Abstract
Background:
The aim of the study was to compare the efficacy of chlorhexidine gluconate 2.5 mg (Periochip) and Minocycline hydrochloride 1 mg (Arestin) as local drug delivery agents in the management of chronic periodontitis.
Materials and Methods:
Twenty patients in the age group of 30–50 years suffering from chronic periodontitis (12 males and 8 females), with almost identical probing depth bilaterally (5–8 mm), and exhibiting bleeding on probing were selected and divided into two groups: Group I consisted of periodontal pockets on the left side and received Periochip and group II consisted of periodontal pockets on the right side and received Arestin. Patients were recalled after 6 weeks and 3 months intervals from the baseline visit to record plaque index, gingival index, and probing depth.
Results:
There was reduction in all the parameters in both the groups at 6 weeks and 3 months as compared to baseline.
Conclusion:
From the results of the present study, it was concluded that both the drugs were equally effective in reduction of plaque scores as well as gingival scores. It was further observed that Arestin resulted in better results at 6 weeks while Periochip showed better results at 3 months with respect to probing depth reduction.
Keywords: Antimicrobial agents, chlorhexidine gluconate (Periochip), chronic periodontitis, local drug delivery agents, Minocycline microspheres (Arestin)
INTRODUCTION
Periodontal disease is a multifactorial disease in which the etiological role of bacteria is an established fact.[1,2,3] Elevated numbers of subgingival microbial species have been associated with destructive periodontal disease activity. The prevention of periodontal disease requires a reduction of subgingival microbial plaque mass or at least a suppression of periodontopathic bacteria. Successful treatment is dependent on the stoppage of tissue destruction by elimination or control of etiologic agents, together with a microbial shift toward one typically present in health. In early periodontitis with pockets of ≤5 mm depth, scaling and root planing (SRP) is usually effective in removing the calculus and plaque and, therefore, reduces the bacterial load and probing pocket depth. However, due to poor access to the base of deep pockets and anatomical complexities of teeth and furcation involvement, SRP alone may not always result in the complete elimination of the disease, which results in exacerbation of the disease. A significant number of periodontal pathogenic bacteria remain on the root surfaces and within the dentinal tubules of the teeth associated with these pockets. This encouraged the systemic use of antibiotics as an adjunct to mechanical therapy.
Systemic antibiotics control the bacterial infection because bacteria can invade periodontal tissues, making mechanical therapy ineffective. Systemic antibiotic therapy has an additional effect, i.e. suppression of periodontal pathogens including those present on the tongue and mucosal surfaces and, hence, delaying subgingival recolonization of the pathogens.[4] On the other hand, to obtain an effective concentration of the antimicrobial drug in the periodontal pocket after systemic administration, repeated intakes over a prolonged period of time may be required. Moreover, systemically administered antibacterial agents achieve relatively low concentrations in the pocket even at high dosage. Also, the unwanted effects such as development of resistant strains and superimposed infections preclude the use of these agents as the sole treatment modality. In addition, the discontinuation of the therapy results in return of pathogens to periodontal pockets.
To overcome the disadvantages of systemic antibiotic therapy, the concept of local delivery of antibacterial agents into periodontal pockets was developed.[5] The development of modern carrier systems has brought about a crucial improvement in the pharmacokinetics of the applied antibiotic.[6] This development resulted in adequate drug concentration at the site of action which was maintained for a sufficient duration of time. As a lower dose is required, the adverse effects are also minimized. The purpose of the present study was to compare the efficacy of chlorhexidine gluconate 2.5 mg (Periochip) and Minocycline hydrochloride 1 mg (Arestin) as local drug delivery agents in the management of chronic periodontitis.
MATERIALS AND METHODS
Selection of patients
Inclusion criteria
Twenty patients in the age group of 30–50 years (12 males, 8 females) who fulfilled the following criteria were included in the study:
Patients suffering from chronic periodontitis with almost similar probing depth bilaterally (5–8 mm) at the selected sites and exhibiting bleeding on probing
Patients with no caries and restorations on the selected teeth
Patients showing effective individual oral hygiene.
A written informed consent was taken from the patients prior to the study.
Exclusion criteria
The following patients were excluded from the study:
Patients presenting with history of intake of local and/or systemic antibiotic therapy for the last 1 month
Patients with known systemic and debilitating diseases
Patients presenting with known adverse reactions to any component of the test agent
Patients on anticoagulant therapy
Pregnant and lactating females
Smokers.
Each patient was recalled 4 weeks after completion of supragingival scaling, which formed the baseline visit. Plaque scores were brought to zero, and the probing pocket depth and gingival index (Loe and Silness, 1963) were recorded on the proforma specially prepared for the purpose.
The selected treatment sites were then divided into two groups, and subgingival SRP was performed prior to the delivery of the drugs at the test sites.
Group I: Consisted of periodontal pockets on the left side of maxillary or mandibular arch and received chlorhexidine gluconate 2.5 mg (Periochip). Periochip was placed into the periodontal pocket with the help of plastic filling instrument after drying and isolating the area using cotton swabs [Figures 1 and 2a, b]
Group II: Consisted of periodontal pockets on the right side of maxillary or mandibular arch and received Minocycline hydrochloride 1 mg (Arestin). Subgingival administration of Arestin was accomplished by inserting the unit-dose cartridge into the base of the periodontal pocket and pressing the thumb ring in the handle mechanism to expel the powder while gradually withdrawing the tip from the base of the pocket [Figures 3 and 4].
Figure 1.
Recording of plaque and gingival scores (group I)
Figure 2.
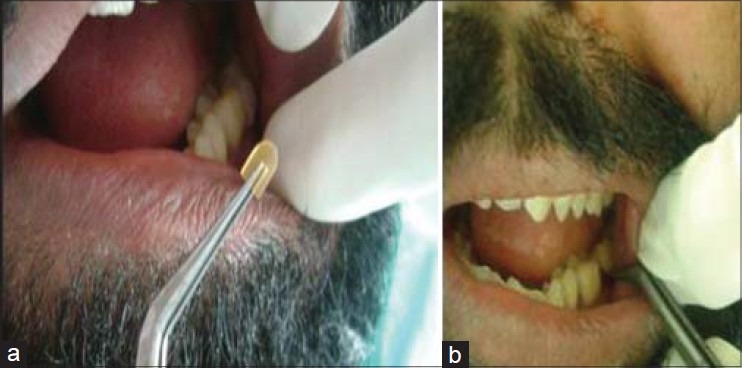
(a and b) Insertion of Periochip
Figure 3.
Recording of plaque and gingival scores (group II)
Figure 4.
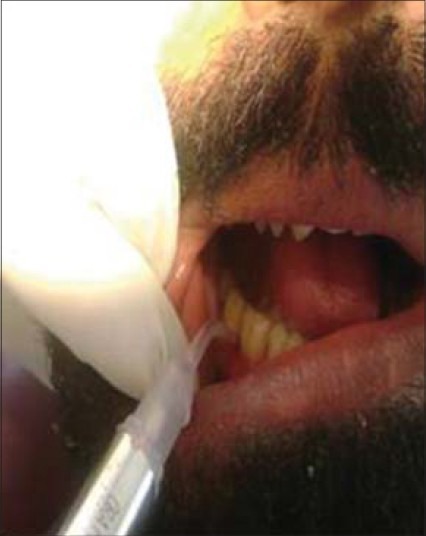
Administration of Arestin
Both the drugs were inserted at the same sitting.
Patients were recalled after 6 weeks and 3 months intervals from the baseline visit to record the plaque index (Turesky-Gilmore-Glickman modification of Quigley-Hein Index, 1970), gingival index (Loe and Silness, 1963), and probing depth (measured using William's periodontal probe).
The data thus obtained were compiled and subjected to statistical analysis.
RESULTS
The results of the study are as follows:
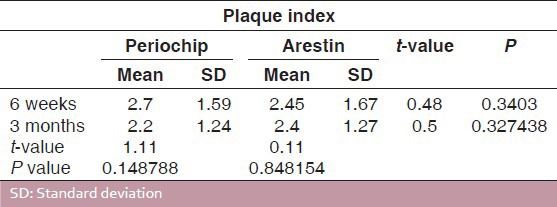
The values of plaque scores for group I and group II obtained at 6 weeks and 3 months intervals are presented in Tables 1 and 2. The difference between the mean plaque scores was subjected to statistical analysis to know its significance by employing Student's “t” test. The difference in the mean plaque scores between group I and group II was found to be statistically insignificant at 5% level of probability [Table 3 and Graph 1].
Table 1.
Plaque scores for Group I at baseline, 6 weeks and 3 months

Table 2.
Plaque scores for Group II at baseline, 6 weeks and 3 months
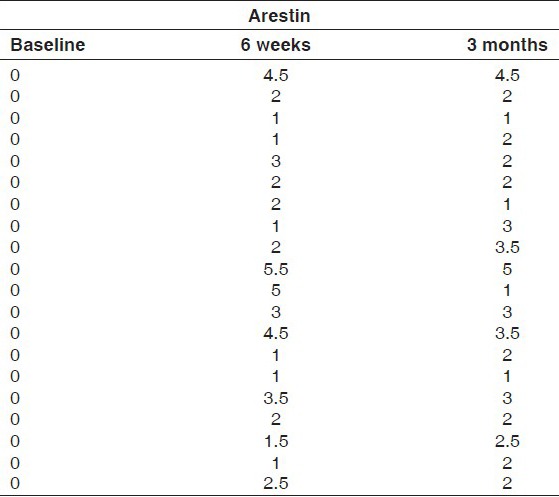
Table 3.
Comparison of plaque scores for Group I (periochip) and Group II (arestin)
Graph 1.
Comparison of plaque scores for group 1 (Periochip) and group 2 (Arestin)
The gingival index scores of group I and group II at 6 weeks and 3 months intervals are presented in Tables 4 and 5. For both the groups, the difference in the mean gingival scores obtained at 6 weeks and 3 months intervals was found to be statistically insignificant at 5% level of probability, although there was a statistically significant reduction in gingival index scores obtained at baseline and those obtained at 6 weeks and 3 months for both the groups [Table 6 and Graph 2].
Table 4.
Gingival index scores for Group I at baseline, 6 weeks and 3 months
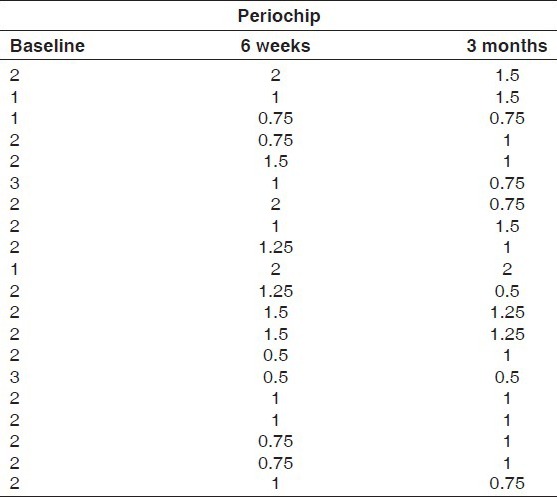
Table 5.
Gingival index scores for Group II at baseline, 6 weeks and 3 months
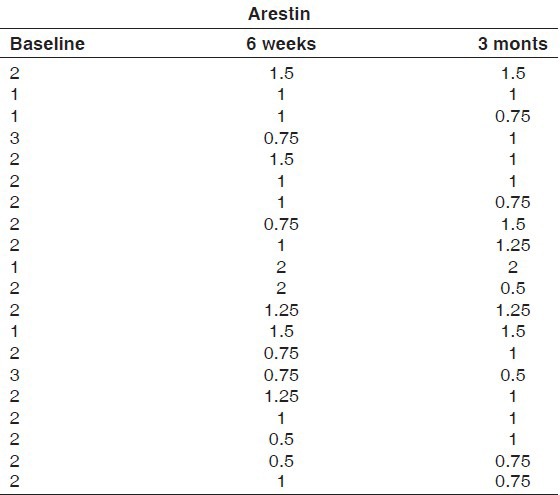
Table 6.
Comparison of gingival index scores for Group I (periochip) and Group II (arestin)
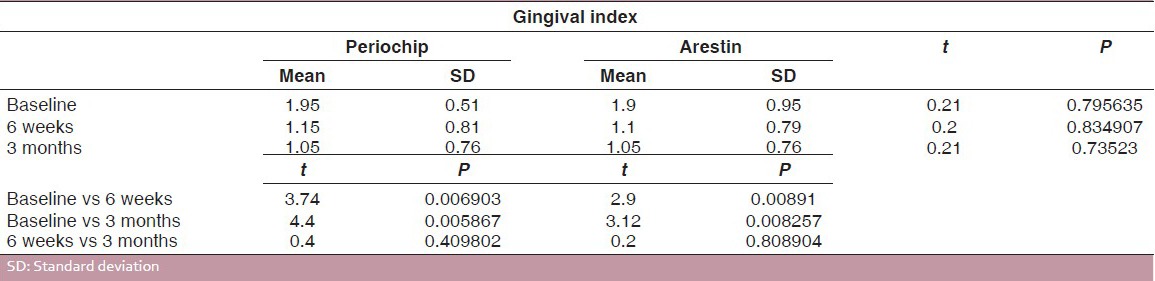
Graph 2.

Comparison of gingival scores for group 1 (Periochip) and group 2 (Arestin)
Tables 7 and 8 show the pocket probing depths of group I and group II at baseline, 6 weeks, and 3 months intervals. The difference in the mean probing depth between group I and group II at baseline and at 3 months was found to be statistically insignificant at 5% level of probability. There was a statistically significant reduction in mean probing depth from baseline to 6 weeks and 3 months intervals in both the groups. The difference in the mean probing depth obtained at 6 weeks and 3 months intervals for group I was found to statistically significant, whereas for group II, it was found to be insignificant [Table 9 and Graph 3].
Table 7.
Probing depths for Group I at baseline, 6 weeks and 3 months
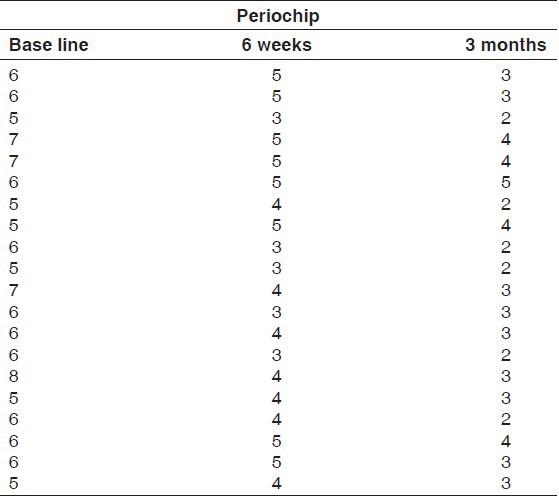
Table 8.
Probing depths for Group II at baseline, 6 weeks and 3 months
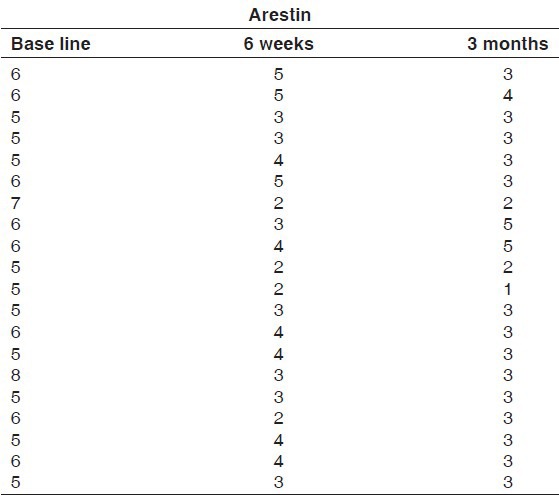
Table 9.
Comparison of probing depths for Group I (periochip) and Group II (Arestin)
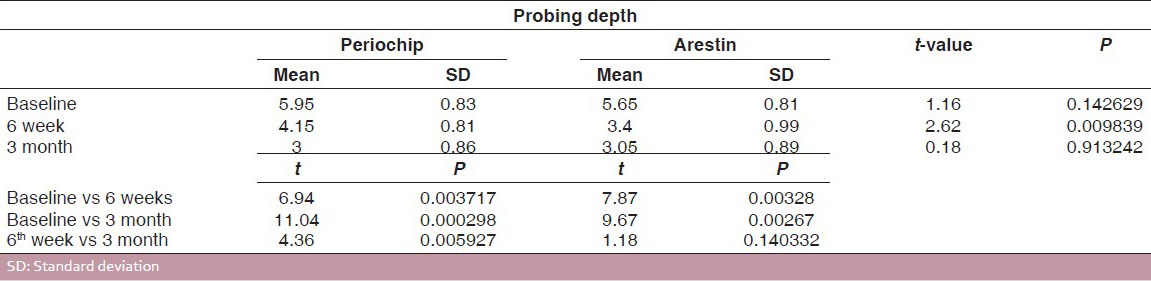
Graph 3.
Comparison of probing depth for group 1 (Periochip) and group 2 (Arestin)
DISCUSSION
Dental research has provided us with a better understanding of the microbial etiology and the nature of periodontitis. Periodontitis, initiated by bacteria, frequently appears in localized areas in the patient's mouth or is confined to localized areas by treatment. These infected localized areas lend themselves well to treatment using an antimicrobial agent. Antimicrobial agents may be used systemically or can be applied directly to the pocket. However, to obtain an effective concentration of the antimicrobial drug in the periodontal pocket after systemic administration, repeated intakes over a prolonged period of time may be required. In addition, unwanted effects such as development of resistant strains and superimposed infections preclude the use of these agents as the sole treatment modality. Therefore, nonresorbable and resorbable intrapocket drug delivery systems have been used, as they eliminate many of the adverse side effects associated with systemic delivery of antibiotics.[7]
The main objective of the periodontal therapy is the reduction or elimination of the periodontal pocket, which can be carried out by non-surgical or surgical methods. Twenty years ago, Goodson et al. (1979) first proposed the concept of “controlled delivery of antibiotics” in the treatment of periodontitis. Malathi et al. concluded that locally delivered antimicrobial agents are administered to prevent plaque accumulation and to disinfect the root surface and adjacent periodontal tissues. They are designed to enhance the healing following periodontal therapy.[8]
The present study was intended to compare two commercially available local drug delivery agents – Periochip (chlorhexidine gluconate 2.5 mg) and Arestin (Minocycline hydrochloride 1 mg) – in the treatment of chronic periodontitis.
Chlorhexidine (CHX) is a widely used broad-spectrum antimicrobial agent to inhibit bacterial growth and, thus, an adjunctive mean to control oral hygiene in patients with periodontal disease.[9] Periochip used in the present study is a bioresorbable CHX chip that enables slow subgingival release of 2.5 mg chlorhexidine gluconate. It maintains an average concentration of >125 mg/ml for 7–10 days in the crevicular fluid (Soskolne et al., 1998 as quoted by Mizrak et al.),[10] reported to be above the minimum inhibitory concentration of >99% of the subgingival microorganisms isolated from periodontal pockets. It has an added advantage of inducing negligible bacterial resistance as compared to antibiotics.[11] Arestin is a subgingival sustained-release product containing the antibiotic Minocycline hydrochloride (1 mg) incorporated into a bioresorbable polymer, poly (glycolide-co-Dl-lactide) or PGLA. This delivery system releases the antibiotic over a 2-week period at the site of administration at concentrations >300 μg/ml as measured in the gingival crevicular fluid.[12] Three months had been selected as the treatment interval for the placement of CHX because it corresponds to the typical recall interval for periodontal patients in practice.[13] A previous study has reported that CHX can inhibit the collagenolytic activity of MMP-8 and the oxidative activation of matrix metallo proteinases-8 by triggering degranulating neutrophils [polymorphonuclear neutrophils (PMNs)] in vitro (Gendron et al. in 1996 and Sorsa et al. in 1990, as quoted by Azmak et al.)[14] Minocycline hydrochloride, the semi-synthetic derivative of tetracycline, is one of the most active antibiotics against periodontal pathogens.
In the present study, the efficacy of Periochip and Arestin was compared in terms of amount of plaque accumulated, gingival index scores, and pocket probing depths at 6 weeks and 3 months. It was observed that there was no statistically significant difference in plaque scores for both the drugs, when recorded at 6 weeks and at 3 months. Similar findings were reported by Grisi et al. in 2002,[15] who evaluated the effects of a controlled-release CHX chip on the clinical and microbiological parameters of periodontal syndrome. They found no significant difference between the plaque scores over the entire study period of 9 months, but the gingival index scores at 6 weeks as well as at 3 months were found to be statistically significant; however, there was no statistically significant difference observed between the two groups. These findings are in accordance with the results of Cortelli et al.,[16] who clinically evaluated adjunctive Minocycline in the treatment of chronic periodontitis in 2006. They observed a significant reduction in gingival scores. Similar findings were also observed by Renvert et al. in 2006,[17] who evaluated topical Minocycline microspheres versus topical CHX gel as an adjunct to mechanical debridement of incipient peri-implant infections. With respect to probing depth, a statistically significant difference was observed between the two groups from baseline to 6 weeks for both the drugs. The findings of the present study are in accordance with the results of Jeffcoat et al.,[18] who used biodegradable CHX chip in the treatment of adult periodontitis and concluded that CHX chip when used as an adjunct to SRP significantly reduces loss of alveolar bone. Grisi et al.,[15] Azmak et al.,[9] Heasman et al., and Rodrigues et al.[19] studied the effect of CHX chip in periodontal maintenance therapy. The patients were assessed for plaque index, bleeding on probing, probing depth, clinical attachment level, and gingival recession at baseline, 6 weeks, 3 and 6 months. They concluded that CHX chip was more effective than SRP alone in reducing probing depth. Jeffcoat et al.[20] also evaluated the adjunctive use of a subgingival controlled-release CHX chip and concluded that when it was used as an adjunct to SRP, it resulted in the reduction of probing depth and improvement in clinical attachment level, when compared with SRP alone. The results of the present study are in accordance with Williams et al.,[21] who evaluated the local delivery of Minocycline microspheres in the treatment of periodontitis. They observed statistical significant reduction in probing depth in the test group when measured at 1, 3, 6, and 9 months.
CONCLUSION
From the present study, it can be concluded that both the drugs were equally effective in reducing the plaque scores as well as gingival scores. It was further observed that Arestin resulted in better results at 6 weeks while Periochip showed better results at 3 months, with respect to probing depth reduction. However, longitudinal studies in a larger population are suggested to confirm the findings of the present study.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Genco RJ. Current view of risk factors for periodontal diseases. J Periodontol. 1996;67(Suppl):1041–9. doi: 10.1902/jop.1996.67.10s.1041. [DOI] [PubMed] [Google Scholar]
- 2.Grossi SG, Zambon JJ, Ho AW, Koch G, Dunford RG, Machtei EE, et al. Assessment of risk for periodontal disease I Risk indicators for attachment loss. J Periodontol. 1994;65:260–7. doi: 10.1902/jop.1994.65.3.260. [DOI] [PubMed] [Google Scholar]
- 3.Socransky SS. Relationship of bacteria to the etiology of periodontal disease. J Dent Res. 1970;49:203–22. doi: 10.1177/00220345700490020401. [DOI] [PubMed] [Google Scholar]
- 4.Moreno Villagrana AP, Gómez Clavel JF. Antimicrobial or subantimicrobial antibiotic therapy as an adjunct to the nonsurgical periodontal treatment: A Meta-Analysis. ISRN Dent 2012. 2012 doi: 10.5402/2012/581207. 581207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nair SC, Anoop KR. Intraperiodontal pocket: An ideal route for local antimicrobial drug delivery. J Adv Pharm Technol Res. 2012;3:9–15. doi: 10.4103/2231-4040.93558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tiwari G, Tiwari R, Sriwastawa B, Bhati L, Pandey S, Pandey P, et al. Drug delivery systems: An updated review. Int J Pharm Investig. 2012;2:2–11. doi: 10.4103/2230-973X.96920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryan ME. Nonsurgical approaches for the treatment of periodontal diseases. Dent Clin N Am. 2005;49:611–36. doi: 10.1016/j.cden.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 8.Malathi K, Jeevarekha M, Prem M, Singh A. Local Drug Delivery – A Targeted Approach. Int J Med Biosci. 2014;3:29–34. [Google Scholar]
- 9.Azmak N, Atilla G, Luoto H, Sorsa T. The effect of subgingival controlled- release delivery of chlorhexidine chip on clinical parameters and matrix metalloproteinase-8 levels in gingival crevicular fluid. J Periodontol. 2002;73:608–15. doi: 10.1902/jop.2002.73.6.608. [DOI] [PubMed] [Google Scholar]
- 10.Mizrak T, Güncü GN, Caglayan F, Balci TA, Aktar GS, Ipek F. Effect of a controlled- release chlorhexidine chip on clinical and microbiological parameters and prostaglandin E2 levels in gingival crevicular fluid. J Periodontol. 2006;77:437–43. doi: 10.1902/jop.2006.050105. [DOI] [PubMed] [Google Scholar]
- 11.Greenstein G, Berman C, Jaffin R. Chlorhexidine: An adjunct to periodontal therapy. J Periodontol. 1986;57:370–7. doi: 10.1902/jop.1986.57.6.370. [DOI] [PubMed] [Google Scholar]
- 12.Williams RC, Paquette DW, Offenbacher S, Adams DF, Armitage GC, Bray K, et al. Treatment of periodontitis by local administration of Minocycline Microspheres: A controlled trial. J Periodontol. 2001;72:1535–44. doi: 10.1902/jop.2001.72.11.1535. [DOI] [PubMed] [Google Scholar]
- 13.Jeffcoat MK, Bray KS, Ciancio SG, Dentino AR, Fine DH, Gordon JM, et al. Adjunctive use of a subgingival controlled-release chlorhexidine chip reduces probing depth and improves attachment level compared with scaling and root planing alone. J Periodontol. 1998;69:989–97. doi: 10.1902/jop.1998.69.9.989. [DOI] [PubMed] [Google Scholar]
- 14.Azmak N, Atilla G, Luoto H, Sorsa T. The effect of subgingival controlled- release delivery of chlorhexidine chip on clinical parameters and matrix metalloproteinase-8 levels in gingival crevicular fluid. J Periodontol. 2002;73:608–15. doi: 10.1902/jop.2002.73.6.608. [DOI] [PubMed] [Google Scholar]
- 15.Grisi DC, Salvador SL, Figueiredo LC, Souza SL, Novaes AB, Grisi MF. Effect of a controlled-release chlorhexidine chip on clinical and microbiological parameters of periodontal syndrome. J Clin Periodontol. 2002;29:875–81. doi: 10.1034/j.1600-051x.2002.291001.x. [DOI] [PubMed] [Google Scholar]
- 16.Cortelli JR, Querido SM, Aquino DR, Ricardo LH, Pallos D. Longitudinal clinical evaluation of adjunct Minocycline in the treatment of chronic periodontitis. J Periodontol. 2006;77:161–6. doi: 10.1902/jop.2006.040409. [DOI] [PubMed] [Google Scholar]
- 17.Renvert S, Lessem J, Dahlén G, Lindahl C, Svensson M. Topical minocycline microspheres versus topical chlorhexidine gel as an adjunct to mechanical debridement of incipient peri-implant infections: A randomized clinical trial. J Clin Periodontol. 2006;33:362–9. doi: 10.1111/j.1600-051X.2006.00919.x. [DOI] [PubMed] [Google Scholar]
- 18.Jeffcoat M, Palcanis K, Weatherford T, Reese M, Geurs N, Flashner M. Use of a biodegradable chlorhexidine chip in the treatment of adult periodontitis: Clinical and radiographic findings. J Periodontol. 2002;71:256–62. doi: 10.1902/jop.2000.71.2.256. [DOI] [PubMed] [Google Scholar]
- 19.Rodrigues IF, Machion L, Casati MZ, Nociti FH, Jr, de Toledo S, Sallum AW, et al. Clinical evaluation of the use of locally delivered chlorhexidine in periodontal maintenance therapy. J Periodontol. 2007;78:624–8. doi: 10.1902/jop.2007.060317. [DOI] [PubMed] [Google Scholar]
- 20.Jeffcoat MK, Bray KS, Ciancio SG, Dentino AR, Fine DH, Gordon JM, et al. Adjunctive use of a subgingival controlled –Release chlorhexidine chip reduces probing depth and improves attachment level compared with scaling and root planing alone. J Periodontol. 1998;69:989–97. doi: 10.1902/jop.1998.69.9.989. [DOI] [PubMed] [Google Scholar]
- 21.Williams RC, Paquette DW, Offenbacher S, Adams DF, Armitage GC, Bray K, et al. Treatment of periodontitis by local administration of Minocycline Microspheres: A controlled trial. J Periodontol. 2001;72:1535–44. doi: 10.1902/jop.2001.72.11.1535. [DOI] [PubMed] [Google Scholar]


