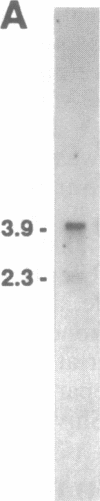
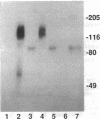
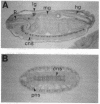
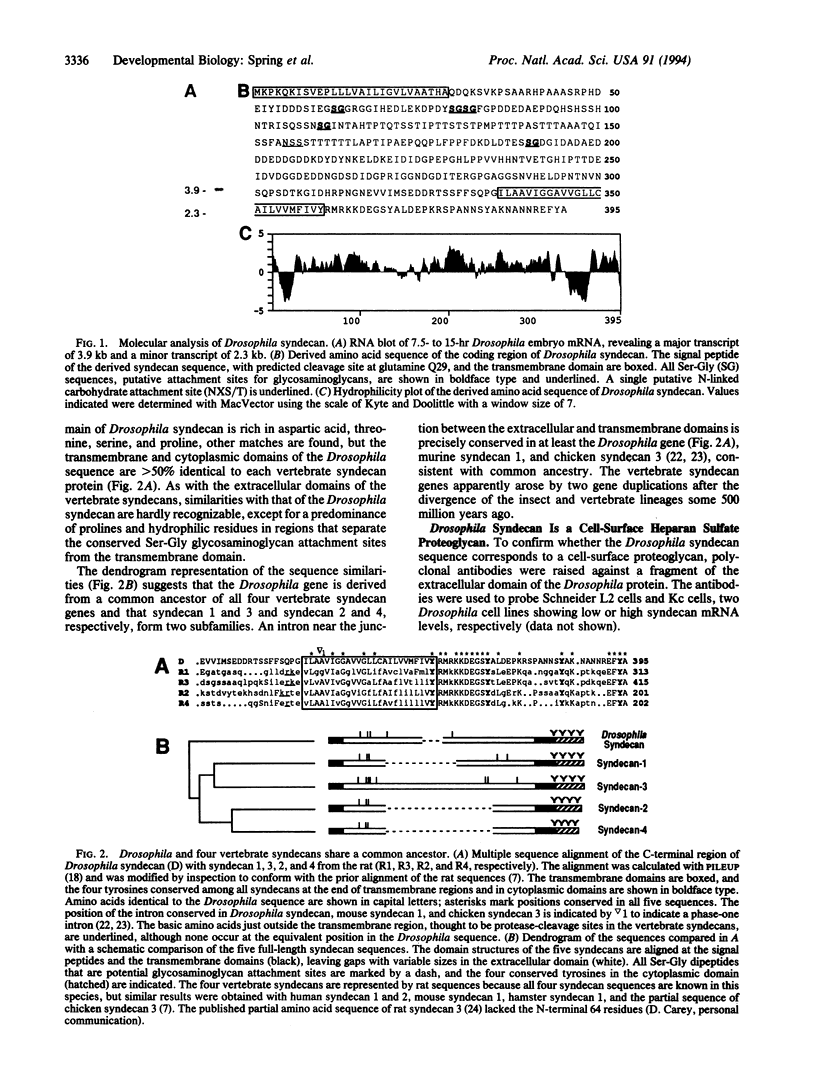
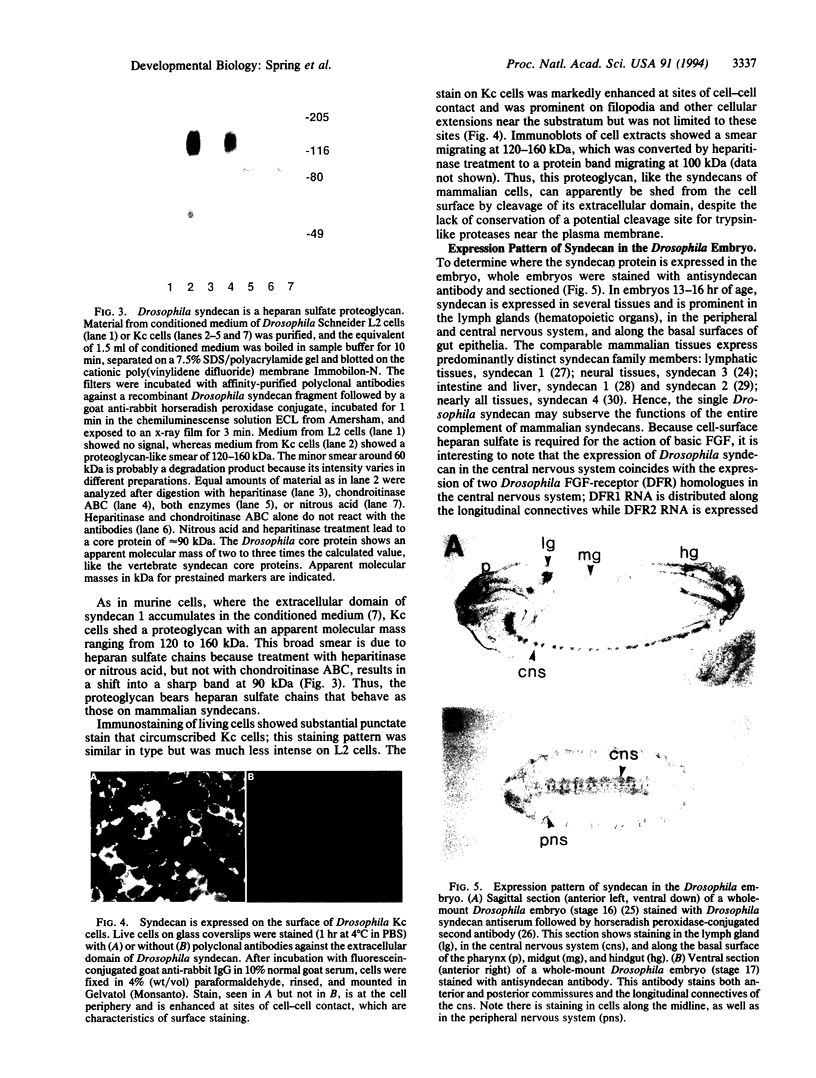
Abstract
In mammals, cell-surface heparan sulfate is required for the action of basic fibroblast growth factor, fibronectin, antithrombin III, as well as other effectors. The syndecans, a gene family of four transmembrane proteoglycans that participates in these interactions, are the major source of this heparan sulfate. Based on the conserved transmembrane and cytoplasmic domains of the mammalian syndecans, a single syndecan-like gene was detected and localized in the Drosophila genome. As in mammals, Drosophila syndecan is a heparan sulfate proteoglycan expressed at the cell surface that can be shed from cultured cells. The single Drosophila syndecan is expressed in embryonic tissues that correspond with those tissues in mammals that express distinct members of the syndecan family predominantly. Conservation of this class of molecules suggests that Drosophila, like mammals, uses cell-surface heparan sulfate as a receptor or coreceptor for extracellular effector molecules.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Artavanis-Tsakonas S., Simpson P. Choosing a cell fate: a view from the Notch locus. Trends Genet. 1991 Nov-Dec;7(11-12):403–408. doi: 10.1016/0168-9525(91)90264-q. [DOI] [PubMed] [Google Scholar]
- Bernfield M., Kokenyesi R., Kato M., Hinkes M. T., Spring J., Gallo R. L., Lose E. J. Biology of the syndecans: a family of transmembrane heparan sulfate proteoglycans. Annu Rev Cell Biol. 1992;8:365–393. doi: 10.1146/annurev.cb.08.110192.002053. [DOI] [PubMed] [Google Scholar]
- Cambiazo V., Inestrosa N. C. Proteoglycan production in Drosophila egg development: effect of beta-D-xyloside on proteoglycan synthesis and larvae motility. Comp Biochem Physiol B. 1990;97(2):307–314. doi: 10.1016/0305-0491(90)90286-3. [DOI] [PubMed] [Google Scholar]
- Carey D. J., Evans D. M., Stahl R. C., Asundi V. K., Conner K. J., Garbes P., Cizmeci-Smith G. Molecular cloning and characterization of N-syndecan, a novel transmembrane heparan sulfate proteoglycan. J Cell Biol. 1992 Apr;117(1):191–201. doi: 10.1083/jcb.117.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cássaro C. M., Dietrich C. P. Distribution of sulfated mucopolysaccharides in invertebrates. J Biol Chem. 1977 Apr 10;252(7):2254–2261. [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould S. E., Upholt W. B., Kosher R. A. Syndecan 3: a member of the syndecan family of membrane-intercalated proteoglycans that is expressed in high amounts at the onset of chicken limb cartilage differentiation. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3271–3275. doi: 10.1073/pnas.89.8.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald I., Rubin G. M. Making a difference: the role of cell-cell interactions in establishing separate identities for equivalent cells. Cell. 1992 Jan 24;68(2):271–281. doi: 10.1016/0092-8674(92)90470-w. [DOI] [PubMed] [Google Scholar]
- Hayashi K., Hayashi M., Jalkanen M., Firestone J. H., Trelstad R. L., Bernfield M. Immunocytochemistry of cell surface heparan sulfate proteoglycan in mouse tissues. A light and electron microscopic study. J Histochem Cytochem. 1987 Oct;35(10):1079–1088. doi: 10.1177/35.10.2957423. [DOI] [PubMed] [Google Scholar]
- Hinkes M. T., Goldberger O. A., Neumann P. E., Kokenyesi R., Bernfield M. Organization and promoter activity of the mouse syndecan-1 gene. J Biol Chem. 1993 May 25;268(15):11440–11448. [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Kan M., Wang F., Xu J., Crabb J. W., Hou J., McKeehan W. L. An essential heparin-binding domain in the fibroblast growth factor receptor kinase. Science. 1993 Mar 26;259(5103):1918–1921. doi: 10.1126/science.8456318. [DOI] [PubMed] [Google Scholar]
- Klämbt C., Glazer L., Shilo B. Z. breathless, a Drosophila FGF receptor homolog, is essential for migration of tracheal and specific midline glial cells. Genes Dev. 1992 Sep;6(9):1668–1678. doi: 10.1101/gad.6.9.1668. [DOI] [PubMed] [Google Scholar]
- Marynen P., Zhang J., Cassiman J. J., Van den Berghe H., David G. Partial primary structure of the 48- and 90-kilodalton core proteins of cell surface-associated heparan sulfate proteoglycans of lung fibroblasts. Prediction of an integral membrane domain and evidence for multiple distinct core proteins at the cell surface of human lung fibroblasts. J Biol Chem. 1989 Apr 25;264(12):7017–7024. [PubMed] [Google Scholar]
- Nurcombe V., Ford M. D., Wildschut J. A., Bartlett P. F. Developmental regulation of neural response to FGF-1 and FGF-2 by heparan sulfate proteoglycan. Science. 1993 Apr 2;260(5104):103–106. doi: 10.1126/science.7682010. [DOI] [PubMed] [Google Scholar]
- Patel N. H., Snow P. M., Goodman C. S. Characterization and cloning of fasciclin III: a glycoprotein expressed on a subset of neurons and axon pathways in Drosophila. Cell. 1987 Mar 27;48(6):975–988. doi: 10.1016/0092-8674(87)90706-9. [DOI] [PubMed] [Google Scholar]
- Pierce A., Lyon M., Hampson I. N., Cowling G. J., Gallagher J. T. Molecular cloning of the major cell surface heparan sulfate proteoglycan from rat liver. J Biol Chem. 1992 Feb 25;267(6):3894–3900. [PubMed] [Google Scholar]
- Rapraeger A. C., Krufka A., Olwin B. B. Requirement of heparan sulfate for bFGF-mediated fibroblast growth and myoblast differentiation. Science. 1991 Jun 21;252(5013):1705–1708. doi: 10.1126/science.1646484. [DOI] [PubMed] [Google Scholar]
- Roth J., Kempf A., Reuter G., Schauer R., Gehring W. J. Occurrence of sialic acids in Drosophila melanogaster. Science. 1992 May 1;256(5057):673–675. doi: 10.1126/science.1585182. [DOI] [PubMed] [Google Scholar]
- Rubin G. M. Drosophila melanogaster as an experimental organism. Science. 1988 Jun 10;240(4858):1453–1459. doi: 10.1126/science.3131880. [DOI] [PubMed] [Google Scholar]
- Sanderson R. D., Lalor P., Bernfield M. B lymphocytes express and lose syndecan at specific stages of differentiation. Cell Regul. 1989 Nov;1(1):27–35. doi: 10.1091/mbc.1.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders S., Jalkanen M., O'Farrell S., Bernfield M. Molecular cloning of syndecan, an integral membrane proteoglycan. J Cell Biol. 1989 Apr;108(4):1547–1556. doi: 10.1083/jcb.108.4.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüpbach T., Wieschaus E. Female sterile mutations on the second chromosome of Drosophila melanogaster. II. Mutations blocking oogenesis or altering egg morphology. Genetics. 1991 Dec;129(4):1119–1136. doi: 10.1093/genetics/129.4.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shishido E., Higashijima S., Emori Y., Saigo K. Two FGF-receptor homologues of Drosophila: one is expressed in mesodermal primordium in early embryos. Development. 1993 Feb;117(2):751–761. doi: 10.1242/dev.117.2.751. [DOI] [PubMed] [Google Scholar]
- Shively J. E., Conrad H. E. Nearest neighbor analysis of heparin: identification and quantitation of the products formed by selective depolymerization procedures. Biochemistry. 1976 Sep 7;15(18):3943–3950. doi: 10.1021/bi00663a006. [DOI] [PubMed] [Google Scholar]
- Wang L., Denburg J. L. A role for proteoglycans in the guidance of a subset of pioneer axons in cultured embryos of the cockroach. Neuron. 1992 Apr;8(4):701–714. doi: 10.1016/0896-6273(92)90091-q. [DOI] [PubMed] [Google Scholar]
- Yayon A., Klagsbrun M., Esko J. D., Leder P., Ornitz D. M. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell. 1991 Feb 22;64(4):841–848. doi: 10.1016/0092-8674(91)90512-w. [DOI] [PubMed] [Google Scholar]
- Zusman S., Grinblat Y., Yee G., Kafatos F. C., Hynes R. O. Analyses of PS integrin functions during Drosophila development. Development. 1993 Jul;118(3):737–750. doi: 10.1242/dev.118.3.737. [DOI] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]