Abstract
Pretreatment of nerve allografts by exposure to irradiation or green tea polyphenols can eliminate neuroimmunogenicity, inhibit early immunological rejection, encourage nerve regeneration and functional recovery, improve tissue preservation, and minimize postoperative infection. In the present study, we investigate which intervention achieves better results. We produced a 1.0 cm sciatic nerve defect in rats, and divided the rats into four treatment groups: autograft, fresh nerve allograft, green tea polyphenol-pretreated (1 mg/mL, 4°C) nerve allograft, and irradiation-pretreated nerve allograft (26.39 Gy/min for 12 hours; total 19 kGy). The animals were observed, and sciatic nerve electrophysiology, histology, and transmission electron microscopy were carried out at 6 and 12 weeks after grafting. The circumference and structure of the transplanted nerve in rats that received autografts or green tea polyphenol-pretreated nerve allografts were similar to those of the host sciatic nerve. Compared with the groups that received fresh or irradiation-pretreated nerve allografts, motor nerve conduction velocity in the autograft and fresh nerve allograft groups was greater, more neurites grew into the allografts, Schwann cell proliferation was evident, and a large number of new blood vessels was observed; in addition, massive myelinated nerve fibers formed, and abundant microfilaments and microtubules were present in the axoplasm. Our findings indicate that nerve allografts pretreated by green tea polyphenols are equivalent to transplanting autologous nerves in the repair of sciatic nerve defects, and promote nerve regeneration. Pretreatment using green tea polyphenols is better than pretreatment with irradiation.
Keywords: nerve regeneration, peripheral nerve injury, allograft, green tea polyphenols, irradiation, sciatic nerve, transplantation, nerve defects, nerve repair, alternative, nerual regeneration
Introduction
It is estimated that peripheral nerve injury accounts for 1.5–4.0% of new trauma cases worldwide each year (Taylor et al., 2008), and the repair and reconstruction of peripheral nerve defects remains a major clinical problem (Gu et al., 2011). Autologous nerve transplantation is the preferred treatment at present, but its use is limited by difficulties in finding sources for the repair of long-segment nerve defects, and a risk of complications for the donor (Chang et al., 2014). The use of nerve allografts, however, is precluded by immunological rejection. Many attempts have been made to minimize immunological rejection, including hypothermia, freeze-drying, freeze-thawing, plasma immersion, and irradiation of the donor tissue (Marmor et al., 1964). The mechanism underlying the effects of irradiation may be that X-rays destroy the antigen-secreting function of Schwann cells, thus promoting peripheral nerve regeneration through the retained neurilemma and neural canal. In addition, irradiation of the allograft can prevent the spread of pathogens after transplantation; however, the technique cannot prevent post-transplantation infection altogether, and postoperative infection is the leading cause of nerve allograft failure (Hu, 1995).
There is growing evidence that green tea lowers blood sugar and cholesterol, and has anti-radiation, anti-tumor, anti-inflammatory, and neuroprotective effects (Assuncao et al., 2010). Green tea polyphenol (GTP, C17H19N30) are the main active component of green tea (Khan et al., 2007; Mandel et al., 2008). It has been proposed that nerve allografts stored in GTP solution are equal to autografts in promoting nerve regeneration and restoration. Ikeguchi et al., (2005) showed that GTP-preserved nerve allografts attenuated ischemic injury and immunological rejection after transplantation.
To find the optimal intervention for nerve transplantation, we pretreated nerve allografts with GTP solution or irradiation, and transplanted the pretreated allografts into rat models of sciatic nerve defects.
Materials and Methods
Animals
Sixty-six inbred male Wistar rats, aged 6 months and weighing 200–220 g, were provided by the Experimental Animal Center of Lanzhou University (Lanzhou, Gansu Province, China; license No. SCXK (Gan) 20050007). Experiments were approved by the Experimental Animal Ethics Committee of Lanzhou General Hospital, Lanzhou Military Area Command of Chinese PLA (Lanzhou, Gansu Province, China).
Drug
GTP (purity > 98%, batch No. 20071210) was purchased from Zhejiang Orient Tea Development Co., Ltd., Hangzhou, Zhejiang Province, China.
Sciatic nerve defect model establishment and experimental grouping
Forty-eight rats were anesthetized with 2% sodium pentobarbital (30–40 mg/kg intraperitoneally). The femoral posterolateral area was shaved and sterilized using 8% sodium sulfide solution and 5% povidone-iodine. A curved incision was made in the femoral posterolateral area and the sciatic nerve was exposed. A 1.0 cm length of nerve trunk was completely resected 0.5 cm below the piriformis muscle. The 48 rats with sciatic nerve defects were then randomly assigned to four groups of 12 rats, to undergo nerve autograft, fresh nerve allograft, irradiation-pretreated nerve allograft, or GTP-pretreated nerve allograft.
Preparation and pretreatment of nerve grafts
Sciatic nerves of 18 Wistar rats were harvested bilaterally under anesthesia, resulting in 36 donor nerves for transplantation into the damaged sciatic nerve of rats in the three allograft groups. In addition, the sciatic nerve contralateral to the injury site in the 12 model rats in the autograft group was obtained for autologous transplantation.
Irradiation pretreatment
The donor sciatic nerves were trimmed to 1.0 cm to match the host nerve defect, and rinsed with cold (4°C) saline and stored in a refrigerator. Donor nerves were then exposed to irradiation at a dose of 26.39 Gy/min for 12 hours (total 19 kGy; Lanzhou Municipal Irradiation Center, Lanzhou, Gansu Province, China), and served as nerve allografts in subsequent experiments.
GTP pretreatment
The donor sciatic nerves were trimmed to 1.0 cm and preserved in DMEM solution containing 1 mg/mL GTP at 4°C for 7 days, then transferred to DMEM solution without GTP at 4°C for 23 days (Ikeguchi et al., 2003), and served as nerve allografts in subsequent experiments.
Transplantation
The donor nerves were transplanted by end-to-end anastomosis. For the autografts and fresh nerve allografts, the nerve segments were trimmed and transplanted without any further treatment. The incisions were then sutured under a microscope (10× magnification) using 11-0 noninvasive thread, and thoroughly disinfected. All surgical procedures were performed by the same investigator.
General observation
Mental state, feeding, activity, wound healing and foot ulcers were monitored in the sciatic nerve defect rats 4, 7, 10 and 12 weeks after transplantation.
Gross observation
Six rats in each group were selected 6 and 12 weeks after transplantation, to observe the thickness, structure and color of the nerve grafts, their adhesion to adjacent tissue, and the distribution of surface vessels.
Electrophysiological examination
Six rats in each group were selected 6 and 12 weeks after transplantation, to measure sciatic nerve conduction velocity using the Keypoint EMG evoked potential device (Copenhagen, Denmark) in Lanzhou Municipal Rehabilitation Hospital (Lanzhou, Gansu Province, China). The rats were anesthetized via intraperitoneal injection of 2% pentobarbital (30–40 mg/kg). The sciatic nerve was exposed, two stimulating electrodes were placed at the proximal end of the nerve allograft and common peroneal nerve across the peroneus muscle at a distance of 2.0 cm. Recording electrodes were obliquely inserted into the belly of the anterior tibial muscle at a 45° angle. Stimulation (6 mA per second) was applied and the motor evoked potentials were observed and recorded. Motor nerve conduction velocity (m/s) was also measured.
Histological examination
After electrophysiological examination, nerve grafts and surrounding tissue (5 mm at the proximal and distal ends of the host sciatic nerves) were harvested and subject to Masson trichrome and Masson-fontana silver staining (kits provided by Beijing Solarbio Science & Technology Co., Ltd., Beijing, China). The morphology of the regenerated nerve was observed under an optical microscope (type PH-2, Olympus, Tokyo, Japan).
Transmission electron microscopy
At 12 weeks post-surgery, the middle segments of the nerve grafts in each group were harvested and fixed with 3% glutaraldehyde and 1% osmic acid, then cut into superthin (< 0.1 μm) sections. The sections were stained with uranyl acetate and lead citrate, and the ultrastructure of axons and myelin in the regenerating nerves was observed under a JEM-1230 transmission electron microscope (Japan Electron Optics Laboratory Co., Ltd., Japan).
Statistical analysis
Data were expressed as the mean ± SD and analyzed using SPSS 11.0 software (SPSS, Chicago, IL, USA). Groups were compared using one-way analysis of variance and pairwise comparisons were performed with the least significant difference test. A significance level of α = 0.05 was defined.
Results
Ulcer healing
Limb dragging, and swelling and ulceration of the ankle, appeared 4 weeks after transplantation. In the autologous nerve graft and GTP-pretreated nerve allograft groups, the swelling disappeared and ulcers healed by 7 weeks post-surgery, and at 12 weeks the ipsilateral hindpaws responded well to stimulation. In the fresh nerve allograft group, ulcers were healed by 12 weeks post-surgery, but toe deformities were observed. However, in the irradiation-pretreated nerve allograft group, ulcers were healed at 10 weeks post-surgery and residual scars were visible; ipsilateral hindpaws responded slightly to stimulation at 12 weeks post-surgery.
Motor nerve conduction
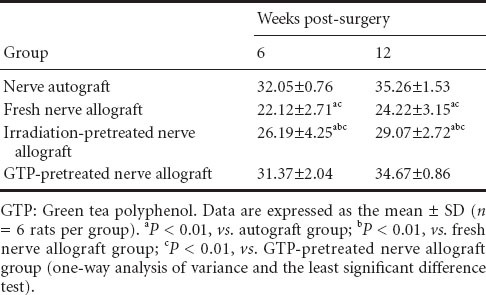
Electrophysiological examination revealed that the motor nerve conduction velocity after transplantation of autologous nerve grafts or GTP-pretreated nerve allografts was higher than that after fresh nerve or irradiation-pretreated nerve allografts at 6 and 12 weeks post-surgery (P < 0.01; Table 1).
Table 1.
Motor nerve conduction in velocity (m/s) rats with sciatic nerve defects after autograft, fresh nerve allograft, or irradiation- or GTP-pretreated nerve allograft transplantation
Tissue observations
Fresh sciatic nerves were flexible. After irradiation pretreatment, nerve allografts were paler in color than fresh nerve. Nerve allografts stored in GTP solution were yellow and slightly harder than normal nerve, conducive to surgical suturing. At 6 weeks postoperatively, all nerve grafts showed edema and adhered tightly to the surrounding soft tissue. Autografts and GTP-pretreated allografts were lighter in weight than the fresh or irradiation-pretreated allografts. At 12 weeks, the autografts and GTP-pretreated nerve allografts had similar circumferences to the host sciatic nerve, and appeared normal in structure, adhering slightly to the surrounding tissue but remaining easy to separate. In addition, nerve allografts stored in GTP solution were close to the color of normal nerve. Atrophy was noted after fresh nerve allografts transplantation and extensive adhesion to the surrounding tissue, which was difficult to separate. The irradiated nerve allografts were thinner than the host sciatic nerve and adhered tightly to surrounding tissue, with capillaries forming at the surface.
Sciatic nerve morphology in rats after irradiation- and GTP-pretreated nerve allograft transplantation
At 6 weeks postoperatively, morphological changes were similar between the autograft and GTP-pretreated allograft groups; new axons were observed growing into the grafts, Schwann cells proliferated noticeably, nerve fibers showed mild swelling, and a large number of blood vessels had formed. In the fresh nerve allograft group, fewer nerve fibers regenerated and were arranged in a disorderly manner, and massive vacuolar degeneration was observed. The irradiation-pretreated nerve allograft group showed an orderly arrangement of nerve fibers, but still with a large degree of vacuolar degeneration; the regenerated nerve fibers were localized at the edge of the grafts, and there were fewer regenerated fibers than in the GTP-pretreated nerve allograft group (Figure 1).
Figure 1.
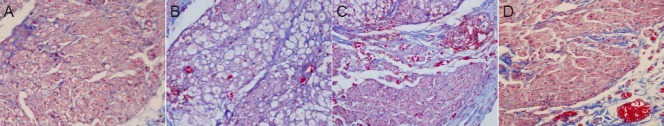
Morphology of the middle segment of nerve grafts in rats with sciatic nerve defects 6 weeks after autograft, fresh nerve allograft, or irradiation- or GTP-pretreated nerve allograft transplantation (Masson staining, × 400).
(A) Autograft group: orderly arrangement of new axons growing into the graft; no apparent nerve fiber swelling. (B) Fresh nerve allograft group: large amount of fibrous tissue; notable vacuolar degeneration; less nerve fiber regeneration than in the autograft group; disorderly nerve fiber arrangement. (C) Irradiation-pretreated nerve allograft group: massive vacuolar degeneration; few new axons or blood vessels; regenerated nerve fibers localized around the grafts. (D) GTP-pretreated nerve allograft group: abundant new axons; orderly arrangement of nerve fibers. GTP: Green tea polyphenol.
At 12 weeks post-surgery, in the autograft and GTP-pretreated nerve allograft groups, the regenerated axons crossed through the grafts, and myelin sheath formation and Schwann cell regeneration were observed. In the fresh nerve allograft group, the majority of regenerating axons were not able to cross the graft. There were more regenerating nerve fibers in the irradiated nerve allograft group than in the fresh nerve allograft group, and some regenerating peripheral axons crossed through the graft (Figure 2).
Figure 2.
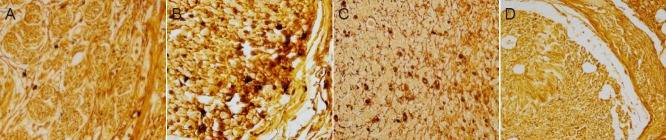
Morphology of the middle segment of nerve grafts in rats with sciatic nerve defects 12 weeks after autograft, fresh nerve allograft, or irradiation- or GTP-pretreated nerve allograft transplantation (silver staining, × 400).
(A) Autograft group: extensive myelination, evident Schwann cell regeneration, orderly arrangement of nerve fibers. (B) Fresh nerve allograft group: proliferation of connective tissue and less nerve fiber regeneration than in the autograft group. (C) Irradiation-pretreated nerve allograft group: few new axons, sparsely arranged. (D) GTP-pretreated nerve allograft group: evident myelination and regeneration of Schwann cells. GTP: Green tea polyphenol.
Ultramicroscopic morphology
Transmission electron microscopy revealed that neuronal morphology was similar in the autograft and GTP-pretreated nerve allograft groups 12 weeks after surgery. Myelinated nerve fibers had regenerated, Schwann cells wrapped the regenerating axonal bud, the wall of the myelin sheath was thick, the lamella was darkly stained and clearly visible, and there were abundant microfilaments and microtubules in the axoplasm. In the fresh nerve allograft group, the majority of visible tissue was fibrous connective tissue; a small number of nerve fibers had regenerated but showed irregular shapes and uneven myelin sheath thickness; some Schwann cells appeared edematous, showing mitochondrial vacuolar necrosis and disintegration. In the irradiated-nerve allograft group, some fibers had regenerated but were sparsely distributed, and the regenerated axons were poorly developed compared with those in the autograft and GTP-pretreated nerve allograft groups (Figure 3).
Figure 3.
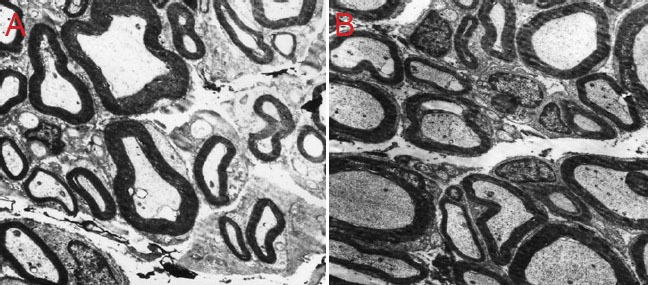
Neuronal morphology in rats with sciatic nerve defects 12 weeks after irradiation- and GTP-pretreated nerve allograft transplantation (transmission electron microscope, × 2,500).
(A) Uneven thickness and irregular arrangement of myelin sheath in GTP-pretreated nerve allograft group. (B) Orderly arrangement and good development of myelin sheath in fresh nerve allograft group. GTP: Green tea polyphenol.
Discussion
Peripheral nerve defects are frequently observed in the clinic (Nakajima et al., 2012; Wang et al., 2013). Common treatments include nerve autografts, allografts or artificial bridging grafts (Glaus et al., 2011; Siemionow et al., 2011; Bayraktar et al., 2012; Deal et al., 2012; McCormick et al., 2012; Jiao et al., 2014; Zhang et al., 2014). Autografts are considered the gold standard for the restoration of peripheral nerve defects (Fujioka et al., 2007), but despite their effects, limited donor tissue and resulting impairment restrict their use. Therefore, there is growing interest in the allograft, although a number of problems remain, including the elimination of antigenicity, maintenance of physical properties, sterilization, nerve regeneration and functional recovery. To minimize the antigenicity and immune rejection of the nerve allograft, and to promote nerve regeneration after transplantation, a variety of methods have been proposed to decellularize nerve allografts (Hudson et al., 2004a, b); for example, physical methods such as freezing, freeze-drying, freeze-thawing, ethanol, neural pre-collapse and irradiation; or chemical methods using detergent or sodium deoxycholate (Dumont et al., 1997; Sondell et al., 1998). Myelin sheath staining, immunohistochemical staining and scanning electron microscopy studies have shown that such chemical extraction methods clear apoptotic cells, myelin and debris, while retaining the cell membrane and cytoplasm, but the ideal processing methods remain debated.
In the present study, we compared the effects of autologous nerve grafts with those of fresh, irradiated, and GTP-pretreated nerve allografts. Using immunohistochemical staining, we showed that nerve allografts preserved in GTP solution for 1 month maintained neuronal membrane and cellular structures, contributing to Schwann cell migration and growth. Before transplantation, the nerve membrane and cellular structures in the GTP-pretreated nerve allograft group were intact and appeared bright under the light microscope, whereas in the irradiated nerve allograft group, neurons were deeply stained. This discrepancy can be explained by the survival of some Schwann cells in the nerves that had been preserved in GTP solution. Electrophysiological tests revealed that good motor nerve function was regained after transplantation of autografts and GTP-pretreated nerve allografts. Histological detection showed that the number of myelinated nerve fibers, and the amount of Schwann cell proliferation, nerve regeneration and vascularization, were improved in the autograft and GTP-pretreated nerve allograft groups.
In summary, transplantation of GTP-pretreated nerve allografts obtains better functional and structural results than irradiated or fresh nerve allografts, and is equal to autologous nerve grafting. The present study demonstrated that the basement membrane and Schwann cells are the two essential elements for nerve regeneration. However, donor Schwann cells secrete a variety of active substances and promote nerve regeneration after peripheral nerve injury (Evans et al., 1994). Therefore, we speculate that the bioactivity of some Schwann cells should be maintained while reducing nerve allograft antigenicity. Many methods (such as irradiation) reduce the antigenicity of nerve allografts, but also kill Schwann cells (Moore et al., 2004). GTP pretreatment not only retains the nerve membrane and canal structure in the allograft, allowing the promotion and guidance of peripheral nerve regeneration, but also allows the survival of some Schwann cells at the donor site; thus, neurites grow into the distal end of the defect with the assistance of the donor Schwann cells. The conditions required for GTP pretreatment are relatively simple and inexpensive to obtain, meaning that GTP preservation of nerve allografts is a promising method for the treatment of peripheral nerve injury.
Acknowledgments
We appreciate all staff from Orthopedics Center, Lanzhou General Hospital of Lanzhou Military Area Command of Chinese PLA for participation in the present experiment.
Footnotes
Funding: This study was supported by grants from Research Fund of Lanzhou Military Area Command of Chinese PLA, No. CLZ12JA07; Gansu Provincial Science and Technology Program, No. 1208RJZA108.
Conflicts of interest: None declared.
Copyedited by Murphy JS, Norman C, Wang J, Yang Y, Li CH, Song LP, Zhao M
References
- Assunção M, Santos-Marques MJ, Carvalho F, Andrade JP. Green tea averts age-ependent decline of hippocampal signailing systems related to antioxidant defenses and survival. Free Radical Biol Med. 2010;48:831–838. doi: 10.1016/j.freeradbiomed.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Bayraktar AM, Ozbek S, Ozcan M, Noyan B, Cavuşoğlu I. The effect of catheter use on vein grafting of a peripheral nerve defect: an experimental study. Ulus Travma Acil Cerrahi Derg. 2012;18:367–375. doi: 10.5505/tjtes.2012.59932. [DOI] [PubMed] [Google Scholar]
- Chang R, Yin XL, Shang BS, He P. Combination of olfactory ensheathing cells and chitosan in treatment of peripheral nerve injury. Zhongguo Zuzhi Gongcheng Yanjiu. 2014;18:3361–3366. [Google Scholar]
- Deal DN, Griffin JW, Hogan MV. Nerve conduits for nerve repair orreconstruction. J Am Acad Orthop Surg. 2012;20:63–68. doi: 10.5435/JAAOS-20-02-063. [DOI] [PubMed] [Google Scholar]
- Dumont CE, Hentz VR. Enhancement of axon growth by detergent-extracted nerve grafts. Transplantation. 1997;63:1210–1215. doi: 10.1097/00007890-199705150-00004. [DOI] [PubMed] [Google Scholar]
- Evans PJ, Mdha R, Mackinnon SE. The peripheral nerve allograft: a comprehensive review of regeneration and neuroimmunology. Prog Neurobiol. 1994;43:187–233. doi: 10.1016/0301-0082(94)90001-9. [DOI] [PubMed] [Google Scholar]
- Fujioka M, Tasaki I, Kitamura R, Yakabe A, Hayashi M, Matsuya F, Miyaguchi T, Tsuruta J. Cavernous nerve graft reconstruction using an autologous nerve guide to restore potency. BJU Int. 2007;100:1107–1109. doi: 10.1111/j.1464-410X.2007.07068.x. [DOI] [PubMed] [Google Scholar]
- Glaus SW, Johnson PJ, Mackinnon SE. Clinical strategies to enhance nerve regeneration in composite tissue allotransplantation. Hand Clin. 2011;27:495–509. doi: 10.1016/j.hcl.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Ding F, Yang Y, Liu J. Construction of tissue engineerednerve grafts and their application in peripheral nerve regeneration. Prog Neurobiol. 2011;93:204–230. doi: 10.1016/j.pneurobio.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Hu YY. Establishment and management of bone bank. Zhonghua Guke Zazhi. 1995;15:54–58. [Google Scholar]
- Hudson TW, Liu SY, Schmidt CE. Engineering an improved acellular nerve graft via optimized chemical processing. Tissue Eng. 2004a;10:1346–1358. doi: 10.1089/ten.2004.10.1641. [DOI] [PubMed] [Google Scholar]
- Hudson TW, Zawko S, Deister C, Lundy S, Hu CY, Lee K, Schmidt CE. Optimized acellular nerve graft is Immunologically tolerated and supports regeneration. Tissue Eng. 2004b;10:1641–1651. doi: 10.1089/ten.2004.10.1641. [DOI] [PubMed] [Google Scholar]
- Ikeguchi R, Kakinoki R, Okamoto T, Matsumoto T, Hyon SH, Nakamura T. Successful storage of peripheral nerve before transplantation using greentea polyphenol: an experimental study in rats. Exp Neurol. 2003;184:688–696. doi: 10.1016/S0014-4886(03)00344-3. [DOI] [PubMed] [Google Scholar]
- Ikeguchi R, Kakinoki R, Matsumoto T, Hyon SH, Nakamura T. Peripheral nerve allografts stored in green tea polyphenol solution. Transplantation. 2005;79:688–695. doi: 10.1097/01.tp.0000155417.87823.17. [DOI] [PubMed] [Google Scholar]
- Jiao WeD, Li YH, Ji AY, Xia YJ. Sodium alginate film promotes the regeneration of injured sciatic nerve in rats. Zhongguo Zuzhi Gongcheng Yanjiu. 2014;18:3973–3979. [Google Scholar]
- Khan N, Mukhtar Tea Polyphenols for health promotion. Life Sei. 2007;81:519–533. doi: 10.1016/j.lfs.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel SA, Amit T, Kalfon L, Reznichenko L, Youdim MB. Targeting multiple neurodegenerative disease etiologies with multimodal-acting green tea cacechins. J Nutr. 2008;138:1578S–1583S. doi: 10.1093/jn/138.8.1578S. [DOI] [PubMed] [Google Scholar]
- Marmor J. Regeneration of peripheral nerve by irradiated honografts. J Bone Joint Surg. 1964;46:383. [PubMed] [Google Scholar]
- McCormick AM, Leipzig ND. Neural regenerative strategies incorporating biomolecular axon guidance signals. Ann Biomed Eng. 2012;40:578–597. doi: 10.1007/s10439-011-0505-0. [DOI] [PubMed] [Google Scholar]
- Moore TM, Gendler E. Viruses absorbed on musculoakeletal allografts are inactivated by terminal ethyleneoxide disinfection. Orthop Res. 2004;22:1358–1361. doi: 10.1016/j.orthres.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Nakajima Y, Nishiura Y, Hara Y, Sharula, Ochiai N. Simultaneous gradual lengthening of proximal and distal nerve stumps for repair of chronic peripheral nerve defect in rats. Hand Surg. 2012;17:1–11. doi: 10.1142/S0218810412500013. [DOI] [PubMed] [Google Scholar]
- Siemionow M, Duggan W, Brzezicki G, Klimczak A, Grykien C, Gatherwright J, Nair D. Peripheral nerve defect repair with epineural tubes supported with bone marrow stromal cells: a preliminary report. Ann Plast Surg. 2011;67:73–84. doi: 10.1097/SAP.0b013e318223c2db. [DOI] [PubMed] [Google Scholar]
- Sondell M, Lundborg G, Kanje M. Regeneration of the rat sciatic nerve into allografts made acellular through chemical extraction. Brain Res. 1998;795:44–54. doi: 10.1016/s0006-8993(98)00251-0. [DOI] [PubMed] [Google Scholar]
- Taylor CA, Braza D, Rice JB, Dillingham T. The incidence of peripheral nerve injury in extremity trauma. Am J Phys Med Rehabil. 2008;87:381–385. doi: 10.1097/PHM.0b013e31815e6370. [DOI] [PubMed] [Google Scholar]
- Wang KL, Lu LJ, Zhang JL, Li XJ, Jing XB. Pilose antler polypeptide composite membrane supports a suitable microenvironment for peripheral nerve regeneration. Zhongguo Zuzhi Gongcheng Yanjiu. 2013;17:4652–4659. [Google Scholar]
- Zhang CS, Lv G. Tissue engineered artificial nerves repair sciatic nerve defect in rats: an evaluation using horseradish peroxidase retrograde tracer technique. Zhongguo Zuzhi Gongcheng Yanjiu. 2014;18:4658–4662. [Google Scholar]


