Glia-derived axonal growth inhibitory proteins limit functional repair following damage to the adult central nervous system (CNS). Nogo proteins, myelin-associated glycoprotein (MAG), oligodendrocyte myelin glycoprotein (OMgp) and B lymphocyte stimulator (BLyS), are 4 inhibitors that commonly interact with the neuronal receptor, Nogo receptor-1 (NgR1), leading to inhibition of axonal growth. Here, we demonstrate that lateral olfactory tract usher substance (LOTUS) binds to NgR1 and blocks the binding of all four ligands to NgR1, resulting in the suppression of axonal growth inhibition induced by these NgR1 ligands. LOTUS allows neurons to overcome NgR1-mediated axonal growth inhibition, raising the possibility that LOTUS may be useful in future therapeutic approaches as an endogenous potent inhibitor of NgR1 for promoting neuronal regeneration.
Damaged axons in the adult CNS are unable to regrow to their original targets. This inability has been attributed to the non-permissive CNS environment that includes myelin-associated inhibitory molecules such as Nogo proteins, myelin-associated glycoprotein (MAG) and oligodendrocyte myelin glycoprotein (OMgp) (Schwab, 2010). These axonal growth inhibitors commonly bind to Nogo receptor-1 (NgR1) which is expressed in many types of CNS neurons and their axons (Schwab, 2010). B lymphocyte stimulator (BLyS) which is expressed in astrocytes (Krumbholz et al., 2005) has also been identified as a functional ligand for NgR1 (Schwab, 2010). The interaction of these four glial components with NgR1 induces growth cone collapse and neurite outgrowth inhibition, which limits the capability of injured neurons to be functionally restored in the CNS (Schwab, 2010). Numerous studies have indicated that inhibition of NgR1 or its ligands improves histological and behavioral recovery after CNS lesion (Schwab, 2010). Moreover, triple deletion of Nogo, MAG and OMgp results in a higher degree of histological and behavioral regeneration of injured CNS axons, as compared to single deletion of Nogo (Schwab, 2010). This report suggests that suppression of multiple NgR1 ligands is more effective in promoting neuronal regeneration of damaged axons in the CNS.
LOTUS antagonizes Nogo, MAG, OMgp and BLyS- activated NgR1: We identified lateral olfactory tract usher substance (LOTUS) as a novel protein which contributes to axonal bundle formation in lateral olfactory tract development by antagonizing NgR1 activation by Nogo (Sato et al., 2011). We further examined whether LOTUS exerts a similar NgR1 antagonism with regards to MAG, OMgp and BLyS. The following observations were made: (1) Overexpression of LOTUS with NgR1 in COS7 cells blocked the binding of these three NgR1 ligands to NgR1. (2) In cultured dorsal root ganglion neurons where endogenous LOTUS is only weakly expressed, LOTUS overexpression suppressed the growth cone collapse and neurite outgrowth inhibition normally induced by these NgR1 ligands. (3) In cultured olfactory bulb neurons which endogenously express LOTUS, LOTUS suppressed the growth cone collapse normally induced by NgR1 ligands. Conversely, growth cone collapse was induced by NgR1 ligands in lotus-deficient mice (Kurihara et al., 2014). Collectively, our data suggest that LOTUS suppresses NgR1-mediated axonal growth inhibition by blocking the interaction of NgR1 with its four ligands (Figure 1).
Figure 1.
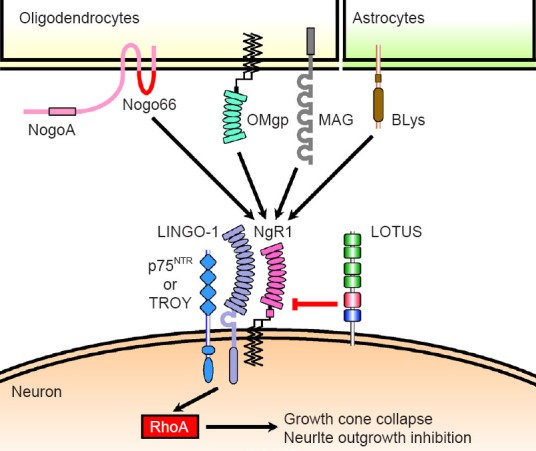
Schematic drawing of molecular mechanism associated with Nogo signaling.
NogoA, myelin-associated glycoprotein (MAG) and oligodendrocyte myelin glycoprotein (OMgp) are axonal growth inhibitors derived from oligodendrocytes. B lymphocyte stimulator (BLyS) is also an axonal growth inhibitor derived from astrocytes. These four glial inhibitors bind to neuronal receptor Nogo receptor-1 (NgR1), which activates the intracellular molecule RhoA via the NgR1 co-receptors, leucine-rich repeat and immunoglobulin domain-containing Nogo receptor-interacting protein 1 (LINGO-1) and either the 75-kDa neurotrophin re-ceptor (p75NTR) or tumor necrosis factor receptor superfamily member 19 (TROY), resulting in induction of growth cone collapse and neurite outgrowth inhibition. Lateral olfactory tractusher substance (LOTUS) interacts with NgR1 and interrupts the binding of Nogo66, which is the functional ectodomain of NogoA that binds to NgR1, thereby inducing axonal growth inhibition. Similarly, LOTUS interrupts the binding of MAG, OMgp and BLyS to NgR1 and in this way, suppresses all of the four NgR1 ligands-induced axonal growth inhibition.
NogoA extracellular peptide residues 1–40 (NEP1–40), leucine-rich glioma inactivated 1 and soluble NgR1 peptide can also interrupt the interaction of NgR1 with its ligand(s) (Schwab, 2010). NEP1–40 has been shown to specifically block the binding of Nogo66 to NgR1, where Nogo66 is the functional domain of NogoA that binds to NgR1 and induces axonal growth inhibition. NEP1–40 does not block the binding of MAG or OMgp. leucine-rich glioma inactivated 1 has been shown to interfere with Nogo66-binding to NgR1. It remains unknown whether NEP1–40 interrupts BLyS-binding to NgR1 and whether leucine-rich glioma inactivated 1 interrupts the binding of MAG, OMgp or BLyS to NgR1. However, LOTUS does inhibit the interaction of NgR1 with all of the four ligands (Sato et al., 2011; Kurihara et al., 2014). It has been known that Nogo66 competes with MAG and OMgp for the binding to NgR1, indicating that the Nogo66 binding site on NgR1 overlaps those of MAG and OMgp. From these studies, it is suspected that a single soluble NgR1 peptide cannot simultaneously bind to Nogo66, MAG and OMgp. LOTUS may have the benefit of simultaneously antagonizing NgR1 activation by all four ligands although whether LOTUS antagonizes NgR1 in equal stoichiometry is still undetermined. The overlap of Nogo66, MAG or OMgp binding sites on NgR1 raises the possibility that LOTUS may interfere with the binding of Nogo66, MAG and OMgp to NgR1 in a competitive manner. It is unknown which region of NgR1 interacts with BLyS and whether BLyS competes with the other ligands for the binding to NgR1. It is also possible that LOTUS may exert its inhibitory effect via an allosteric mechanism that only interferes with BLyS-binding toNgR1, or this allosteric inhibition may extend to all four ligands. To elucidate the molecular mechanism by which LOTUS antagonizes NgR1, further investigations in structural biology are required.
Identification of a novel NgR1 ligand, chondroitin sulfate proteoglycans: Recently, chondroitin sulfate proteoglycans, which are abundant in reactive astrocytes derived from glial scars, have been identified as a functional ligand for NgR1 and Nogo receptor-3, an NgR1 homologue (Dickendesher et al., 2012). Genetic deletion of chondroitin sulfate-synthesizing enzyme (Takeuchi et al., 2013) or NgR1 (Schwab, 2010) promotes the ability of damaged CNS axons to be re-elongated, suggesting that the binding of chondroitin sulfate proteoglycans to NgR1 may be involved in the failure of damaged CNS axons to regenerate. Moreover, double administration of antibodies neutralizing NogoA and chondroitinase ABC, which catalyzes the degradation of the glycosaminoglycan chains on the chondroitin sulfate proteoglycans, is more effective in enhancing the histological and behavioral recovery after CNS injuries, compared with single administration (Zhao et al., 2013). This report suggests that concurrent inhibition of NgR1 ligands may be more effective in overcoming the failure of damaged CNS axons to regenerate. To clarify whether LOTUS is also able to inhibit chondroitin sulfate proteoglycans-mediated activation of NgR1, further investigation is required to ascertain whether LOTUS can also suppress chondroitin sulfate proteoglycans-binding to NgR1 and chondroitin sulfate proteoglycans-induced axonal growth inhibition as shown for the other four ligands. We previously revealed that the carboxyl-terminal region of LOTUS antagonizes NgR1 activation by Nogo66 (Kurihara et al., 2012). It will be interesting to explore whether this region would exert similar antagonistic effects on NgR1 with regards to MAG, OMgp and BLyS and which region of LOTUS is necessary and sufficient to exert the antagonistic activity on NgR1 with regards to all of the four ligands.
Future perspectives and challenges: It has often been established in animal models of spinal cord injury that negatively regulating NgR1 or its ligands enhances the histological and behavioral repair after CNS lesion in vivo. In addition, these molecules are associated with the neurological disorder multiple sclerosis. NogoA, BLyS and NgR1 are up-regulated in the lesioned brains of multiple sclerosis patients (Krumbholz et al., 2005; Satoh et al., 2005). Antibodies neutralizing NogoA and genetic deletion of NgR1 alleviate the symptoms of experimental autoimmune encephalomyelitis, an animal model of multiple sclerosis(Petratos et al., 2012). Consequently, Nogo or BLyS function through binding to NgR1 may impede relief of multiple sclerosis symptoms. We have recently found that LOTUS is down-regulated in the cerebral spinal fluid of multiple sclerosis patients (Takahashi et al., 2014). This finding suggests that decrease of LOTUS concentration is associated with the pathological conditions of multiple sclerosis.
The binding of Nogo, MAG, OMgp and BLyS to NgR1 transduces signals through the NgR1 co-receptors, leucine-rich repeat and immunoglobulin domain-containing Nogo receptor-interacting protein 1 (LINGO-1) and either the 75-kDa neurotrophin receptor (p75NTR) or tumor necrosis factor receptor superfamily member 19, to intracellular molecules, RhoA and its effect or, Rho-associated, coiled-coil containing protein kinase (Schwab, 2010). Soluble LINGO-1 peptides, a RhoA inactivator and a coiled-coil containing protein kinase inhibitor also improve the histological and behavioral recovery following CNS lesion (Schwab, 2010). However, the interaction of NgR1 with its ligands can also mediate signal transduction through p75NTR and tumor necrosis factor receptor superfamily member 19. Furthermore, NgR1 ligands also activate protein kinase C independently of RhoA-mediated signaling, which induces growth cone collapse and neurite outgrowth inhibition (Hasegawa et al., 2004). Therefore, it is likely that these inhibitors may be not sufficient to completely inhibit the signal transduction induced by the interaction of NgR1 with its ligands. LOTUS can completely block the interaction of NgR1 with all four ligands and therefore LOTUS can completely shut down NgR1-mediated axonal growth inhibition. Therefore, further studies are required to elucidate whether therapeutic approaches using LOTUS (for example, implantation of LOTUS-overexpressing cells and/or administration of LOTUS recombinant protein) can promote neuronal regeneration in neurological disorders such as spinal cord injury and multiple sclerosis.
This work was supported by a grant-in-aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan and by grants for Research and Development project of Yokohama City University.
References
- Dickendesher TL, Baldwin KT, Mironova YA, Koriyama Y, Raiker SJ, Askew KL, Wood A, Geoffroy CG, Zheng B, Liepmann CD, Katagiri Y, Benowitz LI, Geller HM, Giger RJ. NgR1 and NgR3 are receptors for chondroitin sulfate proteoglycans. Nat Neurosci. 2012;15:703–712. doi: 10.1038/nn.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa Y, Fujitani M, Hata K, Tohyama M, Yamagishi S, Yamashita T. Promotion of axon regeneration by myelin-associated glycoprotein and Nogo through divergent signals downstream of Gi/G. J Neurosci. 2004;24:6826–6832. doi: 10.1523/JNEUROSCI.1856-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumbholz M, Theil D, Derfuss T, Rosenwald A, Schrader F, Monoranu CM, Kalled SL, Hess DM, Serafini B, Aloisi F, Wekerle H, Hohlfeld R, Meinl E. BAFF is produced by astrocytes and up-regulated in multiple sclerosis lesions and primary central nervous system lymphoma. J Exp Med. 2005;201:195–200. doi: 10.1084/jem.20041674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara Y, Arie Y, Iketani M, Ito H, Nishiyama K, Sato Y, Nakamura F, Mizuki N, Goshima Y, Takei K. The carboxyl-terminal region of Crtac1B/LOTUS acts as a functional domain in endogenous antagonism to Nogo receptor-1. Biochem Biophys Res Commun. 2012;418:390–395. doi: 10.1016/j.bbrc.2012.01.033. [DOI] [PubMed] [Google Scholar]
- Kurihara Y, Iketani M, Ito H, Nishiyama K, Sakakibara Y, Goshima Y, Takei K. LOTUS suppresses axon growth inhibition by blocking interaction between Nogo receptor-1 and all four types of its ligand. Mol Cell Neurosci. 2014;61:211–218. doi: 10.1016/j.mcn.2014.07.001. [DOI] [PubMed] [Google Scholar]
- Petratos S, Ozturk E, Azari MF, Kenny R, Lee JY, Magee KA, Harvey AR, McDonald C, Taghian K, Moussa L, Mun Aui P, Siatskas C, Litwak S, Fehlings MG, Strittmatter SM, Bernard CC. Limiting multiple sclerosis related axonopathy by blocking Nogo receptor and CRMP-2 phosphorylation. Brain. 2012;135:1794–1818. doi: 10.1093/brain/aws100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Iketani M, Kurihara Y, Yamaguchi M, Yamashita N, Nakamura F, Arie Y, Kawasaki T, Hirata T, Abe T, Kiyonari H, Strittmatter SM, Goshima Y, Takei K. Cartilage acidic protein-1B (LOTUS), an endogenous Nogo receptor antagonist for axon tract formation. Science. 2011;333:769–773. doi: 10.1126/science.1204144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh J, Onoue H, Arima K, Yamamura T. Nogo-A and nogo receptor expression in demyelinating lesions of multiple sclerosis. J Neuropathol Exp Neurol. 2005;64:129–138. doi: 10.1093/jnen/64.2.129. [DOI] [PubMed] [Google Scholar]
- Schwab ME. Functions of Nogo proteins and their receptors in the nervous system. Nat Rev Neurosci. 2010;11:799–811. doi: 10.1038/nrn2936. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Kurihara Y, Suzuki Y, Goshima Y, Tanaka F, Takei K. Cerebrospinal fluid levels of LOTUS protein, an endogenous Nogo receptor-1 antagonist, as a possible biomarker for disease activity of multiple sclerosis. JAMA Neurol. 2014 doi: 10.1001/jamaneurol.2014.3613. doi: 10.1001/jamaneurol.20143613. [DOI] [PubMed] [Google Scholar]
- Takeuchi K, Yoshioka N, Higa Onaga S, Watanabe Y, Miyata S, Wada Y, Kudo C, Okada M, Ohko K, Oda K, Sato T, Yokoyama M, Matsushita N, Nakamura M, Okano H, Sakimura K, Kawano H, Kitagawa H, Igarashi M. Chondroitin sulphate N-acetylgalactosaminyl-transferase-1 inhibits recovery from neural injury. Nat Commun. 2013;4:2740–2750. doi: 10.1038/ncomms3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao RR, Andrews MR, Wang D, Warren P, Gullo M, Schnell L, Schwab ME, Fawcett JW. Combination treatment with anti-Nogo-A and chondroitinase ABC is more effective than single treatments at enhancing functional recovery after spinal cord injury. Eur J Neurosci. 2013;38:2946–2961. doi: 10.1111/ejn.12276. [DOI] [PubMed] [Google Scholar]


