While in one hand, due to genetic alterations, lifestyle changes, infections or injuries, or sudden turn of life events, we get health problems, on the other, we have been endowed with enormous natural remedies to take care our health. Cinnamon, the brown bark of cinnamon tree, is one such natural product that has already been being used for centuries throughout the world as spice or flavoring agent. In addition, medieval physicians used cinnamon for medical purposes to treat a variety of disorders including arthritis, coughing, hoarseness, sore throats, etc. It was once so highly-prized that several wars were fought over it.
Recently, this natural product is being considered for its use in a new territory – Parkinson's disease (PD). PD is the most common neurodegenerative movement disorder, which is caused by the death of dopaminergic neurons in the substantia nigra pars compacta (SNpc) and associated loss of dopamine in the striatum (Dauer and Przedborski, 2003; Roy and Pahan, 2011). Despite intense investigations, until now no effective therapy is available to stop the progression of this devastating disease. It is becoming clear that chronic neuroinflammation, loss of supportive molecules in the brain and accumulation of α-synuclein (α-syn) are critical for the manifestation of nigrostriatal pathology in PD (Dauer and Przedborski, 2003). Interestingly, cinnamon can modify these aspects of pathology.
Cinnamon and neuroinflammation: The major compound in cinnamon is cinnamaldehyde, which is converted into cinnamic acid by oxidation. In the liver, this cinnamic acid is β-oxidized to benzoate that exists as sodium benzoate (NaB) or benzoyl-CoA. A minor amount of benzoate, a direct metabolite of cinnamic acid, is also excreted in the urine of humans. Recently, we have demonstrated that oral administration of ground cinnamon increases the level of NaB in serum and brain of mice (Jana et al., 2013). NaB is of medical importance as it is a component of Ucephan, a FDA-approved drug used in the treatment for hepatic metabolic defects associated with hyperammonemia such as urea cycle disorder in children. It is also widely used as a preservative in broad range of foods and cosmetic products. Although at higher concentrations, NaB could be toxic, it has been reported that 2% solution of NaB in drinking water is safe for lifelong treatment in mice without any noticeable side effects (Toth, 1984).
Glial activation and associated neuroinflammation participate in the pathogenesis of several neurodegenerative disorders including PD (Ghosh et al., 2007). Interestingly, it has been found that NaB is capable of inhibiting the expression of proinflammatory molecules in cultured astrocytes and microglia (Brahmachari et al., 2009). Reversal of NaB-mediated inhibition of NF-κB activation and inducible nitric oxide synthase (iNOS) expression by mevalonate, 3-hydroxy-3-methylglutaryl-coenzyme A and farnesyl pyrophosphate in activated astrocytes suggests that NaB exhibits anti-inflammatory effect via inhibiting the cholesterol-biosynthetic pathway (Brahmachari et al., 2009). However, although NaB reduces the level of cholesterol, cholesterol is not involved in NaB-mediated inhibition of iNOS (Brahmachari et al., 2009). Suppression of p21ras activation by NaB (Brahmachari et al., 2009) and inhibition of nuclear factor-κB (NF-κB) activation and iNOS expression by dominant-negative mutant of p21ras suggest that NaB attenuates the activation of NF-κB and the expression of iNOS in glial cells via reducing the activation of p21ras (Figure 1). Accordingly, oral administration of cinnamon powder and NaB suppresses the expression of iNOS and glial fibrillary acidic protein (GFAP), an astroglial marker, in vivo in the SNpc of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of PD (Khasnavis and Pahan, 2014), suggesting that cinnamon is capable of reducing inflammation in vivo in the brain.
Figure 1.
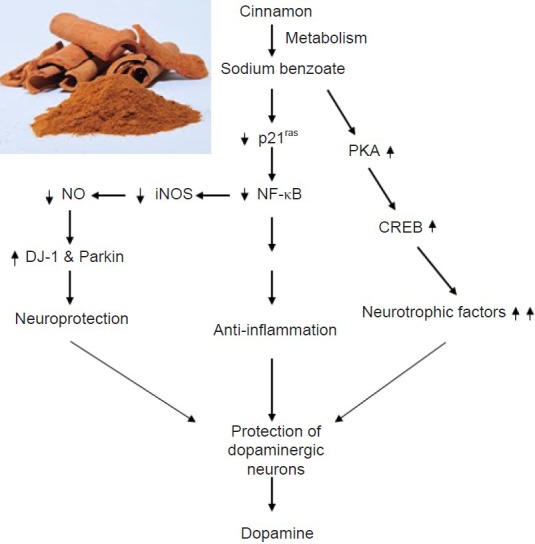
Schematic diagram representing cinnamon-mediated protection of dopaminergic neurons.
Cinnamon is metabolized into sodium benzoate (NaB) in the liver. Once NaB enters into the brain, it increases the production of neurotrophic factors via activation of protein kinase A (PKA) – cAMP response element binding (CREB) pathway. NaB also inhibits the activation of p21ras, a small G protein, and thereby attenuates the activation of nuclear factor-κB (NF-κB) and the expression of NF-κB-dependent proinflammatory molecules leading to anti-inflammation. Following the suppression of NF-κB activation, the expression of inducible nitric oxide synthase (iNOS) also goes down and the production of nitric oxide is reduced, which ultimately restores/upregulates the level of neuroprotective proteins like DJ-1 and Parkin. Together, cinnamon-mediated neurotrophism, anti-inflammation and neuroprotection protect dopaminergic neurons. NO: Nitric oxide.
Cinnamon and brain-derived neurotrophic factor (BDNF): BDNF, a member of the neurotrophin family of growth factor, is probably the most important supportive molecule for neuronal survival and growth. Several studies have shown that the expression of BDNF is significantly reduced in the SNpc of patients with PD and that BDNF protects dopaminergic neurons from MPTP- and 6-hydroxy dopamine (6-OHDA)-induced toxicity (Klein et al., 1999). BDNF is also capable of promoting dopaminergic axonal sprouting. Alternatively, inhibiting BDNF expression by antisense oligonucleotide infusion causes loss of nigral dopaminergic neurons (Porritt et al., 2005). Taken together, there are reasons to believe that induction of BDNF expression in the brain may negatively regulate the loss of dopaminergic neurons. Interestingly, NaB increases the production of BDNF and neurotrophin-3 (NT-3) in cultured astrocytes and neurons (Jana et al., 2013). Activation of protein kinase A (PKA) and cAMP response element binding (CREB) by NaB, recruitment of CREB to the promoter of BDNF by NaB, and abrogation of NaB-induced expression of BDNF and NT-3 by pharmacological inhibition of PKA and siRNA knockdown of CREB in neurons and astrocytes suggest that NaB induces neurotrophic factors in brain cells via the PKA-CREB pathway (Figure 1) (Jana et al., 2013).
Protection of DJ-1 and Parkin by cinnamon: Parkin and DJ-1 are known to stimulate and support the survival of existing dopaminergic neurons. Consistently, in PD, Parkin and DJ-1 have been suggested as rescuers of these vulnerable cells (Ng et al., 2009). However, clinical application of these molecules in PD has been limited because of difficulties in delivery. These small proteins do not readily diffuse across the blood-brain barrier (BBB) or ventricular lining and have limited or unstable bioavailability. Gene delivery and/or protein delivery by stereotactic injection is definitely an option but it has several limitations. It seems from the therapeutic angle, the best option is to stimulate/induce the production of Parkin and DJ-1 within the SNpc of PD patients.
Interestingly, cinnamon and NaB are capable of upregulating DJ-1 and Parkin (Khasnavis and Pahan, 2014). While the level of Parkin and DJ-1 decreases in activated astrocytes, NaB treatment protects these proteins (Khasnavis and Pahan, 2012, 2014). We have also demonstrated that NO is a negative regulator of Parkin/DJ-1 and that NaB protects Parkin/DJ-1 in activated astrocytes via suppressing iNOS-mediated NO production (Figure 1) (Khasnavis and Pahan, 2014). Accordingly, oral administration of cinnamon and NaB inhibited the expression of iNOS and protected DJ-1 and Parkin in the nigra of MPTP mouse model of PD (Khasnavis and Pahan, 2014).
Cinnamon and α-syn: Lewy body formation is a pathological hallmark of PD and aggregated α-syn is an important component of Lewy body. Aggregated α-syn is deposited in the nigra possibly due to a defect in autophagic clearance. Although mechanisms by which aggregation of α-syn occurs are poorly understood, it has been demonstrated that cinnamon extract precipitation (CEppt) affects the process of aggregation of α-syn without changing its secondary structure and suggest that increasing amounts of CEppt slow this process by stabilizing the soluble oligomeric phase (Shaltiel-Karyo et al., 2012). Accordingly, when administered to Drosophila fly model expressing mutant A53T α-syn in the nervous system, CEppt reduced α-syn aggregation in their brain and ameliorated behavioral abnormalities of the flies (Shaltiel-Karyo et al., 2012).
Cinnamon and nigrostriatal degeneration: Since cinnamon and its metabolite NaB reduce glial inflammation, upregulate BDNF, protect DJ-1 and Parkin, and inhibit aggregation of α-syn, the therapeutic efficacy of cinnamon and NaB was tested in MPTP mouse model. While nigral tyroxine hydroxylase (TH)-positive neurons and striatal TH fibers disappeared in MPTP-intoxicated mice, oral administration of cinnamon powder and NaB protected TH-positive neurons and fibers from MPTP toxicity (Khasnavis and Pahan, 2014). Accordingly, cinnamon treatment also restored the level of neurotransmitters in the striatum (Khasnavis and Pahan, 2014). The ultimate therapeutic goal of neuroprotection is to decrease functional impairment. While MPTP intoxication caused hypolocomotion in mice, cinnamon treatment improved locomotor activities (Khasnavis and Pahan, 2014).
Administration of a dopamine agonist or levodopa/carbidopa has been the standard treatment for PD. However, these drugs basically take care of the symptoms by either supplying or imitating dopamine. With time, these drugs cause a number of side effects including dyskinesia, thus creating more problems for PD patients. Furthermore, these drugs fail to target disease progression as these drugs are unable to protect neuroprotective molecules in a neurodegenerating microenvironment, attenuate neuroinflammation and suppress α-syn pathology. Although some drugs exhibit efficacy in cell culture systems, very few of these are in fact capable of crossing the blood-brain barrier and entering into the nigra. After oral administration, cinnamon is metabolized into NaB in the liver, which then readily enters into the brain (Jana et al., 2013). Another important issue is toxicity. It is difficult to find a pharmacological drug that does not have side effects. On the other hand, cinnamon has a long track record as a non-toxic natural product. Therefore, cinnamon fits well as a possible natural medicine to halt the progression of PD. However, before recommending cinnamon to PD patients, it is important to know the long-term effect of cinnamon and NaB in chronic MPTP mouse model and other transgenic animal models of PD. Finally, a clinical trial either as an add-on with L-dopa/carbidopa or monotherapy is needed to attest the fragrance of cinnamon in PD.
This study was supported by grants from National Institutes of Health (AT6681 and NS83054).
References
- Brahmachari S, Jana A, Pahan K. Sodium benzoate, a metabolite of cinnamon and a food additive, reduces microglial and astroglial inflammatory responses. J Immunol. 2009;183:5917–5927. doi: 10.4049/jimmunol.0803336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauer W, Przedborski S. Parkinson's disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Roy A, Liu X, Kordower JH, Mufson EJ, Hartley DM, Ghosh S, Mosley RL, Gendelman HE, Pahan K. Selective inhibition of NF-kappaB activation prevents dopaminergic neuronal loss in a mouse model of Parkinson's disease. Proc Natl Acad Sci U S A. 2007;104:18754–18759. doi: 10.1073/pnas.0704908104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jana A, Modi KK, Roy A, Anderson JA, van Breemen RB, Pahan K. Up-regulation of neurotrophic factors by cinnamon and its metabolite sodium benzoate: therapeutic implications for neurodegenerative disorders. J Neuroimmune Pharmacol. 2013;8:739–755. doi: 10.1007/s11481-013-9447-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasnavis S, Pahan K. Sodium benzoate, a metabolite of cinnamon and a food additive, upregulates neuroprotective parkinson disease protein DJ-1 in astrocytes and neurons. J Neuroimmune Pharmacol. 2012;7:424–435. doi: 10.1007/s11481-011-9286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasnavis S, Pahan K. Cinnamon treatment upregulates neuroprotective proteins Parkin and DJ-1 and protects dopaminergic neurons in a mouse model of Parkinson's disease. J Neuroimmune Pharmacol. 2014;9:569–581. doi: 10.1007/s11481-014-9552-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RL, Lewis MH, Muzyczka N, Meyer EM. Prevention of 6-hydroxydopamine-induced rotational behavior by BDNF somatic gene transfer. Brain Res. 1999;847:314–320. doi: 10.1016/s0006-8993(99)02116-2. [DOI] [PubMed] [Google Scholar]
- Ng CH, Mok SZ, Koh C, Ouyang X, Fivaz ML, Tan EK, Dawson VL, Dawson TM, Yu F, Lim KL. Parkin protects against LRRK2 G2019S mutant-induced dopaminergic neurodegeneration in Drosophila. J Neurosci. 2009;29:11257–11262. doi: 10.1523/JNEUROSCI.2375-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porritt MJ, Batchelor PE, Howells DW. Inhibiting BDNF expression by antisense oligonucleotide infusion causes loss of nigral dopaminergic neurons. Exp Neurol. 2005;192:226–234. doi: 10.1016/j.expneurol.2004.11.030. [DOI] [PubMed] [Google Scholar]
- Roy A, Pahan K. Prospects of statins in Parkinson disease. Neuroscientist. 2011;17:244–255. doi: 10.1177/1073858410385006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaltiel-Karyo R, Davidi D, Frenkel-Pinter M, Ovadia M, Segal D, Gazit E. Differential inhibition of alpha-synuclein oligomeric and fibrillar assembly in parkinson's disease model by cinnamon extract. Biochim Biophys Acta. 2012;1820:1628–1635. doi: 10.1016/j.bbagen.2012.04.021. [DOI] [PubMed] [Google Scholar]
- Toth B. Lack of tumorigenicity of sodium benzoate in mice. Fundam Appl Toxicol. 1984;4:494–496. doi: 10.1016/0272-0590(84)90208-2. [DOI] [PubMed] [Google Scholar]


