing the peripheral leukocyte counts. Lymphocytes appeared to be the most sensitive type of leukocytes in response to either type of SPE radiation. It is particularly noteworthy that following exposure to pSPE radiation at the skin doses >5 Gy, the neutrophils do not recover from the radiation damage at times up to 30 days, and the neutrophils have not recovered to their baseline levels even at 90 days post-irradiation. These results suggest a marked difference in the ability of the neutrophils to recover from pSPE radiation compared the results observed for eSPE radiation.
Keywords: solar particle event radiation, leukocytes, proton radiation
Introduction
During a major solar particle event (SPE), astronauts in space are potentially at significant risk of increased radiation exposure, which could result in acute radiation sickness, skin injury, and/or a compromised immune defense. SPEs originate from magnetically disturbed regions of the sun, which sporadically emit bursts of energetic charged particles [1, 2]. Large SPEs are expected to occur only once or twice in a solar cycle; however, during the 22nd solar cycle, four extremely large SPEs of comparable magnitude occurred within a 4-month period. The types of radiation emitted during SPEs are predominately protons [3]. The majority of protons in an SPE are at or below 50 MeV, but each event varies in energy spectra and proton fluence. At these lower energies, the distribution of the absorbed proton dose received during an SPE is expected to be inhomogeneous, with a significantly higher absorbed dose delivered to the skin and subcutaneous tissues than to the internal organs [4, 5]. The inhomogeneous dose distribution makes it difficult to determine the effectiveness of the SPE radiation relative to the effect of conventional radiation commonly available on Earth for radiation biology research. To overcome this problem, a novel concept of using megavoltage electron beam radiation to simulate the total dose and dose distribution of SPE protons has been explored in animal models [6]. Electron radiation with mixed energies of 6 MeV + 12 MeV (6+12 MeV), which simulate the whole body dose distribution of SPE radiation [6], can be used as a reference radiation to determine the relative biological effectiveness (RBE) of proton simulated SPE radiation.
The doses used in our studies to mimic SPE radiation exposure in animal studies have utilized relatively high doses of radiation that astronauts could have received from exposure to SPE radiation during historical SPEs. The total skin doses estimated for possible astronaut exposure to SPE radiation during extravehicular activity, as predicted from modeling 3 different historical SPEs, would have been >5 Gy (specific estimations for total skin doses were as follows for these 3 SPEs: 7.68, 25.99 and 32.15 Gy) [5]. It is recognized that these levels of exposure to SPE radiation would represent “worst-case” scenarios, and that the probability of astronaut exposure to skin doses of this magnitude is very low [5]. The SPE-like radiation skin doses used in the studies reported here are within these worst-case scenario skin doses estimated for potential astronaut exposure to SPE radiation.
We have previously evaluated the dose responses of SPE-like proton radiation in Yucatan minipigs [7]. Using 6+12 MeV electrons as a reference radiation, we have also determined the RBE for the SPE-like proton radiation with respect to the hematological effects in Yucatan minipigs [8] . In the present study, the minipig peripheral leukocyte count data obtained from the proton radiation study [7] and the 6+12 MeV electron radiation study [8] were analyzed to compare and contrast the time course differences between the hematological effects of the SPE-like proton radiation and 6+12 MeV electron radiation.
Materials and Methods
Animals
As previously described [7, 8], male Yucatan minipigs aged 8-12 weeks were purchased from Sinclair Bio Resources, LLC (Auxvasse, MO). The animals were housed individually with ad lib access to water and fed standard chow twice daily. After acclimation for 7 days, the animals were randomized into different treatment groups with 3 animals per group and exposed to proton simulated SPE radiation at skin doses of 5, 7.7 and 10 Gy or electron simulated SPE radiation at skin doses of 5, 7.5, 10 and 20 Gy.
The animal care and treatment procedures were approved by the Institutional Animal Care and Use Committee of Loma Linda University Medical Center (LLUMC) and the University of Pennsylvania (Penn).
Irradiation
The radiation experiments with SPE-like protons and 6+12 MeV electrons were described in detail previously [7, 8]. For the proton radiation experiment, the animals were exposed to beams comprised of protons with energy distribution up to 155 MeV and a custom depth dose profile similar to what is expected for SPE radiation; specifically, the August 1989 SPE. The source, modulation and characterization of the proton beams, as well as the proton dose calibration, delivery and monitoring were described in detail previously [9]. For the electron radiation experiment, the animals were irradiated with 6+12 MeV electron beams composed of a mixture of 80% 6 MeV electrons and 20% 12 MeV electrons, which closely mimics the whole body dose distribution of the August 1989 SPE [10]. Throughout the remainder of manuscript, proton simulated SPE radiation and electron simulated SPE radiation will be referred to as pSPE and eSPE, respectively.
Blood cell count analyses
Prior to irradiation and at 4 hours and 1, 4, 14, 30 and 90 days after the proton irradiation or 4 hours and 1, 7, 14, 30 and 85 (for all but the 5 Gy electron dose group, in which the time point at 85 days was not collected) days after electron irradiation, a whole blood sample was collected from each animal (cranial vena cava). The blood samples (maintained at 4°C) were sent to Antech Diagnostics (Irvine, CA) and analyzed using a Bayer Advia 120 Hematology Analyzer within 24 hours of the blood sample collection, as previously described [7, 8]. It has been previously determined that the blood cell counts are stable and reliable when comparing blood cell counts analyzed immediately after collection or within/up to 24 hours after collection utilizing the automated analyzer used in this study [11].
Data and statistical analyses
The minipig peripheral leukocyte count data obtained from the proton radiation study [7] and the 6+12 MeV electron radiation study were analyzed to compare and contrast the time course differences between the hematological effects of the SPE-like proton radiation and 6+12 MeV electron radiation. The mean counts of WBCs, lymphocytes, neutrophils, monocytes and eosinophils of all animals prior to the radiation exposure were calculated and used as the baseline values for the respective blood cell types in the same experiment. For each animal at each time point after irradiation, the count of each blood cell type was divided by the respective baseline value, and the result was expressed as fraction of control for further analyses.
The mean results and standard errors for WBCs, lymphocytes, neutrophils, monocytes and eosinophils were calculated for each treatment group at each time point. The mean results for each type of leukocytes were plotted against the time (days) elapsed after radiation exposure to illustrate the time course of leukocyte count changes after irradiation. The results for the different time points after irradiation were analyzed by one-way ANOVA followed by the Holm-Sidak test for a comparison with the respective baseline values.
Results
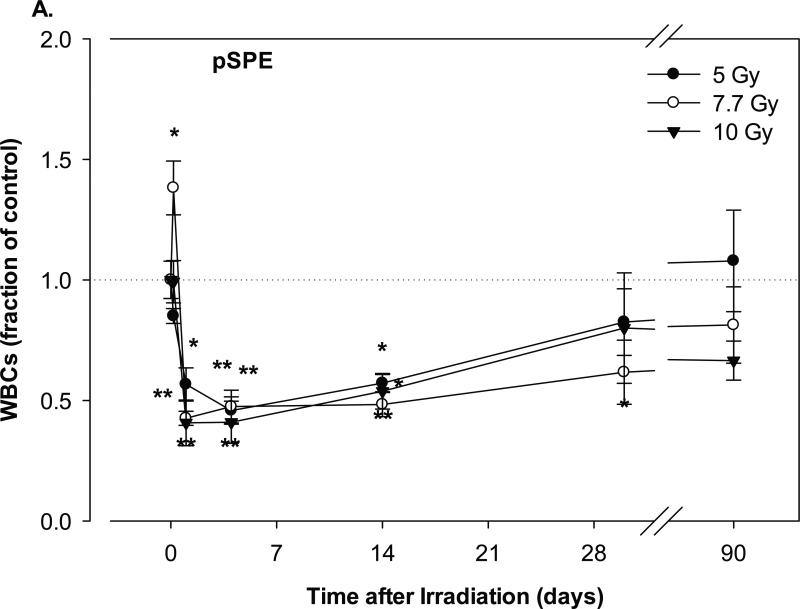
The time courses for the change in the leukocyte counts were compared between Yucatan minipigs exposed to pSPE and eSPE radiation; eSPE radiation was used as the reference radiation. In the minipigs exposed to the pSPE radiation, the WBC count decreased significantly by 54.2% (p < 0.01) on day-4 after irradiation at a single dose of 5 Gy and by 57.4% (p < 0.01) and 59.3% (p < 0.01) on day-1 after irradiation at a single dose of 7.7 and 10 Gy, respectively (Figure 1A).
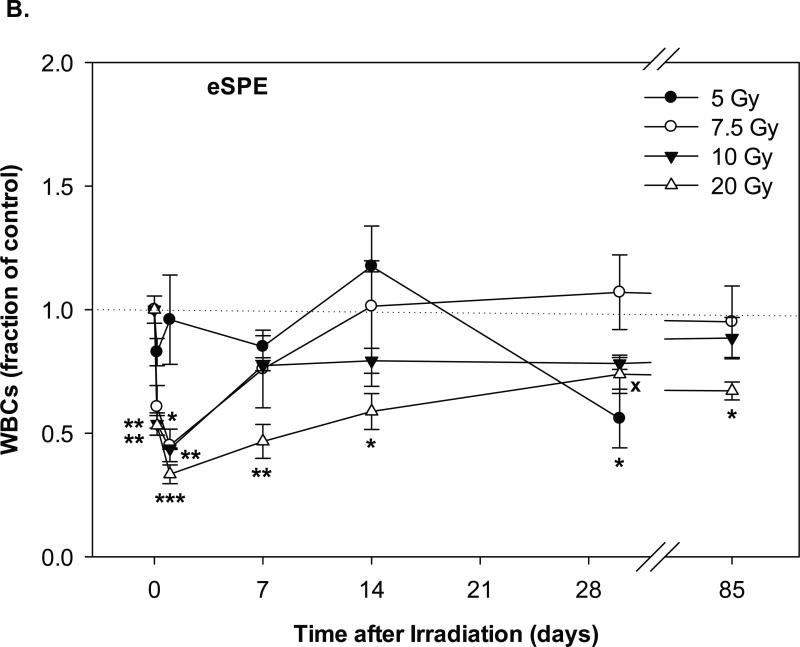
Fig. 1.
Change of WBC count in Yucatan minipigs exposed to pSPE radiation or eSPE radiation. A minimum of 3 animals per group were exposed to pSPE radiation (A) at a single skin dose of 5 (solid circle), 7.7 (unfilled circle) or 10 (filled triangle) Gy or eSPE radiation (B) at a single skin dose of 5 (solid circle), 7.5 (unfilled circle), 10 (filled triangle), and 20 Gy (unfilled triangle). The WBC count was determined at the indicated time points. The results are expressed as fraction of control and compared with the pre-irradiation baseline value (indicated by dashed line). Statistical significance of the comparison is indicated by an x (p is between 0.005 and 0.10, and considered as marginally significant), * (p < 0.05), ** (p < 0.01) and *** (p < 0.001), as appropriate. Each data point represents a mean of three animals, and the associated standard error is indicated by the error bar.
The WBC count recovered slowly thereafter. By day-30 after irradiation, the WBC count for the pSPE irradiated groups remained 17.5% (p = 0.57) to 38.3% (p < 0.05) below the baseline level, although the decrease was only significant for the 7.7 Gy pSPE dose group. By day-90 after the pSPE irradiation, the WBC count recovered fully in the 5 Gy pSPE dose group, but was still 18.7% (p = 0.20) and 33.5% (p = 0.11) below, although no longer significantly different from, the baseline value for the 7.7 and 10 Gy pSPE dose groups, respectively. Despite the observed changes in the differential blood cell counts, all animals were reported stable upon a daily health check post-irradiation and throughout the experimental period. The daily health check included inspection of eyes, skin, overall body wellness and breathing, responsiveness to socialization, food check, and water intake. .
In the animals exposed to eSPE radiation, the WBC count for the 5 Gy electron dose group did not decrease significantly after irradiation, except for day-30 after irradiation when the WBC count decreased by 44.1% (p < 0.05) as compared with the baseline level (Figure 1B). In the 7.5 Gy eSPE dose group, the WBC count decreased significantly by 54.9% (p < 0.05) within a day after irradiation, then recovered quickly within 7 days after irradiation to a level that was no more than 24.0% below the baseline value (p ≥ 0.53). In the 10 Gy eSPE dose group, the WBC count decreased significantly by 56.2% (p < 0.01) within a day after irradiation, then recovered within 7 days to levels that were no more than 22.7% (p ≥ 0.26) below the baseline value for the remainder of the observation period up to 85 days after the eSPE irradiation. In the 20 Gy eSPE dose group, the WBC count decreased significantly by 66.6% (p < 0.001) within a day after irradiation, then recovered slowly but was still 26.2% (p = 0.07) and 32.9% (p < 0.05) below the baseline value on day-30 and day-85, respectively, after irradiation. Overall, the WBC count in the 5, 7.5 and 10 Gy electron dose groups decreased to lesser extents and recovered faster as compared to the WBC count in the corresponding pSPE dose groups, whereas the time course of the WBC count change in the 20 Gy eSPE dose group was similar to that of the 10 Gy pSPE dose group.
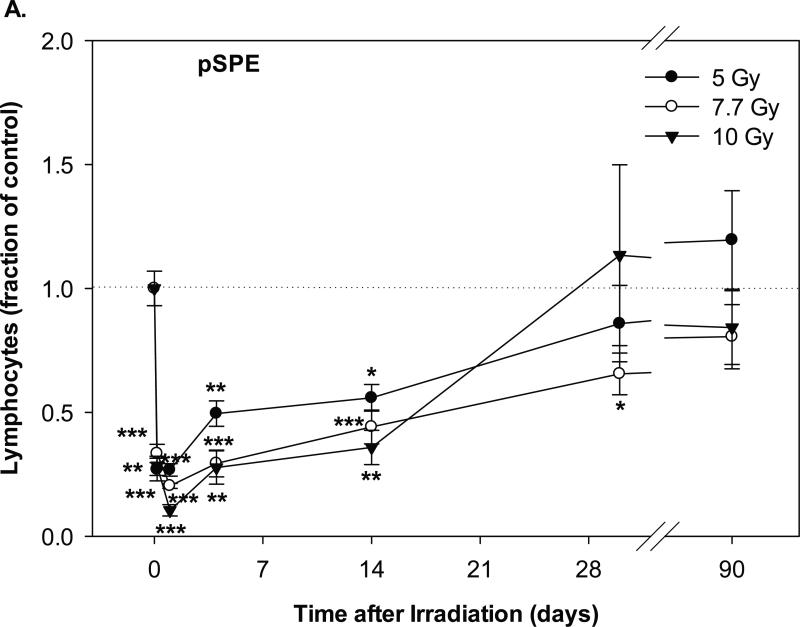
The lymphocyte count in the groups irradiated with pSPE radiation decreased significantly as early as 4 hours after irradiation (Figure 2A). On day-1 after irradiation, the lymphocyte count reached the lowest level, which was 73.2% (p < 0.001), 79.7% (p < 0.001) and 89.5% (p < 0.001) below the baseline level for the 5, 7.7 and 10 Gy proton dose groups, respectively. The lymphocyte count recovered slowly and was still 44.1% (p < 0.05) to 64.1% (p < 0.01) below the baseline value on day-14 after irradiation, and the decrease was statistically significant. On day-30 and day-90 after the pSPE irradiation, the lymphocyte count in the pSPE irradiated animals recovered to levels that were not more than 34.5% (p < 0.05) and 19.5% (p = 0.13) below the baseline value and the decrease was not statistically significant except for the 7.7 Gy pSPE dose group on day-30 after irradiation.
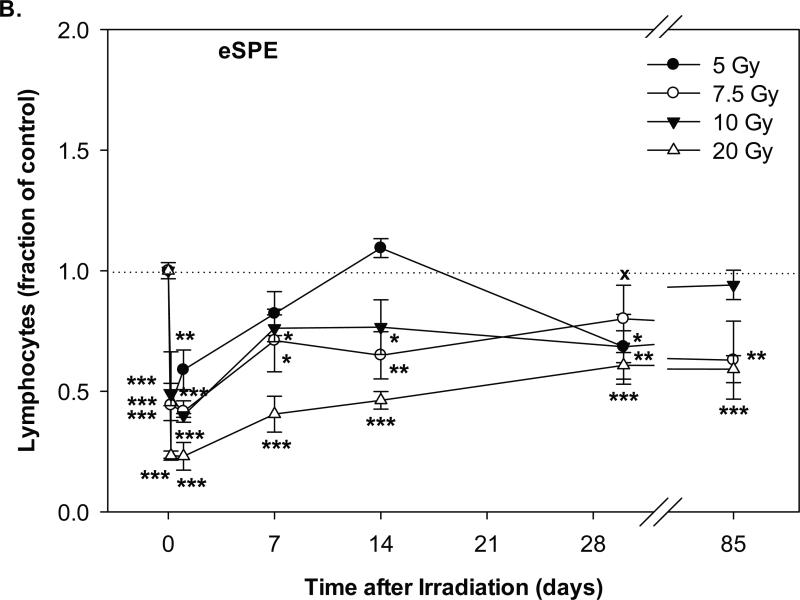
Fig. 2.
Change of lymphocyte count in Yucatan minipigs exposed to pSPE radiation or eSPE radiation. A minimum of 3 animals per group were exposed to pSPE radiation (A) at a single skin dose of 5 , 7.7 or 10 Gy or eSPE radiation (B) at a single skin dose of 5, 7.5, 10, or 20 Gy. The lymphocyte count was determined at the indicated time points. The results are expressed as fraction of control and compared with the pre-irradiation baseline value (indicated by dashed line). Statistical significance of the comparison is indicated by an x (p is between 0.005 and 0.10, and considered as marginally significant), * (p < 0.05), ** (p < 0.01) and *** (p < 0.001), as appropriate. Each data point represents a mean of three animals, and the associated standard error is indicated by the error bar.
In the animals exposed to eSPE radiation, the lymphocyte count also decreased significantly within 4 hours after irradiation and reached the lowest level at 4 hours after irradiation for the 5 Gy eSPE dose group and on day-1 after irradiation for the 7.5, 10 and 20 Gy eSPE dose groups (Figure 2B). At the lowest level, the lymphocyte count was 55.7%, 58.4%, 60.0% and 77.0% below the baseline level (p < 0.001) for the 5, 7.5, 10 and 20 eSPE dose groups, respectively. Overall, the lymphocyte count in the 5, 7.5 and 10 Gy eSPE dose groups decreased to lesser extents and/or recovered faster than the lymphocyte count in the 5 and 7.7 Gy pSPE dose groups, whereas the time course of the lymphocyte count change in the 20 Gy eSPE dose group was similar to that of the 10 Gy pSPE dose group during the first 14 days after irradiation.
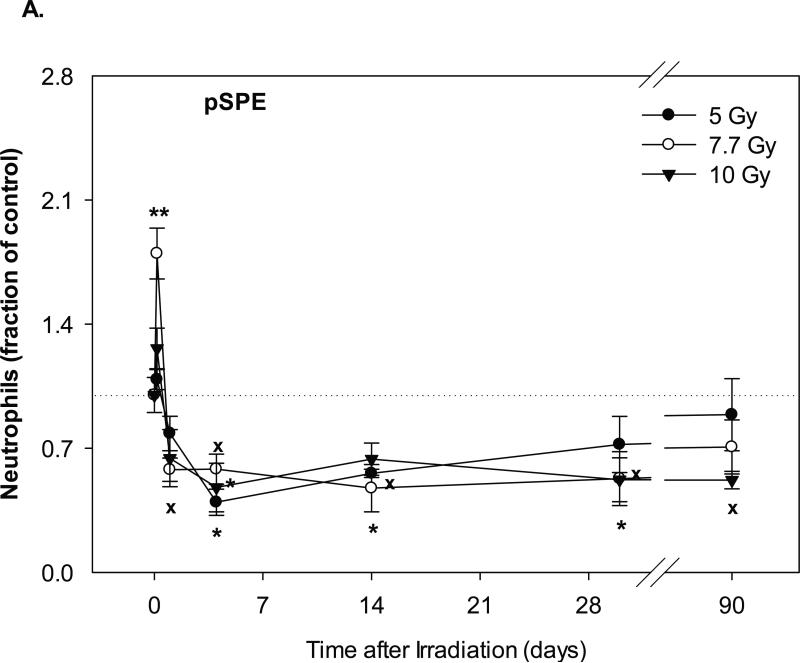
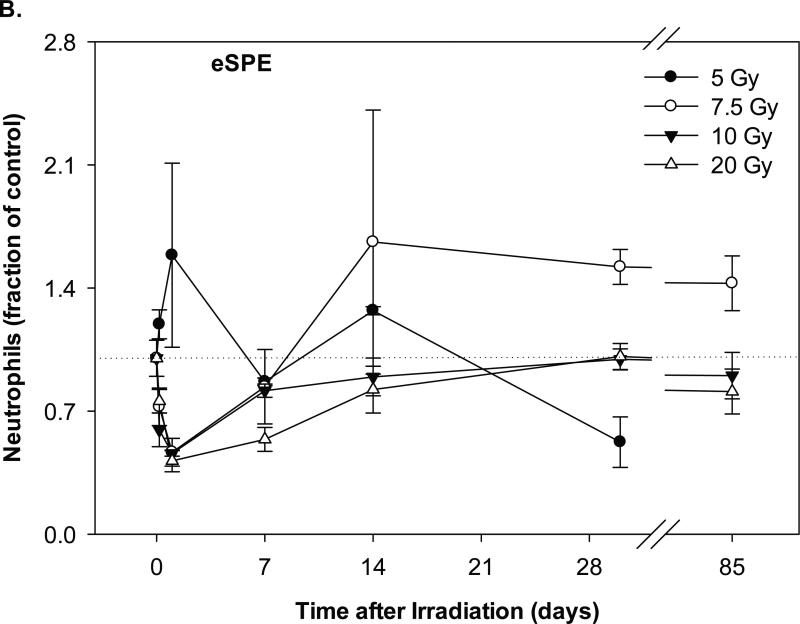
The neutrophil counts in the pSPE irradiated animals increased by 8.7% (p = 0.62), 79.8% (p < 0.01) and 26.3% (p = 0.15) at 4 hours after irradiation with 5, 7.7 and 10 Gy pSPE, respectively (Figure 3A). Following the initial increase, the neutrophil count decreased by up to 60.4% (p < 0.05), 52.5% (p < 0.05) and 52.1% (p < 0.05) for the 5, 7.7 and 10 Gy pSPE dose groups, respectively, between day-4 and day-14 after irradiation. The neutrophil count in the 5 and 7.7 Gy pSPE dose groups recovered slowly thereafter but was still 27.9% (p = 0.41) and 47.2% (p = 0.06) below the baseline level on day-30 and 11.1% (p = 0.78) and 29.3% (p = 0.11) below the baseline level on day-90 after irradiation. The neutrophil count in the 10 Gy pSPE dose group did not show an appreciable recovery and remained 47.8% (p < 0.05) and 48.0% (p = 0.06) below the baseline level on day-30 and day-90, respectively, after irradiation.
Fig. 3.
Change of neutrophil count in Yucatan minipigs exposed to pSPE or eSPE radiation. A minimum of 3 animals per group were exposed to pSPE radiation (A) at a single skin dose of 5 , 7.7 or 10 Gy or eSPE radiation (B) at a single skin dose of 5, 7.5, 10, or 20 Gy. The neutrophil count was determined at the indicated time points. The results are expressed as fraction of control and compared with the pre-irradiation baseline value (indicated by dashed line). Statistical significance of the comparison is indicated by an x (p is between 0.005 and 0.10, and considered as marginally significant), * (p < 0.05), and ** (p < 0.01). Each data point represents a mean of three animals, and the associated standard error is indicated by the error bar.
In the animals irradiated with eSPE, the neutrophil count increased by 58.6% (p = 0.25) within a day after irradiation for the 5 Gy eSPE dose group but decreased by 53.4% (p = 0.43), 54.0% (p = 0.22) and 58.4% (p = 0.16) within a day after irradiation for the 7.5, 10 and 20 Gy eSPE dose groups, respectively (Figure 3B). For the remainder of the observation period up to 85 days after eSPE irradiation at doses of 5 to 20 Gy, the neutrophil count fluctuated between a broad range of +66.1% (p = 0.26) and -47.7% ( p = 0.39) of the baseline value. Due to the large variation within the treatment groups, the change in the neutrophil count as compared to the baseline value was not statistically significant for any of the eSPE irradiated groups (p ≥ 0.16). Overall, the neutrophil count in the groups irradiated with electrons at doses up to 20 Gy decreased to lesser extents and recovered faster than the neutrophil count in the groups irradiated with protons at doses up to 10 Gy.
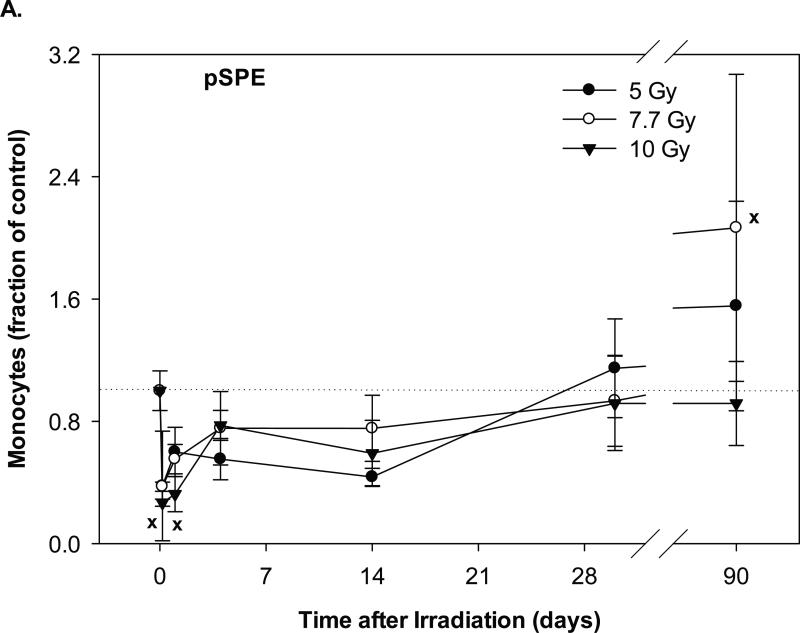
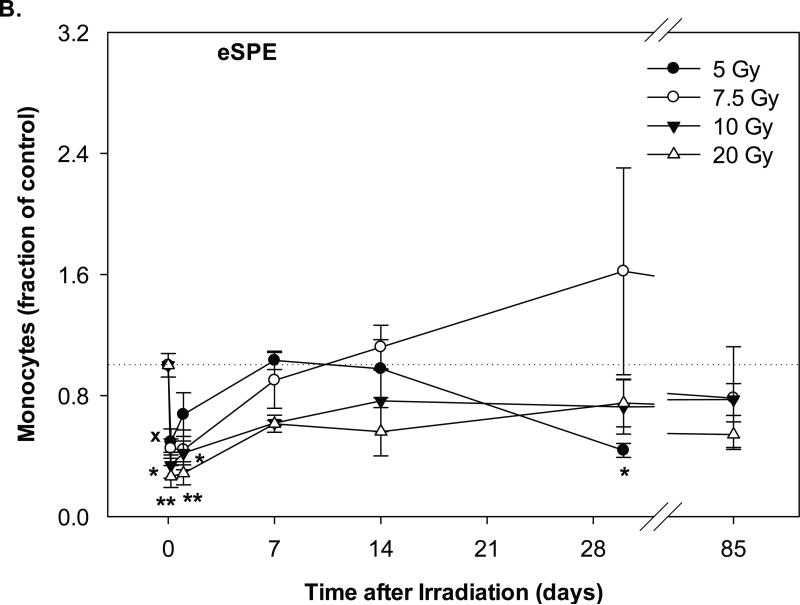
In the animals exposed to pSPE radiation, the monocyte count reached the lowest level at 4 hours after irradiation and was 62.3% (p = 0.45), 62.7% (p = 0.54) and 73.2% (p = 0.05) below the baseline values for the 5, 7.7 and 10 Gy pSPE dose groups, respectively (Figure 4A). The monocyte count recovered gradually thereafter. By day-30 after irradiation, the monocyte count in the pSPE irradiated animals was not more than 8.2% below the baseline level (p ≥ 0.75). By day-90 after irradiation, the monocyte count in the 5 and 7.7 Gy pSPE dose group increased by 55.4% (p = 0.47) and 106.5% (p = 0.10) as compared to the baseline value whereas the monocyte count in the 10 Gy proton dose group was 8.3% (p = 0.94) below the baseline value. Due to the relatively large variation within the treatment groups, the decrease in the monocyte count after irradiation was not statistically significant for the 5 and 7.7 Gy pSPE dose groups at any time points evaluated and was only marginally significant for the 10 Gy pSPE dose group at 4 hours (p = 0.05) and on day-1 (p = 0.07) after irradiation.
Fig. 4.
Change of monocyte count in Yucatan minipigs exposed to pSPE or eSPE radiation. A minimum of 3 animals per group were exposed to pSPE radiation (A) at a single skin dose of 5, 7.7 or 10 Gy or eSPE radiation (B) at a single skin dose of 5, 7.5, 10, or 20 Gy. The monocyte count was determined at the indicated time points. The results are expressed as fraction of control and compared with the pre-irradiation baseline value (indicated by dashed line). Statistical significance of the comparison is indicted by x (p is between 0.005 and 0.10, and considered as marginally significant), * (p < 0.05), and ** (p < 0.01). Each data point represents a mean of three animals, and the associated standard error is indicated by the error bar.
In the animals exposed to eSPE radiation, the monocyte count reached the lowest level within a day after irradiation and was 50.6% (p = 0.08), 55.9% (p = 0.24), 66.1% (p < 0.05) and 73.6% (p < 0.01) below the baseline values for the 5, 7.5, 10 and 20 Gy eSPE dose groups, respectively (Figure 4B). By day-7 and thereafter after irradiation, the monocyte count recovered but fluctuated between a broad range of +62.1% (p = 0.18) and -56.3% (p = 0.52) of the baseline value. Overall, the change in the monocyte count followed a similar pattern between the corresponding proton and electron dose groups except for the high monocyte count observed in the 5 and 7.7 Gy proton dose group on day-90 and in the 7.5 Gy electron dose group on day-30 after irradiation.
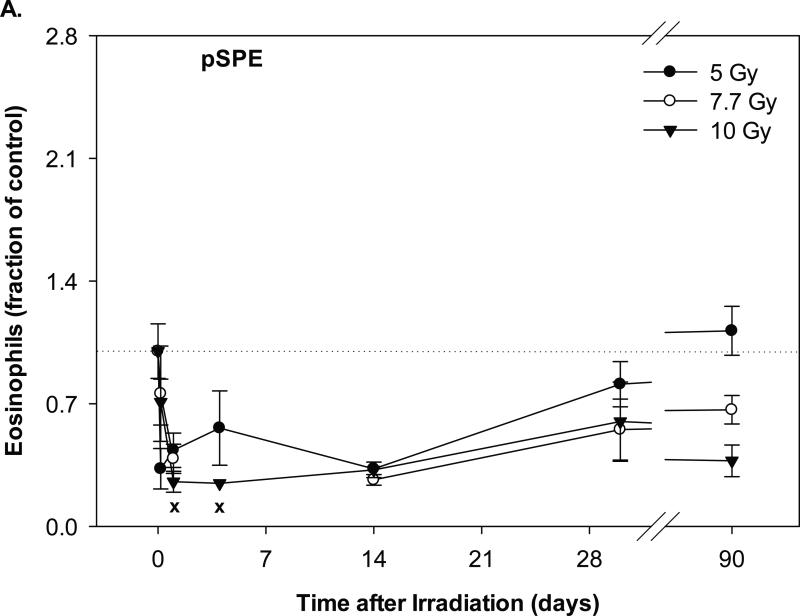
Eosinophil counts in the animals exposed to pSPE radiation decreased by 67.2% (p = 0.30), 61.4% (p = 0.16) and 74.5% (p = 0.07) within a day after irradiation for the 5, 7.7 and 10 Gy pSPE dose groups, respectively (Figure 5A). Between day-1 and day-14 days after the pSPE irradiation, the eosinophil count remained 43.9% (p = 0.49), 61.4% (p = 0.16) and 67.8% (0.10) below the baseline level, but the decrease was only marginally significant for the 10 Gy proton dose group on day-1 and day-14 after irradiation. By day-30 after irradiation and thereafter, the eosinophil count in the 5 Gy proton dose group recovered to levels that were not more than 18.8% (p = 0.76) below the baseline level, whereas the eosinophil level in the 7.7 and 10 Gy pSPE dose groups was still at least 33.5% (p = 0.44) and 40.2% (p = 0.28) below the baseline level.
Fig. 5.
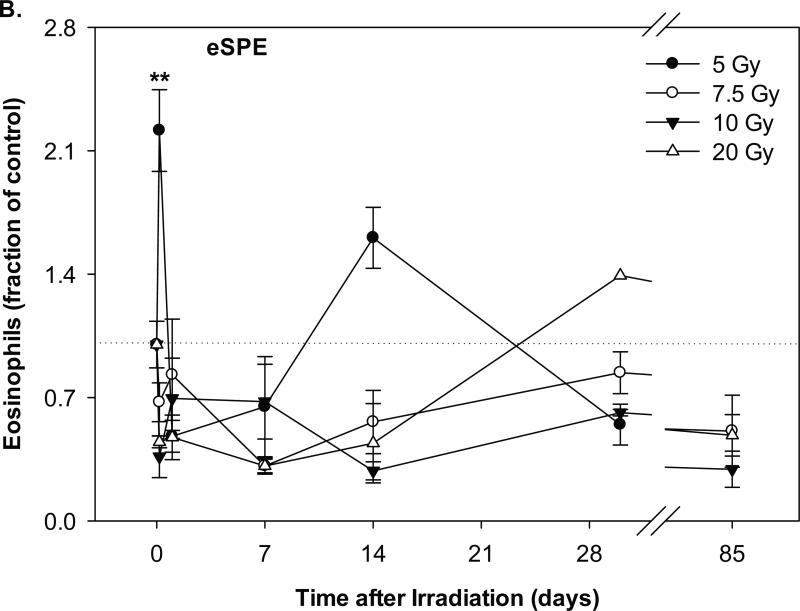
Change of eosinophil count in Yucatan minipigs exposed to pSPE or eSPE radiation. A minimum of 3 animals per group were exposed to pSPE radiation (A) at a single skin dose of 5, 7.7 or 10 Gy or eSPE radiation (B) at a single skin dose of 5, 7.5, 10, or 20 Gy. The eosinophil count was determined at the indicated time points. The results are expressed as fraction of control and compared with the pre-irradiation baseline value (indicated by dashed line). Statistical significance of the comparison is indicated by an x (p is between 0.005 and 0.10, and considered as marginally significant) and ** (p < 0.01). Each data point represents a mean of three animals, and the associated standard error is indicated by the error bar.
In the animals exposed to eSPE radiation, the eosinophil count increased by 121.4% (p < 0.01) for the 5 Gy eSPE dose group, but decreased by 32.7% (p = 0.72), 63.6% (p = 0.24) and 55.2% (p = 0.38) for the 7.5, 10 and 20 Gy eSPE dose groups, respectively, at 4 hours after irradiation (Figure 5B). Between 1 and 14 days after the eSPE irradiation, the eosinophil count reached the lowest level, which was 52.0% (p = 0.38) to 71.6% (p = 0.22) below the baseline value for the groups irradiated with 5 to 20 Gy eSPE radiation. Between day 14 and day 85 after irradiation, the eosinophil count fluctuated within a broad range of +60.6% (p = 0.32) to -71.6% (p = 0.21) for the eSPE irradiated groups. Due to the relatively large variation within the treatment groups, the change in the eosinophil count did not reach a statistical significance for any of the proton or electron dose groups except for the 121.4% increase observed in the 5 Gy electron dose group at 4 hours after irradiation.
Discussion
We have previously evaluated the radiation dose response in Yucatan minipigs irradiated with SPE-like proton radiation [7] or 6+12 MeV electrons [8] and determined the RBE for the SPE-like protons using the 6+12 MeV electrons as a reference radiation [8]. In the present study, the time course for the change in WBC, lymphocyte, neutrophil, monocyte and eosinophil counts were compared in Yucatan minipigs exposed to pSPE radiation at skin doses of 5, 7.7 and 10 Gy or eSPE radiation at skin doses of 5, 7.5, 10 and 20 Gy. For the proton irradiated animals, the lowest leukocyte counts were generally observed at 4 hours after irradiation for monocytes, on day-1 for lymphocytes, on day-4 for neutrophils, between 4 hours and day-14 for eosinophils, and between day-1 and day-4 for total WBCs. At the lowest level of the leukocyte counts, the WBCs, lymphocytes, neutrophils, monocytes and eosinophils in the proton irradiated animals decreased by up to 59.3%, 89.5%, 60.4%, 73.2% and 75.5%, respectively, from the respective baseline values. For the eSPE radiation exposed animals, the lowest leukocyte counts occurred most frequently at 4 hours after irradiation for monocytes, on day-1 for lymphocytes, between day-1 and day-7 for neutrophils, between 4 hours and day-14 for eosinophils, and between day-1 and day-7 for total WBCs. At the lowest level of the leukocyte counts, the WBCs, lymphocytes, neutrophils, monocytes and eosinophils in the electron irradiated animals decreased by up to 66.6%, 77.0%, 58.4%, 73.6% and 77.1%, respectively, from the respective baseline values. The time required after irradiation to reach the lowest leukocyte counts was generally comparable between the animals exposed to pSPE and eSPE radiation. Based on the magnitude of the decrease and the time required to reach the lowest leukocyte counts after irradiation, lymphocytes appeared to be the most sensitive to either proton or electron irradiation, which is consistent with what was previously reported in Rhesus monkeys exposed to 200 MeV protons at absorbed doses of 175 to 500 rads [12] or exposed to 146.8-MeV protons at doses of 275 to 900 rads [13].
The time courses of leukocyte count changes after irradiation were determined separately for WBCs, lymphocytes, neutrophils, monocytes and eosinophils in the present study. The results demonstrated that the time course of the leukocyte count changes were not synchronized among the cell types compared and increases in the neutrophil, monocyte and eosinophil counts were observed at some time points after irradiation. Striking differences were observed in the time courses between the changes in lymphocyte count, which declined quickly after irradiation, and the changes in neutrophil, monocyte and eosinophil counts, which increased initially in some of the irradiated groups before declining. Such differences were also observed previously in ferrets [14] and mice [15, 16] irradiated with proton radiation and γ-ray radiation. Since the increase in the neutrophil, monocyte and eosinophil counts is expected to counter the decrease in the lymphocyte count when the total WBC count is calculated for a given time point, the time course of the WBC count change after irradiation does not necessarily reflect the time course of the change of the counts of individual leukocyte types after irradiation, especially at the lower radiation doses when the increases in the neutrophil, monocyte and eosinophil counts were observed more frequently.
One major difference observed was for the decreases in neutrophil counts in animals exposed to pSPE or eSPE radiation. None of the decreases in neutrophil counts in the eSPE irradiated animals were statistically significant, while most of the decreases in the neutrophil counts in the pSPE irradiated animals were statistically significant. It is particularly noteworthy that following exposure to pSPE radiation at the skin doses evaluated at >5 Gy, the neutrophils do not appear to recover from the radiation damage at times up to 30 days, and they have not recovered to their baseline levels even at 90 days post-irradiation.
In conclusion, the magnitude of the decreases in the leukocyte counts was comparable between the proton and electron irradiated minipigs despite the higher doses of electron doses used in the experiment; however, the leukocyte count often appeared to recover faster in the eSPE irradiated animals than in the animals exposed to pSPE radiation at the corresponding doses. Taken together, these data suggest that the pSPE radiation was more effective than eSPE radiation in reducing the peripheral leukocyte counts. It is widely accepted that the mechanism of radiation induced hematopoietic cell death is apoptosis [17]. Apoptosis was not measured in the cells of the bone marrow or the peripheral blood cells of the animals in these experiments to confirm the mechanism involved in the effects produced by exposure to inhomogeneous solar particle event radiation.
Acknowledgements
This work was supported by the Center of Acute Radiation Research (CARR) grant from the National Space Biomedical Research Institute (NSBRI) through NASA NCC 9-58 and NIH Training Grant 2T32CA009677. We would like to thank Dr. Casey Maks, Ms. Molly Peterlin, and Dr. Gabriel Krigsfeld for their assistance with the animal procedures. We are grateful to Drs. Daila Gridley and Andrew Wroe, Mr. Steven Rightnar, Mr. Celso Perez, and the Accelerator Team at LLUMC for the planning and assistance with the proton irradiations. We are also grateful to Dr. Keith Cengel and Dr. Eric Diffenderfer for the planning and assistance with the animal irradiation experiments performed at Penn.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wilson JW, Cucinotta FA, Shinn JL, Simonsen LC, et al. Shielding from solar particle event exposures in deep space. Radiat Res. 1999;30:361–82. doi: 10.1016/s1350-4487(99)00063-3. [DOI] [PubMed] [Google Scholar]
- 2.Smart DF, Shea MA. The local time dependence of the anisotropic solar cosmic ray flux. Adv Space Res. 2003;32:109–14. doi: 10.1016/s0273-1177(03)90377-2. [DOI] [PubMed] [Google Scholar]
- 3.Hellweg CE, Baumstark-Khan C. Getting ready for the manned mission to Mars: the astronauts' risk from space radiation. Naturwissenschaften. 2007;94:517–26. doi: 10.1007/s00114-006-0204-0. [DOI] [PubMed] [Google Scholar]
- 4.Coutrakon GB, Benton ER, Gridley DS, Hickey T, et al. Simulation of a 36 h solar particle event at LLUMC using a proton beam scanning system. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms. 2007;261:791–794. [Google Scholar]
- 5.Hu S, Kim M-HY, McClellan GE, Cucinotta FA. Modelling the acute health effects of astronauts from exposure to large solar particle events. Health Phys. 2009;96:465–476. doi: 10.1097/01.HP.0000339020.92837.61. [DOI] [PubMed] [Google Scholar]
- 6.Cengel KA, Diffenderfer ES, Avery S, Kennedy AR, et al. Using electron beam radiation to simulate the dose distribution for whole body solar particle event proton exposure. Radiat Environ Biophys. 2010;49:715–21. doi: 10.1007/s00411-010-0315-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanzari JK, Wan XS, Wroe AJ, Rightnar S, et al. Acute hematological effects of solar particle event proton radiation in the porcine model. Radiation Research. 2013;180:7–16. doi: 10.1667/RR3027.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanzari JK, Wan SX, Diffenderfer ES, Cengel KA, et al. Relative biological effectiveness of simulated solar particle event proton radiation to induce acute hematological change in the porcine model. J Radiat Res. 2013 doi: 10.1093/jrr/rrt108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanzari JK, Wan XS, Krigsfeld GS, King GL, et al. Effects of solar particle event proton radiation on parameters related to ferret emesis. Radiat Res. 2013;180:166–76. doi: 10.1667/RR3173.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diffenderfer ES, Dolney D, Schaettler M, Sanzari JK, et al. Monte Carlo modeling in CT-based geometries: dosimetry for biological modeling experiments with particle beam radiation. J Radiat Res. 2013 doi: 10.1093/jrr/rrt118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Romero-Weaver AL, Kennedy AR. Comparison of Two Methods for the Determination of the Effects of Ionizing Radiation on Blood Cell Counts in Mice. Int J Biomed Sci. 2012;8:7–15. [PMC free article] [PubMed] [Google Scholar]
- 12.Taketa ST, Castle BL, Howard WH, Conley CC, et al. Effects of acute exposure to high-energy protons on primates. Radiat Res Suppl. 1967;7:336–59. [PubMed] [Google Scholar]
- 13.Traynor JE, Siegal AM. Early effects of 150-MEV proton irradiation in rhesus monkeys. SAM-TR-68-87. Tech Rep SAM-TR. 1968:1–7. [PubMed] [Google Scholar]
- 14.Sanzari JK, Wan XS, Krigsfeld GS, Wroe AJ, et al. The effects of gamma and proton radiation exposure on hematopoietic cell counts in the ferret model. Gravit and Space Biol. 2013 in press. [PMC free article] [PubMed] [Google Scholar]
- 15.Romero-Weaver AL, Wan XS, Diffenderfer ES, Lin L, et al. Kinetics of Neutrophils in Mice Exposed to Radiation and/or Granulocyte Colony-Stimulating Factor Treatment. Radiat Res. 2013;180:177–88. doi: 10.1667/RR3055.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wambi C, Sanzari J, Wan XS, Nuth M, et al. Dietary antioxidants protect hematopoietic cells and improve animal survival after total-body irradiation. Radiat Res. 2008;169:384–96. doi: 10.1667/RR1204.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall EJ, Giaccia AJ. Radiobiology for the Radiologist. 7 ed Lippincott Williams & Wilkins; 2012. p. 576. [Google Scholar]