Abstract
Background and Objectives:
Oral lichen planus (OLP) is a relatively common, chronic inflammatory condition that frequently presents with symptoms of pain and burning sensation. It is generally a very unrelenting disorder despite several kinds of treatment. Only symptomatic OLP requires treatment, and it remains a challenging predicament. Efforts are made in a sustained manner for searching for novel therapies for symptomatic OLP. Therefore, this study was aimed to compare the efficacy of treatment with topical pimecrolimus cream 1% with that of triamcinolone acetonide oral paste 0.1% in subjects with symptomatic OLP.
Materials and Methods:
A prospective, parallel-group, randomized, active control clinical study was conducted among 30 symptomatic OLP subjects (20 females and 10 males, with 15 patients in each treatment group) treated with topical pimecrolimus 1% cream and triamcinolone acetonide 0.1% oral paste four times daily for two consecutive months and treatment-free follow-up was performed for 2 months. Pain or burning sensation, mean clinical score and presence of erythematous areas were assessed. The data obtained were statistically analyzed using Wilcoxon's Rank test and the Mann Whitney test.
Results:
Subjects in both the groups showed significant improvement in symptom scores; however, the overall treatment response was higher in the pimecrolimus group compared with the triamcinolone acetonide group. On intergroup comparison, there was no statistically significant difference between the groups in the reduction in burning sensation (P = 0.18) and erythematous area (P = 0.07), but there was a statistically highly significant improvement in reduction of clinical scoring (P < 0.01%). Following the termination of the treatment, sustained remission of symptoms and long-lasting therapeutic effects was detected in 93.3% of the patients treated with pimecrolimus.
Interpretation and Conclusion:
Topical pimecrolimus 1% cream showed better therapeutic response compared with triamcinolone acetonide 0.1% oral paste in subjects with symptomatic OLP.
Keywords: Oral lichen planus, pimecrolimus, triamcinolone acetonide
INTRODUCTION
Lichen planus (LP) is a chronic inflammatory dermatosis of the skin and mucous membranes. In 1869, Erasmus Wilson delineated and named the condition Lichen planus. LP is derived from the Greek word “Lichen,” which means “tree moss” and the Latin word “planus,” which means “flat.”[1] The prevalence of LP in various populations has been found to be 0.1–4%, with peak incidence between the ages of 30 and 60 years and more commonly affecting women; children are rarely affected.[2,3]
Clinically, oral lichen planus (OLP) presents as white striations, papules, plaques, erythema, erosions or blisters, affecting predominantly the buccal mucosa, tongue and gingival, etc.[4] The reticular form is most common and asymptomatic, but the erosive, atrophic and bullous forms are typically the most symptomatic forms usually seeking urgent treatment.[5]
Multiple topical and systemic treatments have been reported to be effective, including systemic and topical immunosuppressants such as corticosteroids, griseofulvin, dapsone, hydroxychloroquine, cyclosprin, tarcolimus, etc.[5] But, till date, no fully resolutive and effective treatment has been found.[6] Therefore, the management of symptomatic OLP represents a confounding therapeutic challenge. Despite numerous existing remedies, there are many treatment failures and there is no definite cure for this oral lesion.
Topical corticosteroids remain the first line of therapy in OLP and triamcinolone acetonide paste is the most widely used commercial preparation for OLP; unfortunately, some patients are refractory to corticosteroids and often unable to tolerate their long-term side-effects. Therefore, supplementary, efficacious treatments are considered necessary for patients with OLP.[5]
Pimecrolimus, a new, nonsteroidal topical immunomodulator, is finding increasing application in recalcitrant inflammatory skin and mucosal disease and have been tried for the treatment of OLP, showing promising results. They act by binding to macrophillin12 and subsequently inhibit dephosphorylation of nuclear factor of activated T cells by calcineurin. This markedly reduces the production of TH1 cytokines and inhibits the mast cell production of pro-inflammatory cytokines. The efficacy of these treatments is likely attributable to the inhibition of the T-cell mediated-pathogenesis of OLP.[5]
MATERIALS AND METHODS
Study setting
The present prospective, parallel-group, randomized, active control clinical study was conducted in 30 patients (20 females, 10 males; age range 20–64 years) with a confirmed diagnosis of symptomatic OLP based on clinical and histopathological features in the Department of Oral Medicine and Radiology, College of Dental Sciences, Davangere, Karnataka, India.
Inclusion criteria of the study was patients with symptomatic OLP (pain and or burning sensation) who were agreeing for the biopsy and were ready to apply the medication supplied. Patients with a history of malignancy, immunocompromised diseases, current systemic or generalized infections, history of pregnancy or breast feeding, received topical or systemic immunosuppressants, retinoids or any other systemic therapies known to cause or suspected to have an effect on OLP within the last 4 weeks and patients allergic to the drugs supplied were excluded from the study.
All the participants were explained the need and design of the study and the possible adverse effects, and only those patients who gave a signed informed consent on an institutionally approved document were included in the study. The study was reviewed and approved by our ethical committee.
The patients were divided into two groups, 15 patients in Group “A” were instructed to apply a thin layer of pimecrolimus cream 1% (10 g, pacroma 1%; Ajanta Pharmaceuticals Ltd.) four times a day for a total of 2 months and 15 patients in Group “B” were instructed to apply triamcinolone acetonide 0.1% oral paste (5 g, kenacort 0.1%: Mepromax Life Sciences P.Ltd) for four times a day on symptomatic OLP lesions for 2 months. Patients were instructed to gently dry the area of application with clean dry cotton just before the application of the drug and to restrain from eating, drinking or rinsing their mouth for 30 min after each application. All the patients were assessed monthly for 2 months during the treatment course (visits 1–3) and followed-up with 2 months of treatment-free observation (visit 4). The clinical parameters were recorded upon clinical examination at baseline and at each subsequent visit.
All patients had four visits for the study; they were reviewed on the 0, 1st, 2nd and 4th months, and each visit consisted of measuring the intensity of pain and/or burning sensation by using a Visual Analogue Scale (VAS) of 0–10 (with 1 mm divisions, where “0” is no burning sensation and “10” is worst possible burning sensation). The patients were asked to mark VAS at a point that best represented the level of the symptoms. The size of the lesion was recorded by clinical scoring of the lesion using the criteria scale described by Thongprason et al. Erythematous areas were recorded by the symbols “+” for presence or “_” for absence. All the scores were recorded at baseline and at each subsequent visit after the administration of the drug therapy and during the treatment-free follow-up, and relevant data collected were entered in a proforma and subjected to appropriate statistical analysis.
The results are presented as mean ± SD for quantitative data and number and percentages for categorical data. Because the data are in scores, nonparametric tests were used for intra- (Wilcoxon's Sign Rank test) and inter- (Mann Whitney test) group comparisons. For all the tests, a P value of 0.05 or less was considered as statistically significant.
RESULTS
A total of 30 adult subjects were enrolled in the study, 20 females and 10 males, suggesting female predilection with a male to female ratio of 2:1. The age of the patients ranged from 20 to 64 years, and the mean age of the subjects was 36.7 ± 13.4 years. All the patients were symptomatic with erosive, ulcerative lesions and with additional reticular lesions as well.
In both Group A and Group B, reduction in mean scores of burning sensation was observed during the treatment period. However, this reduction was higher in the pimecrolimus group than that in the triamcinolone acetonide group at the end of the 1st month, i.e. 57% and 49%, respectively, and at the end of the 2nd month (93% and 92%, respectively) Reduction in burning sensation was higher in Group A than in Group B, and it was statistically highly significant (P < 0.01). During the treatment-free follow-up period, at the end of the 4th month, reduction of burning sensation was 98% and 89%, respectively, and it was statistically highly significant (P < 0.01). But, on intergroup comparison, at the end of the 1st, 2nd and 4th months, it was statistically nonsignificant [Table 1 and Figure 1].
Table 1.
Intra and inter group comparision of burning sensation (before and after treatment)
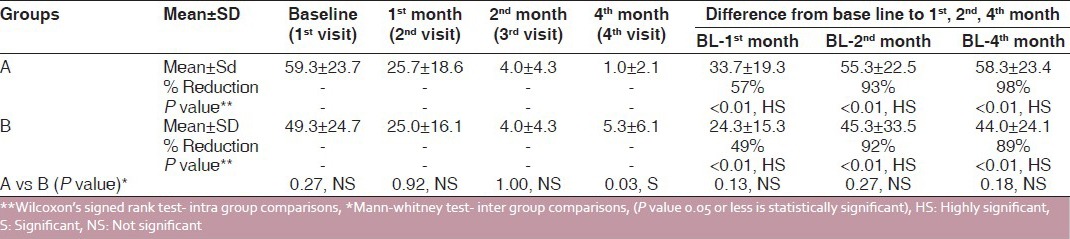
Figure 1.
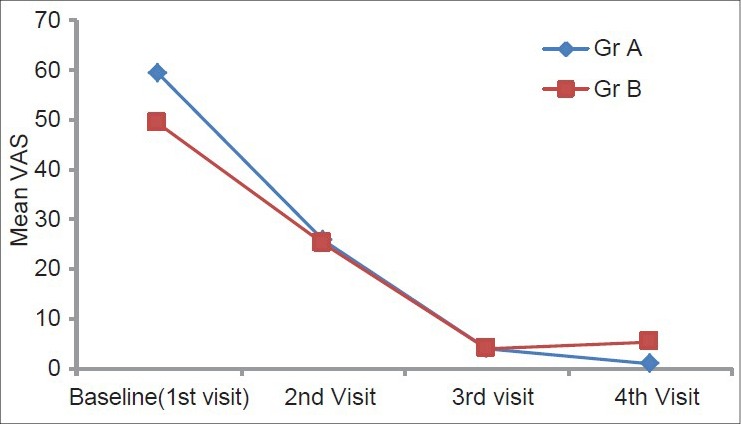
Intergroup comparison of burning sensation
There was reduction in mean clinical scores in both the groups after the treatment. However, this reduction during the treatment was higher in the pimecrolimus group than in the triamcinolone acetonide group, i.e. 49% and 44%, respectively, at the end of the 1st month (2nd visit). Similar reductions were seen at the end of the 2nd month, 80% and 85%, respectively, and this was statistically highly significant (P < 0.01). During the posttreatment follow-up of 2 months, it was 95% and 80%, respectively, which was statistically highly significant (P < 0.01). On intergroup comparison, at the end of the 1st and 2nd months, it was statistically nonsignificant, but at the 4th month during treatment-free follow-up it was highly significant (P < 0.01) [Table 2 and Figure 2].
Table 2.
Inter and intra group comparison of clinical scores (before and after treatment)
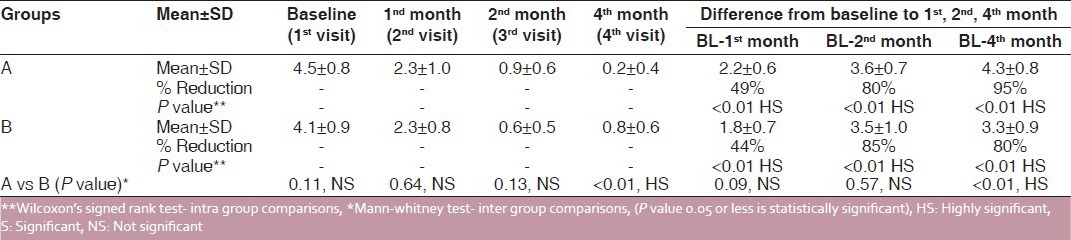
Figure 2.
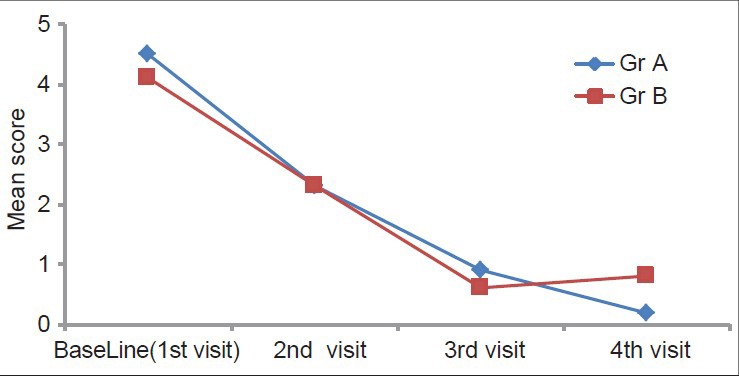
Intergroup comparison of clinical score
Improvement in resolution of erythematous areas during the treatment at the end of the 1st month was 80% and 20%, respectively in the pimecrolimus and triamcinolone acetate groups, which was statistically highly significant (P < 0.01). At the end of the 2nd month, none of the patients had existence of erythematous areas in both Group A and Group B and no statistical difference was found. After the posttreatment follow-up of 2 months, the erythematous areas reappeared in 6.7% and 33.3% subjects in the pimecrolimus and triamcinolone acetonide groups, respectively, but no statistical difference was found (P = 0.07) [Table 3 and Figure 3].
Table 3.
Inter and intra group comparison of erythematous areas (before and after treatment)
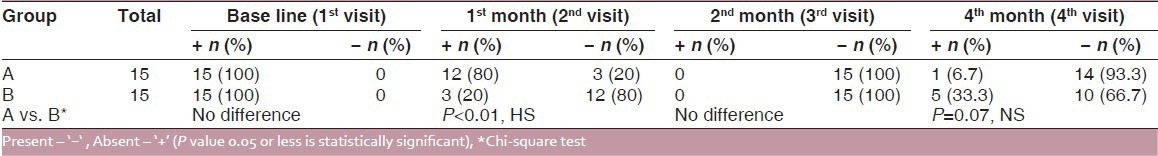
Figure 3.
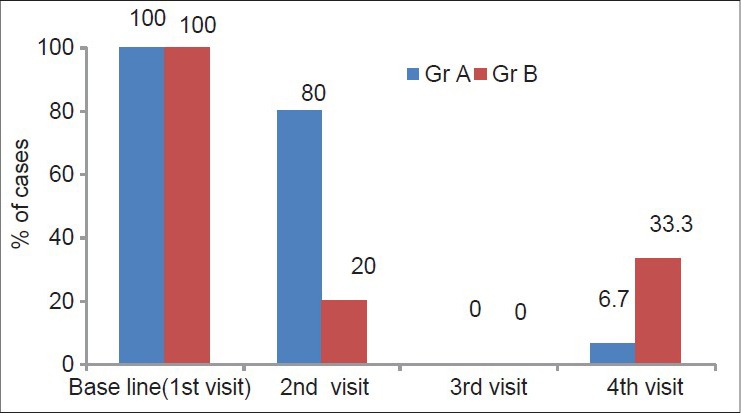
Intergroup comparison of the erythematous area
DISCUSSION
In the present study, both the drugs resulted in reduction of burning sensation, clinical scores and erythematous areas. Improved patient's quality of life was observed during the 2 months of treatment period. At the end of the study, difference of clinical scores between groups was highly statistically significant, whereas burning sensation and erythematous areas were statistically nonsignificant. However, better results in all the parameters were observed in the pimecrolimus group compared with the triamcinolone group. These clinical changes are seen in the Figures 4 and 5. This is consistent with the study conducted by Gourouhi et al.[7]
Figure 4.
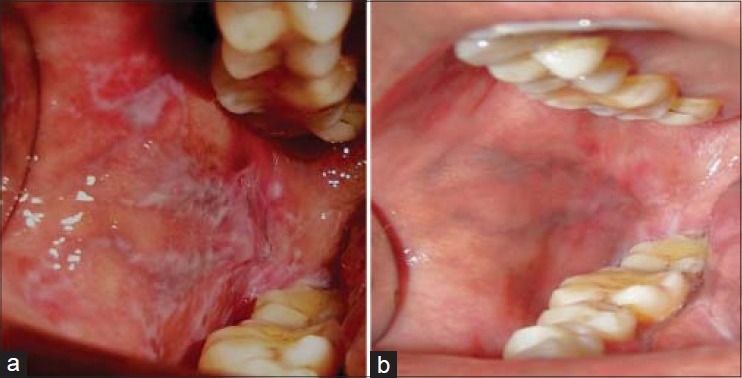
Pimecrolimus group (4a- 1st visit) (4b- 4th visit)
Figure 5.
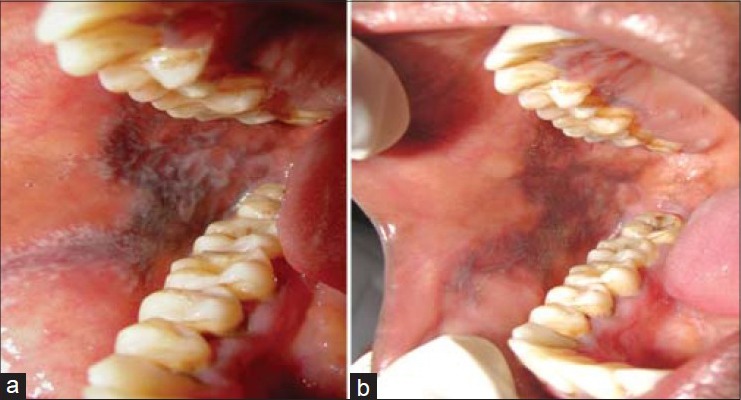
Triamcinolone acetonide group (5a- 1st visit) (5b- 4th visit)
Despite various alternative treatment options, corticosteroids remain the treatment of choice for symptomatic OLP because of the autoimmune nature of OLP and its effects on the epithelial and connective tissues.[8] The efficacy of topical triamcinolone acetonide is primarily due to the local anti-inflammatory properties of suppressing T-cell function.[9] The search for new topical anti-inflammatory agents without the adverse effects of topical corticosteroid has resulted in the development of an topical immunomodulator, i.e. pimecrolimus.[4] Therefore, in this present study, we have tried this topical drug to manage symptomatic OLP.
Pimecrolimus (SDZ ASM 981), an ascomycin derivative, is a new addition to dermatologic therapeutics. It is one of the new classes of immunomodulating macrolactams and was specifically developed for the treatment of inflammatory skin diseases.[10] The clinical efficacy of pimecrolimus was confirmed after topical application in patients with atopic dermatitis and allergic contact dermatitis. The safety and tolerability of pimecrolimus after repeated topical application of the 1% cream formulation were observed in moderate to severe atopic dermatitis and were shown to be well tolerated and safe even after repeated topical application, in contrast to corticosteroids, with no potential to induce skin atrophy. The interest in pimecrolimus has been substantial because of its significant anti-inflammatory activity, immunomodulatory capabilities and its low systemic immunosuppressive potential.[11]
Pimecrolimus is derived from Streptomyces hygropicus var, ascomycetus; like all ascomycins, it is an immunophilin ligand that binds specifically to the cytosolic receptor, immunophilin macrophilin-12. This pimecrolimus–macrophilin complex effectively inhibits the protein phosphatase calcineurin by preventing calcineurin from dephosphorylating the nuclear factor of activated T cells (NF-AT), a transcription factor. This results in the blockage of signal transduction pathways in T cells and inhibits the synthesis of inflammatory cytokines, specially Th1- and Th2-type cytokines. Furthermore, it has also been shown to prevent the release of cytokines and pro-inflammatory mediators from mast cells. Studies on the effectiveness of pimecrolimus cream compared with steroids in many dermatologic diseases showed no associated side-effects such as local atrophy, fragility and telangiectasias, and to promote infections, including acute candidiasis characteristic of a topical steroids.[10]
In our study, the higher reduction in VAS score seen in the pimocrolimus group can be attributed to its efficacy in OLP due to its immunophilin ligand binding specifically to the cytosolic receptor and acting by inhibition of the release of numerous inflammatory cytokines, thereby preventing the cascade of immune and inflammatory signals.[7]
The efficacy of triamcinolone acetonide paste can be attributed to its local anti-inflammatory and antimmunological properties of suppressing T-cell function, which is in accordance with a prospective, randomized comparative study, where twice-daily application of 0.1% triamcinolone acetonide was compared with 0.03% tacrolimus.[12]
In the present study, pimecrolimus has shown significant improvement in all the clinical parameters. This is similar to the studies reported by Swift et al.,[4] Pedraza et al.,[13] Taebunpakul et al.,[14] Passerron et al.,[15] Volz et al.[16] and McCaughey et al.,[17] where pimecrolimus has shown superior results compared with placebo.
Pimecrolimus is available only in a cream form; therefore, it was recommended to apply the drug four times daily. In a case report, pimecrolimus was successfully used in a hydrophilic adhesive gel base with effective and tolerable results. By virtue of higher absorption in the form of oral paste or any other appropriate form, we can suggest that by replacing it with a pimecrolimus orabase, we may find even higher efficacy in terms of short-term and maintenance effects.[7,18]
All the subjects were followed-up for 2 months with treatment-free observation (visit 4), and the symptoms and erythematous areas reappeared in one subject in Group A and five subjects in Group B, which was statistically nonsignificant (P = 0.07). This is in accordance with the recent randomized vehicle-controlled study that showed that topical pimecrolimus was effective in controlling the symptoms of OLP up to 30 days after cessation of therapy[19] because it was associated with less impairment of langerhans cell function, offering a better long-term safety profile. At the completion of the treatment, none of the patients had any serious adverse effects locally or systemically to both the medications.
From our study, it can be concluded that topical pimecrolimus 1% cream, applied four times daily for 2 months, has a better therapeutic advantage than triamcinolone acetonide 0.1%, with less or no local or systemic adverse effects. However, this drug may be an important addition to previously existing treatment modalities for symptomatic OLP.
However, in our study, pimecrolimus was used in a cream form and adherence of drug to the mucosal surface was not satisfactory; therefore, patients were instructed to apply it for four times a day repeated application. If it is used in a hydrophilic adhesive gel base, more therapeutic benefits may be achieved and it can find even higher efficacy in terms of short-term and maintenance effects.
CONCLUSION
Topical pimecrolimus has an enormous potential as a new therapy for symptomatic erosive or ulcerative OLP refractory to other topical and systemic therapies, and the results from the present study are highly encouraging. The use of topical pimecrolimus 1% cream applied four times showed salutary results than triamcinolone acetonide 0.1% paste and substantial reduction in all the parameters were noted. But, there is a need for appropriate studies to establish the efficacy of pimecrolimus with orabase for its prolonged long-term efficacy and to determine its malignant potential. The present study was a parallel-group, randomized, active control clinical study, but the study would have been still more valid if it had been a double-blinded, randomized clinical control study instead. However, because the sample size is small, further studies are recommended on a larger sample size with a long-term follow-up to confirm its efficacy and safety with the immunologic markers, in order to minimize the effects of confounding factors and to maximize the sensitivity for detecting subtle changes of the mucosa during the course of the treatment.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ. 7th ed. Vol. 1. US of America: 2008. Fitzpatrick's Text book of dermatology in general medicine; pp. 463–76. [Google Scholar]
- 2.Edwards P, Kelsch R. Oral Lichen Planus: Clinical Presentation and Management. J Can Dent Assoc. 2002;68:494–9. [PubMed] [Google Scholar]
- 3.Sugerman PB, Savge NW. Oral lichen planus: Causes, diagnosis and management. Aust Dent J. 2002;47:290–7. doi: 10.1111/j.1834-7819.2002.tb00540.x. [DOI] [PubMed] [Google Scholar]
- 4.McCaughey C, Machan M, Bennet R, Zone JJ, Hull CM. Pimecrolimus 1% cream for oral erosive lichen planus-A 6 weeks randomized, double blind, vehicle controlled study with a 6 week open label extension to assess efficacy and safety. J Eur Acad Dermatol Venereol. 2011;25:1061–7. doi: 10.1111/j.1468-3083.2010.03923.x. [DOI] [PubMed] [Google Scholar]
- 5.Swift JC, Rees TD, Plemons JM, Hallmon WW, Wright JC. The effectiveness of 1% Pimecrolimus cream in the treatment of Oral Lichen Planus. J Periodontol. 2005;76:627–35. doi: 10.1902/jop.2005.76.4.627. [DOI] [PubMed] [Google Scholar]
- 6.Salazar-Sánchez N, López-Jornet P, Camacho-Alonso F, Sánchez-Siles M. Efficacy of topical aloe vera in patients with oral lichen planus: A randomized double blind study. J Oral Pathol Med. 2010;39:735–40. doi: 10.1111/j.1600-0714.2010.00947.x. [DOI] [PubMed] [Google Scholar]
- 7.Gorouhi F, Solhpour A, Beitollahi JM, Afshar S, Davari P, Hashemi P, et al. Randomized trial of Pimecrolimus cream Vs Triamcinolone acetonide paste in the treatment of OLP. J Am Acad Dermatol. 2007;57:806–13. doi: 10.1016/j.jaad.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez-Garcia A, Diniz-Freitas M, Gandara-Vila P, Blanco-Carrion A, Garcia-Garcia A, Gandara-Rey J. Triamcinolone acetonide mouth rinses for treatment of erosive oral lichen planus: Efficacy and risk of fungal over-infection. Oral Dis. 2006;12:559–65. doi: 10.1111/j.1601-0825.2006.01238.x. [DOI] [PubMed] [Google Scholar]
- 9.Xia J, Li C, Hong Y, Yang L, Huang Y, Cheng B. Short –term clinical evaluation of intralesional triamcinolone acetonide injection for ulcerative oral lichen planus. J Oral Pathol Med. 2006;35:327–31. doi: 10.1111/j.1600-0714.2006.00441.x. [DOI] [PubMed] [Google Scholar]
- 10.Swift JC, Rees TD, Plemons JM, Hallmon WW, Wright JC. The effectiveness of 1% Pimecrolimus cream in the treatment of Oral Lichen Planus. J Periodontol. 2005;76:627–35. doi: 10.1902/jop.2005.76.4.627. [DOI] [PubMed] [Google Scholar]
- 11.Gupta AK, Chow M. Pimecrolimus: A review. J Eur Acad Dermatol Venerel. 2003;17:493–503. doi: 10.1046/j.1468-3083.2003.00692.x. [DOI] [PubMed] [Google Scholar]
- 12.Allen BR, Lakhanpaul M, Morris A, Lateo S, Davies T, Scott G, et al. Systemic exposure, tolerability, and efficacy of Pimecrolimus cream 1% in atopic dermatitis patient. Arch Dis Child. 2003;88:969–73. doi: 10.1136/adc.88.11.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swarna YM, Ali M, Rajeshwari AG, Devarasa GM. A comaparative evaluation of tacrolimus and triamcinolone acetonide in the management of Symptomatic Oral Lichen Planus. J Indian Acad Oral Med Radiol. 2011;23:184–9. [Google Scholar]
- 14.Chatchatee T, Piboonratanakit, Thomprasong K. Topical Pimecrolimus 1% cream in the treatment of OLP. Thai J Pharmacol. 2005;27:2–3. [Google Scholar]
- 15.Pesseron T, Lacour JP, Fontas E, Ortonne JP. Treatment of OLP with 1% Pimecrolimus cream. Arch Dermatol. 2007;143:470–6. doi: 10.1001/archderm.143.4.472. [DOI] [PubMed] [Google Scholar]
- 16.Volz T, Caroli U, Lüdtke H, Bräutigam M, Kohler-Späth H, Röcken M, et al. Pimecrolimus cream 1% in erosive lichen planus- A prospective randomized double blind vehicle controlled study. Br J Dermatol. 2008;159:936–41. doi: 10.1111/j.1365-2133.2008.08726.x. [DOI] [PubMed] [Google Scholar]
- 17.Bogaert H, Sanchez E. Lichen planus: Treatment of thirty cases with systemic and topical Phenytoin. Int J Dermatol. 1990;29:157–8. doi: 10.1111/j.1365-4362.1990.tb04100.x. [DOI] [PubMed] [Google Scholar]
- 18.Pedraza LE, Fernandez-Cuevas L, Ortiz-Pedroza G, Reyes-Gutierrez E, Orozco-Topete R. Treatment of OLP with topical Pimecrolimus 1% cream. Br J Dermatol. 2004;150:771–3. doi: 10.1111/j.0007-0963.2004.05875.x. [DOI] [PubMed] [Google Scholar]
- 19.Al Johani KA, Hegarty AM, Porter SR, Fedele S. Calcineurin inhibitors in oral medicine. J Am Acad Dermatol. 2009;61:829–40. doi: 10.1016/j.jaad.2009.03.012. [DOI] [PubMed] [Google Scholar]


