Abstract
Controversial results of the association between coffee consumption and bladder cancer (BC) risk were reported among epidemiological studies. Therefore, we conducted this meta-analysis to clarify the association. Relevant studies were identified according to the inclusion criteria. Totally, 34 case-control studies and 6 cohort studies were included in our meta-analysis. The overall odds ratio (OR) with 95% confidence interval (CI) between coffee consumption and BC risk was 1.33 (95% CI 1.19 to 1.48). The summary ORs of BC for an increase of 1 cup of coffee per day were 1.05 (95% CI 1.03 to 1.06) for case-control studies and 1.03 (95% CI 0.99 to 1.06) for cohort studies. The overall ORs for male coffee drinkers, female coffee drinkers and coffee drinkers of both gender were 1.31 (95% CI: 1.08 to 1.59), 1.30 (95% CI: 0.87 to 1.96) and 1.35 (95% CI: 1.20 to 1.51). Compared with smokers (OR = 1.24, 95% CI: 0.91 to 1.70), non-smokers had a higher risk (OR = 1.72, 95% CI: 1.25 to 2.35) for BC. Results of this meta-analysis suggested that there was an increased risk between coffee consumption and BC. Male coffee drinkers and non-smoking coffee drinkers were more likely to develop BC.
Bladder cancer is the most common cancer of the urinary tract and the ninth most common cancer among men, accounting for approximately 330,000 new cases and 130,000 deaths per year worldwide1. BC is a torturous disease which is hard to be cured and easy to relapse2. Additionally, it was reported that the cost per patient of BC from diagnosis to death was the highest of all cancers, ranging from US$96,000–187,000 due to long-term survival and the need for lifelong routine monitoring and treatment3. Moreover, the incidence of BC will probably increase in the next decades since the world population is increasing and the problem of the ageing is getting worse in the world. However, feasible measures for the prevention of BC remain limited due to the unknown cause of BC. Therefore, more etiology researches of BC should be conducted to provide theoretical basis for the prevention of BC.
Coffee is a popular drink worldwide, especially among western countries, which also possess high incidence of BC1,4. Researches on coffee-induced BC mechanisms had reported that several compounds in coffee, including caffeine, polycyclic aromatic hydrocarbons (PAHs), and nitrosamines could increase the risk of BC5,6. However, chemicals in coffee like diterpenes cafestol and kahweol had been found to reduce the chance of having BC7,8,9. Since the early 1970s, coffee consumption had been considered to be related with the risk of BC by a case-control study10 conducted by Cole. Several studies had been published thereafter, with inconsistent results reported11,12,13,14,15. Recently, a pooled analysis of the studies conducted in Europe showed non-smokers who were heavy coffee drinkers might have a small excess risk of BC16.
In order to clarify the association between coffee consumption and the risk of BC and provide theoretical basis for the prevention of BC, we conducted a meta-analysis of all published case-control and cohort studies.
Results
Study characteristics
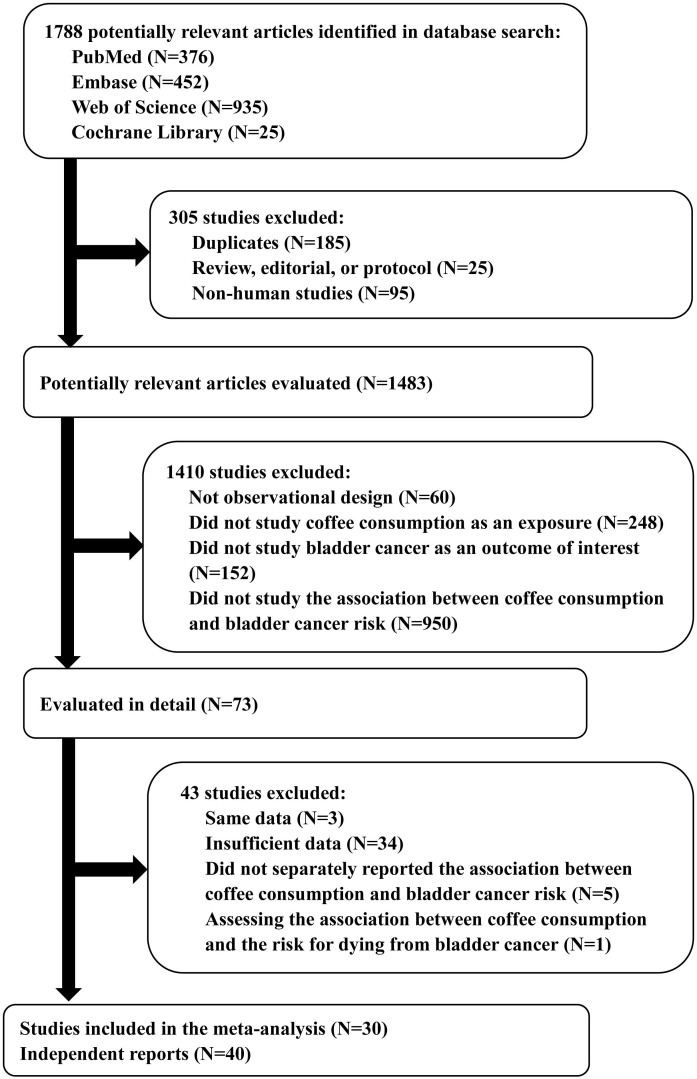
The study identification and selection progression was summarized in Figure 1. Totally, we identified 25 case-control studies10,11,12,13,14,15,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35 including 15,419 cases and 23,585 controls, and 5 prospective cohort studies36,37,38,39,40 including 753 cases and 236,343 participants in our meta-analysis. Among the 25 case-control studies, 9 studies reported 2 separate outcomes stratified by gender (male and female). As for the 5 cohort studies, 1 study reported 2 separate outcomes stratified by gender (male and female). Thus, there were 40 independent reports included in this meta-analysis. General characteristics of the included studies had been shown in Supplementary Table S1 and Table S2. Among these studies, 14 were conducted in America, 5 in Asia and 22 in Europe. Besides, 17 studies included both male and female, 12 studies male only and 11 studies female only. According to the year of publication, 26 studies were published before 2000 (including 2000), while the remaining 14 studies were published after 2000. Among the 34 case-control studies, data of the types of control were also extracted, revealing that 20 studies were hospital-based, 12 studies were population-based and 2 studies were mixed with population-based and hospital-based controls. The estimated quality of all included studies was in the range of 3–7 scores. The ratings had been reported in the Supplementary Table S3 and Table S4.
Figure 1. Flow chart showing the relevant observational studies of coffee consumption in relation to bladder cancer.
Association between coffee consumption and bladder cancer risk
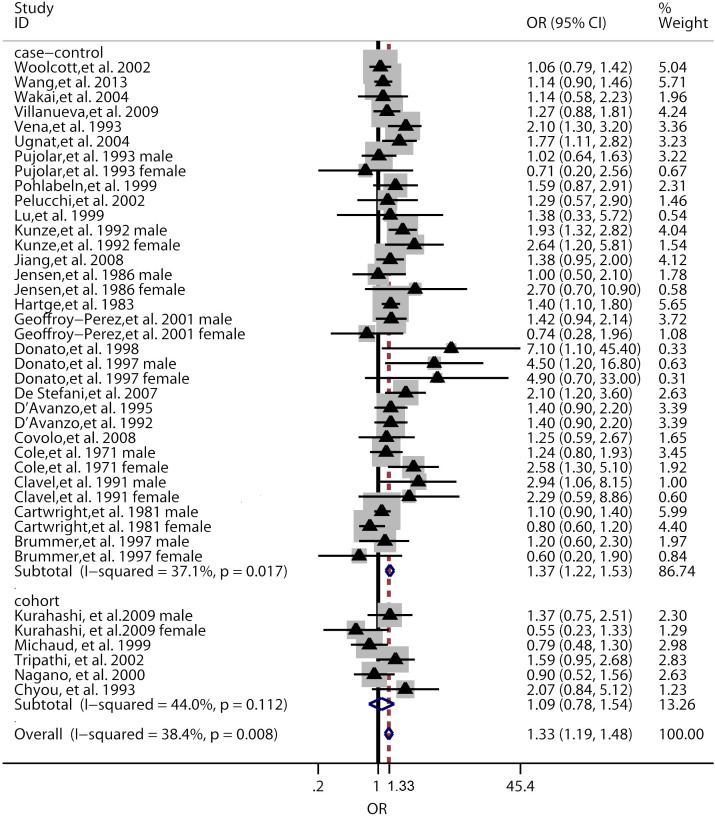
The results from the random-effect model combining the ORs for the risk of BC in relation to coffee consumption had been shown in Figure 2. A statistically significant association between the coffee intake and the risk of BC was found (OR = 1.33, 95% CI 1.19 to 1.48), and a moderate heterogeneity was detected (P = 0.008 for heterogeneity, I2 = 38.4%). For case-control studies, the combined OR was 1.37 (95% CI 1.22 to 1.53), with a moderate heterogeneity of P = 0.017 and I2 = 37.1%. For cohort studies, the combined OR was 1.10 (95% CI 0.78 to 1.54), with a moderate heterogeneity of P = 0.112 and I2 = 44.0%.
Figure 2. Pooled random effects OR and 95% CIs for the association of coffee consumption and bladder cancer.
The triangles and horizontal lines correspond to the study-specific ORs and 95% CIs. The gray areas reflect the study-specific weight. The diamonds represent the pooled ORs and 95% CIs of each subgroup and overall population. The vertical solid line shows the OR of 1 and the vertical dashed line indicates the overall pooled OR of 1.33.
Dose-response meta-analysis
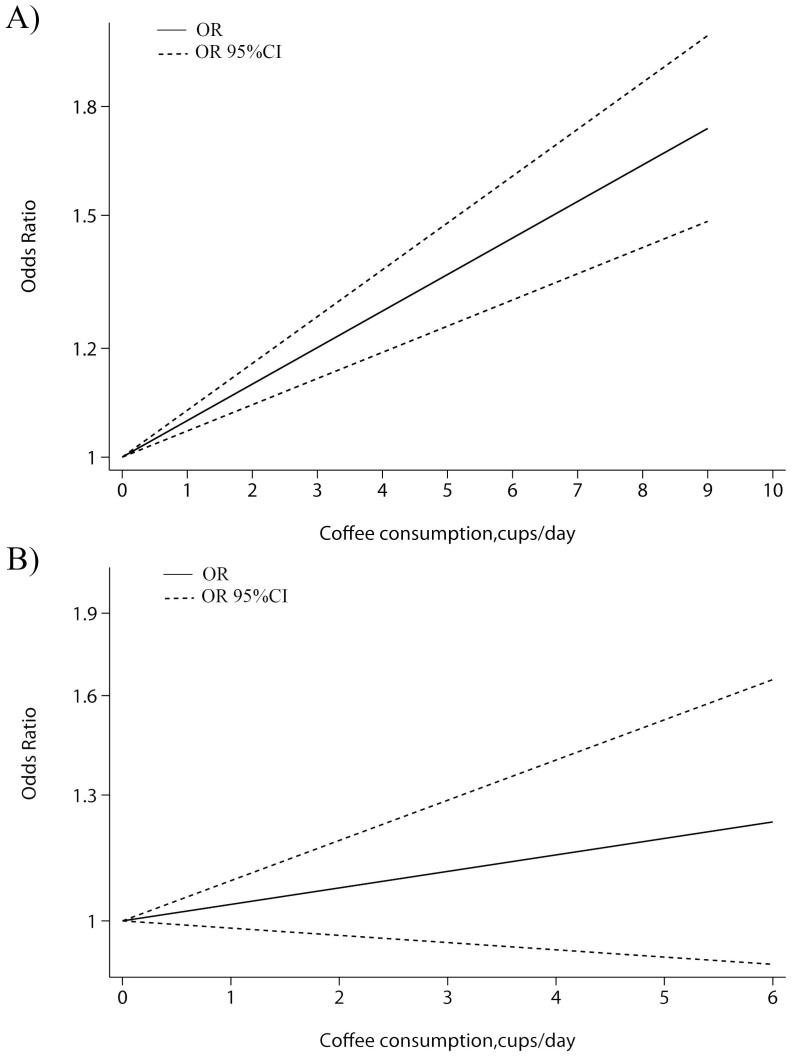
Studies10,11,12,25 reporting less than three categories of coffee consumption were excluded in dose-response meta-analysis. Totally, 28 case-control studies and 6 cohort studies were included. The results had been shown in Figure 3. For case-control studies, we found no evidence of a nonlinearity association between coffee consumption and BC risk in case-control studies (P = 0.073 for nonlinearity). Thus, linear model had been applied among case-control studies. The summary relative risk of bladder cancer for an increase of one cup of coffee per day was 1.05 (95% CI 1.03 to 1.06; P = 0.001 for linear trend). As for cohort studies, a linear association was confirmed (P = 0.433 for nonlinearity). The summary relative risk of bladder cancer for an increase of one cup of coffee per day was 1.03 (95% CI 0.99 to 1.06; P = 0.001 for linear trend).
Figure 3. Dose-response relationship between coffee consumption and the risk of bladder cancer among A) case-control studies and B) cohort studies.
The solid lines represent the linear trend. The dashed lines dashes represent the pointwise 95% confidence intervals for the linear trend.
Subgroup analyses
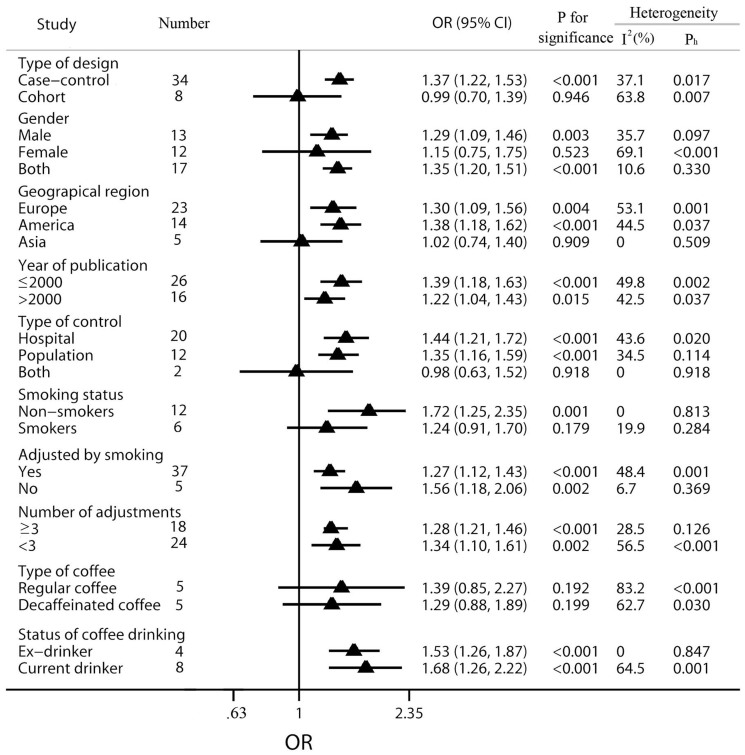
Results of subgroup analyses had been shown in Figure 4. To assess the potential effects of specific study characteristics on the association between coffee consumption and BC risk, we pooled the ORs and 95% CIs from the subgroups of study design, gender, geographical region, year of publication, type of control (for case-control studies), smoking status and adjustments. In most subgroups, coffee consumption was associated with an increased risk of BC. In the subgroup of study design, a significantly increased risk was observed in the case-control studies (OR = 1.37, 95% CI: 1.22 to 1.53), while a non-significant association was found within the cohort studies (OR = 1.10, 95% CI: 0.78 to 1.54), with a P value for test of 0.603. When stratified by gender, the studies on male and both gender had shown a significantly increased association (male: OR = 1.31, 95% CI: 1.08 to 1.59, both: OR = 1.35, 95% CI: 1.20 to 1.51). However, the association among female group was lack of statistical significance (OR = 1.30, 95% CI: 0.87 to 1.96), with the P value of 0.204. According to geographical region, positive relations were detected in Europe (OR = 1.36, 95% CI: 1.15 to 1.62) and America (OR = 1.38, 95% CI: 1.18 to 1.62), but non-association was noted in Asia (OR = 1.02, 95% CI: 0.74 to 1.40). Moreover, in order to avoid residual confounding variables by smoking, we also conducted the subgroup analyses of whether the pooled ORs were adjusted for smoking or not and extracted 18 independent reports from the included 40 studies to evaluate the difference of the studied association between non-smokers and smokers. The results had shown that there was a greater increased risk among the smoking non-adjusted group (OR = 1.56, 95% CI: 1.18 to 2.06) than the smoking adjusted group (OR = 1.30, 95% CI: 1.16 to 1.46). Interestingly, we found a moderate increased risk in the non-smokers group (OR = 1.72, 95% CI: 1.25 to 2.35), but no significance in the smokers group (OR = 1.24, 95% CI: 0.91 to 1.70). Besides, high coffee consumption was found to be associated with BC risk among the subgroups of publication year (≤2000 or >2000) and adjustments (≥3 or <3). In order to further explore the potential association between different aspects of coffee consumption and the risk of BC, subgroup analyses of type of coffee and coffee drinking status had been conducted. The pooled OR for regular coffee group was 1.39 (95% CI 0.85 to 2.27), while the combined OR for decaffeinated group was 1.29 (95% CI 0.88 to 1.89). When stratified by coffee drinking status, significantly increased associations were found in both groups of ex-coffee drinker (OR = 1.53, 95% CI 1.26 to 1.87) and current coffee drinker (OR = 1.68, 95% CI 1.26 to 2.22).
Figure 4. Subgroup analysis of OR of bladder cancer according to coffee consumption.
The triangles and horizontal lines correspond to the subgroup-specific ORs and 95% CIs. The vertical solid line shows the OR of 1. “Ph” represents the P value for heterogeneity from Q-test. Especially, the subgroup of type of control includes only the case-control studies, and “Both” indicates the study contains both hospital-based and population-based controls. For the subgroup stratified by the number of adjustments, “≥3” indicates the study should be adjusted at least three of the following factors: BMI, age, gender, smoking and other fluid intake.
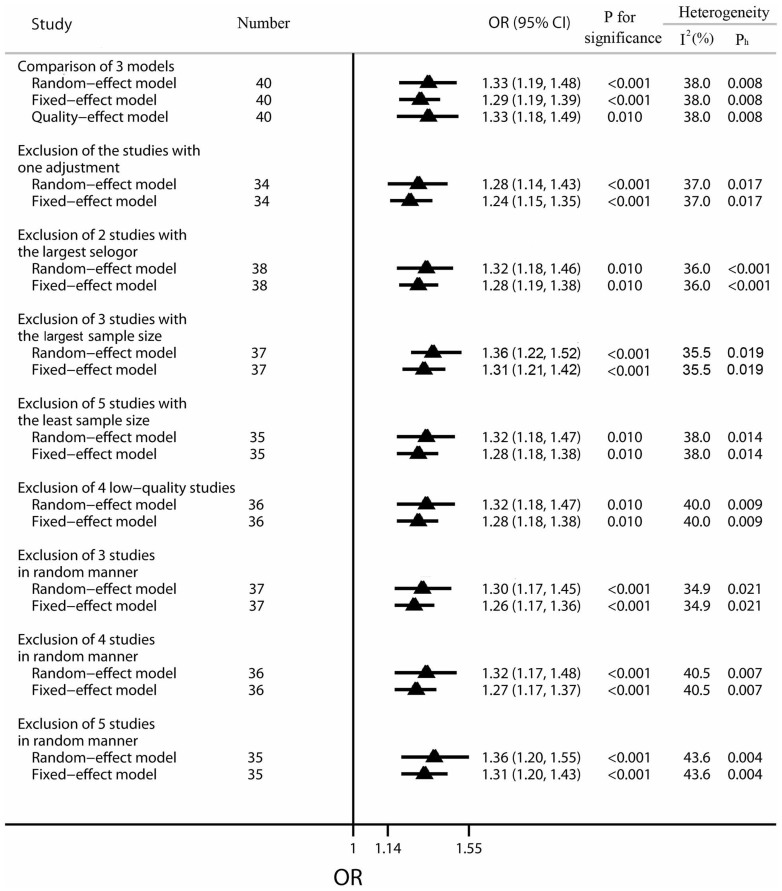
Sensitivity analyses
Sensitivity analyses were performed to evaluate the sensitivity of our conclusions about the overall effect of coffee consumption on the risk of BC. Firstly, the results of fixed-effect model, random-effect model and quality-effect model (Supplementary Figure S1) were compared to each other, and the results were robust (fixed-effects model pooled OR = 1.29, 95% CI 1.19 to 1.39; random-effects model pooled OR = 1.33, 95% CI 1.19 to 1.48; quality-effect model pooled OR = 1.33, 95% CI 1.18 to 1.49). Secondly, the leave-one-out analysis was performed by omitting one study in turn. The positive association was not drastically changed in the leave-one-out analysis, with pooled ORs ranging from 1.27 (95% CI 1.09 to 1.48) to 1.32 (95% CI 1.13 to 1.54). The results of the leave-one-out analysis had been displayed in Supplementary Figure S2. Thirdly, specific studies were excluded to further evaluate the reliability and stability of our conclusions. Exclusion of the studies with one adjustment, exclusion of the two studies possessing the largest standard error of effect estimates, exclusion of 3 studies with the largest sample size, exclusion of 5 studies with the least sample size and exclusion of 4 low-quality studies had been taken into account. Besides, we also omitted 3, 4 and 5 studies in random manner for further study. All the analyses had been performed applying both fixed-effect and random-effect models and the results were clearly shown in Figure 5.
Figure 5. Results of sensitivity analysis on the association between coffee consumption and the risk of bladder cancer.
The triangles and horizontal lines represent the corresponding ORs and 95% CIs. The vertical solid line shows the OR of 1. “Ph” represents the P value for heterogeneity from Q-test. Especially, “studies with one adjustment” contains Tripathi, et al. 2002, Kunze, et al. 1992 male, Kunze, et al. 1992 female, Jensen, et al. 1986 male, Jensen, et al. 1986 female and Pohlabeln, et al. 1999; “2 studies possessing the largest selogor” represents the two studies with the largest standard error of OR (Donato, et al. 1998 and Donato, et al. 1997 female); “3 studies with the largest sample size” represents Kurahashi, et al.2009 female, Kurahashi, et al.2009 male and Michaud, et al. 1999; “5 studies with the least sample size” includes Clavel, et al. 1991 female, Pujolar, et al. 1993 female, Cole, et al. 1971 female, Geoffroy-Perez, et al. 2001 female and Donato, et al. 1997 female; “ 4 low-quality studies” indicates Pelucchi, et al. 2002, Donato, et al. 1997 male, Covolo, et al. 2008 and Donato, et al. 1997 female.
Publication bias
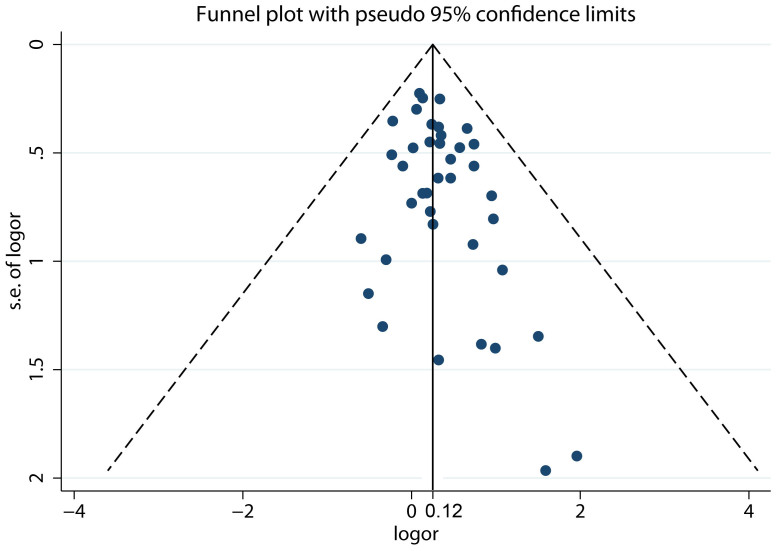
Visual inspection of the funnel plot showed little asymmetry (Figure 6). The Egger test and Begg test did not suggest evidence of publication bias (Egger, P = 0.051; Begg, P = 0.139). Thus, no publication bias was observed in this study.
Figure 6. Funnel plot for studies of coffee consumption in relation to bladder cancer risk.
The vertical solid line represents the summary effect estimates, and the dotted lines are pseudo 95% CIs.
Discussion
Drinking coffee can both facilitate and inhibit the occurrence and the development of BC in several mechanisms21,22,29. On the one side, coffee is a complex drink that contains many chemical compounds, including known or possible bladder carcinogens such as PAHs, nitrosamines and heterocyclic amines5. Besides, caffeine, mainly from coffee intake, has been demonstrated to affect DNA repair through the modification of the apoptotic response and perturbation of the cell cycle checkpoint integrity6. Moreover, the lethality of DNA-damaging agents, such as ionizing radiation or alkylating agents, is often potentiated when human cells are treated with caffeine41. However, on the other side, it should be noted that special compounds in coffee, including diterpenes cafestol and kahweol, polyphenols, caffeic acid and chlorogeni acid, can produce biological effects compatible with anti-carcinogenic properties to inhibit the occurrence and development of cancer7,8,9,42. In a research published in 2014, a hypothesis had been given that an increase of fluid intake would lead to the expansion of the bladder wall which would facilitate the direct actions of the carcinogens to deeper layer of the bladder urothelium43. However, meanwhile, a higher level of fluid consumption could increase urination frequency and reduce the exposure time of the carcinogens in turn, which can prevent the occurrence of cancer.
Potential bias is the major challenge for the meta-analysis of observational studies44. In the subgroup analyses of the type of control, the group of population-based controls (OR = 1.35, 95% CI: 1.16 to 1.59) had indicated a lower risk than the group of hospital-based controls (OR = 1.44, 95% CI: 1.21 to 1.72). This difference should be due to the various recall bias for the two types of controls. When considering the coffee is not good for health, cases and hospital-based controls might overstate their actual coffee consumption, while most of the population-based controls would properly evaluate their coffee intake. Thus, results from the population-based controls was thought to be more reliable. Besides, we also assessed the selection bias and investigation bias through the process of quality assessment (Supplementary Table S3 and Table S4).
In most cases, coffee drinkers tended to smoke, lack enough exercises and possess high body mass index (BMI), which had been reported to be the potential risk factors of the BC45,46,47. Owing to the correlations, the effect of coffee will be magnified in the presence of confounders. Therefore, adjustments should be conducted to reduce the potential confounding effect to get a more reliable conclusion. In the present meta-analysis, only studies with adjusted estimates were extracted. Influence of the potential confounding factors including smoking and BMI had been studied, and the result had shown that group with 3 or more adjustments (OR = 1.29, 95% CI: 1.11 to 1.49) exhibited a lower risk than the group with less than 3 adjustments (OR = 1.39, 95% CI: 1.18 to 1.64). This result had further confirmed the reliability of our findings, because the association remained unchanged even after the adjustments of the major confounding factors. Interestingly, there was a distinct difference in findings between studies published at or before 2000 (OR = 1.39, 95% CI: 1.18 to 1.63) and after 2000 (OR = 1.27, 95% CI: 1.12 to 1.43). The difference might result from better design of the studies published after 2000, which adjusted with more confounding factors, including BMI, tea consumption, physical activity and so on.
In particular, the interaction between coffee and smoking had been studied with more details within the present meta-analysis. Study between smokers and non-smokers had revealed that the risk of the BC among non-smokers (OR = 1.72, 95% CI: 1.25 to 2.35) was higher than smokers (OR = 1.24, 95% CI: 0.91 to 1.70). The observed outcome could be partly explained by coffee-induced BC mechanisms researches. It was reported that caffeine was the substrate of cytochrome P450 (CYP) enzymes in the liver, such as CYP1A2 or NAT2, and these enzymes might increase the metabolic activation of carcinogens like polycyclic aromatic hydrocarbons in cigarette smoking6. In the presence of smoke compounds, metabolism of caffeine could be faster48. Besides, experimental studies reported caffeine metabolism was induced by approximately 60 to 70% by cigarette smoke49. Thus, the adverse effects of coffee and caffeine may be more clearly expressed among never or former smokers and caffeine may modify the increased BC risk caused by smoking.
Bladder cancer is a complex disease resulting from interactions between genetic factors and environment50. The slow-acetylator phenotype of N-acetyltransferase and the polymorphisms of cytochrome P4501A2 has been found to be associated with a higher risk of BC within some epidemiological studies51. Recently, it was reported that the distribution of phenotypes of N-acetyltransferase varied between Asian and Caucasian population37,52, which could partly explain the different findings between Europe (OR = 1.36, 95% CI: 1.15 to 1.62) and America (OR = 1.38, 95% CI: 1.18 to 1.62), and Asia (OR = 1.02, 95% CI: 0.74 to 1.40). However, it should be noted that there were only 5 Asian studies included in our meta-analysis. More studies should be conducted in Asian countries to reveal the effect of genetic phenotypes in the relationship between coffee intake and BC.
There were several limitations in the present meta-analysis. First, we did not search for unpublished studies or original data, thus publication bias might be inevitable, even though no significant evidence of publication bias was observed. Second, categories of coffee consumption varied from studies, which might be responsible for the heterogeneity among studies in this analysis. Third, owing to the small number of Asian studies, the selection bias was unavoidable and the association among different countries remained unclear. Besides, due to the lack of relevant studies, important aspects of coffee drinking, including duration of coffee consumption, type of coffee and the status of coffee drinking, had not been studied enough. Furthermore, major risk factors including high risk occupations, disinfection byproducts, arsenic in the drinking water and genotype for the major metabolic enzymes were hard to be avoided.
In summary, the present meta-analysis, consisting of 34 case-control studies and 6 cohort studies, had suggested a significantly increased risk between coffee consumption and the risk of BC. Subgroup analyses indicated that male drinkers and non-smokers with high coffee consumption were more likely to develop BC. However, caution is needed in interpreting the findings from our meta-analysis because of the inevitable heterogeneity. Further well-designed large-scaled studies are warranted to provide more definitive conclusions.
Methods
Search strategy
In this paper, we conducted a literature search of PubMed (Medline), Embase, Web of Science and Cochrane Library from January 1966 through August 2014 for observational studies examining the association between coffee consumption and risk of BC. Search terms including “coffee”, “caffeine”, “drink”, “beverage” or “fluid” combined with “bladder cancer”, “bladder tumor”, “bladder neoplasm” or “bladder carcinoma” had been applied in the database search. Besides, reference lists from retrieved articles were also reviewed. Only articles published in English were considered. We followed the standard criteria for conducting meta-analyses of observational studies and reporting the results53.
Inclusion criteria
Studies meeting the following criteria were included in the meta-analysis: (1) the study design was observational, (2) the exposure of interest was coffee consumption, (3) the outcome of interest was bladder cancer, (4) the study reported adjusted risk estimates (relative risks or hazard risks or odds risks) with their 95% CI for the association between coffee consumption and BC or provided sufficient information to allow their calculations, (5) the study provided the frequency of every category of coffee consumption. Additionally, we excluded reviews, editorials, non-human studies, and letters without sufficient data. When multiple reports based on the same study were published, only the most recent or complete report would be used.
Data extraction
Eligibility evaluation and data extraction were carried out independently by two reviewers (W.W. and Y.T.) according to the guidelines for meta-analysis53. Discrepancies were adjudicated by discussions with a third reviewer (Q.L.). The following information were extracted from all the identified studies: name of first author, year of publication, country where the study was performed, type of study design, characteristics of the study population, duration of follow-up (for cohort study), number of cases or controls (participants and person-years for cohort studies), variables adjusted for in the analysis, as well as multivariate adjusted ORs (HRs or RRs) and 95% CIs for each category of coffee consumption. Only risk estimates adjusted with covariates were extracted. For studies that applied different models for the calculation of estimate risks, we chose the results adjusted with more potential confounders. In particular, we extracted ORs (RRs or HRs) adjusted for smoking when they were available, as smoking was a major confounder for the possible relationship between coffee consumption and risk of BC. Besides, ORs (RRs or HRs) among non-smokers and smokers were also extracted for further studying the confounding effect of smoking.
Quality assessment
Two reviewers (W.W. and Y.T.) independently performed the quality assessment using the Newcastle-Ottawa Scale54 (for the cohort and case–control study), which is a nine-point scale allocating points based on the selection process of studies (0–4 points), the comparability of studies (0–2 points) and the identification of the exposure and the outcomes of study participants (0–3 points). The quality of the articles was first evaluated according to the established questions, which were scored according to the follows: 1 point if the item was considered in the study, 0 point if the item was not considered or it was impossible to determine whether it was considered or not. We assigned scores of 0–3, 4–6 and 7–9 for low, moderate and high quality of studies, respectively.
Statistical analysis
The statistical analyses for the overall association between coffee consumption and BC risk were based on comparisons of the highest intake category of coffee consumption with the reference. In most cases, the reference refer to the lowest consumption of coffee, including the non-drinkers. Among the 30 identified studies, 10 studies10,11,13,15,22,26,28,29,31,37 did not offer pooled risk estimates for the whole research population but they provided separate estimates for each group by gender. Under this circumstance, each of these studies would be considered as two independent reports. Thus, 40 independent reports were included in our meta-analysis. It was reported that when the outcome was rare, ORs, HRs and RRs would provide similar estimates of risk55. In this work, ORs were chosen as a common measure of the association between coffee consumption and BC risk.
The median or mean coffee consumption in each category was used as the corresponding dose of consumption. Different measurements of coffee consumption in the original studies were converted into approximate cups/day. We defined 240 ml or 8 oz of coffee equal to one cup. When the median or mean consumption per category was not reported in the article, we assigned the midpoint of the upper and lower boundaries in each category as the average consumption. If the highest category was open-ended, we set the midpoint of the category at 1.5 times the lower boundary. When the lowest category was open-ended, the lower boundary was set to zero.
In the dose-response analysis, the number of cases and the total number of objects were used as covariates among case-control studies, while the number of cases and person-years were applied among cohort studies. Thus, analyses had been conducted for case-control studies and cohort studies, respectively. We performed the dose–response analysis to examine a potential nonlinear relationship. This was done by modeling coffee consumption using restricted cubic splines with 3 knots at fixed percentiles 10%, 50%, and 95% of the distribution. A P value for nonlinearity would be calculated by testing the null hypothesis that the coefficient of the second spline was equal to zero56.
The heterogeneity among studies was estimated by the Cochran Q test and I2 statistic44. Heterogeneity was confirmed with a significance level of P ≤ 0.10. For the I2 metric, low, moderate, and high I2 values were considered to be 25%, 50%, and 75%, respectively44. Fixed effect model would be applied when heterogeneity was negligible, otherwise random effect model would be used57. Besides, quality-effect model58 based on quality scores was employed and the results were compared with those from random-effect model and fixed-effect model.
To explore the potential heterogeneity among studies, subgroup analyses were conducted according to study design, gender, geographical region, publication year, adjustments. Besides, subgroup analyses of type of coffee and coffee drinking status had been conducted to further explore the potential association between different aspects of coffee consumption and the risk of BC. Sensitivity analyses were employed to find potential origins of heterogeneity and to examine the influence of various exclusions on the combined OR. Publication bias was assessed through the visual inspection of funnel plots and with tests of Begg rank correlation59 and Egger regression asymmetry60. P < 0.05 was considered to be representative of a significant statistical publication bias. Forest plots were applied to assess the overall association between coffee consumption and BC.
In addition to quality-effect modeling (conducted with MetaXL version 2.2 software), all statistical analyses were performed with STATA version 12.0 software (Stata Corporation, College Station, Texas, United States). All reported probabilities (P values) were two-sided, with P < 0.05 considered statistically significant.
Author Contributions
All authors contributed significantly to this work. W.W., Y.T. and Q.L. designed the research study; W.W., Y.T., Q.Z., G.Y. and X.W. performed the research study and collected the data; W.W., Y.T. and Q.Z. analyzed the data; W.W., Y.T., Q.L. wrote the paper; Q.Z., G.Y. and X.W. prepared Figures 1–6, Figure S1–S2 and Supplemental Table S1–S4. All authors reviewed the manuscript. In addition, all authors approved the final draft.
Supplementary Material
Supplementary Information
Acknowledgments
The financial supports from National Basic Research Program of China (973 Program, No. 2015CB352100), Program for New Century Excellent Talents in University (No. NCET-13-0232), National Natural Science Foundation of China (NSFC, No. 81302497) and China Postdoctoral Scientific Foundation (No. 2014M550394) are greatly acknowledged.
References
- Jemal A. et al. Global cancer statistics. CA Cancer J Clin 61, 69–90 (2011). [DOI] [PubMed] [Google Scholar]
- Leppert J. T. et al. Prevention of bladder cancer: a review. Eur Urol 49, 226–234 (2006). [DOI] [PubMed] [Google Scholar]
- Botteman M. F., Pashos C. L., Redaelli A., Laskin B. & Hauser R. The health economics of bladder cancer. Pharmacoeconomics 21, 1315–1330 (2003). [DOI] [PubMed] [Google Scholar]
- Boyle P. & Levin B. World cancer report 2008. (IARC Press, International Agency for Research on Cancer, 2008). [Google Scholar]
- Burger M. et al. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol 63, 234–241 (2013). [DOI] [PubMed] [Google Scholar]
- Porta M. et al. Coffee drinking: the rationale for treating it as a potential effect modifier of carcinogenic exposures. Eur J Epidemiol 18, 289–298 (2003). [DOI] [PubMed] [Google Scholar]
- Huber W. W. et al. Coffee and its chemopreventive components Kahweol and Cafestol increase the activity of O6-methylguanine-DNA methyltransferase in rat liver--comparison with phase II xenobiotic metabolism. Mutat Res 522, 57–68 (2003). [DOI] [PubMed] [Google Scholar]
- Cavin C. et al. Induction of Nrf2-mediated cellular defenses and alteration of phase I activities as mechanisms of chemoprotective effects of coffee in the liver. Food Chem Toxicol 46, 1239–1248 (2008). [DOI] [PubMed] [Google Scholar]
- Ramos S. Cancer chemoprevention and chemotherapy: dietary polyphenols and signalling pathways. Mol Nutr Food Res 52, 507–526 (2008). [DOI] [PubMed] [Google Scholar]
- Cole P. Coffee-drinking and cancer of the lower urinary tract. Lancet 1, 1335–1337 (1971). [DOI] [PubMed] [Google Scholar]
- Cartwright R. A., Adib R., Glashan R. & Gray B. K. The epidemiology of bladder cancer in West Yorkshire. A preliminary report on non-occupational aetiologies. Carcinogenesis 2, 343–347 (1981). [DOI] [PubMed] [Google Scholar]
- Hartge P., Hoover R., West D. W. & Lyon J. L. Coffee drinking and risk of bladder cancer. J Natl Cancer Inst 70, 1021–1026 (1983). [PubMed] [Google Scholar]
- Clavel J. & Cordier S. Coffee consumption and bladder cancer risk. Int J Cancer 47, 207–212 (1991). [DOI] [PubMed] [Google Scholar]
- Vena J. E. et al. Coffee, cigarette smoking, and bladder cancer in western New York. Ann Epidemiol 3, 586–591 (1993). [DOI] [PubMed] [Google Scholar]
- Bruemmer B., White E., Vaughan T. L. & Cheney C. L. Fluid intake and the incidence of bladder cancer among middle-aged men and women in a three-county area of Western Washington. Nutr Cancer 29, 163–168 (1997). [DOI] [PubMed] [Google Scholar]
- Sala M. et al. Coffee consumption and bladder cancer in nonsmokers: a pooled analysis of case–control studies in European countries. Cancer Cause Control 11, 925–931 (2000). [DOI] [PubMed] [Google Scholar]
- Woolcott C. G., King W. D. & Marrett L. D. Coffee and tea consumption and cancers of the bladder, colon and rectum. Eur J Cancer Prev 11, 137–145 (2002). [DOI] [PubMed] [Google Scholar]
- Wang J. et al. Fluid intake, genetic variants of UDP-glucuronosyltransferases, and bladder cancer risk. Br J Cancer 108, 2372–2380 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakai K. et al. Foods and beverages in relation to urothelial cancer: Case-control study in Japan. Int J Urol 11, 11–19 (2004). [DOI] [PubMed] [Google Scholar]
- Villanueva C. M. et al. Coffee consumption, genetic susceptibility and bladder cancer risk. Cancer Cause Control 20, 121–127 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugnat A. M., Luo W., Semenciw R. & Mao Y. Occupational exposure to chemical and petrochemical industries and bladder cancer risk in four western Canadian provinces. Chronic Dis Can 25, 7–15 (2004). [PubMed] [Google Scholar]
- Pujolar A. E. et al. Bladder cancer and coffee consumption in smokers and non-smokers in Spain. Int J Epidemiol 22, 38–44 (1993). [DOI] [PubMed] [Google Scholar]
- Pohlabeln H., Jockel K. H. & Bolm-Audorff U. Non-occupational risk factors for cancer of the lower urinary tract in Germany. Eur J Epidemiol 15, 411–419 (1999). [DOI] [PubMed] [Google Scholar]
- Pelucchi C., La Vecchia C., Negri E., Dal Maso L. & Franceschi S. Smoking and other risk factors for bladder cancer in women. Prev Med 35, 114–120 (2002). [DOI] [PubMed] [Google Scholar]
- Lu C. M. et al. Tea consumption: Fluid intake and bladder cancer risk in southern Taiwan. Urology 54, 823–828 (1999). [DOI] [PubMed] [Google Scholar]
- Kunze E., Chang-Claude J. & Frentzel-Beyme R. Life style and occupational risk factors for bladder cancer in Germany: A case-control study. Cancer 69, 1776–1790 (1992). [DOI] [PubMed] [Google Scholar]
- Jiang X. et al. Water intake and bladder cancer risk in Los Angeles County. Int J Cancer 123, 1649–1656 (2008). [DOI] [PubMed] [Google Scholar]
- Jensen O. M., Wahrendorf J., Knudsen J. B. & Sorensen B. L. The copenhagen case-control study of bladder cancer. II. Effect of coffee and other beverages. Int J Cancer 37, 651–657 (1986). [DOI] [PubMed] [Google Scholar]
- Geoffroy-Perez B. & Cordier S. Fluid consumption and the risk of bladder cancer: Results of a multicenter case-control study. Int J Cancer 93, 880–887 (2001). [DOI] [PubMed] [Google Scholar]
- Donato F., Boffetta P., Fazioli R., Gelatti U. & Porru S. Reliability of data on smoking habit and coffee drinking collected by personal interview in a hospital-based case-control study. Eur J Epidemiol 14, 259–267 (1998). [DOI] [PubMed] [Google Scholar]
- Donato F. et al. Bladder cancer, tobacco smoking, coffee and alcohol drinking in Brescia, northern Italy. Eur J Epidemiol 13, 795–800 (1997). [DOI] [PubMed] [Google Scholar]
- De Stefani E. et al. Non-alcoholic beverages and risk of bladder cancer in Uruguay. BMC Cancer 7 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Avanzo B., La Vecchia C., Negri E., Decarli A. & Benichou J. Attributable risks for bladder cancer in Northern Italy. Ann Epidemiol 5, 427–431 (1995). [DOI] [PubMed] [Google Scholar]
- D'Avanzo B. et al. Coffee consumption and bladder cancer risk. Eur J Cancer 28, 1480–1484 (1992). [DOI] [PubMed] [Google Scholar]
- Covolo L. et al. Bladder cancer, GSTs, NAT1, NAT2, SULT1A1, XRCC1, XRCC3, XPD genetic polymorphisms and coffee consumption: A case-control study. Eur J Epidemiol 23, 355–362 (2008). [DOI] [PubMed] [Google Scholar]
- Chyou P. H., Nomura A. M. & Stemmermann G. N. A prospective study of diet, smoking, and lower urinary tract cancer. Ann Epidemiol 3, 211–216 (1993). [DOI] [PubMed] [Google Scholar]
- Kurahashi N., Inoue M., Iwasaki M., Sasazuki S. & Tsugane S. Coffee, green tea, and caffeine consumption and subsequent risk of bladder cancer in relation to smoking status: A prospective study in Japan. Cancer Sci 100, 284–291 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud D. S. et al. Fluid intake and the risk of bladder cancer in men. N Engl J Med 340, 1390–1397 (1999). [DOI] [PubMed] [Google Scholar]
- Nagano J. et al. Bladder-cancer incidence in relation to vegetable and fruit consumption: a prospective study of atomic-bomb survivors. Int J Cancer 86, 132–138 (2000). [DOI] [PubMed] [Google Scholar]
- Tripathi A., Folsom A. R. & Anderson K. E. Risk factors for urinary bladder carcinoma in postmenopausal women - The Iowa women's health study. Cancer 95, 2316–2323 (2002). [DOI] [PubMed] [Google Scholar]
- Link C. J. Jr et al. Caffeine inhibits gene-specific repair of UV-induced DNA damage in hamster cells and in human xeroderma pigmentosum group C cells. Carcinogenesis 16, 1149–1155 (1995). [DOI] [PubMed] [Google Scholar]
- Vucic E. A., Brown C. J. & Lam W. L. Epigenetics of cancer progression. Pharmacogenomics 9, 215–234 (2008). [DOI] [PubMed] [Google Scholar]
- Bai Y. et al. Relationship between bladder cancer and total fluid intake: a meta-analysis of epidemiological evidence. World J Surg Oncol 12, 223 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J. P., Thompson S. G., Deeks J. J. & Altman D. G. Measuring inconsistency in meta-analyses. BMJ 327, 557–560 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison A. S. Epidemiology and environmental factors in urologic cancer. Cancer 60, 632–634 (1987). [DOI] [PubMed] [Google Scholar]
- Chung C. J. et al. The effect of cigarette smoke and arsenic exposure on urothelial carcinoma risk is modified by glutathione S-transferase M1 gene null genotype. Toxicol Appl Pharm 266, 254–259 (2013). [DOI] [PubMed] [Google Scholar]
- Tao L. et al. Environmental tobacco smoke in relation to bladder cancer risk--the Shanghai bladder cancer study. Cancer Epidem Biomar 19, 3087–3095 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurahashi N., Inoue M., Iwasaki M., Sasazuki S. & Tsugane S. Coffee, green tea, and caffeine consumption and subsequent risk of bladder cancer in relation to smoking status: a prospective study in Japan. Cancer Sci 100, 294–291 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zevin S. & Benowitz N. L. Drug interactions with tobacco smoking. Clin Pharmacokinet 36, 425–438 (1999). [DOI] [PubMed] [Google Scholar]
- Johansson S. L. & Cohen S. M. Epidemiology and etiology of bladder cancer. Semin Surg Oncol 13, 291–298 (1997). [DOI] [PubMed] [Google Scholar]
- Fanlo A. et al. Urinary mutagenicity, CYP1A2 and NAT2 activity in textile industry workers. J Occup Health 46, 440–447 (2004). [DOI] [PubMed] [Google Scholar]
- Hickman D. & Sim E. N-acetyltransferase polymorphism. Comparison of phenotype and genotype in humans. Biochem Pharmacol 42, 1007–1014 (1991). [DOI] [PubMed] [Google Scholar]
- Stroup D. F. et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283, 2008–2012 (2000). [DOI] [PubMed] [Google Scholar]
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25, 603–605 (2010). [DOI] [PubMed] [Google Scholar]
- Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev 9, 1–30 (1987). [DOI] [PubMed] [Google Scholar]
- Orsini N. et al. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol 175, 66–73 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau J. et al. Quantitative Synthesis in Systematic Reviews. Ann Intern Med 127, 820–826 (1997). [DOI] [PubMed] [Google Scholar]
- Doi S. A. et al. A quality-effects model for meta-analysis. Epidemiology 19, 94–100 (2008). [DOI] [PubMed] [Google Scholar]
- Begg C. B. & Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–1101 (1994). [PubMed] [Google Scholar]
- Egger M., Davey Smith G., Schneider M. & Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information