Abstract
Objective
To determine the skeletal effects of alendronate therapy in men with primary hyperparathyroidism (PHPT) in comparison with those in postmenopausal women.
Methods
There essentially are no published data on the effects of bisphosphonate therapy in men with PHPT. We previously conducted a double-blind, randomized, single- crossover trial of alendronate, 10 mg daily, in PHPT and reported that alendronate significantly increases bone mineral density (BMD) at 12 months relative to baseline values. That study sample included both women (n = 28) and men (n = 9) and both premenopausal (n = 4) and postmenopausal (n = 24) women. Study subjects were randomly assigned to receive either alendronate or placebo during the first year, and all subjects received alendronate during the second year. Among the men, 3 received alendronate and 6 received placebo during the first year. The current analysis focuses on the skeletal effects of alendronate therapy in the 9 men during their first year of treatment versus the 6 men during their first year while receiving placebo as well as the 24 postmenopausal women during their first year of alendronate therapy. Paired t tests comparing baseline and 12-month data were performed for the 9 treated men and the 6 control subjects; unpaired t tests were used to compare the 9 treated men and the 24 treated women.
Results
Alendronate therapy for 1 year (n = 9) resulted in a 4.8% increase in BMD at the lumbar spine (P = .1) in comparison with the men who received 1 year of placebo (n = 6). Relative to baseline, men receiving alendronate showed a significant 4.4% gain in BMD at the lumbar spine (P = .009) and a 2.95% gain in total hip BMD (P = .027). A 47% decline in serum levels of bone-specific alkaline phosphatase activity was also noted with alendronate therapy (P = .003). Changes in BMD in the male population were similar to previously reported effects of alendronate therapy in postmenopausal women with PHPT.
Conclusion
Alendronate therapy in men with PHPT is associated with improvements in BMD and reductions in bone turnover. These data, similar to the findings in postmenopausal women with PHPT, suggest that aminobisphosphonates may be of value in providing skeletal protection for men with PHPT. Further study is needed to confirm skeletal protection and fracture efficacy in this population.
INTRODUCTION
Primary hyperparathyroidism (PHPT) affects approximately 1 in 400 to 1 in 1,000 individuals, with a female-to- male ratio of approximately 3 to 1 (1–3). The mean age at presentation is between 50 and 60 years (1). Although PHPT formerly was a disorder associated with overt target organ involvement, such as osteitis fibrosa cystica or renal stones, approximately 80% of patients with PHPT are currently asymptomatic at the time of presentation (4). Although parathyroidectomy is always a suitable option for patients with asymptomatic disease if no medical contraindications exist, a subset of such patients has been shown to have a stable condition for up to 15 years (5). The recently revised recommendations for parathyroidectomy in asymptomatic patients define this population further and suggest parathyroidectomy for those with osteoporosis (T-score of −2.5 or less at the hip, spine, or one-third distal radial site) (5), hypercalcemia >1 mg/dL (>0.25 mmol/L) above normal, creatinine clearance below 60 mL/min, or age <50 years (6).
Among those patients who do not meet guidelines for surgical treatment, approximately 35% will demonstrate evidence of progressive disease during a 15-year period (5). Recently, there have been attempts to manage PHPT medically, including with use of raloxifene (7), cinacalcet (8,9) (a calcimimetic), and bisphosphonates—namely, alendronate (10–12). We have published the results of a double-blind, randomized, crossover trial of alendronate, 10 mg daily for 1 year, in patients with PHPT (10). Alendronate was found to increase bone mineral density (BMD) significantly at 12 months relative to baseline values (10). That study sample included 28 women (4 premenopausal and 24 postmenopausal [PMP]) and 9 men. The purpose of the current report is to present the results of a subgroup analysis, examining the skeletal effects of alendronate in the 9 men included in our original study. We have undertaken this analysis because of the limited information available on the treatment of men with metabolic bone diseases in general and PHPT in particular. The primary hypotheses are that a similar protective effect of alendronate in PHPT will be observed in men as in PMP women and that men receiving alendronate will demonstrate an increased BMD in comparison with men given placebo.
PATIENTS AND METHODS
Study Cohort
Methodologic details of the original study have been published elsewhere (10). Briefly, the multicenter study involved 3 investigative sites—McMaster University (Hamilton, Ontario), Columbia University (New York, New York), and the University of Hong Kong (Hong Kong, China). Across the 3 centers, 44 patients with confirmed PHPT were enrolled, and 37 patients (28 female; 9 male) completed the study. All study participants met a priori inclusion criteria: confirmed hypercalcemia and elevated levels of parathyroid hormone (PTH) by immunoradiometric assay on 3 separate occasions as well as reduced bone density (T-score of less than −1.0) at one or more skeletal sites (lumbar spine, hip, or distal radius). Patients with a T-score of less than −3.5 were advised to undergo surgical treatment, but they were included if they declined that advice and still wished to participate in the study. Otherwise eligible patients were excluded from analysis if they met any guideline for surgical treatment as established by the 2002 National Institutes of Health consensus guidelines on PHPT: were taking concomitant antiresorptive therapy; were premenopausal women who still planned a future pregnancy or who were not using effective birth control (or both); had any other metabolic bone disease; had used hormone replacement therapy for less than 2 years; had impaired renal function, as defined by a serum creatinine level of more than 177 μmol/L (2 mg/dL); had familial hypocalciuric hypercalcemia; reported a history of allergy or intolerance to bisphosphonates; reported active upper gastrointestinal symptoms; or had severe PHPT and a serum calcium concentration of greater than 3.12 mmol/L (12.5 mg/dL). Patients were advised of the benefits of parathyroidectomy, and those wishing to proceed with surgical treatment were able to do so.
Study Protocol
Patients were assigned to 1 of 2 treatment groups by means of random-number tables. The active treatment group received 10 mg of alendronate daily for 1 year. Control subjects received a placebo tablet, identical in appearance and in taste to the alendronate-containing tablet. After 1 year, the placebo group was switched to active drug, and the active drug was continued for a second year in those who had received alendronate initially. Allocation during the first year remained blinded from the beginning until the end of the 2-year trial period. Data on BMD were not blinded. Moderate calcium intake was recommended, along with adequate hydration and ambulation. Patients did not receive additional supplemental calcium or vitamin D. Stratification was by sex, ensuring a roughly equal number of men and women with PHPT in the year 1 treatment and placebo arms.
Total and ionized serum calcium, phosphorus, PTH, 25-hydroxyvitamin D [25(OH)D], 1,25-dihydroxyvitamin D [1,25(OH)2D], and bone-specific alkaline phosphatase (BSAP) activity were measured every 3 months. The 24-hour urine calcium and morning urine N-terminal telopeptide of cross-linked collagen type I (NTX) levels also were measured every 3 months. A central laboratory at Mt. Sinai Hospital (Toronto, Ontario, Canada) measured PTH, 25(OH)D, 1,25(OH)2D, BSAP activity, and urinary NTX. Dual-energy x-ray absorptiometry assessments with use of the Hologic QDR 4500 bone densitometer system (Hologic, Inc., Bedford, Massachusetts) were performed at the L1–L4 lumbar spine (posteroanterior projection), total hip, femoral neck, and one-third distal radial sites every 6 months. The precision of the densitometers at the 3 investigative sites was similar; the precision results for the lumbar spine, total hip, and one-third distal radius were as follows: 1.3%, 1.5%, and 1.7% (Canada); 0.7%, 1.3%, and 0.6% (United States); and 1.2%, 1.8%, and 1.6% (China), respectively. T-scores were calculated on the basis of sex-specific referent databases. Patients were seen at 3-month intervals for 2 years at all investigative sites. Primary end points were BMD, bone markers, and serum calcium. In addition, patients were monitored for adverse effects to study drug as well as for fractures. Any reported fracture was confirmed radiographically. Screening radiographs to detect subclinical vertebral compression fractures were not done.
Before initiation of the study, the protocol had been approved by each local institutional review board. Informed consent was obtained from each patient before participation in the study.
All values reported in the “Results” section are in the following format: percent change ± standard error; P value.
Statistical Analysis
For the purposes of comparing alendronate year 1 treatment differences by sex, active treatment and control treatment groups were combined as follows. First-year data on the 3 men and 12 PMP women in the active treatment group (who received alendronate during the first year of the study) were combined with second-year data on the 6 men and 12 PMP women from the control group (who received alendronate during the second year of the study). Thus, a group was formed of 9 treated men and 24 PMP women who had received alendronate for a 1-year period.
Change in BMD over the first year of treatment was established as the primary outcome variable for the lumbar spine, total hip, and distal radius. Paired t tests were used to detect differences in BMD within the male and the PMP female groups. In similar fashion, biochemical serum analyses [BSAP, NTX, serum PTH, 25(OH)D, 1,25(OH)2D, total calcium, urinary calcium, ionized calcium, and phosphate] were also compared. For comparison of BMD and biochemical serum changes over 1 year during alendronate therapy, we used unpaired t tests to compare therapeutic effect differences between men and PMP women.
We also performed subgroup analyses on the 6 control group men who received placebo during year 1 and alendronate during year 2. Paired t tests were used to compare the differences between year 1 change scores while receiving placebo with year 2 change scores while receiving alendronate therapy.
Because these were secondary analyses with limited data available, no corrections were made to account for multiple comparisons to maximize the opportunity to detect differences. All statistical analyses were conducted by using SPSS software version 15.0 for Windows (SPSS, Inc., Chicago, Illinois), 2-tailed tests, and P<.05 as the threshold for statistical significance.
RESULTS
Demographic and Baseline Characteristics
Demographic characteristics (ethnicity and age) were similar between the male and PMP female groups. Ethnically, both the male and PMP female groups had similar proportions of white (male, 56%; PMP female, 50%) and Chinese (male, 44%; PMP female, 42%) patients. The PMP female group also included African American patients (male, 0%; PMP female, 16%). Baseline age was also similar between the male and PMP female groups. The mean age was 67.7 years for the men and 69.7 years for the PMP women (P = .55). The baseline and 12-month characteristics for the 9 men and 24 PMP women who received alendronate for 1 year are presented in Table 1. There were no clinically meaningful differences at baseline between the 2 groups, other than the distal radius BMD being lower among the PMP women than among the men (0.500 g/cm2 versus 0.625 g/cm2, respectively; P = .004).
Table 1.
Comparison of 12-Month Bone Mineral Density and Biochemical Changes in Men Receiving Alendronate, in Postmenopausal Women Receiving Alendronate, and Between Groupsa
| Factor | Group 1: men (N = 9)
|
Group 2: postmenopausal women (N = 24)
|
Comparison between groups (N = 33): absolute difference
|
Reference range | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12 mo | Change: % (value) | P value | Baseline | 12 mo | Change:% (value) | P value | % | P value | ||
| Bone mineral density | |||||||||||
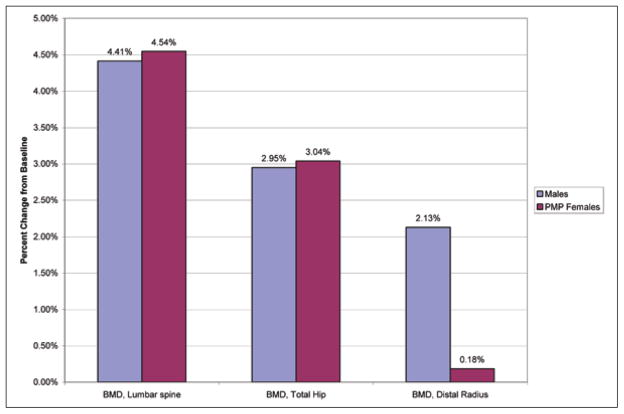
| Lumbar spine (g/cm2) | 0.802 | 0.838 | 4.41 (0.035) | .009 | 0.799 | 0.835 | 4.54 (0.036) | <.001 | −0.1 | .941 | … |
| Total hip (g/cm2) | 0.736 | 0.758 | 2.95 (0.022) | .027 | 0.686 | 0.707 | 3.04 (0.021) | <.001 | −0.1 | .932 | … |
| Distal radius (g/cm2) | 0.625 | 0.639 | 2.13 (0.013) | .194 | 0.500 | 0.501 | 0.18 (0.001) | .727 | 1.9 | .089 | … |
| Bone-specific alkaline phosphatase (μg/L) | 29.43 | 15.71 | −46.60 (−13.71) | .003 | 34.29 | 19.01 | −44.55 (−15.27) | <.001 | −2.1 | .753 | b |
| NTX (BCE nmol/mmol creatinine) | 60.14 | 23.71 | −60.57 (−36.43) | .071 | 99.56 | 52.94 | −46.82 (−46.61) | .109 | −13.8 | .826 | <85 |
| Serum parathyroid hormone (pmol/L) | 20.36 | 27.50 | 35.09 (7.14) | .062 | 15.30 | 13.87 | −9.37 (−1.43) | .285 | 44.5 | .006 | 1.3–7.6 |
| 1,25(OH)2D (pmol/L) | 133.29 | 162.86 | 22.19 (29.57) | .122 | 130.05 | 122.52 | −5.79 (−7.52) | .430 | 28.0 | .059 | 40–140 |
| 25(OH)D (nmol/L) | 40.57 | 48.57 | 19.72 (8.00) | .086 | 48.67 | 51.05 | 4.89 (2.38) | .461 | 14.8 | .354 | c |
| Urinary calcium (mmol/d) | 5.76 | 4.88 | −15.28 (−0.88) | .228 | 4.79 | 4.06 | −15.34 (−0.73) | .018 | 0.1 | .815 | 1.2–6.2 |
| Total calcium (mmol/L) | 2.66 | 2.57 | −3.36 (−0.09) | .072 | 2.66 | 2.62 | −1.48 (−0.04) | .188 | −1.9 | .371 | 2.2–2.6 |
| Ionized calcium (mmol/L) | 1.39 | 1.33 | −4.09 (−0.06) | .230 | 1.32 | 1.36 | 2.61 (0.03) | .375 | −6.7 | .166 | 1.17–1.33 |
| Phosphate (mmol/L) | 1.13 | 1.00 | −11.56 (−0.13) | .050 | 1.32 | 1.28 | −2.94 (−0.04) | .350 | −8.6 | .221 | 0.90–1.45 |
BCE = bone collagen equivalents; NTX = N-terminal telopeptide of cross-linked collagen type I; 1,25(OH)2D = 1,25-dihydroxyvitamin D; 25(OH)D = 25- hydroxyvitamin D.
Women >45 years old, postmenopausal: 14.2–42.7; men >25 years old: 15.0–41.3.
<25 deficient, <50 insufficient, >250 toxic.
Treatment Effects
Relative to baseline values, men receiving alendronate for 12 months had a clinically and statistically significant increase in BMD at both the lumbar spine (+4.4% ± 1.27%; P = .009) and the total hip (+2.95% ± 1.09%; P = .027). An increase (2.13% ± 1.49%; P = .19) in BMD was measured at the distal radius. In comparison with baseline, men receiving alendronate for 1 year had a decline in BSAP (−46.6% ± 9.57%; P = .003) and a decrease in serum phosphate values (−11.6% ± 5.01%; P = .05). Urinary NTX (−60.6% ± 27.7%; P = .07) and total calcium levels tended to decline also (−3.4% ± 1.59%; P = .07). Increases for 25(OH)D (+19.7% ± 9.59%; P = .086), 1,25(OH)2D (+22.2% ± 12.33%; P = .122), and PTH levels (+35.1% ± 15.36%; P = .062) all approached statistical significance. A complete summary of results is shown in Table 1.
Men who received placebo exhibited no differences or trends in BMD or any of the biochemical markers of bone turnover.
Paired Comparison (Placebo Versus Alendronate)
The paired comparison of the 6 control group men who received placebo during year 1 and alendronate during year 2 is displayed in Table 2. Among those male patients who received placebo for 1 year, BMD essentially remained unchanged at the lumbar spine (−0.36%), total hip (−0.09%), and distal radius (−1.24%). In comparison with the year 1 differences while placebo was given, men who received alendronate during year 2 had clinical increases in BMD that were statistically insignificant for the lumbar spine (+4.4% versus −0.4%; P = .099), as well as at the total hip (+1.95% versus −0.1%; P = .45) and the distal radius (+1.4% versus −1.2%; P = .28) (Fig. 1). Other alendronate versus placebo differences for men included a statistically significant decline in BSAP (−52.0% versus 23.5%; P = .037). Furthermore, decreases in serum phosphate (−15.19% versus 9.18%; P = .054) and ionized calcium (−7.02% versus 5.45%; P = .087) and increases for both 25(OH)D (20.13% versus 1.17%; P = .099) and 1,25(OH)2D (27.59% versus −7.62%; P = .068) all approached statistical significance.
Table 2.
Paired Comparison of Control Group Men (n = 6)
During Year 1 While Receiving Placebo With Alendronate Treatment in Year 2 Changea
| Factor | Year 1 change (T12-T0): % (value) | Year 2 change (T24-T12): % (value) | Absolute difference (year2–year1)
|
|
|---|---|---|---|---|
| % (value) | P value | |||
| Bone mineral density | ||||
| Lumbar spine (g/cm2) | −0.36 (−0.003) | 4.41 (0.037) | 4.77 (0.04) | .099 |
| Total hip (g/cm2) | −0.09 (−0.001) | 1.95 (0.015) | 2.04 (0.02) | .446 |
| Distal radius (g/cm2) | −1.24 (−0.008) | 1.38 (0.009) | 2.62 (0.02) | .281 |
| Bone-specific alkaline phosphatase (μg/L) | 23.46 (6.333) | −52.00 (−17.333) | −75.46 (−23.67) | .037 |
| NTX (BCE nmol/mmol creatinine) | −34.88 (−26.567) | −56.45 (−28.000) | −21.57 (−1.43) | .938 |
| Serum parathyroid hormone (pmol/L) | −30.16 (−5.363) | 51.37 (6.380) | 81.53 (11.74) | .311 |
| 1,25(OH)2D (pmol/L) | −7.62 (−10.867) | 27.59 (36.367) | 35.21 (47.23) | .068 |
| 25(OH)D (nmol/L) | 1.17 (0.533) | 20.13 (9.300) | 18.96 (8.77) | .099 |
| Urinary calcium (mmol/d) | −5.53 (−0.271) | −23.60 (−1.092) | −18.07 (−0.82) | .449 |
| Total calcium (mmol/L) | 1.88 (0.049) | −3.97 (−0.106) | −5.85 (−0.15) | .113 |
| Ionized calcium (mmol/L) | 5.45 (0.070) | −7.02 (−0.095) | −12.47 (−0.17) | .087 |
| Phosphate (mmol/L) | 9.18 (0.095) | −15.19 (−0.172) | −24.37 (−0.27) | .054 |
BCE = bone collagen equivalents; NTX = N-terminal telopeptide of cross-linked collagen type I; 1,25(OH)2D = 1,25-dihydroxyvitamin D; 25(OH)D = 25-hydroxyvitamin D.
Fig. 1.
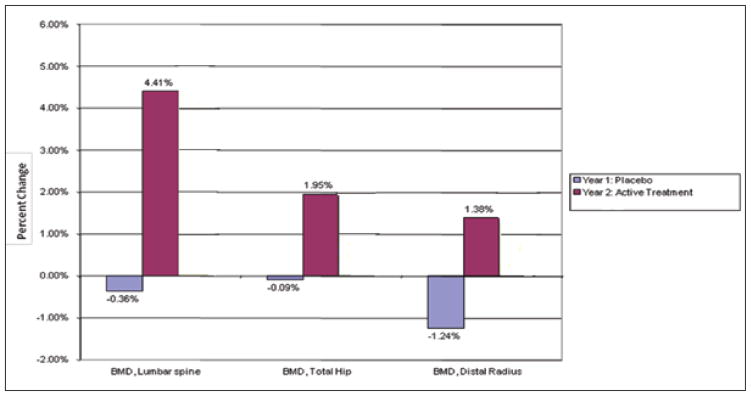
Percentage change in bone mineral density (BMD) at 3 anatomic sites in men receiving placebo in comparison with men receiving alendronate (“active treatment”).
Comparison of Alendronate Treatment Effects Between Men and PMP Women
The increases in BMD at the lumbar spine for men (4.41% ± 1.27%; P = .009) and for PMP women (4.54% ± 1.61%; P<.001) as well as at the total hip for men (2.95% ± 1.09%; P = .027) and for PMP women (3.04% ± 1.42%; P<.001) were almost identical and were not statistically different between the treated men and women (Table 1). Interestingly, men had a BMD change that was 1.95% greater than that for the PMP female group at the distal radius (for men: 2.13% ± 1.49%; P = .194; for PMP women: 0.18% ± 1.40%; P = .727), and this difference approached statistical significance (P = .089). In response to alendronate therapy, the serum PTH level increased in men (35.1%) whereas it declined in women (−9.4%), an absolute percentage difference of 44.5% (for men: 35.1% ± 15.4%; P = .062; for PMP women: −9.4% ± 18.75%; P = .285). There was also a relatively large difference in 1,25(OH)2D values between men (+22.2% ± 12.3%; P = .122) and PMP women (−5.8% ± 14.4%; P = .430). This difference also approached statistical significance (P = .059). The overall comparison is presented in Table 1 and Figure 2.
Fig. 2.
Percentage change in bone mineral density (BMD) at 3 anatomic sites after alendronate treatment in men in comparison with in postmenopausal (PMP) women.
DISCUSSION
We have previously demonstrated the densitometric benefits of alendronate in PHPT among PMP women and in a combined sample of men and women (10). In the current report, we demonstrate, for the first time, a beneficial densitometric effect of alendronate in men with PHPT when they are considered separate from women. Improvements were evident in BMD at both the lumbar spine and the total hip skeletal sites. This pattern was similar to previous observations in the original study sample of 37 subjects, among whom those receiving alendronate experienced the largest increases in BMD in the lumbar spine (4.9%) and total hip (4.0%), versus a smaller significant increase in the femoral neck (2.1%) and no significant increase in the radius (10).
Because of the crossover design, paired analysis was feasible. Statistically significant differences in BMD versus baseline were evident for the lumbar spine and total hip in the active treatment (alendronate) group. When the treatment group was compared with the placebo group, there was a clinically significant larger increase in BMD in the alendronate group.
In the current study, we observed an increase in PTH levels in men (in comparison with the relatively stable values seen in women), which approached statistical significance. It is possible that this result was attributable to the lower mean baseline 25(OH)D level in men at 40.57 nmol/L in comparison with the value of 48.67 nmol/L noted in the PMP women. The lower vitamin D level could have contributed to secondary increases in PTH after initiation of bisphosphonate therapy. The importance of maintaining vitamin D levels close to the reference range was recognized at the Third International Workshop on Asymptomatic PHPT, at which time it was recommended that vitamin D levels be maintained at or above 50 nmol/L in those patients with PHPT (6,13,14).
In addition to our previous study, other groups have demonstrated a beneficial effect of alendronate in PHPT (11,12,15) (Table 3), but none has included sufficient men and completed a randomized controlled trial evaluating the effects of alendronate in comparison with placebo. Hassani et al (11) conducted an open-label, nonrandomized, observational study involving 45 patients, 19 of whom received alendronate in comparison with 26 patients who were part of the control group. Those patients receiving alendronate experienced a 3.4% gain in lumbar spine BMD and a 3.1% gain in femoral neck BMD. The difference between the patients who took alendronate and those who did not (there was no placebo control) was about 4.8% in the lumbar spine and 3.9% at the femoral neck, which is similar to our results. There was no significant change at the radius among the 8 patients who had measurements at that site.
Table 3.
Summary of Published Data Evaluating Aminobisphosphonate Therapy in Men With Primary Hyperparathyroidisma
| Reference | Study design | No. of patients | No. of men | No. of men
|
Changes in BMD for male patients versus control subjects
|
||
|---|---|---|---|---|---|---|---|
| Receiving alendronate (10 mg control regularly) | In control group | Lumbar spine | Femoral neck | ||||
| Chow et al (16), 2003 | Randomized, controlled | 40 | 0 | 0 | 0 | NA | NA |
| Parker et al (15), 2002 | Prospective, observational | 32 | 5 | NP | NP | NA | NA |
| Rossini et al (12), 2001 | Prospective, observational | 26 | 0 | 0 | 0 | NA | NA |
| Hassani et al (11), 2001 | Prospective, observational | 45 | 19 | 9 | 10 | NP | NP |
| Khan et al (10), 2004 | Double-blind, randomized, controlled | 44 | 9 | 6 | 3 | 6.85% | −0.001 ± 0.76% |
BMD = bone mineral density; NA = not applicable; NP = not published.
Rossini et al (12) demonstrated statistically significant increases (mean ± SD) over 2 years in BMD at the lumbar spine (+8.6% ± 3.0%), total hip (+4.8% ± 3.9%), and total body (+1.2% ± 1.4%) when alendronate was administered to 26 patients with mild PHPT, between the ages of 67 and 81 years, in doses of 10 mg given on alternate days. Similarly, Parker et al (15) reported gains in BMD of between 3.1% and 7.3% after 2 years of alendronate treatment (10 mg daily) in 27 female and 5 male patients with PHPT. Nonsignificant trends toward improvement at the femoral neck, total hip, and midradius also were noted. The results of a randomized controlled trial of alendronate (10 mg daily) versus placebo during a period of 48 weeks in 40 PMP women (mean age, 70 years) were reported by Chow et al (16), in which active treatment was associated with statistically significant increases in BMD at the lumbar spine (3.8% to 4.0%) and femoral neck (4.2% to 6.0%). Again, however, no significant change in BMD was observed involving the distal third of the radius. Although the design of these studies differs from ours, all generally report results that are consistent with our findings in both our total sample (10) and the current male subgroup. The current analysis is the first published study to devote itself specifically to evaluation of the effects in men with PHPT.
Men have been the subject of far fewer studies evaluating skeletal health in comparison with women. In fact, the published data assessing etiologic factors, incidence, and treatment of osteoporosis in men are disproportionately lower than can be justified on the basis of what is known regarding the incidence of osteoporosis in men. This sex bias favoring studies in women has extended to studies of bisphosphonates in men with osteoporosis. The numbers of men in the studies evaluating the bisphosphonates alendronate, risedronate, and zoledronate are in the hundreds in comparison with the tens of thousands of women who have been studied with these agents. Moreover, this aforementioned sex bias continues in the application of these agents to subgroups of subjects. For example, alendronate has been studied in African American women but not in African American men. Similarly, studies with alendronate in PHPT have been rather predominantly weighted toward women. Our work has facilitated, for the first time, a subgroup analysis of data that provides insight into the potential efficacy of alendronate in men with PHPT.
Limitations of the current analysis include the relatively small number of men receiving active treatment and placebo. We did not correct for the multiple t tests performed; thus, a possibility exists that we may have committed one or more type I errors—that is, accepted the alternative hypothesis that a statistically significant difference exists between the sample and true population means, when in fact there is no difference. Additionally, although a priori hypotheses for this post hoc analysis were created, the fact that this was a post hoc analysis increases the chance of type I errors. For some of the test P values, the degree of significance is robust enough to have confidence in the results. Nevertheless, there are some results with significance levels approaching the .05 threshold, and these should be interpreted with caution. Furthermore, many of the t test P values were between .05 and .10. Because of the small sample size, it is possible that we have committed type II errors—that is, accepted the null hypothesis (that there is no difference) when in fact differences existed. To account for this possibility, we have highlighted differences in the “Results” section for results with t test P values between .05 and .1. Another limitation of this study is the fact that we do not have the fracture data to confirm the skeletal protective effects of alendronate.
CONCLUSION
This study is the first to document a positive effect of alendronate therapy on BMD in men with PHPT. In the aggregate, it adds to our body of knowledge regarding the value of aminobisphosphonates in lowering bone turnover and improving BMD in men with PHPT. The effect on bone turnover and BMD has not been associated with any influence on serum calcium or PTH levels. Currently, no fracture data with bisphosphonate therapy are available; therefore, further studies are needed.
Other pharmacologic options have been studied in PHPT and include cinacalcet, which has been noted to lower serum calcium and PTH levels in patients with PHPT. Cinacalcet, however, has not been shown to improve BMD or lower biochemical markers of bone turnover (17) and also requires additional study with respect to fracture efficacy in PHPT.
Pharmacologic therapies may be of value in the medical management of asymptomatic PHPT. Nevertheless, further studies are needed before these agents can be recommended as alternative options to surgical treatment. In those patients who are unable or unwilling to proceed with parathyroidectomy or those who pose increased operative risks, these medical options may be considered with close follow-up (18).
Acknowledgments
Funding for this study was received from the Investigator-Initiated Trials Fund, Merck Research Laboratories as well as the National Institutes of Health grant DK32333.
Abbreviations
- BMD
bone mineral density
- BSAP
bone-specific alkaline phosphatase
- NTX
N-terminal telopeptide of cross-linked collagen type I
- 1, 25(OH)2D
1,25-dihydroxyvitamin D
- 25(OH)D
25-hydroxyvitamin D
- PHPT
primary hyperparathyroidism
- PMP
postmenopausal
- PTH
parathyroid hormone
Footnotes
DISCLOSURE
The authors have no multiplicity of interest to disclose.
References
- 1.Bilezikian J. the American Society for Bone and Mineral Research, editor. Primary hyperparathyroidism. In: Favus MJ, editor. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. 4. Hagerstown, MD: Lippincott Williams & Wilkins; 1999. pp. 187–192. [Google Scholar]
- 2.Adami S, Marcocci C, Gatti D. Epidemiology of primary hyperparathyroidism in Europe. J Bone Miner Res. 2002;17(suppl 2):N18–N23. [PubMed] [Google Scholar]
- 3.Melton LJ., III The epidemiology of primary hyperparathyroidism in North America. J Bone Miner Res. 2002;17(suppl 2):N12–N17. [PubMed] [Google Scholar]
- 4.Silverberg SJ, Bilezikian JP. Primary hyperparathyroidism. In: Rosen CJ, editor. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. 7. Chapter 66 Washington, DC: American Society for Bone and Mineral Research; 2008. pp. 302–306. [Google Scholar]
- 5.Rubin MR, Bilezikian JP, McMahon DJ, et al. The natural history of primary hyperparathyroidism with or without parathyroid surgery after 15 years. J Clin Endocrinol Metab. 2008;93:3462–3470. doi: 10.1210/jc.2007-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bilezikian JP, Khan AA, Potts JT, Jr Third International Workshop on the Management of Asymptomatic Primary Hyperparathyroidism. Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the Third International Workshop. J Clin Endocrinol Metab. 2009;94:335–339. doi: 10.1210/jc.2008-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubin MR, Lee KH, McMahon DJ, Silverberg SJ. Raloxifene lowers serum calcium and markers of bone turnover in postmenopausal women with primary hyperparathyroidism. J Clin Endocrinol Metab. 2003;88:1174–1178. doi: 10.1210/jc.2002-020667. [DOI] [PubMed] [Google Scholar]
- 8.Peacock M, Bilezikian JP, Klassen PS, Guo MD, Turner SA, Shoback DM. Cinacalcet hydrochloride maintains long-term normocalcemia in patients with primary hyperparathyroidism. J Clin Endocrinol Metab. 2005;90:135–141. doi: 10.1210/jc.2004-0842. [DOI] [PubMed] [Google Scholar]
- 9.Wüthrich RP, Martin D, Bilezikian JP. The role of calcimimetics in the treatment of hyperparathyroidism. Eur J Clin Invest. 2007;37:915–922. doi: 10.1111/j.1365-2362.2007.01874.x. [DOI] [PubMed] [Google Scholar]
- 10.Khan AA, Bilezikian JP, Kung AW, et al. Alendronate in primary hyperparathyroidism: a double blind, randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2004;89:3319–3325. doi: 10.1210/jc.2003-030908. [DOI] [PubMed] [Google Scholar]
- 11.Hassani S, Braunstein GD, Seibel MJ, et al. Alendronate therapy of primary hyperparathyroidism. Endocrinologist. 2001;11:459–464. [Google Scholar]
- 12.Rossini M, Gatti D, Isaia G, Sartori L, Braga V, Adami S. Effects of oral alendronate in elderly patients with osteoporosis and mild primary hyperparathyroidism. J Bone Miner Res. 2001;16:113–119. doi: 10.1359/jbmr.2001.16.1.113. [DOI] [PubMed] [Google Scholar]
- 13.Eastell R, Arnold A, Brandi ML, et al. Diagnosis of asymptomatic primary hyperparathyroidism: proceedings of the Third International Workshop. J Clin Endocrinol Metab. 2009;94:340–350. doi: 10.1210/jc.2008-1758. [DOI] [PubMed] [Google Scholar]
- 14.Khan AA, Bilezikian JP, Potts JT., Jr Asymptomatic primary hyperparathyroidism: a commentary on the revised guidelines. Endocr Pract. 2009;15:494–498. doi: 10.4158/EP09162.CO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parker CR, Blackwell PJ, Fairbairn KJ, Hosking DJ. Alendronate in the treatment of primary hyperparathyroid-related osteoporosis: a 2-year study. J Clin Endocrinol Metab. 2002;87:4482–4489. doi: 10.1210/jc.2001-010385. [DOI] [PubMed] [Google Scholar]
- 16.Chow CC, Chan WB, Li JK, et al. Oral alendronate increases bone mineral density in postmenopausal women with primary hyperparathyroidism. J Clin Endocrinol Metab. 2003;88:581–587. doi: 10.1210/jc.2002-020890. [DOI] [PubMed] [Google Scholar]
- 17.Marcocci C, Chanson P, Shoback D, et al. Cinacalcet reduces serum calcium concentrations in patients with intractable primary hyperparathyroidism. J Clin Endocrinol Metab. 2009;94:2766–2772. doi: 10.1210/jc.2008-2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khan A, Grey A, Shoback D. Medical management of asymptomatic primary hyperparathyroidism: proceedings of the Third International Workshop. J Clin Endocrinol Metab. 2009;94:373–381. doi: 10.1210/jc.2008-1762. [DOI] [PubMed] [Google Scholar]