Abstract
The greatest challenge to successful treatment of spinal cord injury is the limited regenerative capacity of the central nervous system and its inability to replace lost neurons and severed axons following injury. Neural stem cell grafts derived from fetal central nervous system tissue or embryonic stem cells have shown therapeutic promise by differentiation into neurons and glia that have the potential to form functional neuronal relays across injured spinal cord segments. However, implementation of fetal-derived or embryonic stem cell-derived neural stem cell therapies for patients with spinal cord injury raises ethical concerns. Induced pluripotent stem cells can be generated from adult somatic cells and differentiated into neural stem cells suitable for therapeutic use, thereby providing an ethical source of implantable cells that can be made in an autologous fashion to avoid problems of immune rejection. This review discusses the therapeutic potential of human induced pluripotent stem cell-derived neural stem cell transplantation for treatment of spinal cord injury, as well as addressing potential mechanisms, future perspectives and challenges.
Keywords: transplantation, axonal growth, axonal regeneration, neuroprotection, remyelination, differentiation, neuronal relay, human, astrocytes, neurons, oligodendrocytes, secondary degeneration
Stem cell therapies for treatment of spinal cord injury
The greatest challenge to recovery of function following spinal cord injury (SCI) is the limited regenerative capacity of the central nervous system (CNS), such that it cannot replace lost neurons and severed axons following injury. Transplantation of stem cells, especially neural stem cells (NSCs), can replace lost neurons and glia and enhance regeneration by providing a permissive growth environment. We previously found that rat and human fetal spinal cord tissue-derived NSCs survive implantation, fill even large lesion cavities after severe SCI and differentiate into neurons and glia. Host axon growth into the NSC graft and extensive graft-derived axon growth into host tissue forms a neuronal relay across the injury site, leading to functional recovery (Lu et al., 2012). However, use of fetal-derived or embryonic stem (ES) cell-derived NSCs for treatment of SCI is limited by ethical concerns and the need for immunosuppression regimens that may further compromise the general health of patients with SCI.
Induced pluripotent stem cells (iPSCs), generated by reprogramming adult somatic cells to a self-renewing, pluripotent state (Takahashi et al., 2007), allay ethical concerns associated with use of human fetal/embryonic tissue and may also reduce the risk of immune rejection of implanted cells. Clinical iPSC-based therapy would allow skin cells taken from a patient with SCI to be reprogrammed into autologous iPSCs that would be further differentiated into NSCs for implantation into the lesion site of that individual (Figure 1A). Survival, proliferation and differentiation of implanted iPSC-derived NSCs into neurons would be expected to give rise to extensive axon growth throughout the white and grey matter (Figure 1B), as has been shown in preclinical studies (Lu et al., 2014), thereby making functional improvement possible through neuronal relays that bridge the injured spinal cord segments (Lu et al., 2012).
Figure 1.
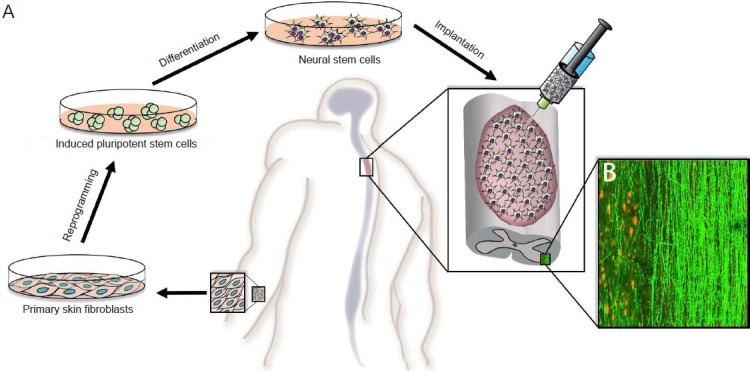
Schematic illustration of the potential clinical application of iPSC-derived NSC therapies in patients with SCI.
(A) Patient-derived fibroblasts would be reprogrammed to iPSCs, differentiated into NSCs and implanted into the SCI site as an autologous graft. (B) A representative image illustrating robust axon outgrowth (shown in green, expression of GFP) along white matter tracts and projections into host grey matter (indicated by NeuN labeling of host neurons in red) by iPSC-derived NSCs that have differentiated into neurons in our rat study. A target of clinical translation may be to use iPSC-derived NSCs to generate neuronal relays to yield functional recovery.
iPSC: Induced pluripotent stem cell; NSCs: neural stem cells; SCI: spinal cord injury; GFP: green fluorescent protein.
Generation of iPSC-derived NSCs
The reprogramming of human somatic cells into iPSCs is accomplished by inducing these cells to express four transcription factors: octamer-binding transcription factor 4 (Oct4), sex-determining region Y box 2 (Sox2), Krüppel-like factor (Klf4) and c-Myc (Takahashi et al., 2007). These transgenes have commonly been expressed via viral delivery, but this approach leads to multiple genomic insertions and carries a high risk of mutagenesis and tumorigenicity (Han et al., 2011). Strategies to eliminate or reduce genome integration include development of excision systems, such as the cre-lox (Chang et al., 2009) and PiggyBac transposon systems (Kaji et al., 2009), or use of non-integrating delivery approaches, such as episomal vectors (Yu et al., 2009), Sendai virus (Nakanishi and Otsu, 2012), plasmid DNA (Okita et al., 2008), mRNA (Schott et al., 2011) or direct protein delivery (Zhou et al., 2009). Further research has focused on improving the method of reprogramming with the goal of efficiently, rapidly and inexpensively generating non-tumorigenic iPSCs without use of oncogenes, such as c-Myc, or animal-derived products (Kramer et al., 2013).
iPSCs can be driven to become neural-restricted lineages by using the same protocols that have been developed for neural differentiation of ES cells. Differentiation to NSCs occurs via embryoid body formation, adherent monolayer culture or co-culture with stromal feeder cells in the presence of growth factors and cytokines. Neural progenitors can be further differentiated to distinct neural fates using sonic hedgehog, retinoic acid, fibroblast growth factor 8 or Wnts, factors that are involved in dorsoventral and rostrocaudal patterning of the developing nervous system (Han et al., 2011). Use of these techniques can yield oligodendrocytes, astrocytes, and specific neuronal subtypes including glutamatergic, GABAergic, motor and peripheral sensory neurons [for a comprehensive review see (Kramer et al., 2013)]. Further research will be required to identify what types of neuronal precursors yield greatest therapeutic benefit for treatment of SCI.
Direct conversion of fibroblasts to neurons or NSCs
In addition to using iPSCs as a source of NSCs, neurons or NSCs can be directly re-programmed/converted from embryonic and adult somatic cells. A combination of three neural lineage-specific transcription factors, Ascl1, Brn2 and Myt1l, can be used to convert mouse fibroblasts into induced neurons that exhibit typical neuronal morphologies and express multiple neuronal markers (Vierbuchen et al., 2010). The same three factors, with addition of NeuroD1, have been shown to convert human fetal and postnatal fibroblasts into induced neurons (Pang et al., 2011). Direct conversion can also be used to specifically generate dopaminergic and motor neurons (Caiazzo et al., 2011; Pfisterer et al., 2011; Son et al., 2011). However, because induced neurons are post-mitotic and not expandable, their therapeutic potential may be limited, particularly if implanted into the harsh SCI lesion environment in which initial cell transplant survival may be low (Hermann and Storch, 2013). Thus far, cell transplantation of induced neurons into models of SCI has not been reported.
Induced neural stem cell (iNSC) generation from mouse fibroblasts has been reported using either pluripotency factors alone or in combination with neural lineage-specific transcription factors (Kim et al., 2011; Han et al., 2012; Lujan et al., 2012; Thier et al., 2012; Kim et al., 2014). These protocols yield NSCs with the potential to differentiate into neurons, astrocytes and oligodendrocytes. Human fibroblasts have been converted to iNSCs using either Sox2 or Oct4, yielding NSCs that gave rise to neurons, astrocytes and oligodendrocytes (Ring et al., 2012; Mitchell et al., 2014). A recent study demonstrated limited graft survival but improved locomotor outcomes following implantation of mouse-derived iNSCs into a rat model of spinal contusion (Hong et al., 2014), demonstrating the therapeutic potential of this approach.
The advantage of induced neurons and iNSCs over iPSC-derived NSCs is that somatic cells do not go through a pluripotent cell state, thus reducing the risk for tumor formation after transplantation (Kim et al., 2014). Furthermore, production of iNSCs and induced neurons takes less time than generation of iPSCs, and may be more clinically useful for generating autologous cells for early (acute or sub-acute) treatment of SCI.
Application of iPSC-derived NSCs in experimental models of SCI
Though iPSC-derived therapies have been used in various in vivo models of neurodegenerative disorders, few studies have evaluated efficacy of iPSC-derived NSC transplantation in models of SCI (Table 1).
Table 1.
Overview of studies reporting use of human iPSC-derived NSCs for treatment of SCI
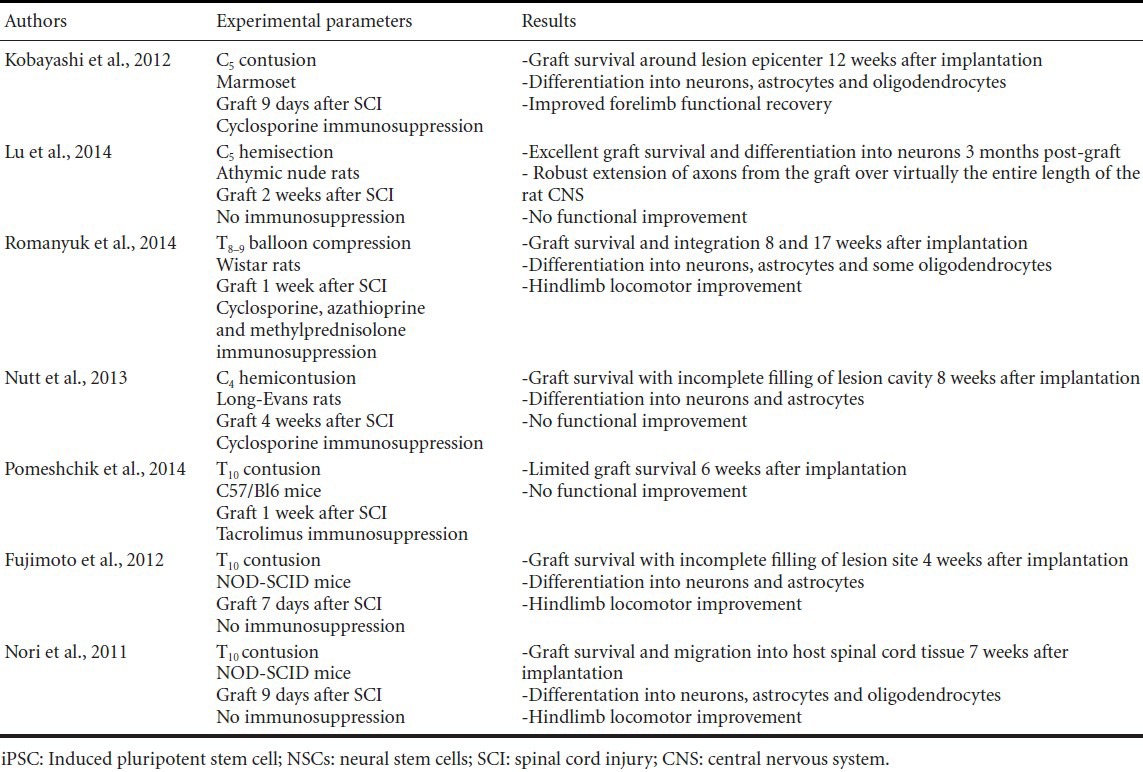
Mouse iPSC-derived NSCs grafted as neurospheres into a C57BL/6J mouse model of lower thoracic contusion differentiated into neurons, astrocytes and oligodendrocytes and yielded functional recovery comparable to that obtained following grafting of mouse ES cell-derived neurospheres despite low cell survival (Tsuji et al., 2010).
Improved locomotor function was also observed following transplantation of human iPSC-derived NSCs into NOD-SCID mice 7–9 days after lower thoracic contusion injury (Nori et al., 2011; Fujimoto et al., 2012). The human iPSC-derived neurospheres implanted in these studies differentiated primarily into astrocytes and neurons, forming synapses with host neurons and increasing host axon regeneration. Graft-derived oligodendrocytes were observed infrequently (< 1–3 %). Transplantation of the same human iPSC-derived NSCs described in Nori et al. (2011) into a marmoset model of C5 contusion 9 days after injury was also shown to have functional benefits, with cells differentiating in neurons (52 %), astrocytes (31 %) and oligodendrocytes (26 %) (Kobayashi et al., 2012).
Human iPSC-derived neural progenitor cells grafted into a Wistar rat model of thoracic balloon compression with use of a triple immunosuppression regimen showed robust survival within the lesion cavity and led to hindlimb locomotor improvement that was associated with improved white and grey matter sparing around the lesion site (Romanyuk et al., 2014). In contrast, human iPSC-derived NSCs that were implanted into a Long-Evans rat model of cervical hemi-contusion or C57Bl/6J mouse model of low thoracic contusion did not lead to functional improvement (Nutt et al., 2013; Pomeshchik et al., 2014). This may be because transplanted cell survival and/or filling of the lesion site was low in these studies, complicating interpretation of results.
We recently showed robust human iPSC-derived NSC survival, with implants forming a graft that filled most of the lesion cavity at the site of a C5 hemisection lesion (Lu et al., 2014). The iPSC-derived NSCs generated from the skin cells of an 86 year-old man were grafted into nude rats to eliminate the confound of immunosuppression on cell differentiation and axon outgrowth. A fibrin matrix containing growth factor cocktail was used to support cell retention, survival and differentiation (Lu et al., 2012). Cells not only survived, but the majority (71.2%) differentiated into neurons expressing the mature neuronal marker NeuN, while 17.2% differentiated into GFAP positive astrocytes. Neurons within the graft extended thousands of axons that traveled along the white matter tracts and projected into host grey matter, reaching as far rostrally as the olfactory bulb and as far caudally as the lumbar spinal cord. Host axon regeneration into the graft was also observed, with serotonergic and reticulospinal projections both penetrating the graft. The presence of both host-to-graft and graft-to-host synaptic contacts suggests a potential for relay formation across the graft. Nevertheless, no graft-derived improvement was observed in tests of forelimb function. This may be due to the presence of collagenous rifts or attenuated cell density in the center of most grafts, which may have resulted in discontinuous grafts with incomplete relay formation. Alternatively, as the majority of axons did not express neurofilament, a marker of mature axons, and were not myelinated by host oligodendrocytes, neural conduction may not have been optimal. The absence of neurofilament expression and myelination of graft-derived axons may be due to the greater time scale of neuronal maturation and myelination in human development compared to rat (Semple et al., 2013), and may complicate detection of putative functional improvements derived by relay formation. Experiments using human iPSC-derived NSCs may need to be conducted over longer durations to adequately detect functional improvements derived from relay circuit formation. Nevertheless, our data demonstrate that iPSC-derived NSCs have an equivalent capacity to survive, differentiate and send out axons throughout the neuraxis as do human fetal-derived and human ES cell-derived NSCs (Lu et al., 2012, 2014).
Potential mechanisms by which iPSCs provide therapeutic benefit
iPSC-derived NSC transplantation into SCI is expected to provide therapeutic benefit via the same mechanisms as fetal CNS-derived or ES cell-derived NSCs. These include neuronal relay formation across the injury site, improved host axon regeneration, remyelination of demyelinated axons around the lesion site and reduction of secondary damage through immunomodulation and neurotrophic effects.
Our previous study of embryonic CNS tissue-derived NSCs for treatment of SCI supported the idea that NSC transplants can be used to bridge the injured spinal cord (Lu et al., 2012). Neuronal relays are formed when host axons grow into the graft and synapse on graft-derived neurons that in turn send axons to distant targets, thereby allowing information to be passed across the lesion site (Jakeman and Reier, 1991). In our most recent study using iPSC-derived NSCs, we observe both growth and synapsing of host axons into the graft and extension of axons from graft-derived neurons to distant synaptic targets throughout the neuraxis (Lu et al., 2014). These observations support relay formation as a plausible mechanism for expected therapeutic improvement following iPSC-derived NSC graft into SCI, with the caveat that the long developmental time course of the human nervous system relative to the rat nervous system (Semple et al., 2013) may preclude observation of functional improvement derived from mature relay formation.
iPSC-derived cells, particularly oligodendrocyte precursor cells (OPCs), could also potentially be used to remyelinate host axons following SCI, which is associated with prolonged and dispersed oligodendrocyte cell death (Plemel et al., 2014). OPCs derived from human ES cells were found to remyelinate axons and modestly improve locomotor function in a rat model of lower thoracic contusion injury (Keirstead et al., 2005). Human iPSC-derived OPCs have recently been shown to remyelinate axons in a mouse model of congenital hypomyelination (Wang et al., 2013), suggesting that iPSC-derived OPCs may be a promising therapeutic approach for treatment of demyelination following SCI. Mouse iPSC-derived NSC grafts increased myelination in a mouse model of thoracic contusion injury (Tsuji et al., 2010), but, thus far, studies of human iPSC-derived NSC grafts in rodent models of SCI have not demonstrated extensive differentiation into oligodendrocytes (Nori et al., 2011; Fujimoto et al., 2012; Nutt et al., 2013; Lu et al., 2014).
Secondary damage can lead to ongoing loss of neurons and glia adjacent to a site of SCI that can persist for weeks to months following the initial injury (Silva et al., 2014). Embryonic tissue-derived NSC transplants are proposed to reduce secondary damage via immunomodulation and secretion of neurotrophic factors (Mothe and Tator, 2013). NSC transplants can promote neuroprotection by reducing T-cell activation and inhibiting signaling of inflammatory cytokines that are implicated in secondary degeneration (Fainstein et al., 2008; Cusimano et al., 2012), though these mechanisms have not yet been demonstrated using iPSC-derived NSCs. Embryonic-derived NSC transplants have been shown to express neurotrophic factors (Lu et al., 2003), and human iPSC-derived NSCs grafted into a mouse model of contusion injury were found to release neurotrophic factors including nerve growth factor, brain-derived neurotrophic factor and hepatocyte growth factor (Nori et al., 2011). This was associated with enhanced angiogenesis and tissue sparing, and may explain the observation of improved locomotor function despite low transplant survival (Nori et al., 2011). Likewise, the enhanced tissue sparing following implantation of human iPSC-derived neural progenitor cells in a rat model of balloon compression may be due to graft-derived neurotrophins (Romanyuk et al, 2014). Sustained expression of neurotrophic factors by iPSC-derived NSCs could also have therapeutic effects by enhancing host axon growth as has been shown following grafts of neurotrophin-secreting embryonic tissue-derived NSCs (Lu et al., 2003).
Future perspectives and challenges
Despite promising data indicating the clinical potential of iPSC-derived NSCs for treatment of SCI, several questions and challenges remain to be addressed as this therapy proceeds along the translational path. Methods of iPSC-generation need to be better understood, both to be more clinically practical and to yield cell types that will optimize therapeutic outcomes with minimal undesirable side effects. The impact of the lesion site itself, both on cell survival and differentiation, will need to be minimized so that cell grafts behave consistently across a variety of injury types. Finally, the extensive axon outgrowth that we have observed may result in innervation of ectopic targets, which could potentially be manipulated and shaped with axon guidance strategies.
Even though iPSC technology has undergone rapid advances, many aspects of iPSC generation will need to be refined before iPSC-derived NSCs can be translated for use in the clinic. In particular, it remains unclear whether iPSC-derived NSCs will prove to be a perfect replacement for ES cell-derived NSCs. Though initial studies demonstrated that iPSCs have similar pluripotency, gene expression and DNA methylation as ES cells (Takahashi et al., 2007), some differences in genetic signature have been detected (Chin et al., 2009). It is also unknown whether differences in the parent cell, method of iPSC generation or protocol used to differentiate iPSCs into NSCs will impact variables such as cell survival, differentiation or axon outgrowth after implantation. Current methods of iPSC generation are too inefficient or time-consuming to be of practical clinical use, particularly allowing for either generation of safer non-DNA integrating methods or adequate screening of tumorigenicity (Kramer et al., 2013). Induced neuron or iNSC approaches may partially resolve these concerns, but future experiments will need to evaluate the clinical potential of these cell types for treatment of SCI. Furthermore, despite assumptions that autologous iPSC grafts will not be rejected by the host, too few studies have been conducted to conclude this with certainty (Cao et al., 2014). These variables will need to be addressed before iPSC-derived NSCs can be translated for use in the clinic.
Though our studies have shown promising results with remarkable survival, proliferation and differentiation of implanted iPSC-derived NSCs, survival of grafted cells in the hostile SCI lesion environment remains a problem in a majority of studies. Direct reprogramming of somatic cells in vitro and then activation of these transferred genes after transplantation (Torper et al., 2013) may improve graft survival compared to direct implantation of iPSC-derived NSCs, as somatic cells such as fibroblasts are more resistant to the harsh environment of the lesion site. In situ reprogramming of astrocytes into neurons, as has been described in a mouse model of SCI (Su et al., 2014), may also bypass problems with initial graft survival by transforming the reactive astrocyte layer that is normally inhibitory to growth into functional neurons that could bridge the injured spinal cord and contribute to functional recovery.
In addition to impacting cell survival, the injured spinal cord environment may affect iPSC-derived NSC proliferation and differentiation. It is likely that the grafting milieu impacts the fate of iPSC-derived NSCs, as it has been reported that iPSC-derived neurospheres differentiated into neurons, astrocytes and oligodendrocytes when grafted into a marmoset model of cervical contusion injury, while the same cells differentiated primarily into neurons when cultured in vitro (Kobayashi et al., 2012). Similarly, neurospheres derived from the same iPSC clone primarily became neurons and astrocytes with very few (< 3 %) oligodendrocytes when grafted into a mouse model of thoracic contusion injury (Nori et al., 2011). Systematic studies may be required to address how the lesion environment impacts iPSC-derived NSC differentiation and to identify which cell types and conditions yield optimum therapeutic benefit.
The impressive ability of human iPSC-derived NSCs to extend axons over long distances throughout the rat nervous system (Lu et al., 2014) also raises questions as to how those connections will behave. Growth of axons onto ectopic targets seems inevitable in the absence of supplied axon guidance, and it is not yet known whether these axons will result in adverse behavioral consequences such as pain, as has been reported in some studies of NSC transplants into SCI (Hofstetter et al., 2005), and spasticity. However, it is also possible that aberrant projections will undergo pruning over time to refine functional plasticity and restore spinal cord function, particularly if locomotor or rehabilitative training is applied (Ichiyama et al., 2008). There is also great potential for the development of strategies that will optimize use of iPSC-derived NSCs for treatment of SCI. For example, guidance of axons coming out of the graft either by using scaffolds (Sakiyama-Elbert et al., 2012) or guidance molecules (Giger et al., 2010) may help target axons to appropriate termini. Likewise, the limited regeneration of host spinal tracts into the graft that we have observed could be enhanced by secretion of growth-promoting factors within the graft (Lu et al., 2007), or through the use of chemotropic guidance to support growth of host tracts through the permissive environment of the graft and directly back on to their original targets (Alto et al., 2009). However, questions relating to the functional effects of grafted human iPSC-derived NSCs may be difficult to evaluate in rodent pre-clinical models of SCI if the maturation time is on the human scale and may necessitate use of primate models of SCI evaluated over longer durations.
Conclusions
iPSCs bypass ethical concerns about use of embryonic/fetal-derived cells, and are a promising source of NSCs for treatment of patients with SCI. Preclinical work has demonstrated promising survival, differentiation and therapeutic effects following implantation of human iPSC-derived NSCs into pre-clinical models of SCI. Beneficial effects can be derived from neuronal relay formation, re-myelination or reduction of secondary damage cascades. Nevertheless, ideal methods of iPSC reprogramming and differentiation remain to be developed for clinical translation, and further experimentation is necessary at the pre-clinical level to better understand the impact of different methods of iPSC reprogramming on survival, differentiation and therapeutic outcomes.
Footnotes
Funding: This work was supported by grants from the Veterans Administration and the California Institute for Regenerative Medicine.
Conflicts of interest: None declared.
References
- Alto LT, Havton LA, Conner JM, Hollis ER, 2nd, Blesch A, Tuszynski MH. Chemotropic guidance facilitates axonal regeneration and synapse formation after spinal cord injury. Nat Neurosci. 2009;12:1106–1113. doi: 10.1038/nn.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caiazzo M, Dell’Anno MT, Dvoretskova E, Lazarevic D, Taverna S, Leo D, Sotnikova TD, Menegon A, Roncaglia P, Colciago G, Russo G, Carninci P, Pezzoli G, Gainetdinov RR, Gustincich S, Dityatev A, Broccoli V. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature. 2011;476:224–227. doi: 10.1038/nature10284. [DOI] [PubMed] [Google Scholar]
- Cao J, Li X, Lu X, Zhang C, Yu H, Zhao T. Cells derived from iPSC can be immunogenic - yes or no? Protein Cell. 2014;5:1–3. doi: 10.1007/s13238-013-0003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CW, Lai YS, Pawlik KM, Liu K, Sun CW, Li C, Schoeb TR, Townes TM. Polycistronic lentiviral vector for “hit and run” reprogramming of adult skin fibroblasts to induced pluripotent stem cells. Stem Cells. 2009;27:1042–1049. doi: 10.1002/stem.39. [DOI] [PubMed] [Google Scholar]
- Chin MH, et al. Induced pluripotent stem cells and embryonic stem cells are distinguished by gene expression signatures. Cell Stem Cell. 2009;5:111–123. doi: 10.1016/j.stem.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusimano M, Biziato D, Brambilla E, Donega M, Alfaro-Cervello C, Snider S, Salani G, Pucci F, Comi G, Garcia-Verdugo JM, De Palma M, Martino G, Pluchino S. Transplanted neural stem/precursor cells instruct phagocytes and reduce secondary tissue damage in the injured spinal cord. Brain. 2012;135:447–460. doi: 10.1093/brain/awr339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fainstein N, Vaknin I, Einstein O, Zisman P, Ben Sasson SZ, Baniyash M, Ben-Hur T. Neural precursor cells inhibit multiple inflammatory signals. Mol Cell Neurosci. 2008;39:335–341. doi: 10.1016/j.mcn.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Fujimoto Y, Abematsu M, Falk A, Tsujimura K, Sanosaka T, Juliandi B, Semi K, Namihira M, Komiya S, Smith A, Nakashima K. Treatment of a mouse model of spinal cord injury by transplantation of human induced pluripotent stem cell-derived long-term self-renewing neuroepithelial-like stem cells. Stem Cells. 2012;30:1163–1173. doi: 10.1002/stem.1083. [DOI] [PubMed] [Google Scholar]
- Giger RJ, Hollis ER, 2nd, Tuszynski MH. Guidance molecules in axon regeneration. Cold Spring Harb Perspect Biol. 2010;2:a001867. doi: 10.1101/cshperspect.a001867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han DW, Tapia N, Hermann A, Hemmer K, Hoing S, Arauzo-Bravo MJ, Zaehres H, Wu G, Frank S, Moritz S, Greber B, Yang JH, Lee HT, Schwamborn JC, Storch A, Scholer HR. Direct reprogramming of fibroblasts into neural stem cells by defined factors. Cell Stem Cell. 2012;10:465–472. doi: 10.1016/j.stem.2012.02.021. [DOI] [PubMed] [Google Scholar]
- Han SS, Williams LA, Eggan KC. Constructing and deconstructing stem cell models of neurological disease. Neuron. 2011;70:626–644. doi: 10.1016/j.neuron.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Hermann A, Storch A. Induced neural stem cells (iNSCs) in neurodegenerative diseases. J Neural Transm. 2013;120(Suppl 1):S19–25. doi: 10.1007/s00702-013-1042-9. [DOI] [PubMed] [Google Scholar]
- Hofstetter CP, Holmstrom NA, Lilja JA, Schweinhardt P, Hao J, Spenger C, Wiesenfeld-Hallin Z, Kurpad SN, Frisen J, Olson L. Allodynia limits the usefulness of intraspinal neural stem cell grafts; directed differentiation improves outcome. Nat Neurosci. 2005;8:346–353. doi: 10.1038/nn1405. [DOI] [PubMed] [Google Scholar]
- Hong JY, Lee SH, Lee SC, Kim JW, Kim KP, Kim SM, Tapia N, Lim KT, Kim J, Ahn HS, Ko K, Shin CY, Lee HT, Scholer HR, Hyun JK, Han DW. Therapeutic potential of induced neural stem cells for spinal cord injury. J Biol Chem. 2014;289:32512–32525. doi: 10.1074/jbc.M114.588871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichiyama RM, Courtine G, Gerasimenko YP, Yang GJ, van den Brand R, Lavrov IA, Zhong H, Roy RR, Edgerton VR. Step training reinforces specific spinal locomotor circuitry in adult spinal rats. J Neurosci. 2008;28:7370–7375. doi: 10.1523/JNEUROSCI.1881-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakeman LB, Reier PJ. Axonal projections between fetal spinal cord transplants and the adult rat spinal cord: a neuroanatomical tracing study of local interactions. J Comp Neurol. 1991;307:311–334. doi: 10.1002/cne.903070211. [DOI] [PubMed] [Google Scholar]
- Kaji K, Norrby K, Paca A, Mileikovsky M, Mohseni P, Woltjen K. Virus-free induction of pluripotency and subsequent excision of reprogramming factors. Nature. 2009;458:771–775. doi: 10.1038/nature07864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keirstead HS, Nistor G, Bernal G, Totoiu M, Cloutier F, Sharp K, Steward O. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci. 2005;25:4694–4705. doi: 10.1523/JNEUROSCI.0311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Efe JA, Zhu S, Talantova M, Yuan X, Wang S, Lipton SA, Zhang K, Ding S. Direct reprogramming of mouse fibroblasts to neural progenitors. Proc Natl Acad Sci U S A. 2011;108:7838–7843. doi: 10.1073/pnas.1103113108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SM, Flasskamp H, Hermann A, Arauzo-Bravo MJ, Lee SC, Lee SH, Seo EH, Storch A, Lee HT, Scholer HR, Tapia N, Han DW. Direct conversion of mouse fibroblasts into induced neural stem cells. Nat Protoc. 2014;9:871–881. doi: 10.1038/nprot.2014.056. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Okada Y, Itakura G, Iwai H, Nishimura S, Yasuda A, Nori S, Hikishima K, Konomi T, Fujiyoshi K, Tsuji O, Toyama Y, Yamanaka S, Nakamura M, Okano H. Pre-evaluated safe human iPSC-derived neural stem cells promote functional recovery after spinal cord injury in common marmoset without tumorigenicity. PLoS One. 2012;7:e52787. doi: 10.1371/journal.pone.0052787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer AS, Harvey AR, Plant GW, Hodgetts SI. Systematic review of induced pluripotent stem cell technology as a potential clinical therapy for spinal cord injury. Cell Transplant. 2013;22:571–617. doi: 10.3727/096368912X655208. [DOI] [PubMed] [Google Scholar]
- Lu P, Jones LL, Snyder EY, Tuszynski MH. Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Exp Neurol. 2003;181:115–129. doi: 10.1016/s0014-4886(03)00037-2. [DOI] [PubMed] [Google Scholar]
- Lu P, Jones LL, Tuszynski MH. Axon regeneration through scars and into sites of chronic spinal cord injury. Exp Neurol. 2007;203:8–21. doi: 10.1016/j.expneurol.2006.07.030. [DOI] [PubMed] [Google Scholar]
- Lu P, Wang Y, Graham L, McHale K, Gao M, Wu D, Brock J, Blesch A, Rosenzweig ES, Havton LA, Zheng B, Conner JM, Marsala M, Tuszynski MH. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell. 2012;150:1264–1273. doi: 10.1016/j.cell.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Woodruff G, Wang Y, Graham L, Hunt M, Wu D, Boehle E, Ahmad R, Poplawski G, Brock J, Goldstein LS, Tuszynski MH. Long-distance axonal growth from human induced pluripotent stem cells after spinal cord injury. Neuron. 2014;83:789–796. doi: 10.1016/j.neuron.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan E, Chanda S, Ahlenius H, Sudhof TC, Wernig M. Direct conversion of mouse fibroblasts to self-renewing, tripotent neural precursor cells. Proc Natl Acad Sci U S A. 2012;109:2527–2532. doi: 10.1073/pnas.1121003109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell RR, Szabo E, Benoit YD, Case DT, Mechael R, Alamilla J, Lee JH, Fiebig-Comyn A, Gillespie DC, Bhatia M. Activation of neural cell fate programs toward direct conversion of adult human fibroblasts into tri-potent neural progenitors using OCT-4. Stem Cells Dev. 2014;23:1937–1946. doi: 10.1089/scd.2014.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothe AJ, Tator CH. Review of transplantation of neural stem/progenitor cells for spinal cord injury. Int J Dev Neurosci. 2013;31:701–713. doi: 10.1016/j.ijdevneu.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Nakanishi M, Otsu M. Development of Sendai virus vectors and their potential applications in gene therapy and regenerative medicine. Curr Gene Ther. 2012;12:410–416. doi: 10.2174/156652312802762518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nori S, Okada Y, Yasuda A, Tsuji O, Takahashi Y, Kobayashi Y, Fujiyoshi K, Koike M, Uchiyama Y, Ikeda E, Toyama Y, Yamanaka S, Nakamura M, Okano H. Grafted human-induced pluripotent stem-cell-derived neurospheres promote motor functional recovery after spinal cord injury in mice. Proc Natl Acad Sci U S A. 2011;108:16825–16830. doi: 10.1073/pnas.1108077108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt SE, Chang EA, Suhr ST, Schlosser LO, Mondello SE, Moritz CT, Cibelli JB, Horner PJ. Caudalized human iPSC-derived neural progenitor cells produce neurons and glia but fail to restore function in an early chronic spinal cord injury model. Exp Neurol. 2013;248:491–503. doi: 10.1016/j.expneurol.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322:949–953. doi: 10.1126/science.1164270. [DOI] [PubMed] [Google Scholar]
- Pang ZP, Yang N, Vierbuchen T, Ostermeier A, Fuentes DR, Yang TQ, Citri A, Sebastiano V, Marro S, Sudhof TC, Wernig M. Induction of human neuronal cells by defined transcription factors. Nature. 2011;476:220–223. doi: 10.1038/nature10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfisterer U, Kirkeby A, Torper O, Wood J, Nelander J, Dufour A, Bjorklund A, Lindvall O, Jakobsson J, Parmar M. Direct conversion of human fibroblasts to dopaminergic neurons. Proc Natl Acad Sci U S A. 2011;108:10343–10348. doi: 10.1073/pnas.1105135108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plemel JR, Keough MB, Duncan GJ, Sparling JS, Yong VW, Stys PK, Tetzlaff W. Remyelination after spinal cord injury: is it a target for repair? Prog Neurobiol. 2014;117:54–72. doi: 10.1016/j.pneurobio.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Pomeshchik Y, Puttonen KA, Kidin I, Ruponen M, Lehtonen S, Malm T, Akesson E, Hovatta O, Koistinaho J. Transplanted human iPSC-derived neural progenitor cells do not promote functional recovery of pharmacologically immunosuppressed mice with contusion spinal cord injury. Cell Transplant. 2014. doi: http://dx.doi.org/10.3727/096368914X684079 . [DOI] [PubMed]
- Ring KL, Tong LM, Balestra ME, Javier R, Andrews-Zwilling Y, Li G, Walker D, Zhang WR, Kreitzer AC, Huang Y. Direct reprogramming of mouse and human fibroblasts into multipotent neural stem cells with a single factor. Cell Stem Cell. 2012;11:100–109. doi: 10.1016/j.stem.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanyuk N, Amemori T, Turnovcova K, Prochazka P, Onteniente B, Sykova E, Jendelova P. Beneficial effect of human induced pluripotent stem cell-derived neural precursors in spinal cord injury repair. Cell Transplant. 2014. doi: http://dx.doi.org/10.3727/096368914X684042 . [DOI] [PubMed]
- Sakiyama-Elbert S, Johnson PJ, Hodgetts SI, Plant GW, Harvey AR. Scaffolds to promote spinal cord regeneration. Handb Clin Neurol. 2012;109:575–594. doi: 10.1016/B978-0-444-52137-8.00036-X. [DOI] [PubMed] [Google Scholar]
- Schott JW, Galla M, Godinho T, Baum C, Schambach A. Viral and non-viral approaches for transient delivery of mRNA and proteins. Curr Gene Ther. 2011;11:382–398. doi: 10.2174/156652311797415872. [DOI] [PubMed] [Google Scholar]
- Semple BD, Blomgren K, Gimlin K, Ferriero DM, Noble-Haeusslein LJ. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog Neurobiol. 2013;106-107:1–16. doi: 10.1016/j.pneurobio.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva NA, Sousa N, Reis RL, Salgado AJ. From basics to clinical: a comprehensive review on spinal cord injury. Prog Neurobiol. 2014;114:25–57. doi: 10.1016/j.pneurobio.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Son EY, Ichida JK, Wainger BJ, Toma JS, Rafuse VF, Woolf CJ, Eggan K. Conversion of mouse and human fibroblasts into functional spinal motor neurons. Cell Stem Cell. 2011;9:205–218. doi: 10.1016/j.stem.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Z, Niu W, Liu ML, Zou Y, Zhang CL. In vivo conversion of astrocytes to neurons in the injured adult spinal cord. Nat Commun. 2014;5:3338. doi: 10.1038/ncomms4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Thier M, Worsdorfer P, Lakes YB, Gorris R, Herms S, Opitz T, Seiferling D, Quandel T, Hoffmann P, Nothen MM, Brustle O, Edenhofer F. Direct conversion of fibroblasts into stably expandable neural stem cells. Cell Stem Cell. 2012;10:473–479. doi: 10.1016/j.stem.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Torper O, Pfisterer U, Wolf DA, Pereira M, Lau S, Jakobsson J, Bjorklund A, Grealish S, Parmar M. Generation of induced neurons via direct conversion in vivo. Proc Natl Acad Sci U S A. 2013;110:7038–7043. doi: 10.1073/pnas.1303829110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji O, et al. Therapeutic potential of appropriately evaluated safe-induced pluripotent stem cells for spinal cord injury. Proc Natl Acad Sci U S A. 2010;107:12704–12709. doi: 10.1073/pnas.0910106107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Bates J, Li X, Schanz S, Chandler-Militello D, Levine C, Maherali N, Studer L, Hochedlinger K, Windrem M, Goldman SA. Human iPSC-derived oligodendrocyte progenitor cells can myelinate and rescue a mouse model of congenital hypomyelination. Cell Stem Cell. 2013;12:252–264. doi: 10.1016/j.stem.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, Slukvin II, Thomson JA. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324:797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Wu S, Joo JY, Zhu S, Han DW, Lin T, Trauger S, Bien G, Yao S, Zhu Y, Siuzdak G, Scholer HR, Duan L, Ding S. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell. 2009;4:381–384. doi: 10.1016/j.stem.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


