Abstract
Background
A minority of patients with asthma have uncontrolled or partially controlled asthma despite intensive treatment. These patients present a special challenge because of the extensive diagnostic evaluation that they need, insufficient evidence regarding personalized treatments, and their high consumption of health-care resources.
Methods
The definition, diagnosis, and treatment of severe asthma are presented on the basis of a selective literature review and the authors’ clinical experience.
Results
Severe asthma is present, by definition, when adequate control of asthma cannot be achieved by high-dose treatment with inhaled corticosteroids and additional controllers (long-acting inhaled beta 2 agonists, montelukast, and/or theophylline) or by oral corticosteroid treatment (for at least six months per year), or is lost when the treatment is reduced. Before any further treatments are evaluated, differential diagnoses of asthma should be ruled out, comorbidities should be treated, persistent triggers should be eliminated, and patient adherence should be optimized. Moreover, pulmonary rehabilitation is recommended in order to stabilize asthma over the long term and reduce absences from school or work. The additional drugs that can be used include tiotropium, omalizumab (for IgE-mediated asthma), and azithromycin (for non-eosinophilic asthma). Antibodies against interleukin-5 or its receptor will probably be approved soon for the treatment of severe eosinophilic asthma.
Conclusion
The diagnosis and treatment of severe asthma is time consuming and requires special experience. There is a need for competent treatment centers, continuing medical education, and research on the prevalence of severe asthma.
The prevalence of asthma increased significantly in the 20th century and is currently estimated to be 5 to 10% in Europe (1). In the 20th century, the pertinent medical concepts were dominated by the classification of asthma as “allergic asthma” (evidence of allergic sensitization) or “intrinsic asthma” (no evidence of allergic sensitization); this classification was proposed by Francis M. Rackemann in 1918 (2, 3). In the 21st century, this is slowly being replaced by biomarker-based phenotyping of asthma, for targeted treatment of particular subtypes. The concept of asthma severity has also changed: classification by lung function is giving way to classification by degree of asthma control. This concept has been adopted in German (www.versorgungsleitlinien.de) and international (www.ginasthma.com) recommendations.
In clinical practice, asthma control is assessed using questionnaires such as the Asthma Control Test (ACT) (Table 1) and the Asthma Control Questionnaire (ACQ) (4). The majority of patients can be successfully treated with modern standard therapy. As a result, emergency room consultations and hospitalizations of asthma patients have decreased (5). However, the asthma of a minority remains only partially controlled, or even uncontrolled, despite intensive treatment. This asthma, termed severe asthma, is also important in terms of health economics, as this minority of patients accounts for the majority of medical resource use (6, 7).
Table 1. Asthma Control Test (ACT).
| 1 points | 2 points | 3 points | 4 points | 5 points | |
|---|---|---|---|---|---|
| Everyday restriction | In the past 4 weeks. how much of the time did your asthma keep you from getting as much done at work. school or at home? | ||||
| All of the time | Most of the time | Some of the time | A little of the time | None of the time | |
| Daytime complaints | During the past 4 weeks. how often have you had shortness of breath? | ||||
| More than _once a day | Once a day | 3 to 6 times _a week | Once or twice_ a week | Not at all | |
| Nighttime complaints | During the past 4 weeks. how often did your asthma symptoms (wheezing. coughing. shortness of breath. chest tightness or pain) wake you up at night or earlier than usual in the morning? | ||||
| 4 or more nights a week | 2 or 3 nights a week | Once a week | Once or twice | Not at all | |
| Rescue inhaler use | During the past 4 weeks. how often have you used your rescue inhaler or nebulizer medication (such as albuterol)? | ||||
| 3 or more times per day | 1 or 2 times per day | 2 or 3 times per week | Once a week or less | Not at all | |
| Subjective assessment | How would you rate your asthma control during the past 4 weeks? | ||||
| Not controlled at all | Poorly controlled | Somewhat controlled | Well controlled | Completely controlled | |
In the ACT (4) five questions must be answered. and between 1 and 5 points are assigned per answer. There is thus a maximum score of 25. The definition established by the European Respiratory Society and the American Thoracic Society in 2014 defines severe asthma as a score under 20 during high-dose ICS (inhaled corticosteroid) therapy with an additional controller or during oral corticosteroid therapy for more than 6 months per year
Definition
There is no universally accepted definition of the features that constitute severe asthma. In 2010, the World Health Organization (WHO) recommended that severe asthma be divided into three groups (6) (Table 2). The advantage of the WHO classification is its realistic assessment of patients with severe asthma: in most cases severe asthma is not therapy resistant but falls into one of the following three categories (8):
Table 2. Classification of severe asthma according to WHO recommendation (2010) (6).
| WHO class | Name | Explanation |
|---|---|---|
| I | Untreated severe asthma | Uncontrolled. as yet untreated asthma |
| II | Difficult-to-treat asthma | Uncontrolled asthma due to adherence problems. persistent triggers. or comorbidities |
| III | Therapy-resistant asthma | Uncontrolled asthma despite maximum therapy or asthma control that can only be maintained with maximum therapy |
WHO. World Health Organization
Untreated asthma
Incorrectly treated asthma
Difficult-to-treat asthma (as a result of non-adherence, persistent triggers, or comorbidities)
In the current definition (2014) of severe asthma established by a task force of the European Respiratory Society (ERS) and the American Thoracic Society (ATS), untreated patients (who need not necessarily have genuinely severe asthma) are omitted. This definition specifies the criteria for severe asthma (7) (Box 1). It also defines the term “high-dose inhaled corticosteroid (ICS)” (7) (Table 3). In a few cases (e.g. ciclesonide: maximum authorized daily dose 160 μg in Germany), the recommended doses in high-dose ICS therapy can be higher than the highest daily dose established in specific countries. It is important not to forget that the lung function-based criterion (Box 1) (forced expiratory volume in one second [FEV1] <80%) applies only if the Tiffeneau index (FEV1/FVC [forced vital capacity]: a parameter of airway obstruction) is low; this proviso is important, because restrictive lung diseases, which do not automatically make asthma more severe, are also associated with low FEV1.
Box 1. The definition of severe asthma (according to ERS/ATS 2014) (7).
During treatment with:
High-dose ICS + at least one additional controller (LABA, montelukast, or theophylline) or
Oral corticosteroids >6 months/year
…at least one of the following occurs or would occur if treatment would be reduced:
ACT <20 or ACQ >1.5
At least 2 exacerbations in the last 12 months
At least 1 exacerbation treated in hospital or requiring mechanical ventilation in the last 12 months
FEV1 <80% (if FEV1/FVC below the lower limit of normal)
The lower limit of normal (LLN) for FEV1/FVC can be calculated using appropriate spirometer software (www.lungfunction.org). Current recommendations advocate a FEV1/FVC <LLN to detect airway obstruction (40). However, if LLN is unknown, in our opinion the formerly universal limit (FEV1/FVC <70% for adults, FEV1/FVC <75% for children) can still be used.
ICS: Inhaled corticosteroid; ACT, Asthma Control Test; ACQ: Asthma Control Questionnaire; FEV1: Forced expiratory volume in one second; FVC: Forced vital capacity; ERS: European Respiratory Society; ATS: American Thoracic Society; LABA: Long-acting ß2 agonist
Table 3. Definition of high-dose ICS therapy according to ERS/ATS consensus 2014.
| 6 to 12 years | >12 years | |
|---|---|---|
| Beclomethasone | ≥800µg*1 | ≥2000µg*1 |
| ≥320µg*2 | ≥1000 µg*2 | |
| Budesonide | ≥800µg | ≥1600 µg |
| Ciclesonide | ≥160µg*3 | ≥320µg |
| Fluticasone propionate | ≥500µg | ≥1000µg |
| Mometasone furoate | ≥500µg*3 | ≥800µg |
Doses shown are total daily doses of inhaled steroids (ICSs) which are classified as high-dose ICS therapy according to the consensus (7) of the European Respiratory Society (ERS) and the American Thoracic Society (ATS). by age group (patients aged 6 to 12 years and those aged over 12 years).
*1Beclomethasone dose for dry powder inhalers
*2Beclomethasone for hydrofluoroalkane (HFA) metered-dose inhalers
*3Not approved for children under 12 in Germany
Diagnosis
Basic diagnostic procedures (9) includes the following:
Clinical history (Box 2)
Clinical examination
Lung function testing (spirometry or whole-body plethysmography) followed by reversibility testing (in case of airway obstruction) or hyperreactivity testing (if there is no airway obstruction).
Box 2. Content of detailed history taking for asthma.
Nature, duration, and triggers of symptoms
Age at and circumstances of initial onset of symptoms
Relationship between symptoms and physical exertion and between symptoms and occupation
Seasonality and circadian variations in symptoms
Responsiveness of symptoms to asthma-specific therapies
Changes of symptoms on travels
Active and passive smoking
Allergies and comorbidities
Family history (asthma/allergic diseases)
Regular contact with animals
Occupational or private stress factors
Tolerance of cyclooxygenase (COX) 1 inhibitors
Long-term medication, adherence, inhalation technique
Exacerbations/hospitalizations in the last 12 months
Allergy testing should also always be performed (clinical history, skin prick tests, blood tests). Other tests, such as the measurement of exhaled nitric oxide levels (FeNO, in ppb [parts per billion]), are optional (9). Typically, patients with severe asthma have already had a basic assessment of their disease. In the following, we describe how to proceed if a patient presents for therapy optimization.
Confirmation of the diagnosis
If severe asthma is suspected, differential diagnoses that may mimic asthma (Box 3) should first be ruled out. This requires a detailed clinical history (Box 2). Because up to 40% of asthma patients in Europe smoke (10), subacute reversibility testing using systemic steroid therapy (e.g. prednisolone 50 mg for 7 to 14 days) should be performed in addition to acute reversibility testing (using a short-acting bronchodilator) to rule out chronic obstructive pulmonary disease (COPD). If prednisolone therapy largely or completely restores lung function, COPD is unlikely.
Box 3. Diseases that may mimic asthma.
Congenital or acquired immunodeficiency
Primary ciliary dyskinesia
Cystic fibrosis (CF)
Vocal cord dysfunction (VCD)
Central airway obstruction
Recurrent aspiration
Bronchiolitis
Gastroesophageal reflux disease (GERD)
Psychogenic hyperventilation
Chronic obstructive pulmonary disease (COPD)
Heart failure
Drug side effects (e.g. ACE inhibitor-induced cough)
Pulmonary embolism
ACE, angiotensin-converting enzyme
Computed tomography (CT) of the chest may also be useful, as it can rule out many differential diagnoses (including malformations, dysplasias, tumors, bronchiolitis, bronchiectasis, pulmonary embolism, alveolitis, and various interstitial lung diseases). The consensus paper of the ERS/ATS task force (7) therefore gives a conditional recommendation for a chest CT in case of atypical presentation of severe asthma. The following procedures may also be useful:
Bronchoscopy to rule out endobronchial changes, for biopsy, or for diagnostic bronchoalveolar lavage
Echocardiography to rule out heart failure or structural heart disease
24-hour pH measurement to rule out gastroesophageal reflux.
Ruling out diseases associated with asthma
Aspirin-exacerbated respiratory disease (AERD), also known as ASA (acetylsalicylic acid) intolerance, is an intolerance of cyclooxygenase (COX) 1 inhibitors. It is associated with hypersensitivity to ASA, nasal polyps, chronic sinusitis, and asthma (often severe). The exact prevalence of AERD is uncertain and is reported as between 4 and 21% of asthma patients (37). Diagnosis can be confirmed only using ASA provocation as there is no valid skin or laboratory test for AERD (9).
Allergic bronchopulmonary aspergillosis (ABPA) should be suspected in the following cases:
Very high total IgE levels (usually well over 1000 kU/L)
Specific IgG and IgE antibodies to Aspergillus fumigatus (particularly IgE antibodies to recombinant Aspergillus antigens rAsp F4 and rAsp f6)
Fleeting pulmonary opacities
Central bronchiectasis.
Churg–Strauss syndrome (CSS) should be suspected in the following cases:
Blood eosinophils >10%
Migrating pulmonary opacities
Sinusitis
Neuropathy.
Wherever possible, suspected cases of CSS should be further clarified by biopsy (evidence of extravascular eosinophilic infiltrations).
Adherence, triggers, and comorbidities
Common causes of severe asthma are poor treatment adherence and/or persistent triggers (WHO class II: Table 2 (8). Because of this, adherence and triggers should always be systematically investigated (Box 4) before additional medication is prescribed. In addition, comorbidities that affect asthma severity, such as chronic rhinosinusitis, gastroesophageal reflux, sleep-related breathing disorders, or heart disease, must be sought. Obesity can not only adversely affect asthma control but can also be the cause of an asthma misdiagnosis, as both its symptoms and its lung function findings can mimic asthma (7). This requires examination by a respiratory physician.
Box 4. Systematic assessment of adherence and persistent triggers.
Does the patient understand the concept of inhaled therapy for asthma control?
Is the patient receiving basic inhaled therapy according to guidelines and adapted to the severity of his/her asthma?
Does the patient handle his/her inhaler(s) correctly? (If not, who trains the patient and who monitors the success of training?)
Does the patient take inhaled therapy regularly? (If not, how can this be optimized on an individual basis?)
Does the patient avoid active and passive smoking?
Does the patient know his/her allergen spectrum and does he/she effectively avoid these allergens?
Does the patient avoid detrimental medications (e.g. beta blockers for which there are treatment alternatives)?
How often COPD and asthma co-occur is currently being discussed using the term “asthma–COPD overlap syndrome” (ACOS) (www.ginasthma.com). In most unclear cases, however, the clinical history and course of disease indicate either COPD or asthma relatively clearly. Evidence of a psychiatric disorder—depression or an anxiety disorder is present in up to 50% of patients—should be clarified by a specialized physician (11, 12).
Biomarkers
Allergy testing (skin prick test and/or measurement of allergen-specific IgE antibodies) are part of standard assessment. Total serum IgE level is required to evaluate omalizumab therapy (9). A differential blood count is needed to identify the eosinophilic phenotype (this is essential for specific treatment decisions (e.g. anti-interleukin-5 therapy or macrolide therapy) (13). Total eosinophil count in peripheral blood is the key parameter in this regard: cut-off values between 0.15 × 109/L (13, 14) and 0.3 × 109/L (15) are currently being discussed. Systemic corticosteroid therapy makes it impossible to ascertain patients’ “genuine” eosinophil status; a steroid-free interval can be considered in such cases. The ERS/ATS consensus paper gives a conditional recommendation against asthma management using FeNO measurement (7). Persistently high FeNO levels (above 50 ppb) during high-dose ICS therapy, however, may indicate poor adherence, persistent exposure to allergens, or strong intrinsic disease activity (16).
Treatment
The details of standard therapy can be found in the asthma guidelines (17) and the German National Disease Management Guideline. Basic therapy consists of an inhaled corticosteroid (ICS), to which additional controllers are added if asthma control is inadequate: an inhaled long-acting beta 2 agonist (LABA), montelukast, and/or theophylline. If this therapy does not adequately control asthma, oral corticosteroids (e.g. prednisolone) are added. Specific immunotherapy for severe asthma is only a theoretical possibility, for the following reasons (18, 19):
Either there is no detectable allergen or there is polysensitization with no clear relationship between allergen exposure and symptoms.
Lung function is often too poor (immunotherapy requires FEV1 >70% for safety reasons).
There is a lack of randomized clinical trials on severe asthma.
Although patients with severe asthma are often deficient in vitamin D, current evidence does not support a universal recommendation of vitamin D therapy (20). There are specific treatment principles for the diseases associated with asthma (Table 4). Below we outline basic measures and additional treatment options following a diagnosis of severe asthma.
Table 4. Treatment principles for diseases associated with asthma.
| Disease | Abbreviation | Treatment principles |
|---|---|---|
| Aspirin-exacerbated respiratory disease (ASA intolerance) | AERD | Lifelong ASA therapy (at least 300 mg/day); treatment must be begun on an inpatient basis (37) |
| Allergic bronchopulmonary aspergillosis | ABPA | Systemic corticosteroid therapy beginning with 0.5 to 0.75 mg prednisolone equivalent per kg body weight and then tapering down; concomitant antimycotic therapy (itraconazole. alternatively voriconazole if itraconazole not tolerated) in case of recurrent exacerbations (7, 38) |
| Churg–Strauss syndrome | CSS | Corticosteroid plus an additional immunosuppressant (methotrexate. cyclophosphamide. or azathioprine); after high-dose initial therapy. reduce gradually to lowest possible long-term therapy (39) |
ASA. acetylsalicylic acid
Basic therapy
Optimizing inhaled therapy
Poor inhalation technique and poor adherence are common and very straightforward causes of uncontrolled asthma. Where necessary, patients must be re-educated and retrained; resources for this include freely available online videos (approximately 2 to 3 minutes per video) (www.atemwegsliga.de). Whether switching to inhalers with extrafine formulations (average particle size 1 to 3µm) can improve asthma control by means of better ICS deposition in the smaller airways is currently under discussion (21). Optimized patient–inhaler interaction is an essential key to successful inhaled therapy. Therefore, patients should be involved in inhaler selection (e.g. dry powder or metered-dose inhaler). Finally, check whether the maximum daily ICS dose stated in international recommendations has been prescribed (Table 3).
Eliminating persistent triggers
Persistent asthma triggers are a common feature of difficult-to-treat asthma (WHO class II). Identifying persistent (often perennial and/or domestic) sources of allergens can be challenging. Allergens may be uncommon, or exposure may not be obvious and the patient may not consider it worth reporting. Eliminating sources of allergens (e.g. cats) can be even more difficult, due to emotional obstacles. One underestimated challenge is the elimination of occupational allergens, the avoidance of which may threaten patients emotionally as well as economically (e.g. a baker working in a family-owned bakery).
Therapy for comorbidities
For obese patients, weight reduction can have a positive effect on asthma control (22). Chronic rhinosinusitis is an important trigger of asthma and should be investigated and properly treated by a specialized physician; there is a guideline on this subject (ARIA: Allergic Rhinitis and Its Impact on Asthma) (23). Symptomatic gastroesophageal reflux disease (GERD) also requires treatment, as does depression or an anxiety disorder (11, 12).
Additional treatment options
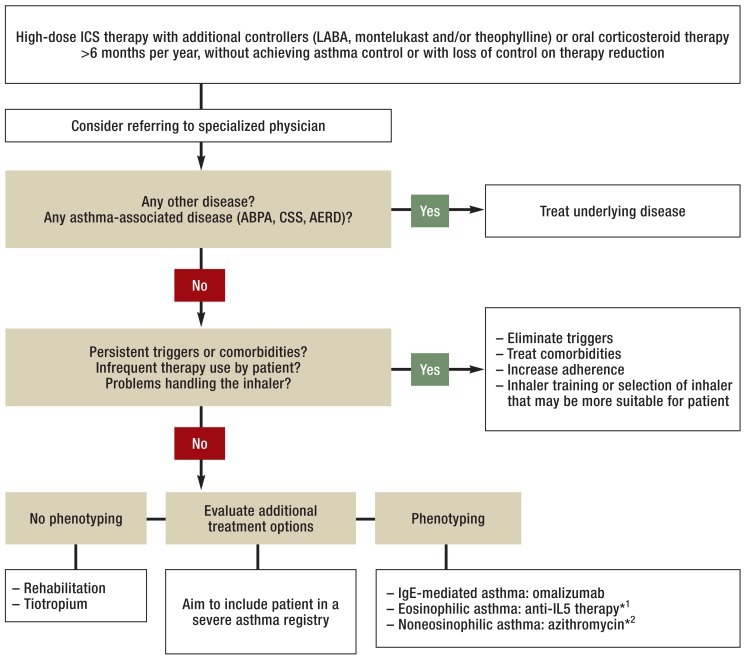
If the basic therapeutic measures mentioned above have been exhausted, additional treatment options that serve in particular to reduce oral and inhaled corticosteroid doses should be evaluated. In general, the evidence advocating these additional options is weak (7), as there are only a few clinical trials on severe asthma that address each one specifically. As a result, in everyday clinical practice treatment decisions can often be made only on the basis of experience and outcomes in similar circumstances. Treatment options can be divided into two groups according to whether or not they can be begun without further phenotyping (Figure 1).
Figure 1.
Diagnosing and treating severe asthma
*1Currently possible in Germany only within clinical trials
*2In case of low eosinophil count (<0.2 × 109/L during cessation of systemic corticosteroid therapy) and considering contraindications for macrolide therapy
ICS, inhaled corticosteroid; LABA, long-acting beta 2 agonist; ABPA, allergic bronchopulmonary aspergillosis; CSS, Churg–Strauss syndrome; AERD, aspirin-exacerbated respiratory disease (ASA intolerance); IgE, immunoglobulin E; IL, interleukin
Rehabilitation
For most patients, asthma requires lifelong medical care which cannot be optimally provided on an outpatient basis or in acute-care hospitals. Inpatient rehabilitation in appropriate specialized facilities is, therefore, recommended. This is particularly important in patients with severe asthma, in whom additional psychosocial or socioeconomic factors often contribute to asthma severity (24, 25). Rehabilitation aids in incorporating therapeutic needs into daily routine and provides patients with knowledge of their disease that allows them to organize their daily lives. Rehabilitation can stabilize asthma in the long term, significantly reduce resource consumption, and result in fewer hospitalizations and days off school or work (26).
Long-acting anticholinergics
The usefulness of long-acting muscarinic antagonists (LAMAs) is obvious, because it is primarily the parasympathetic nervous system that controls airway tone (27) (Figure 2). Three placebo-controlled clinical trials all showed that additional tiotropium therapy improved asthma patients’ lung function (mean FEV1 increase approximately 100 mL greater than the placebo effect) and reduced their number of exacerbations. These patients had had persistent symptoms and at least one exacerbation treated with systemic corticosteroids in the last 12 months despite high-dose ICS/LABA therapy (ICS dose ≥800 ≥g budesonide equivalent) (28, 29). Tiotropium was therefore approved for asthma patients in Germany, with this restriction (medium- or high-dose ICS/LABA combination therapy, at least one exacerbation treated with systemic corticosteroids in the last 12 months), in September 2014. No clinical trials of other anticholinergics (glycopyrronium, aclidinium, umeclidinium) have yet been published for the indication severe asthma.
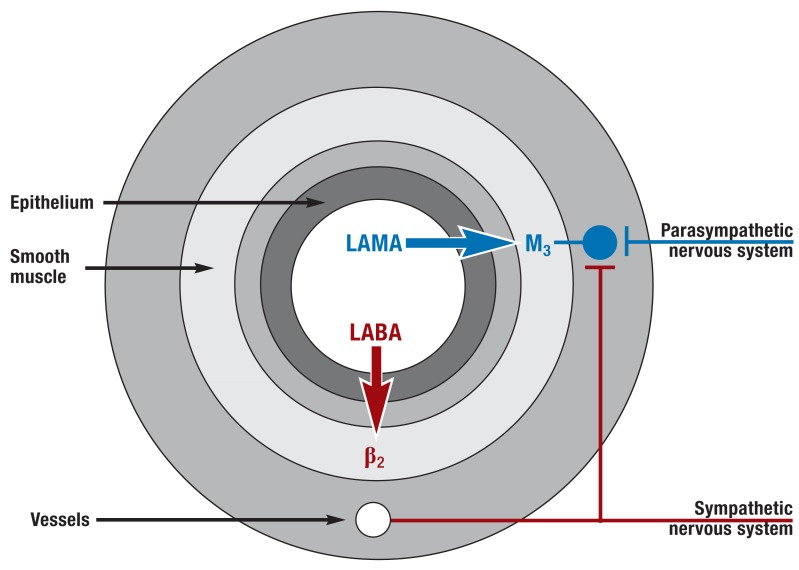
Figure 2.
Neuronal control of airway tone and effect of bronchodilators (simplified).
In parasympathetic ganglia in the airway wall, preganglionic parasympathetic nerve fibers (from the vagus nerve) synapse on postganglionic parasympathetic neurons that lead to bronchoconstriction by releasing acetylcholine (mediated in particular by the M3 receptor on the airway smooth muscle). Postganglionic sympathetic fibers do not innervate the human airway smooth muscle but affect airway tone indirectly (via innervation of the parasympathetic ganglia and control of vessel permeability and catecholamine release). Long-acting muscarinic antagonists (LAMAs) lead to bronchodilation by inhibiting muscarinic receptors (particularly the M3 receptor). Long-acting beta 2 agonists (LABAs) cause bronchodilation in particular by stimulating beta 2 receptors on the smooth muscle of the airways (27)
Anti-IgE therapy
For patients with severe allergic asthma, additional treatment with the anti-IgE antibody omalizumab leads to a reduction (by 50%) in the number of severe exacerbations, improved asthma control and quality of life (30), and reduced need for corticosteroids: the average daily dose of prednisolone falls from 15.5 mg to 5.8 mg (31). Omalizumab therapy is safe (32) but is expensive. Omalizumab is administered subcutaneously every 2 to 4 weeks and is approved for the treatment of asthma, provided the following conditions are all met:
Persistent symptoms and recurrent exacerbations despite high-dose ICS/LABA therapy
FEV1 <80%
Sensitization to perennial airborne allergens
Total serum IgE between 30 and 1500 kU/L (although the upper limit is lower for body weights above 50 kg: see summary of product characteristics).
If there is no improvement within four months of therapy, a subsequent response is unlikely (7). Omalizumab can be as effective in intrinsic asthma (patients with no evidence of allergy) as in allergic asthma (3) but is approved only for the latter. Such off-label use of omalizumab to treat intrinsic asthma should be evaluated on a case-by-case basis in an experienced asthma center.
Macrolides
Because of their immunomodulating effects, the use of macrolides in asthma has been discussed for years. In a clinical trial, azithromycin (3 × 250 mg per week; initially 250 mg per day for five days) reduced the risk of an exacerbation by 46% in severe noneosinophilic asthma, but not in eosinophilic asthma (33). Because this therapy has potential serious side effects (ototoxicity, QT interval prolongation, macrolide resistance), and because there is only one positive trial, the current ERS/ATS consensus paper gives a conditional recommendation against long-term macrolide therapy for severe asthma (7). As there are no specific alternative treatment options for noneosinophilic asthma, however, we believe that azithromycin therapy can be considered as a last resort if a patient with a low eosinophil count (less than 0.2 × 109/L in the absence of systemic corticosteroid therapy) suffers from frequent exacerbations.
Potential future treatment options
The phosphodiesterase 4 inhibitor roflumilast has shown clinical efficacy in asthma (34), but trials exploring the role of roflumilast as an additional treatment option for severe asthma are lacking. Despite some positive clinical studies, thermoplasty (endobronchial radiofrequency ablation through a dedicated catheter) should be performed only within clinical trials or independent registries (7). The use of antibodies targeting interleukin-5 (mepolizumab, reslizumab) or its receptor (benralizumab) is currently being investigated in phase III trials in patients with eosinophilic asthma (14, 15, 35). As yet, patients can only be treated with anti-IL5 antibodies or anti-IL5 receptor antibodies as part of clinical trials. This treatment is expected to be approved in Germany for severe eosinophilic asthma in the near future. Th2 cytokine antagonists (lebrikizumab, tralokinumab, dupilumab) are currently undergoing clinical development (35).
Requirements for treatment optimization
The treatment of patients with severe asthma requires experience, is time-consuming, and often necessitates off-label drug use. As a result, severe asthma is sometimes inadequately diagnosed and treated (36). In our view, this situation makes the following desirable:
Inclusion of as many patients as possible in severe asthma registries such as www.german-asthma-net.de
Better coverage of the diagnosis and treatment of severe asthma in physicians’ training
Assessment of patients with severe asthma in specialized asthma centers, in order to give them the opportunity to participate in clinical trials.
Key Messages.
A precise definition of the term “severe asthma” was proposed by a task force of the ERS and ATS in 2014.
The basic steps in the treatment of severe asthma are confirmation of the diagnosis, treatment for comorbidities, elimination of persistent triggers, and optimization of adherence.
Rehabilitation and tiotropium, omalizumab, or azithromycin therapy are additional treatment options for severe asthma.
It is expected that antibodies targeting interleukin-5 or its receptor will be approved for the treatment of severe eosinophilic asthma in the near future.
There is a need for research on the prevalence of severe asthma, for asthma competence centers, and for continuing education for physicians.
Acknowledgments
Translated from the original German by Caroline Devitt, M.A.
Footnotes
Conflict of interest statement
Prof. Lommatzsch has received consultancy and lecture fees and reimbursement of travel and participation costs from Allergopharma, Astra Zeneca, Bencard, Berlin-Chemie, Boehringer-Ingelheim, Chiesi, GSK, Janssen-Cilag, MSD, Mundipharma, Novartis, Nycomed/Takeda, TEVA, and UCB. He has also received fees for commissioned clinical trials from Astra Zeneca and research funding from GSK.
Prof. Virchow has received consultancy and lecture fees and reimbursement of travel and participation costs from Allergopharma, Astra Zeneca, Avontec, Bayer, Bencard, Berlin-Chemie, Bionorica, Boehringer-Ingelheim, Chiesi, Essex/Schering-Plough, GSK, Janssen-Cilag, Leti, MEDA, Merck, MSD, Mundipharma, Novartis, Nycomed/Takeda, Pfizer, Revotar, Roche, Sanofi-Aventis, Sandoz-Hexal, Stallergens, TEVA, UCB, and Zydus/Cadila. He has also received research funding from GSK and MSD.
References
- 1.Sears MR. Trends in the prevalence of asthma. Chest. 2014;145:219–225. doi: 10.1378/chest.13-2059. [DOI] [PubMed] [Google Scholar]
- 2.Rackemann FM. A clinical study of one hundred and fifty cases of bronchial asthma. Arch Intern Med. 1918;12:517–552. [Google Scholar]
- 3.Lommatzsch M, Korn S, Buhl R, Virchow JC. Against all odds: Anti-IgE for intrinsic asthma? Thorax. 2014;69:94–96. doi: 10.1136/thoraxjnl-2013-203738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jia CE, Zhang HP, Lv Y, et al. The asthma control test and asthma control questionnaire for assessing asthma control: Systematic review and meta-analysis. J Allergy Clin Immunol. 2013;131:695–703. doi: 10.1016/j.jaci.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 5.Rowe BH, Voaklander DC, Wang D, et al. Asthma presentations by adults to emergency departments in Alberta, Canada: A large population-based study. Chest. 2009;135:57–65. doi: 10.1378/chest.07-3041. [DOI] [PubMed] [Google Scholar]
- 6.Bousquet J, Mantzouranis E, Cruz AA, et al. Uniform definition of asthma severity, control, and exacerbations: Document presented for the World Health Organization consultation on severe asthma. J Allergy Clin Immunol. 2010;126:926–938. doi: 10.1016/j.jaci.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 7.Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343–373. doi: 10.1183/09031936.00202013. [DOI] [PubMed] [Google Scholar]
- 8.Bush A, Pavord ID. Omalizumab: NICE to USE you, to LOSE you NICE. Thorax. 2013;68:7–8. doi: 10.1136/thoraxjnl-2012-202969. [DOI] [PubMed] [Google Scholar]
- 9.Lommatzsch M, Virchow JC. Asthma-Diagnostik: aktuelle Konzepte und Algorithmen. Allergologie. 2012;35:454–467. [Google Scholar]
- 10.To T, Stanojevic S, Moores G, et al. Global asthma prevalence in adults: Findings from the cross-sectional world health survey. BMC public health. 2012;12 doi: 10.1186/1471-2458-12-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vamos M, Kolbe J. Psychological factors in severe chronic asthma. Aust N Z J Psychiatry. 1999;33:538–544. doi: 10.1080/j.1440-1614.1999.00591.x. [DOI] [PubMed] [Google Scholar]
- 12.Heaney LG, Conway E, Kelly C, Gamble J. Prevalence of psychiatric morbidity in a difficult asthma population: Relationship to asthma outcome. Respir Med. 2005;99:1152–1159. doi: 10.1016/j.rmed.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 13.Katz LE, Gleich GJ, Hartley BF, Yancey SW, Ortega HG. Blood eosinophil count is a useful biomarker to identify patients with severe eosinophilic asthma. Ann Am Thorac Soc. 2014;11:531–536. doi: 10.1513/AnnalsATS.201310-354OC. [DOI] [PubMed] [Google Scholar]
- 14.Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371:1198–1207. doi: 10.1056/NEJMoa1403290. [DOI] [PubMed] [Google Scholar]
- 15.Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe eosinophilic asthma (DREAM): A multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380:651–659. doi: 10.1016/S0140-6736(12)60988-X. [DOI] [PubMed] [Google Scholar]
- 16.Dweik RA, Boggs PB, Erzurum SC, et al. An official ATS clinical practice guideline: Interpretation of exhaled nitric oxide levels (FeNO) for clinical applications. Am J Respir Crit Care Med. 2011;184:602–615. doi: 10.1164/rccm.9120-11ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buhl R, Berdel D, Criee CP, et al. [Guidelines for diagnosis and treatment of asthma patients] Pneumologie. 2006;60:139–177. doi: 10.1055/s-2005-919153. [DOI] [PubMed] [Google Scholar]
- 18.Abramson MJ, Puy RM, Weiner JM. Injection allergen immunotherapy for asthma. Cochrane Database Syst Rev. 2010 doi: 10.1002/14651858.CD001186.pub2. CD001186. [DOI] [PubMed] [Google Scholar]
- 19.Kleine-Tebbe J, Bufe A, Ebner C, et al. Die spezifische Immuntherapie (Hyposensibilisierung) bei IgE-vermittelten allergischen Erkrankungen. Allergo J. 2009;18:508–537. [Google Scholar]
- 20.Castro M, King TS, Kunselman SJ, et al. Effect of vitamin D3 on asthma treatment failures in adults with symptomatic asthma and lower vitamin D levels: The VIDA randomized clinical trial. JAMA. 2014;311:2083–2091. doi: 10.1001/jama.2014.5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van den Berge M, ten Hacken NH, van der Wiel E, Postma DS. Treatment of the bronchial tree from beginning to end: Targeting small airway inflammation in asthma. Allergy. 2013;68:16–26. doi: 10.1111/all.12062. [DOI] [PubMed] [Google Scholar]
- 22.Dias-Junior SA, Reis M, de Carvalho-Pinto RM, Stelmach R, Halpern A, Cukier A. Effects of weight loss on asthma control in obese patients with severe asthma. Eur Respir J. 2014;43:1368–1777. doi: 10.1183/09031936.00053413. [DOI] [PubMed] [Google Scholar]
- 23.Bousquet J, Schunemann HJ, Samolinski B, et al. Allergic rhinitis and its impact on asthma (ARIA): Achievements in 10 years and future needs. J Allergy Clin Immunol. 2012;130:1049–1062. doi: 10.1016/j.jaci.2012.07.053. [DOI] [PubMed] [Google Scholar]
- 24.Kolbe J, Vamos M, Fergusson W. Socio-economic disadvantage, quality of medical care and admission for acute severe asthma. Aust N Z J Med. 1997;27:294–300. doi: 10.1111/j.1445-5994.1997.tb01981.x. [DOI] [PubMed] [Google Scholar]
- 25.Wainwright NW, Surtees PG, Wareham NJ, Harrison BD. Psychosocial factors and incident asthma hospital admissions in the EPIC-Norfolk cohort study. Allergy. 2007;62:554–560. doi: 10.1111/j.1398-9995.2007.01316.x. [DOI] [PubMed] [Google Scholar]
- 26.Rochester CL, Fairburn C, Crouch RH. Pulmonary rehabilitation for respiratory disorders other than chronic obstructive pulmonary disease. Clin Chest Med. 2014;35:369–389. doi: 10.1016/j.ccm.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 27.Proskocil BJ, Fryer AD. Beta2-agonist and anticholinergic drugs in the treatment of lung disease. Proc Am Thorac Soc. 2005;2:305–310. doi: 10.1513/pats.200504-038SR. discussion 11-2. [DOI] [PubMed] [Google Scholar]
- 28.Kerstjens HA, Disse B, Schroder-Babo W, et al. Tiotropium improves lung function in patients with severe uncontrolled asthma: A randomized controlled trial. J Allergy Clin Immunol. 2011;128:308–314. doi: 10.1016/j.jaci.2011.04.039. [DOI] [PubMed] [Google Scholar]
- 29.Kerstjens HA, Engel M, Dahl R, et al. Tiotropium in asthma poorly controlled with standard combination therapy. N Engl J Med. 2012;367:1198–1207. doi: 10.1056/NEJMoa1208606. [DOI] [PubMed] [Google Scholar]
- 30.Humbert M, Beasley R, Ayres J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy. 2005;60:309–316. doi: 10.1111/j.1398-9995.2004.00772.x. [DOI] [PubMed] [Google Scholar]
- 31.Braunstahl GJ, Chen CW, Maykut R, Georgiou P, Peachey G, Bruce J. The experience registry: the ’real-world’ effectiveness of omalizumab in allergic asthma. Respir Med. 2013;107:1141–1151. doi: 10.1016/j.rmed.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 32.Busse W, Buhl R, Fernandez Vidaurre C, et al. Omalizumab and the risk of malignancy: Results from a pooled analysis. J Allergy Clin Immunol. 2012;129:983–989. doi: 10.1016/j.jaci.2012.01.033. [DOI] [PubMed] [Google Scholar]
- 33.Brusselle GG, Vanderstichele C, Jordens P, et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): A multicentre randomised double-blind placebo-controlled trial. Thorax. 2013;68:322–329. doi: 10.1136/thoraxjnl-2012-202698. [DOI] [PubMed] [Google Scholar]
- 34.Bousquet J, Aubier M, Sastre J, et al. Comparison of roflumilast, an oral anti-inflammatory, with beclomethasone dipropionate in the treatment of persistent asthma. Allergy. 2006;61:72–78. doi: 10.1111/j.1398-9995.2005.00931.x. [DOI] [PubMed] [Google Scholar]
- 35.Hambly N, Nair P. Monoclonal antibodies for the treatment of refractory asthma. Curr Opin Pulm Med. 2014;20:87–94. doi: 10.1097/MCP.0000000000000007. [DOI] [PubMed] [Google Scholar]
- 36.Sachverständigenrat zur Begutachtung der Entwicklung im Gesundheitswesen. www.svr-gesundheit.de, Gutachten 2000/2001, Kurzfassungen Band III (Über-, Unter- und Fehlversorgung), Abschnitt 10.3.2 Asthma [Google Scholar]
- 37.Kowalski ML, Makowska JS, Blanca M, et al. Hypersensitivity to nonsteroidal anti-inflammatory drugs (NSAIDs)—classification, diagnosis and management: Review of the EAACI/ENDA(#) and GA2LEN/HANNA*. Allergy. 2011;66:818–829. doi: 10.1111/j.1398-9995.2011.02557.x. [DOI] [PubMed] [Google Scholar]
- 38.Patterson K, Strek ME. Allergic bronchopulmonary aspergillosis. Proc Am Thorac Soc. 2010;7:237–244. doi: 10.1513/pats.200908-086AL. [DOI] [PubMed] [Google Scholar]
- 39.Bosch X, Guilabert A, Espinosa G, Mirapeix E. Treatment of antineutrophil cytoplasmic antibody associated vasculitis: A systematic review. JAMA. 2007;298:655–669. doi: 10.1001/jama.298.6.655. [DOI] [PubMed] [Google Scholar]
- 40.Quanjer PH, Brazzale DJ, Boros PW, Pretto JJ. Implications of adopting the global lungs initiative 2012 all-age reference equations for spirometry. Eur Respir J. 2013;42:1046–1054. doi: 10.1183/09031936.00195512. [DOI] [PubMed] [Google Scholar]