Abstract
Purpose
The aqueous humor nourishes the avascular tissues of the anterior segment, and the trabecular meshwork (TM) plays a role in the efflux of endogenous substances and xenobiotics from the aqueous humor. ATP (ATP)-binding cassette (ABC) transporter superfamily members respond to stressors such as hypoxia, cytokine signaling, and aging. The innate immune system within the TM, particularly Toll-like receptor 4 (TLR4) and its ligands, e.g., low-molecular-weight hyaluronic acid (LMW-HA) and lipopolysaccharide (LPS), plays a significant role in maintaining a normal environment in the anterior chamber. We hypothesize that the innate immune system may interact with ATP-binding cassette sub-family members ABCB1 (p-glycoprotein and multidrug resistance protein 1) to detoxify xenobiotics from the aqueous humor and in the TM.
Methods
Cell lysates of human TM cells, RAW 264.7 macrophages, and PC12 cells were subjected to western blot analysis. The TM cells were positive for TLR4, ABCB1, and CYP3A5 and were negative for the ABCC1 transporter. Human TM cells and RAW 264.7 macrophages were plated on eight-well chamber slides at 5,000 cells/well overnight in 10% fetal bovine serum (FBS) cell growth medium. The medium was changed to 0.1% FBS 2 h before treatment. Cells were challenged with 1 and 10 mM lactate, 100 ng LMW-HA (20 kDa), 100 ng high-molecular-weight HA (HMW-HA, 1,000 kDa), 100 ng LPS, and/or 100 μM naloxone for 0.5, 1, 2, and 4 h. Calcein acetyoxymethyl ester (calcein AM; 0.25 μM) was added for 30 min as the reporting molecule. After calcein AM was administered, it was cleaved by an esterase into a fluorescent product that is normally transported out of the cell by ABCB1. Positive controls were 100 μM verapamil and 50 μM digoxin. After the challenge, the TM cells were fixed at 4 °C in 3% paraformaldehyde for 15 min, mounted with Vectashield and 4',6-diamidino-2-phenylindole (DAPI) mounting medium, and analyzed by a masked observer using a Leica confocal microscope and software.
Results
Verapamil, an ABCB1 inhibitor, significantly (p<0.001) increased fluorescent calcein retention in the cytoplasm of the TM and RAW 264.7 cells compared to the PBS control. Digoxin, an ABCB1 activator, increased calcein efflux (p<0.001). Lactate reduced ABCB1 activity. HMW-HA significantly (p<0.001) reduced ABCB1 activity, whereas LMW-HA decreased ABCB1 activity, and the HA effects were blocked by naloxone (p<0.001), a TLR4 inhibitor. LPS alone did not change ABCB1 activity whereas dephosphorylated LPS significantly (p<0.001) enhanced ABCB1 activity in the TM cells. β-amyloid significantly reduced ABCB1 activity, and the β-amyloid effects were blocked by naloxone.
Conclusions
TM cells are responsive to ABCB1 inhibitors and activators. ABCB1 functional activity is affected by TLR4 agonists suggesting that modulation of TLR4 is important in ABCB1 function. The innate immune inflammatory response in the TM may play a role in the ABCB1 detoxification of potentially harmful constituents in the aqueous humor.
Introduction
Primary open-angle glaucoma (POAG) is a common neurodegenerative disease characterized clinically by optic nerve cupping and visual field loss [1]. Age, higher intraocular pressure (IOP), and worse visual field status at a patient’s baseline examination are important risk factors for developing blindness in POAG [2]. IOP is regulated primarily by fluid resistance to aqueous humor outflow in the trabecular meshwork (TM) [3]. The TM functions as a one-way, low-flow, self-cleaning filter with an approximate two-third functional reserve [4]. The TM cell population decreases as an individual ages [4]. Dysregulated aqueous humor outflow causes increased IOP [5]. In addition to TM dysregulation, POAG also has systemic features [6]. A recent National Eye Institute goal is to identify biomarkers of POAG and explore new therapeutic approaches [7].
One target area for neurodegenerative diseases is the cellular mechanisms for removing and clearing potentially toxic compounds. The two most important ATP binding cassette (ABC) transporters are ABCB1 (multidrug resistance protein 1, p-glycoprotein) and ABCC1 (multidrug resistance-associated protein 1). ABCB1 and ABCC1 (collectively multidrug resistance [MDR] proteins) transport a wide variety of endogenous substances and xenobiotics across extra- and intracellular membranes [8]. Certain toxic xenobiotics in cells may be hydroxylated or formed into an epoxide by phase 1 enzymes (cytochrome P450) and eliminated by ABCB1 as a metabolite. ABCB1, then, potentially acts to detoxify TM cells. There are 48 ABC genes in the human genome representing seven subfamilies based on the sequence and organization of their ATP-binding domains. The ATP-binding domains have characteristic motifs (Walker A and B) and a signature motif (C). The large number of ABC genes and the strong sequence homology suggest functional redundancy, i.e., substrate specificity overlap [9].
Typically, ABC transporters are unidirectional and move compounds from the cytoplasm to the outside of the cell or into an intercellular compartment, e.g., the endoplasmic reticulum and mitochondria, to detoxify or protect cells from potentially toxic substances [10]. TM cells are likely to remove metabolites and xenobiotics from the aqueous humor and protect intraocular structures from potentially toxic compounds [11]. In circulating leukocytes, upregulation of ABCB1 may play a role in vascular deregulation in the pathogenesis of POAG [12]. MDR proteins respond to stressors such as hypoxia, cytokine signaling, increased pressure, mechanical stretch, and aging [13]. ABCB1 expression and function are controlled by hyaluronic acid (HA) and the HA receptor CD44 [14], both of which are altered in POAG as reported by our laboratory [15-17]. Dysregulation of HA and CD44 interaction could result in decreased MDR protein activity and consequently increased cell vulnerability to stress [18].
The innate immune system [19] within the TM, particularly TLR4 and its ligands—low-molecular-weight hyaluronic acid (LMW-HA) and lipopolysaccharide (LPS)—may play a significant role in maintaining a normal environment in the anterior chamber. We hypothesize that the innate immune system affects MDR proteins, altering the efflux of toxic proteins, such as β-amyloid and soluble CD44, from TM cells and maintaining the normal milieu of the aqueous humor. The purpose of this study was to test whether TM cells respond to several known agonists and inhibitors of TLR4 and MDR proteins in vitro.
Methods
Multidrug resistance proteins
Wagner et al. [20] profiled the gene expression of ten normal ocular tissues—the cornea, TM, iris, lens, sclera, choroid/retinal pigment epithelium, retina, optic nerve head, and optic nerve. The expression values of each tissue are available online in the Ocular Tissue Database. The level of expression of multidrug resistance proteins, their respective ABC transporters, and probe set as determined with Affymetrix Human Exon 1.0 ST arrays (Affymetrix, Inc., Santa Clara, CA), and Probe Logarithmic Intensity Error (PLIER) values are displayed in Table 1. These arrays enable two levels of analyses—gene expression and alternate splicing. The microarrays are useful for determining which gene is present in a tissue by detecting specific mRNA. PLIER values are a means of better expressing the signals of gene expression observed in a microarray; these values are determined by several variables, including the number of perfect match pairs of RNA, the number of mismatch pairs of RNA, the binding affinity of the probe, and the concentration of RNA in the sample [21]. The statistical significance of the PLIER numbers was determined by deriving the z-score as in the methods described by Wagner et al. [20]; thus, any values greater than 30 were deemed statistically significant. The most relevant tissues for POAG are the ciliary body, optic nerve, TM, and retina (Table 1).
Table 1. Gene expression of human ABC transporters in ocular tissues.
| Gene | Alias | Probeset | Trabecular meshwork | Ciliary body | Optic nerve | Optic nerve head |
|---|---|---|---|---|---|---|
| ABCB1 |
P-glycoprotein/MDR1 |
3,060,182 |
33.27 |
23.14 |
30.13 |
45.95 |
| ABCC1 |
MRAP-1 |
3,649,890 |
28.76 |
26.45 |
26.75 |
24.73 |
| CYP3A5 | Cytochrome P450 3A5 | 3,063,406 | 31.92 | 32.63 | 28.74 | 37.14 |
Level of expression of ABC transporters was determined from the Ocular Tissue Database, a collection of data using Affymetrix Human Exon 1.0 ST arrays. Probe Logarithmic Intensity ERror (PLIER) values were used to determine significance of expression for ABCB1 and ABCC1 transporters. *Remaining 46 human ABC transporters were determined to have notable expression using a threshold ≥30 PLIER value.
Cell cultures
The study adhered to the tenets of the Declaration of Helsinki, ARVO statement on human subjects and our institution's guidelines. Human TM cells derived from four healthy, postmortem eyebank (Illinois Eye Bank, Midwest Eyebanks, Chicago, IL) donors (20, 33, 34, and 49 years of age) were obtained and grown in culture and used between the second and fourth passage as previously described [22]. Mouse-transformed macrophage RAW264.7 cells and rat pheochromocytoma cells (PC12) were purchased from American Type Culture Collection (Manassas, VA). Cells were grown to confluency in 10% fetal bovine serum (FBS) in T-25 flasks (Becton Dickinson, Franklin Lakes, NJ), and then harvested for experimentation. Briefly, cells were plated at a density of 5,000 cells in 200 µl Dulbecco's modified Eagle's medium (DMEM, Gibco, Grand Island, NY) with 10% FBS per well on Lab-Tek II eight-well chamber slides (Cole Palmer, Vernon Hills, IL).
Agonist and antagonists
The medium in the TM cell–plated chamber slides was changed from 10% FBS to 0.1% FBS medium 2 h before treatment. Cells were then challenged with 1 and 10 μM lactate (Sigma-Aldrich, St. Louis, MO), 100 ng LMW-HA (20 kDa, Creative PEGWorks, Winston Salem, NC), 100 ng HMW-HA (1000 kDa, Hyalose, Oklahoma City, OK), 100 ng LPS (Sigma-Aldrich), 1 μg/ml β-amyloid 1–42 (Life Technologies, Carlsbad, CA), 0.1, 1, and 10 μg CD44 neutralizing antibody (Ancell, Bayport, MN), and/or 100 μM naloxone (Sigma-Aldrich) for 0.5, 1, 2, and 4 h. Dephosphorylated lipopolysaccharide (LPS-p) was obtained by incubating 5 µg LPS (Sigma-Aldrich) with 30 units of calf intestinal alkaline phosphatase (CIAP; Life Technologies) at 37 °C for 1 h followed by an additional 30 units of CIAP incubation for another 1 h.
Calcein AM assay
ABCB1 activity was determined by the addition of calcein acetyoxymethyl ester (calcein AM; Molecular Probes, Eugene, OR) at a final concentration of 0.25 µM to the culture media for 30 min at 37 °C in 5% CO2 according to Boraldi’s method [23]. Calcein AM is freely diffused into cells and hydrolyzed by esterases into a fluorescent calcein. If the ABCB1 transporter is active, hydrophobic calcein AM is pumped out of the cell before it can be hydrolyzed. Verapamil (100 μM; Life Technologies), a calcium channel blocker, and digoxin (50 μM; Sigma-Aldrich) were used as controls for modulating ABCB1 activity. Verapamil, digoxin, and all other experimental compounds were added 30 min before calcein AM was added. If ABCB1 is less active, calcein fluorescence is increased in the cells. At selected time points, the cells were briefly washed twice with phosphate buffered saline (PBS, 10 mM phosphate buffer, 2.7 mM potassium chloride and 137 mM sodium chloride, pH 7.4, Sigma-Aldrich), fixed in 3% paraformaldehyde in PBS for 15 min, washed twice in PBS, and mounted in medium with 4',6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, CA).
Western blot
Human TM, PC12, and RAW264.7 cells were grown in DMEM containing 10% FBS until confluent. The cells were washed twice with PBS and incubated in DMEM containing 0.1% FBS for 24 h. The media were aspirated. The cells were washed with cold PBS, scraped from the flask, and collected. Cells were subjected to lysis buffer (Sigma-Aldrich) containing 1% Triton X-100 and 1% protease inhibitor cocktail (Sigma-Aldrich). Cell lysates were analyzed for protein content (Pierce, Rockford, IL), resolved with sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE), and then transferred to polyvinylidene fluoride (PVDF) membranes with electrophoresis. Blots were blocked with 5% fat-free dry milk in Tris-buffered saline-Tween (TBST) buffer for 1 h and then incubated overnight with mouse ABCB1 antibody (Santa Cruz Biotechnology, Dallas, TX; 1:1,000 dilution), mouse ABCC1 antibody (Novus Biologics, Littleton, CO; 1:500 dilution), mouse TLR4 antibody (R and D, Minneapolis MN; 1:1,000), or CYP3A5 (Thermo Scientific, Rockford, IL; 1:500). The membranes were washed with TBST and incubated with secondary horseradish conjugated goat anti-mouse antibody (Genetex, Irvine, CA; 1:3,000). Blots were visualized in an imager (ImageQuant 4000, GE Healthcare, Piscaway, NJ) using Clarity Western ECL substrate (Bio-Rad; Hercules, CA, 1:3,000). To ensure equal protein loading, the same blot was developed for β-actin (Sigma-Aldrich; 1:3,000) as a loading control.
Confocal microscopy
Cells were observed in multiple fields by a masked observer under confocal laser scanning microscope (Leica SP2; Wetzlar, Germany). Optical sections of at least 10 cells for each treatment condition were captured in the center of each cell. Single images were obtained using 40× and 63× objectives. Quantification of calcein retention was determined by the fluorescence intensity within a single cell using Leica software. The mean fluorescence intensity in the cytoplasm was outlined on images of individual cells using the Leica software. The mean fluorescence intensity was measured in pixels per unit area per cell minus the background intensity. All treatments were performed in triplicate trials.
Statistical analysis
All data were normalized to PBS-treated controls and expressed as the mean of fluorescence intensity values ± standard error of the mean (SEM). First, one-way ANOVA, followed by Tukey’s multiple comparison test, for each experimental group was used to compare three or more treatments. An unpaired Student t test was used to compare when only two treatments were present. p<0.05 was regarded as statistically significant, and calculated with statistical analysis software packages (Prism 6 [GraphPad Software, LaJolla, CA]).
Results
Western blot
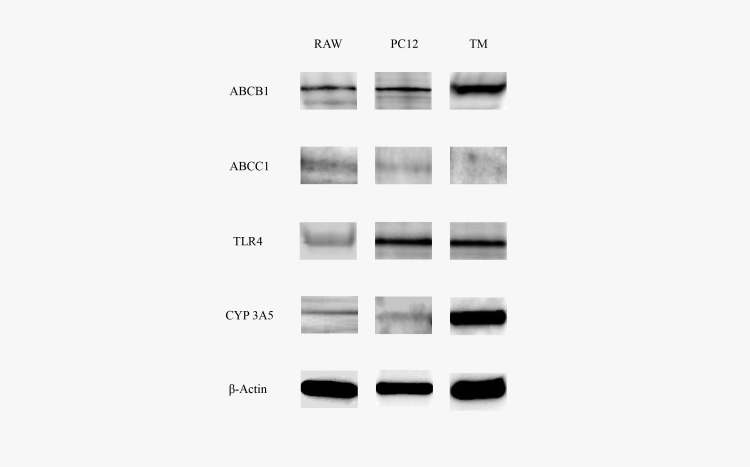
The ABCB1 protein expression was verified with western blot analysis that showed an immunoreactive protein band for ABCB1 in the TM, PC12, and RAW cells at the expected molecular weight of 140 kDa (Figure 1). ABCC1 was not detected with western blot analysis in cultured TM cells. TLR4 protein expression was also verified with western blot analysis that showed a protein band for TLR in TM, PC12, and RAW cells at the expected molecular weight of 95 kDa (Figure 1). CYP3A5 protein expression was detected with western blot analysis in TM cells.
Figure 1.
Western blots of ABCB1, ABCC1, TLR4, and CYP3A5. Cell lysates (10 µg protein load) of RAW 264.7 mouse monocyte cells (RAW), rat pheochromocytoma cells (PC12), and trabecular meshwork (TM) cells were resolved with sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE). β-actin (10 µg protein load) was used as a loading control for each cell lysate. Data represent a representative western blot of replicate samples.
Calcein AM assay
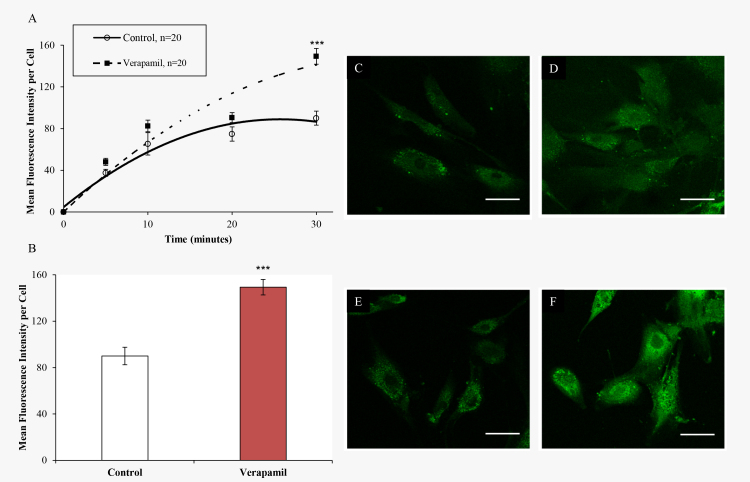
The rate of accumulation of calcein in TM cells was determined with a time course study from 5 to 30 min. In the PBS control, the accumulation of calcein reached a plateau at 30 min (Figure 2). Verapamil, an ABCB1 inhibitor, was used as a negative control in determining the accumulation rate. Treatment with verapamil significantly increased calcein retention in TM cells at 30 min. Based on these results, 30 min was determined to be sufficient for intercellular accumulation of calcein for the rest of the experiments.
Figure 2.
Time course of calcein AM accumulation in TM cells evaluated with confocal microscopy. A: The calcein retention rate within trabecular meshwork (TM) cells following PBS (control) or 100 µM verapamil treatment. B: Mean fluorescence intensity in PBS (control) and verapamil treated cells at the 30 min time point. Confocal microscopy images were obtained in the middle of the TM cells with optical sectioning. C: PBS control at 5 min D: PBS control at 30 min time point. E: Verapamil at 5 min. F: Verapamil at 30 min time point. Error bars are presented as ± standard error of the mean (SEM), ***p<0.001.
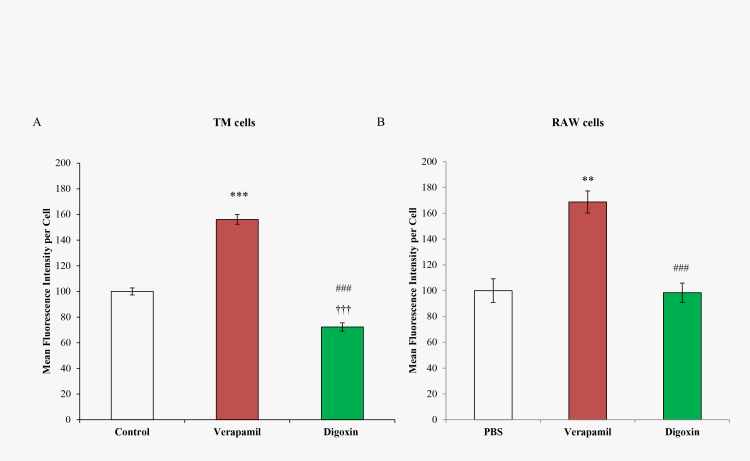
To compare the relative ABCB1 activity, calcein retention was examined in TM and RAW cells under various treatment conditions (see Figure 3). TM cells treated with 100 μM verapamil increased calcein retention to 156.1±3.8 units (p<0.0001) compared to the PBS control, while treatment with 50 μM digoxin, an ABCB1 activator, decreased calcein retention to 72.3±3.2 units (p<0.001). The difference between verapamil and digoxin was significant (p<0.0001). Similarly, RAW cells with verapamil treatment increased calcein retention to 168.8±5.9 units, which was significant compared to the PBS control (p<0.0001). Calcein retention was only slightly decreased after treatment with digoxin to 98.5±5.1 units, but it was significant relative to the verapamil-treated cells (p<0.0001).
Figure 3.
Calcein AM assay following verapamil and digoxin treatment in TM and RAW 264.7 macrophage (RAW) cells. (A) Trabecular meshwork (TM) cells and (B) RAW cells were treated with PBS (control), 100 µM verapamil, and 50 µM digoxin. Error bars are presented as ± standard error of the mean (SEM), **p<0.0005, ***p<0.0001 verapamil compared with PBS (control); †††p<0.001 digoxin compared with PBS (control); ###p<0.0001 digoxin compared with verapamil.
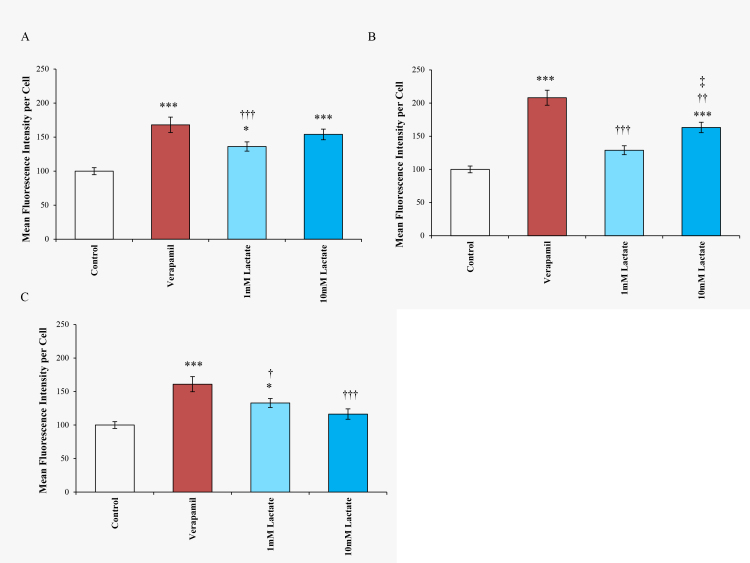
Lactate was added to cells to simulate oxidative stress [24]. Compared to the PBS control, the 1 mM concentration of lactate treatment increased relative calcein retention to a value of 136.2±6.8 units (p<0.005), while 10 mM lactate increased it to 153.9±6.3 units (p<0.0001) at the 1 h time point. After 3 and 6 h of 1 and 10 mM treatment, calcein retention remained greater than the control (see Figure 4).
Figure 4.
Time course of calcein AM assay following lactate treatment in TM cells. The mean fluorescence intensity values of cells treated with 100 µM verapamil, 1 mM, and 10 mM lactate, and PBS (control) for (A) 1 h, (B) 3 h, and (C) 6 h are presented. Verapamil-treated trabecular meshwork (TM) cells yielded higher levels of calcein retention compared to the lactate-treated cells. Error bars are presented as ± standard error of the mean (SEM), *p<0.005, **p<0.001, ***p<0.0001 with respect to PBS (control); †p<0.05, ††p<0.01, ††† p<0.001 lactate compared to verapamil; ‡p<0.05 lactate (10 mM) compared to lactate (1 mM).
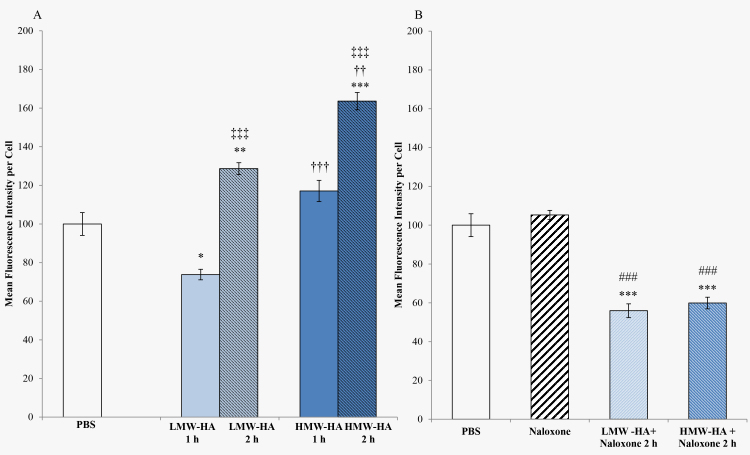
To determine whether TLR4 agonists interact with ABCB1 activity, TM cells were challenged with LMW-HA, a TLR4 agonist, as well as HMW-HA for 1 and 2h (see Figure 5A,B). The fluorescence intensity of the LMW-HA-treated TM cells was significantly lower at 1 h compared to 2 h (p<0.0001). Although HMW-HA is not recognized as a TLR4 agonist, hyaluronidases that are released by stressed or injured cells cleave HMW-HA to become LMW-HA [25]. Calcein retention was increased when TM cells were treated with HMW-HA at the 2 h time point (p<0.0001). Administration of naloxone by itself, a TLR4 antagonist, had no apparent effect on the ABCB1 activity. TLR4 receptor was blocked with coadministration of naloxone and HA, and ABCB1 efflux activity was increased, suggesting that naloxone competed for the binding of HA to the TLR4 complex. The coadministration of LMW-HA with naloxone and HMW-HA with naloxone were highly significant (p<0.0001); see Figure 5B.
Figure 5.
Calcein AM assay of TM cells following treatment with HA and naloxone. A: Trabecular meshwork (TM) cells were treated with the TLR4 agonist low-molecular-weight hyaluronic acid (LMW-HA; 100 ng) and high-molecular-weight HA (HMW-HA; 100 ng) for 1 and 2 h. B: TM cells were treated with a TLR4 inhibitor naloxone (100 µM) alone or in combination. Error bars are presented as ± standard error of the mean (SEM), *p<0.05, **p<0.005, ***p<0.0001 with respect to PBS (control), ††p<0.005, †††p<0.00005 comparing LMW-HA to HMW-HA for each corresponding time point; ‡‡‡p<0.0001 comparing LMW-HA at 1 h to LMW-HA at 2 h or HMW-HA at 1 h to HMW-HA at 2 h, ###p<0.0001 comparing LMW-HA plus naloxone or HMW-HA plus naloxone to naloxone.
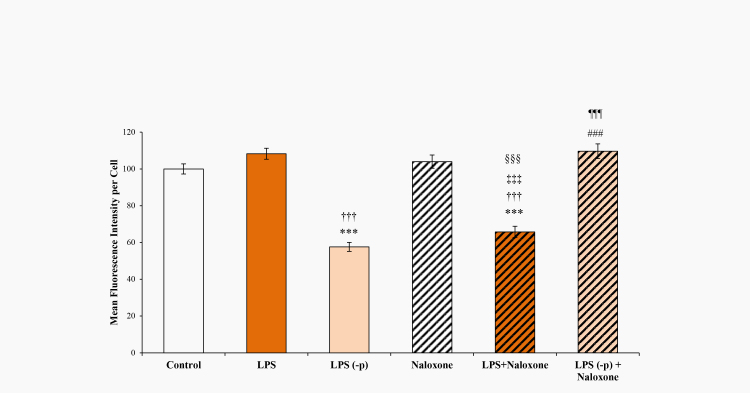
Another TLR4 agonist is LPS; its effect on ABCB1 activity is shown in Figure 6. LPS minimally affected the calcein retention in TM cells. Dephosphorylated LPS-p, however, significantly (p<0.0001) decreased calcein retention. Naloxone alone minimally affected the calcein retention; however, coadministration of LPS with naloxone significantly (p<0.0001) decreased calcein retention. In contrast, coadministration of LPS-p and naloxone reversed the effects of LPS-p; see Figure 6.
Figure 6.
Calcein AM assay of TM cells following treatment with lipopolysaccharide (LPS), dephosphorylated lipopolysaccharide (LPS-p), and naloxone. Trabecular meshwork (TM) cells were treated with TLR4 agonist lipopolysaccharide (LPS; 100 ng), dephosphorylated lipopolysaccharide (LPS-p; 100 ng), and the TLR4 inhibitor naloxone (100 µM) alone or in combination. Error bars are presented as ± standard error of the mean (SEM), ***p<0.0001 with respect to PBS (control), ††† p<0.0001 comparing LPS and LPS-p, ‡‡‡ p<0.0001 comparing LPS and LPS plus naloxone, ### p<0.0001 comparing LPS-p and LPS-p plus naloxone, §§§ p<0.0001 comparing naloxone and LPS plus naloxone, ¶¶¶ p<0.0001 comparing LPS plus naloxone and LPS-p plus naloxone.
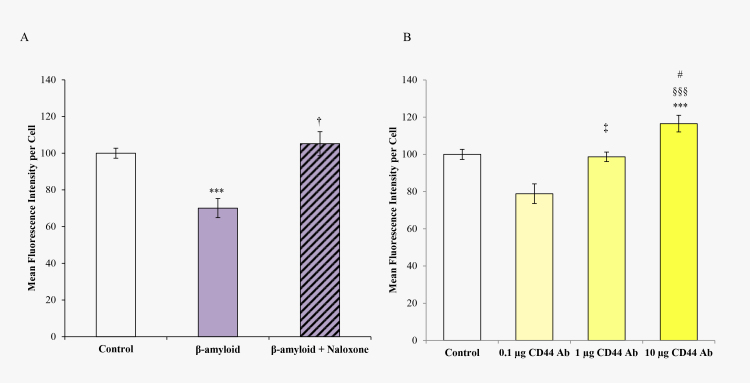
A third TLR4 agonist is β-amyloid [26]. The administration of β-amyloid significantly (p<0.05) decreased calcein retention. The coadministration of β-amyloid and naloxone reversed the effect of β-amyloid on calcein retention (Figure 7A). In addition, neutralizing CD44 antibody treatment in TM cells showed a dose-response effect. With the use of graded amounts of CD44 antibody, the lowest CD44 concentration treatment resulted in a slight decrease in retention whereas the highest CD44 concentration resulted in an increase in calcein retention (p<0.0001); see Figure 7B.
Figure 7.
Calcein AM assay of TM cells following treatment with β-amyloid, naloxone, and CD44 Ab. A: Trabecular meshwork (TM) cells were treated with β-amyloid 1–42 (200 ng) and naloxone alone or in combination B: TM cells were treated with 0.1, 1, and 10 µg CD44 neutralizing antibody (CD44 Ab). Error bars are presented as ± standard error of the mean (SEM), ***p<0.01 with respect to PBS (control), † p<0.05 comparing β-amyloid versus β-amyloid plus naloxone, ‡ p<0.05 comparing 0.1 µg CD44Ab to 1 µg CD44Ab, §§§p<0.0001 comparing 0.1 µg CD44Ab to 10 µg CD44Ab, #p<0.05 comparing 1 µg CD44Ab to 10 µg CD44Ab.
Discussion
ABC transporters are expressed in the TM as determined from the Ocular Tissue Database [20]. The mRNAs of ABCB1, ABCC1, ABCC3, ABCC4, ABCC5, and ABCC6 are expressed for relevant tissues for POAG, including the TM, ciliary body, optic nerve, and retina (Table 1) [20]. However, it is unlikely that all of these transporters are actually translated into proteins. In other studies, ABCC3, ABCC4, and ABCC5 are not present as proteins in many of the ocular tissues, including the cornea, conjunctiva, iris-ciliary body, retina, and choroid [27]. In our study, only the presence of ABCB1 in the cultured TM cells was verified with western blot analysis. To test the functional activity of ABCB1, the TM was evaluated with the calcein AM assay. Verapamil inhibited ABCB1 activity by acting as a substrate for the transporter in TM cells and was used as a positive control. Digoxin upregulates ABCB1 [28,29] efflux activity and decreases calcein retention in TM cells.
The administration of lactate to mimic metabolic stress in TM cells decreased ABCB1 activity resulting in calcein retention similar to that of verapamil. Lactate administration to TM cells decreases human TM cell viability, translocates nuclear factor-kappa B (NF-κB), activates membrane type 1-matrix metalloproteinase 1 (MT1-MMP), and sheds the ectodomain of CD44 [24]. Activation of NF-κB induces ABCB1 activity within 24 h [30,31]. The lactate stimulated release of the ectodomain of CD44, i.e., soluble CD44, may enhance ABCB1 activity [32,33]; however, at higher concentrations, soluble CD44 may inhibit ABCB1 activity. The results of the neutralizing CD44 antibody treatment are in agreement with Toole’s studies [32,33], i.e., at high CD44 antibody concentrations, ABCB1 activity was reduced.
The current study provides support for the role of TM cells in responding to selected agonists and antagonists of the TLR4 receptor of the innate immune system. TLR4 has previously been implicated in the expression of ABCB1 transporters. HA binds to a unique complex of TLR4, MD2, and CD44 in non-infectious inflammation, different from the TLR4, MD2, and CD14 complex that recognizes LPS during infection [34,35]. LMW-HA decreased calcein fluorescence intensity at 1 h but not at the 2 h time point. LMW-HA induces enhanced ABCB1 activity and competes for the CD44 receptor [33]. Although LMW-HA treatment at 1 h was transient, its effect was abated by 2 h presumably by degradation by hyaluronidases. HMW-HA increased calcein retention in TM cells after 1 and 2 h of treatment. ABCB1 is associated with CD44-HA; LMW-HA promotes ABCB1 internalization [18]. HMW-HA decreased ABCB1 activity [36], due at least in part to its degradation by hyaluronidases, thus generating LMW-HA, which decreases ABCB1 activity [4]. Exposure of TM cells to naloxone alone had no significant effect on ABCB1 activity in comparison with controls. Notably, naloxone acts as an inverse agonist or a neutral antagonist [37]. By itself, naloxone acting as a neutral antagonist may not have any effect on TLR4. Coadministration of naloxone with HA reversed the calcein retention effect of HA indicating that HA binds to the TLR4 and MD2-CD44 complex that directly or indirectly alters ABCB1 activity.
LPS is a well-characterized agonist of the TLR4 and MD2-CD14 complex. LPS alone slightly increased calcein retention. Notably, the LPS-TLR4-MD2 complex has a dissociation constant of approximately 3 nM [38]. Removal of the phosphate from LPS [39] resulted in an enhanced ABCB1 activity. Coadministration of LPS and naloxone reversed LPS effect on calcein retention. Naloxone is known to bind to MD-2 of the TLR4 complex with an apparent dissociation constant of approximately 17 µM [40]. A comparison of the dissociation constants indicate that naloxone is more tightly bound to the TLR4 complex compared with LPS [41]. Meanwhile, CD44 neutralizing antibody altered the ABCB1 activity either through HA-CD44-ABCB1 loop [33] or through the TLR4 complex. TLR4 also forms a complex of CD36-TLR4-TLR6 heterodimer that binds β-amyloid 1–42 [42]. Three ABC family members—ABCB1, ABCC1, and ABCG2—export β-amyloid [43,44]. In the current study, β-amyloid treatment led to a reduction in fluorescence intensity, opposite that observed for other agonists. Not surprisingly, β-amyloid has been shown to be cytotoxic to cells and to reduce esterase activity [45]. Coadministration of naloxone and β-amyloid 1–42 reversed this effect indicating that the effects of β-amyloid is through the TLR4-MD-2-CD44 or the TLR4-MD2-CD14 complex. Interestingly, decreased ABCB1 activity has recently been associated with Alzheimer disease [46]. ABCB1 transport of β-amyloid across the blood–brain barrier is responsible for removal of β-amyloid from the brain [43]. In MDR-null mice, β-amyloid is removed at half the rate as in wild-type mice. An inhibitor of ABCB1 causes an increase in β-amyloid concentration in brain interstitial fluid within hours of treatment. Cirrito et al. [47] suggested that decreased MDR protein activity at the blood–brain barrier could affect the risk of developing Alzheimer disease as well as provide a novel therapeutic target.
TLR4 ligands and inhibitors modulate ABCB1 activity, suggesting that the interaction with TLR4 is important in ABCB1 function. The innate immune inflammatory response may thus play a role in the TM through modulation of ABCB1, and ABCB1 is probably the key MDR protein in the TM. As shown in Figure 8, three known TLR4 agonists modulate ABCB1 activity in a calcein AM assay. The coadministration of the TLR4 antagonist blocked the effects of the TLR4 agonists. The current results indicate that TM cells are responsive to TLR4 agonists. Targeting β-amyloid in glaucoma treatment [48], amyloid fibril formation in myocilin mutation [49], and Gram-negative bacteria [50,51] is a potential new therapeutic modality since β-amyloid and LPS are agonists of the CD14-MD2-TLR4 complex. The TLR4 antagonist naloxone may be useful in decreasing the innate immune inflammatory responses as well as modulating ABCB1 function in TM cells.
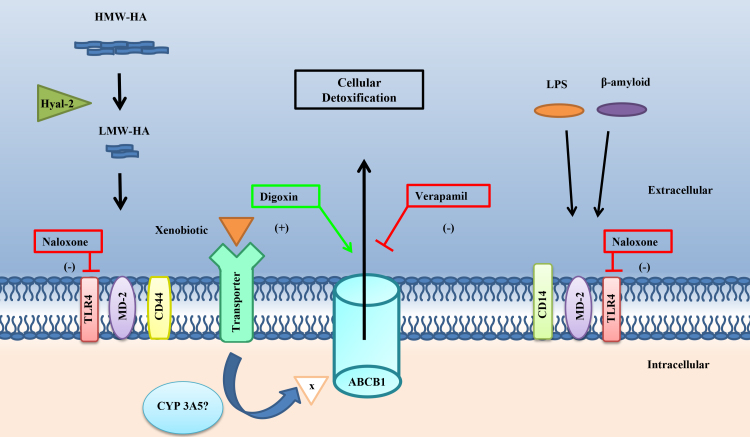
Figure 8.
Diagram depicting the possible activation of TM detoxification of xenobiotics. The schematic illustrates the multiple receptors involved in the uptake and removal of potentially toxic substances in aqueous. First, two separate signaling pathways activate TLR4. The classic CD14-MD2-TLR4 complex is activated by the agonists lipopolysaccharide (LPS) and β-amyloid. The alternate complex of CD44-MD2-TLR4 is modulated by low-molecular-weight hyaluronic acid (LMW-HA). ABCB1 is a first-line defense and is rapidly upregulated by extracellular, e.g., β-amyloid or dephosphorylated lipopolysaccharide (LPS-p), and intracellular stress arms [57]. Xenobiotics enter through several different organic anion transporter proteins including OATP, OAT, and OCT [56]. The xenobiotic is then shuttled to the endoplasmic reticulum where cytochrome P450 3A5 or other cytochrome P450 enzymes process the xenobiotic. The second step of the detoxification pathway is ABCB1 efflux on the inactivated xenobiotic. Prolonged or overwhelming stressors, e.g., lactate accumulation, aging, or cytokine stimulation, may overwhelm the ABCB1 defense mechanism leading to faulted removal of xenobiotic, activation of NF-κB [52], and cell death. Modulators of ABCB1 are digoxin, verapamil, and the TLR4 antagonist, naloxone, which inhibits both signaling pathways.
The innate immune inflammatory response in the TM may have a role in ABCB1 detoxification of potentially harmful metabolites and xenobiotics in the aqueous humor as well as in the TM. Xenobiotics enter a cell through various mechanisms; however, due to their size, many xenobiotics may need to be transported via a protein. Organic anion transporters (OATP and OAT) as well as organic cation transporters (OCTs) are several known non-specific protein transporters responsible for the uptake of various drugs as well as steroids [52]. After entering the cell, xenobiotics can be neutralized by cytochrome P450 enzymes. Within the CYP family, the CYP3A family is the most common in drug and steroid metabolism; approximately 50% of known pharmaceutical drugs are metabolized by these enzymes [53-55]. Interestingly, the CYP3A family shares many of the same metabolites as ABCB1, suggesting that after being deactivated by CYP3A enzymes, xenobiotics are removed by ABCB1 [56]. CYP3A5 was highly expressed in TM cells. One consequence of CYP3A5 activity is the generation of reactive oxidative species (ROS), which can lead to NF-κB activation [52]. Increased levels of ROS and activation of NF-κB could be factors in the progressive TM in aging and POAG. Although ABCB1 is a first line of defense, prolonged or overwhelming stressors may compromise ABCB1 function [57,58]. In addition, although ABCB1 is the primary transporter in TM, further studies are necessary to definitively identify the function of the other ABC transporters. Nonetheless, once ABCB1 defenses are breached in the TM, faulty clearance of toxic substances in the aqueous humor may lead to cellular damage and cell death to other anterior segment tissues, including the ciliary body, iris, lens, corneal endothelium as well as the TM.
In summary, TM cells express the functionally active ABCB1 transporter. The ABC transporter superfamily members respond to stressors such as hypoxia, cytokine signaling, increased pressure, mechanical stretch, and aging [59], which collectively are vital to the function of the TM. The ABCB1 transporter expression and function are regulated, in part, by HA and CD44, both of which are altered in POAG. These results support the hypothesis that MDR proteins are expressed in cells relevant to the POAG disease process and abnormal stress may lead to modified MDR protein activity and TM cell dysfunction and cell death in POAG. The innate immune inflammatory response in the TM may play a role in the ABCB1 detoxification of potentially harmful constituents in the aqueous humor.
Acknowledgments
Supported by BrightFocus Foundation Grant G2011–047, National Eye Institute Grants EY12043 and P30EY01792, Rosemary O'Meara and Kathleen F. Connelly Memorial Funds, Illinois Society for the Prevention of Blindness, Midwest Eye-Banks and Transplantation Center, and an unrestricted grant from the Research to Prevent Blindness.
References
- 1.Medeiros FA, Zangwill LM, Anderson DR, Liebmann JM, Girkin CA, Harwerth RS, Fredette M-J, Weinreb RN. Estimating the rate of retinal ganglion cell loss in glaucoma. Am J Ophthalmol. 2012;154:814–24. doi: 10.1016/j.ajo.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peters D, Boel B, Anders H. Factors associated with lifetime risk of open‐angle glaucoma blindness. Acta Ophthalmol (Copenh) 2014;92:421–5. doi: 10.1111/aos.12203. [DOI] [PubMed] [Google Scholar]
- 3.Acott TS, Kelley MJ. Extracellular matrix in the trabecular meshwork. Exp Eye Res. 2008;86:543–61. doi: 10.1016/j.exer.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knepper PA, Yue BYJT. Abnormal trabecular meshwork outflow pathway. In: Levin LA, Albert DM (editors): Ocular Disease: Mechanisms and Management. London UK, Elsevier; 2010. p.171–177. [Google Scholar]
- 5.Keller KE, Acott TS. The juxtacanalicular region of ocular trabecular meshwork: A tissue with a unique extracellular matrix and specialized function. J Ocul Bio. 2013;1:3–18. [PMC free article] [PubMed] [Google Scholar]
- 6.Knepper PA, Samples JR, Yue BYJT. Biomarkers of primary open-angle glaucoma. Expert Rev Ophthalmol. 2010;5:731–42. doi: 10.1586/EOP.10.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The NEI Audacious Goals Initiative. (http://www.nei.nih.gov/audacious/) identified as a high programmatic priority understanding the intersection of aging and the biological mechanisms of eye disease.
- 8.Rees DC, Johnson E, Lewinson O. ABC transporters: the power to change. Nat Rev Mol Cell Biol. 2009;10:218–27. doi: 10.1038/nrm2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seeger MA, van Veen HW. Molecular basis of multidrug transport by ABC transporters. Biochim Biophys Acta Proteins Proteomics. 2009;1794:725–37. doi: 10.1016/j.bbapap.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Aller SG, Yu J, Ward A, Weng Y, Chittaboina S, Zhuo R, Harrell PM, Trihn YT, Zhang Q, Chang G. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science. 2009;323:1718–22. doi: 10.1126/science.1168750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pattabiraman PP, Pecen PE, Rao PV. MRP4-mediated regulation of intracellular cAMP and cGMP levels in trabecular meshwork cells and homeostasis of intraocular pressure. Invest Ophthalmol Vis Sci. 2013;54:1636–49. doi: 10.1167/iovs.12-11107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wunderlich K, Zimmerman C, Gutmann H, Teuchner B, Flammer J, Drewe J. Vasospastic persons exhibit differential expression of ABC-transport proteins. Mol Vis. 2003;9:756–61. [PubMed] [Google Scholar]
- 13.Golubnitschaja O, Yeghiazaryan K, Flammer J. Glaucomatous optic neuropathy: Risk assessment and potential targets for effective prevention and treatments tailored to the patient. In Neurodegenerative Diseases: Integrative PPPM Approach as the Medicine of the Future. Springer Netherlands. 2013.p.187–201. [Google Scholar]
- 14.Toole BP, Slomiany MG. Hyaluronan, CD44 and Emmprin: partners in cancer cell chemoresistance. Drug Resist Updat. 2008;11:110–21. doi: 10.1016/j.drup.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nolan MJ, Giovingo CC, Miller AM, Wertz RD, Ritch R, Liebmann JM, Allingham RR, Herdon LW, Was MB, Smolyak R, Hasan F, Barnett E, Samples JR, Knepper PA. Aqueous humor sCD44 concentration and visual field loss in primary open-angle glaucoma. J Glaucoma. 2007;16:419–29. doi: 10.1097/IJG.0b013e318050ab4b. [DOI] [PubMed] [Google Scholar]
- 16.Giovingo M, Nolan M, McCarty R, Pang I-H, Clark AF, Beverly RM, Schwartz S, Stamer WD, Walker L, Grybauskas A, Skuran K, Kuprys P, Yue BYJT, Knepper PA. sCD44 overexpress increased intraocular pressure in vivo and increases outflow resistance in vitro. Mol Vis. 2013;19:2151–64. [PMC free article] [PubMed] [Google Scholar]
- 17.Knepper PA, Nolan MJ. CD44 and Primary Open Angle Glaucoma. In The Glaucoma Book, PS Schacknow, Samples JR, editors. Springer New York. 2010.p.939–951. [Google Scholar]
- 18.Zöller M. CD44: can a cancer-initiating cell profit from an abundantly expressed molecule? Nat Rev Cancer. 2011;11:254–67. doi: 10.1038/nrc3023. [DOI] [PubMed] [Google Scholar]
- 19.Streilein JW. Ocular immune privilege: therapeutic opportunities from an experiment of nature. Nat Rev Immunol. 2003;3:879–89. doi: 10.1038/nri1224. [DOI] [PubMed] [Google Scholar]
- 20.Wagner AH, Anand VN, Wang WH, Chatterton JE, Sun D, Shepard AR, Jacobson N, Pang IH, Deluca AP, Casavant TL, Scheetz TE, Mullins RF, Braun TA, Clark AF. Exon-level expression profiling of ocular tissues. Exp Eye Res. 2013;111:105–11. doi: 10.1016/j.exer.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Therneau TM, Ballman KV. What does PLIER really do? Cancer Inform. 2008;6:423–31. [PMC free article] [PubMed] [Google Scholar]
- 22.Choi J, Miller AM, Nolan MJ, Yue BYJT, Thotz ST, Clark AF, Agarwal H, Knepper PA. Soluble CD44 is cytotoxic to trabecular meshwork and retinal ganglion cells in vitro. Invest Ophthalmol Vis Sci. 2005;46:214–22. doi: 10.1167/iovs.04-0765. [DOI] [PubMed] [Google Scholar]
- 23.Boraldi F, Quaglino D, Croce MA, Garcia Fernandez MI, Tiozzo R, Gheduzzi D, Bacchelli I, Ronchetti IP. Multidrug resistance protein-6 (MRP6) in human dermal fibroblasts. Comparison between cells from normal subjects and from Pseudoxanthoma elasticum patients. Matrix Biol. 2003;22:491–500. doi: 10.1016/j.matbio.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 24.Miller AM, Nolan MJ, Choi J, Koga T, Shen X, Yue BY, Knepper PA. Lactate treatment causes NF-κB activation and CD44 shedding in cultured trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2007;48:1615–21. doi: 10.1167/iovs.06-1086. [DOI] [PubMed] [Google Scholar]
- 25.Termeer C, Benedix F, Sleeman J, Fieber C, Voith U, Ahrens T, Miyake K, Freudenberg M, Galanos C, Simon JC. Oligosaccharides of hyaluronan activate dendritic cells via toll-like receptor 4. J Exp Mol Med. 2002;195:99–111. doi: 10.1084/jem.20001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Udan ML, Ajit D, Crouse NR, Nichols MR. Toll‐like receptors 2 and 4 mediate Aβ (1–42) activation of the innate immune response in a human monocytic cell line. J Neurochem. 2008;104:524–33. doi: 10.1111/j.1471-4159.2007.05001.x. [DOI] [PubMed] [Google Scholar]
- 27.Chen P, Chen H, Zang X, Chen M, Jiang H, Han S, Wu X. Expression of efflux transporters in human ocular tissues. Drug Metab Dispos. 2013;41:1934–48. doi: 10.1124/dmd.113.052704. [DOI] [PubMed] [Google Scholar]
- 28.Haslam IS, Jones K, Coleman T, Simmons NL. Rifampin and digoxin induction of MDR1 expression and function in human intestinal (T84) epithelial cells. Br J Pharmacol. 2008;154:246–55. doi: 10.1038/bjp.2008.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rautio J, Humphreys JE, Webster LO, Balakrishnan A, Keogh JP, Kunta JR, Serabjit-Singh C, Polli JW. In vitro p-glycoprotein inhibition assays for assessment of clinical drug interaction potential of new drug candidates: a recommendation for probe substrates. Drug Metab Dispos. 2006;34:786–92. doi: 10.1124/dmd.105.008615. [DOI] [PubMed] [Google Scholar]
- 30.Bentires-Alj M, Barbu V, Fillet M, Chariot A, Relic B, Jacobs N, Bours VNF. -κB transcription factor induces drug resistance through MDR1 expression in cancer cells. Oncogene. 2003;22:90–7. doi: 10.1038/sj.onc.1206056. [DOI] [PubMed] [Google Scholar]
- 31.Gibson CJ, Hossain MM, Richardson JR, Aleksunes LM. Inflammatory regulation of ATP binding cassette efflux transporter expression and function in microglia. J Pharmacol Exp Ther. 2012;343:650–60. doi: 10.1124/jpet.112.196543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer. 2004;4:528–39. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]
- 33.Misra S, Ghatak S, Zoltan-Jones A, Toole BP. Regulation of MDR1 expression and drug resistance by a positive feedback loop involving hyaluronan, phosphoinositide 3-kinase, and ErbB2. J Biol Chem. 2005;280:20310–5. doi: 10.1074/jbc.M500737200. [DOI] [PubMed] [Google Scholar]
- 34.Taylor KR, Yamasaki K, Radek KA, Di Nardo A, Goodarzi H, Golenbock D, Gallo RL. Recognition of hyaluronan released in sterile injury involves a unique receptor complex dependent on Toll-like receptor 4, CD44, and MD-2. J Biol Chem. 2007;282:18265–75. doi: 10.1074/jbc.M606352200. [DOI] [PubMed] [Google Scholar]
- 35.Jiang D, Liang J, Noble PW. Hyaluronan as an immune regulator in human diseases. Physiol Rev. 2011;91:221–64. doi: 10.1152/physrev.00052.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sironen RK, Tammi M, Tammi R, Auvinen PK, Anttila M, Kosma VM. Hyaluronan in human malignancies. Exp Cell Res. 2011;317:383–91. doi: 10.1016/j.yexcr.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 37.Wang D, Raehal KM, Bilsky EJ, Sadée W. Inverse agonists and neutral antagonists at µ opioid receptor (MOR): possible role of basal receptor signaling in narcotic dependence. J Neurochem. 2001;77:1590–600. doi: 10.1046/j.1471-4159.2001.00362.x. [DOI] [PubMed] [Google Scholar]
- 38.Akashi S, Saitoh SI, Wakabayashi Y, Kikuchi T, Takamura N, Nagai Y, Kusumoto Y, Kukase K, Kusumoto S, Adachi Y, Kosugi A, Miyake K. Lipopolysaccharide interaction with cell surface toll-like receptor 4-MD-2 higher affinity than that with MD-2 or CD14. J Exp Med. 2003;198:1035–42. doi: 10.1084/jem.20031076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bentala H, Verweij WR, Huizinga-Van der Vlag A, van Loenen-Weemaes AM, Meijer DK, Poelstra K. Removal of phosphate from lipid A as a strategy to detoxify lipopolysaccharide. Shock. 2002;18:561–6. doi: 10.1097/00024382-200212000-00013. [DOI] [PubMed] [Google Scholar]
- 40.Hutchinson MR, Zhang Y, Shridhar M, Evans JH, Buchanan MM, Zhao TX, Watkins LR. Evidence that opioids may have toll-like receptor 4 and MD-2 effects. Brain Behav Immun. 2010;24:83–95. doi: 10.1016/j.bbi.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stevens CW, Aravind S, Das S, Davis RL. Pharmacological characterization of LPS and opioid interactions at the toll‐like receptor 4. Br J Pharmacol. 2013;168:1421–9. doi: 10.1111/bph.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, Moore KJ. CD36 ligands promote sterile inflammation through assembly of a toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010;11:155–61. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krohn M, Lange C, Hofrichter J, Scheffler K, Stenzel J, Steffen J, Schumacher T, Bruning T, Plath A-S, Alfen F, Schmidt A, Winter F, Rateitschak K, Wree A, Gsponer J, Walker LC, Pahnke J. Cerebral amyloid-β proteostasis is regulated by the membrane transport protein ABCC1 in mice. J Clin Invest. 2011;121:3924–31. doi: 10.1172/JCI57867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharom FJ. The P-glycoprotein multidrug transporter. Essays Biochem. 2011;50:161–78. doi: 10.1042/bse0500161. [DOI] [PubMed] [Google Scholar]
- 45.Liu ML, Hong S. Early phase of amyloid beta42-induced cytotoxicity in neuronal cells is associated with vacuole formation and enhancement of exocytosis. Exp Mol Med. 2005;37:559. doi: 10.1038/emm.2005.69. [DOI] [PubMed] [Google Scholar]
- 46.Chen KD, Chang PT, Ping YH, Lee HC, Yeh CW, Wang PN. Gene expression profiling of peripheral blood leukocytes identifies and validates ABCB1 as a novel biomarker for Alzheimer's disease. Neurobiol Dis. 2011;43:698–705. doi: 10.1016/j.nbd.2011.05.023. [DOI] [PubMed] [Google Scholar]
- 47.Cirrito JR, Deane R, Fagan AM, Spinner ML, Parsadanian M, Finn MB, Jiang H, Prior JL, Sagare A, Bales KR, Paul SM, Ziokovic PV, Piwnica-Worms D, Holtzman DM. P-glycoprotein deficiency at the blood-brain barrier increases amyloid-β deposition in an Alzheimer disease mouse model. J Clin Invest. 2005;115:3285–90. doi: 10.1172/JCI25247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guo L, Salt TE, Luong V, Wood N, Cheung W, Maass A, Ferrari F, Russo-Marie F, Sillito AM, Cheetham ME, Moss SW, Fitzke MF, Cordeiro MF. Targeting amyloid-β in glaucoma treatment. Proc Natl Acad Sci USA. 2007;104:13444–9. doi: 10.1073/pnas.0703707104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Orwig SD, Perry CW, Kim LY, Turnage KC, Zhang R, Vollrath D, Schmidt-Krey I, Lieberman RL. Amyloid fibril formation by the glaucoma-associated olfactomedin domain of myocilin. J Mol Biol. 2012;421:242–55. doi: 10.1016/j.jmb.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Porter KM, Epstein DL, Liton PB. Up-regulated expression of extracellular matrix remodeling genes in phagocytically challenged trabecular meshwork cells. PLoS ONE. 2012;7:e34792. doi: 10.1371/journal.pone.0034792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim JM, Kim SH, Park KH, Han SY, Shim HS. Investigation of the association between Helicobacter pylori infection and normal tension glaucoma. Invest Ophthalmol Vis Sci. 2011;52:665–8. doi: 10.1167/iovs.10-6096. [DOI] [PubMed] [Google Scholar]
- 52.Puntarulo S, Cederbaum AI. Production of reactive oxygen species by microsomes enriched in specific human cytochrome P450 enzymes. Free Radic Biol Med. 1998;24:1324–30. doi: 10.1016/s0891-5849(97)00463-2. [DOI] [PubMed] [Google Scholar]
- 53.Schuetz EG, Relling MV, Kishi S, Yang W, Das S, Chen P, Cook EH, Rosner GL, Pui CH, Blanco JG, Edick MJ, Hancock ML, Winick NJ, Dervieux T, Amylon MD, Bash RO, Behm FG, Camitta BM, Rimondi SC, Goh BC, Lee SC, Wang LZ, Fan L, Guo JY, Lamba J, Lim R, Lim HL, Ong AB, Lee HS, Kuehl P, Zhang J, Lin Y, Assem M, Schuetz J, Watkins PB, Daly A, Wrighton SA, Hall SD, Maurel P, Brimer C, Yasuda K, Venkataramanan R, Strom S, Thummel K, Boguski MS. PharmGKB update: II. CYP3A5, cytochrome P450, family 3, subfamily A, polypeptide 5. Pharmacol Rev. 2004;56:159. doi: 10.1124/pr.56.2.1. [DOI] [PubMed] [Google Scholar]
- 54.Kirn RB, Wandel C, Leake B, Cvetkovic M, Fromm MF, Dempsey PJ, Roden MM, Belas F, Chaudhary AK, Roden DM, Wood AJJ, Wilkinson GR. Interrelationship between substrates and inhibitors of human CYP3A and P-glycoprotein. Pharmacol Res. 1999;16:408–14. doi: 10.1023/a:1018877803319. [DOI] [PubMed] [Google Scholar]
- 55.Thummel KE, Wilkinson GR. In vitro and in vivo drug interactions involving human CYP3A. Annu Rev Pharmacol Toxicol. 1998;38:389–430. doi: 10.1146/annurev.pharmtox.38.1.389. [DOI] [PubMed] [Google Scholar]
- 56.Kim RB. Transporters and xenobiotic disposition. Toxicology. 2002;181-182:291–7. doi: 10.1016/s0300-483x(02)00296-2. [DOI] [PubMed] [Google Scholar]
- 57.Callaghan R, Crowley E, Potter S, Kerr ID. P‐glycoprotein: So Many Ways to Turn It On. J Clin Pharmacol. 2008;48:365–78. doi: 10.1177/0091270007311568. [DOI] [PubMed] [Google Scholar]
- 58.Hotchkiss RS, Moldawer LL. Parallels between Cancer and Infectious Disease. N Engl J Med. 2014;371:380–3. doi: 10.1056/NEJMcibr1404664. [DOI] [PubMed] [Google Scholar]
- 59.Lloberas N, Rama I, Llaudó J, Torras G, Cerezo L, Cassis M, Franquesa M, Merino A, Benitez-Ribas D, Cruzado JM, Herrero-Fresneda I, Bestard O, Grinyo JM. Dendritic cells phenotype fitting under hypoxia or lipopolysaccharide; adenosine 5′‐triphosphate‐binding cassette transporters far beyond an efflux pump. Clin Exp Immunol. 2013;172:444–54. doi: 10.1111/cei.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]