Abstract
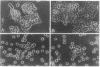
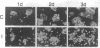
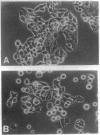
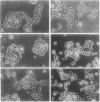
Recombinant baculovirus-derived interleukin 6 (IL-6) disrupts the attachment of human ductal breast carcinoma subline ZR-75-1-Tx cells to neighbors and the substratum in culture without inhibiting the proliferation of the cells. The nonadherent cells lack pseudopodia and do not translocate directionally. These findings stand in contrast to the earlier observations in the Ro subline of ZR-75-1 cells in which IL-6 induces cell-cell separation without detachment of the cells from the substratum, with the cells displaying pseudopodia, increased motility, and decreased proliferation. The IL-6-induced ZR-75-1-Tx cell detachment and rounding are reversible by incubation of the treated cells in IL-6-free medium for several days. The distinctive changes induced by IL-6 in ZR-75-1-Ro cells are similarly reversible. Either acidic fibroblast growth factor or phorbol 12-myristate 13-acetate can replace serum as a cofactor in IL-6-induced ZR-75-1-Tx cell detachment. Our findings indicate that genetic changes can occur in breast carcinoma cells that through cytokine action markedly affect cell structure, adhesiveness, and motility.
Full text
PDF
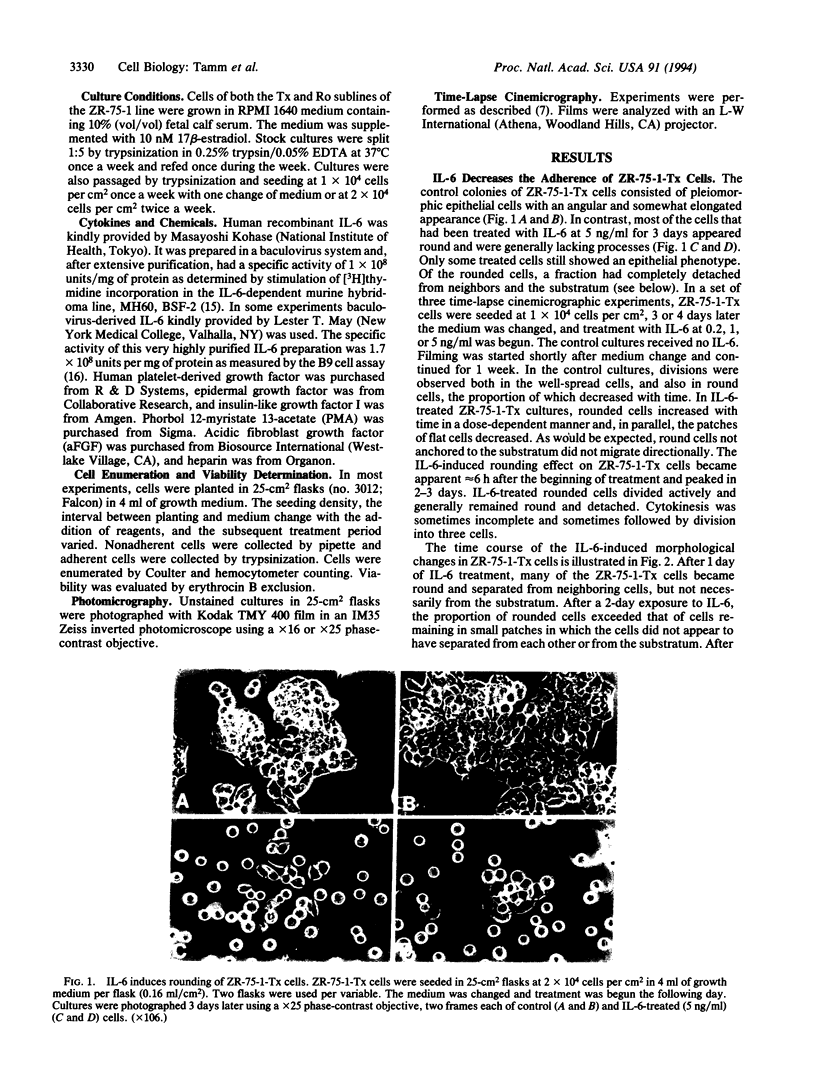
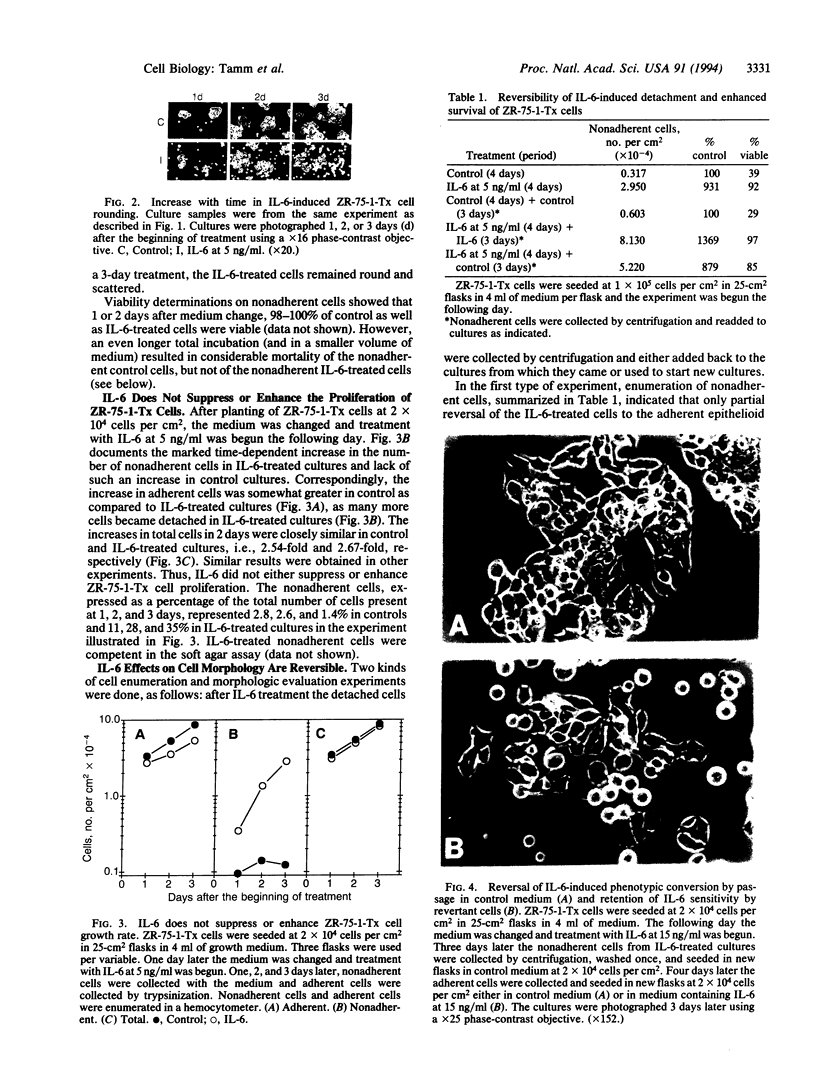


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arteaga C. L., Coronado E., Osborne C. K. Blockade of the epidermal growth factor receptor inhibits transforming growth factor alpha-induced but not estrogen-induced growth of hormone-dependent human breast cancer. Mol Endocrinol. 1988 Nov;2(11):1064–1069. doi: 10.1210/mend-2-11-1064. [DOI] [PubMed] [Google Scholar]
- Chen L., Shulman L. M., Revel M. IL-6 receptors and sensitivity to growth inhibition by IL-6 in clones of human breast carcinoma cells. J Biol Regul Homeost Agents. 1991 Oct-Dec;5(4):125–136. [PubMed] [Google Scholar]
- Engel L. W., Young N. A., Tralka T. S., Lippman M. E., O'Brien S. J., Joyce M. J. Establishment and characterization of three new continuous cell lines derived from human breast carcinomas. Cancer Res. 1978 Oct;38(10):3352–3364. [PubMed] [Google Scholar]
- Huff K. K., Kaufman D., Gabbay K. H., Spencer E. M., Lippman M. E., Dickson R. B. Secretion of an insulin-like growth factor-I-related protein by human breast cancer cells. Cancer Res. 1986 Sep;46(9):4613–4619. [PubMed] [Google Scholar]
- Huff K. K., Lippman M. E. Hormonal control of plasminogen activator secretion in ZR-75-1 human breast cancer cells in culture. Endocrinology. 1984 May;114(5):1702–1710. doi: 10.1210/endo-114-5-1702. [DOI] [PubMed] [Google Scholar]
- Kaibuchi K., Tsuda T., Kikuchi A., Tanimoto T., Yamashita T., Takai Y. Possible involvement of protein kinase C and calcium ion in growth factor-induced expression of c-myc oncogene in Swiss 3T3 fibroblasts. J Biol Chem. 1986 Jan 25;261(3):1187–1192. [PubMed] [Google Scholar]
- Keydar I., Chen L., Karby S., Weiss F. R., Delarea J., Radu M., Chaitcik S., Brenner H. J. Establishment and characterization of a cell line of human breast carcinoma origin. Eur J Cancer. 1979 May;15(5):659–670. doi: 10.1016/0014-2964(79)90139-7. [DOI] [PubMed] [Google Scholar]
- Leslie K. O., Howard P. Oncogenes and antioncogenes in human breast carcinoma. Pathol Annu. 1992;27(Pt 1):321–342. [PubMed] [Google Scholar]
- Magnaldo I., L'Allemain G., Chambard J. C., Moenner M., Barritault D., Pouysségur J. The mitogenic signaling pathway of fibroblast growth factor is not mediated through polyphosphoinositide hydrolysis and protein kinase C activation in hamster fibroblasts. J Biol Chem. 1986 Dec 25;261(36):16916–16922. [PubMed] [Google Scholar]
- Matsuura Y., Tatsumi M., Enami K., Morikawa S., Yamazaki S., Kohase M. Expression of IL-6/IFN-beta 2 in a baculovirus system and its biological function. Ann N Y Acad Sci. 1989;557:122–129. doi: 10.1111/j.1749-6632.1989.tb24005.x. [DOI] [PubMed] [Google Scholar]
- May L. T., Shaw J. E., Khanna A. K., Zabriskie J. B., Sehgal P. B. Marked cell-type-specific differences in glycosylation of human interleukin-6. Cytokine. 1991 May;3(3):204–211. doi: 10.1016/1043-4666(91)90018-9. [DOI] [PubMed] [Google Scholar]
- Naldini L., Weidner K. M., Vigna E., Gaudino G., Bardelli A., Ponzetto C., Narsimhan R. P., Hartmann G., Zarnegar R., Michalopoulos G. K. Scatter factor and hepatocyte growth factor are indistinguishable ligands for the MET receptor. EMBO J. 1991 Oct;10(10):2867–2878. doi: 10.1002/j.1460-2075.1991.tb07836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne C. K., Hamilton B., Nover M., Ziegler J. Antagonism between epidermal growth factor and phorbol ester tumor promoters in human breast cancer cells. J Clin Invest. 1981 Apr;67(4):943–951. doi: 10.1172/JCI110144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm I., Cardinale I., Krueger J., Murphy J. S., May L. T., Sehgal P. B. Interleukin 6 decreases cell-cell association and increases motility of ductal breast carcinoma cells. J Exp Med. 1989 Nov 1;170(5):1649–1669. doi: 10.1084/jem.170.5.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm I., Cardinale I., Murphy J. S. Decreased adherence of interleukin 6-treated breast carcinoma cells can lead to separation from neighbors after mitosis. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4414–4418. doi: 10.1073/pnas.88.10.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm I., Cardinale I., Sehgal P. B. Interleukin-6 and 12-O-tetradecanoyl phorbol-13-acetate act synergistically in inducing cell-cell separation and migration of human breast carcinoma cells. Cytokine. 1991 May;3(3):212–223. doi: 10.1016/1043-4666(91)90019-a. [DOI] [PubMed] [Google Scholar]
- Tsuda T., Kaibuchi K., Kawahara Y., Fukuzaki H., Takai Y. Induction of protein kinase C activation and Ca2+ mobilization by fibroblast growth factor in Swiss 3T3 cells. FEBS Lett. 1985 Oct 28;191(2):205–210. doi: 10.1016/0014-5793(85)80009-0. [DOI] [PubMed] [Google Scholar]
- Van de Vijver M. J., Nusse R. The molecular biology of breast cancer. Biochim Biophys Acta. 1991 Apr 16;1072(1):33–50. doi: 10.1016/0304-419x(91)90005-6. [DOI] [PubMed] [Google Scholar]
- Whang-Peng J., Lee E. C., Kao-Shan C. S., Seibert K., Lippman M. Cytogenetic studies of human breast cancer lines: MCF-7 and derived variant sublines. J Natl Cancer Inst. 1983 Oct;71(4):687–695. [PubMed] [Google Scholar]
- Zankl H., Ludwig B., May G., Zang K. D. Karyotypic variations in human meningioma cell cultures under different in vitro conditions. J Cancer Res Clin Oncol. 1979 Feb 19;93(2):165–172. doi: 10.1007/BF00406574. [DOI] [PubMed] [Google Scholar]
- Zugmaier G., Knabbe C., Fritsch C., Simpson S., Ennis B., Lippman M., Dickson R. B. Tissue culture conditions determine the effects of estrogen and growth factors on the anchorage independent growth of human breast cancer cell lines. J Steroid Biochem Mol Biol. 1991 Nov;39(5A):681–685. doi: 10.1016/0960-0760(91)90367-e. [DOI] [PubMed] [Google Scholar]