Abstract
Background
Convenient dosing, potency, and low toxicity support use of tenofovir disoproxil fumarate (TDF) as preferred nucleotide reverse transcriptase inhibitor (NRTI) for HIV-1 treatment. However, renal and metabolic safety of TDF compared to other NRTIs has not been well described in resource-limited settings.
Methods
This was a secondary analysis examining the occurrence of renal abnormalities (RAs) and renal and metabolic serious non-AIDS-defining events (SNADEs) through study follow-up between participants randomized to zidovudine (ZDV)/lamivudine/efavirenz and TDF/emtricitabine/efavirenz treatment arms within A5175/PEARLS trial. Exact logistic regression explored associations between baseline covariates and RAs. Response profile longitudinal analysis compared creatinine clearance (CrCl) over time between NRTI groups.
Results
Twenty-one of 1,045 participants developed RAs through 192 weeks follow-up; there were 15 out of 21 in the TDF arm (P = .08). Age 41 years or older (odds ratio [OR], 3.35; 95% CI, 1.1–13.1), history of diabetes (OR, 10.7; 95% CI, 2.1–55), and lower baseline CrCl (OR, 3.1 per 25 mL/min decline; 95% CI, 1.7–5.8) were associated with development of RAs. Renal SNADEs occurred in 42 participants; 33 were urinary tract infections and 4 were renal failure/insufficiency; one event was attributed to TDF. Significantly lower CrCl values were maintained among patients receiving TDF compared to ZDV (repeated measures analysis P = .05), however worsening CrCl from baseline was not observed with TDF exposure over time. Metabolic SNADEs were rare, but were higher in the ZDV arm (20 vs 3; P < .001).
Conclusions
TDF is associated with lower serious metabolic toxicities but not higher risk of RAs, serious renal events, or worsening CrCl over time compared to ZDV in this randomized multinational study.
Keywords: antiretrovirals, metabolic toxicity, renal toxicity, tenofovir, zidovudine
Antiretroviral therapy (ART) can effectively reduce HIV-related morbidity and mortality in developed and developing countries.1–4 Differences in population sociodemographics, accessibility, and costs are responsible for the discrepancy in HIV treatment recommendations between rich and poor resource settings.5–12 However, evidence indicating the benefit of ART to prevent HIV transmission and improve survival of non-AIDS-defining diseases supports earlier initiation of HIV treatment and promotes the use of efficacious, less toxic, and conveniently available agents.13
The 2013 World Health Organization’s (WHO) consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection expanded the recommendations on initiation of ART by including all HIV-infected individuals with CD4 cell counts ≤500 cells/μL, active tuberculosis, hepatitis B coinfection, and severe liver disease; in sero-discordant relationships; in pregnant and breastfeeding women; and in children under age 5 years regardless of their CD4 cell count. A combination of tenofovir disoproxil fumarate (TDF) plus emtricitabine (FTC) or lamivudine (3TC) as the nucleoside reverse transcriptase inhibitor (NRTI) backbone with efavirenz (EFV) has been listed as the preferred first-line regimen for treatment of naïve individuals, leaving zidovudine (ZDV) as an alternative.12 A decline in the cost of TDF’s generic form, once-daily dosing, potency and durability of viral suppression, and low toxicity are some of the advantages of a TDF-based regimen. However, evidence supporting the superiority of TDF compared to ZDV is derived from industry-sponsored randomized controlled trials in developed countries, and data from middle- and low-income countries are scarce.14–17
The AIDS Clinical Trials Group (ACTG) 5175 study, based on a population more representative of the global epidemic, showed that ZDV/3TC + EFV was equally effective as TDF/FTC + EFV, but more laboratory abnormalities and side effect symptoms requiring drug substitutions were observed in the ZDV-containing treatment arm. An analysis of the safety profile of both NRTI regimens will help determine the optimum ART regimen in a population in which laboratory monitoring of drug-related toxicity can be limited.18
Serious metabolic abnormalities such as loss or accumulation of subcutaneous body fat, insulin resistance, dyslipidemia, lactic acidosis, myopathy, and pancreatitis have been recognized as complications of long term NRTI use, particularly thymidine analogues.19–22 The incidence of these complications with ZDV use is lower compared to stavudine and didanosine but is higher than with TDF.23–25
In contrast, TDF use is associated with potential bone demineralization and a risk of kidney injury manifested as acute or chronic renal failure, proximal tubular dysfunction, and nephrogenic diabetes insipidus. Nonetheless, postmarketing safety data and meta-analysis have shown that TDF-associated nephrotoxicity is rare and only a modest decline in glomerular filtration rate (GFR) is observed among TDF recipients. Estimating the rates of TDF-associated nephrotoxicity in poor resource settings is important, as prevalence of preexisting renal insufficiency can be higher and a strong pharmacovigilance system is absent. The purpose of this study is to evaluate the occurrence of metabolic and renal adverse events associated with the use of ZDV- and TDF-based regimens among participants of the ACTG 5175 trial.
METHODS
The ACTG 5175 study, also known as Prospective Evaluation of Antiretrovirals in Resource Limited Settings (PEARLS) Study, was a phase IV, prospective, randomized, open-label, noninferiority trial designed to evaluate the efficacy and safety of 3 different drug combinations for initial treatment of HIV-1–infected individuals in resource-limited settings.18 The present study is a safety secondary analysis focusing on the renal and metabolic safety outcomes among participants randomized to ZDV- and TDF-based regimens within the original study. Other secondary safety analyses will be reported elsewhere.
Participants were recruited from 9 selected countries in Africa (Malawi, South Africa, Zimbabwe), Asia (India, Thailand), the Caribbean (Haiti), South America (Brazil, Peru), and North America (United States). Enrollment occurred from May 2005 through July 2007. Study population included individuals ≥18 years of age with documented HIV-1 infection, with CD4+ cell counts <300 cells/mm3 in the preceding 90 days, and who were ARV naïve or had a cumulative ART exposure of less than 7 days prior to study (single-dose nevirapine or ZDV use to prevent mother-to-child HIV transmission was allowed). Individuals with an absolute neutrophil count <750 cells/mL, hemoglobin <7.5 g/dL, platelet count <50,000 cells/mL, estimated creatinine clearance (CrCl) by Cockcroft-Gault method <60 mL/min, total bilirubin >2.5-fold above the upper limit of normal, aspartate or alanine transaminases >5-fold above the upper limit of normal, history of inflamed pancreas within 3 years, active alcohol or drug dependence, who suffered from an untreated (or treated for less than 14 days) serious infections, received radiotherapy or systemic chemotherapy within 45 days prior to study entry, or had a positive pregnancy test, were excluded. Only participants assigned to treatment arm A, ZDV 300 mg and 3TC 150 mg twice daily plus EFV 600 mg daily (ZDV/3TC + EFV), and arm C, TDF 300 mg and FTC 200 mg once daily plus EFV 600 mg once daily (TDF/FTC + EFV), were included in this analysis. Study follow-up closeout visits were conducted between April 1 and May 31, 2010. Further details of the study design and methodology have been published.18
Clinical and laboratory evaluations including serum chemistries and liver function tests were scheduled at screening, study entry, weeks 2, 4, 8, 12, 16, 20, and 24, and then every 8 weeks until closeout visits. Urine dipstick for protein and glucose were recorded every 24 weeks. CrCl was calculated by Cockcroft-Gault equation using the concurrent serum creatinine level and weight every 24 weeks. Lactate, lipase, creatine kinase, and triglycerides values were only obtained when there was a suspected lactic acidosis or pancreatitis diagnosis. Self-reported or physically evident fat deposit changes suggestive of lipoatrophy and lipodystrophy were assessed by anthropometrical measurements at scheduled events. Dual energy x-ray absorptiometry (DEXA) T score to measure bone marrow density (BMD) was not obtained routinely.
This adverse event analysis included occurrence of any renal abnormality and renal or metabolic serious non-AIDS-defining events (SNADEs). Renal abnormality was defined as either a serum creatinine level ≥1.9 mg/dL (grade 3 or 4 serum creatinine levels according to the US Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events) or calculated CrCl < 50 mL/min. The first component of this outcome was part of the primary safety analysis already reported.18 Because renal events defined by this criterion were rare, the second component was added for the current analysis. Renal and metabolic diagnoses that qualified as SNADEs used a standardized definition that has been utilized in other large ACTG studies.26,27 Diagnoses qualifying as renal SNADEs included nephrolithiasis, proximal renal tubular dysfunction, HIV nephropathy, drug-induced nephropathy, and the following diagnoses if approved by blinded central medical review: renal insufficiency/failure, acute renal insufficiency, cystitis/urinary tract infection, calculus of ureter, hydronephrosis, pyelonephritis, renal cyst, unspecified urethral stricture and urethritis. Diagnoses qualifying as metabolic SNADEs included diabetes/glucose intolerance, diabetic ketoacidosis, lipoatrophy/fat loss, fat accumulation, hypogonadism, pancreatitis, lactic acidosis, and additionally thyroid disease if approved by blinded central medical review.
In addition, we explored the association between urine abnormalities (either protein ≥ 2+ or urine glucose ≥ 1+ from dipstick testing) and serum creatinine values in order to assess whether simple urine dipstick might be used as a less invasive and cheaper monitoring parameter for renal abnormalities. Finally, comparisons of CrCl changes over time were performed between participants assigned to TDF versus those assigned to ZDV arm.
Exact logistic regression modeling explored the association between baseline covariates and renal abnormality outcome. Wilcoxon rank-sum tests were used to compare serum creatinine levels with abnormal urine dipstick results every 24 weeks. Response profile repeated measures analysis (using unstructured covariance structure) compared changes in CrCl over time (weeks 24, 48, 96, and 144) by randomized treatment group.
RESULTS
The study population included 1,571 individuals with HIV-1 infection, recruited from 9 countries (including 8 low- and middle-income countries) in 4 continents. A total of 1,045 individuals were randomized to the EFV-containing arms: 519 participants to the ZDV/3TC + EFV arm and 526 to the TDF/FTC + EFV arm. Participant’s baseline demographic and clinical characteristics were comparable in both treatment arms, as expected by randomization. Follow-up was completed in 84% of the participants in the ZDV treatment arm and in 80.5% of the participants in the TDF treatment arm with a median follow-up of 184 weeks. A total of 38 deaths were recorded among study participants (20 deaths in the TDF arm and 18 in the ZDV arm), and inability to reach the clinic was the most common reason for loss to follow-up; details have been reported elsewhere.18
Already published data show that primary safety outcomes (time to grade 3 or higher sign/symptom, laboratory abnormality, or discontinuation of study regimen) occurred in 243 (46%) of participants assigned to TDF/FTC + EFV versus 313 [60%] of participants assigned to ZDV/3TC + EFV (hazard ratio [HR], 0.64; 95% confidence interval [CI], 0.54–0.76).18 Occurrence of a grade 3 or higher sign or symptom was not significantly different between arms (115 vs 116 events; HR, 0.96; 95% CI, 0.74–1.24), whereas the number of a grade 3 or higher laboratory abnormality was significantly higher in ZDV/3TC + EFV arm (154 vs 98 events; HR, 0.55; 95% CI, 0.43–0.71). By 192 weeks, the cumulative probability of developing a grade 3 or 4 laboratory abnormality was 19.7% for the TDF-containing arm versus 40.9% for the ZDV-based regimen. Hematologic abnormalities such as neutropenia and anemia were the most common laboratory abnormalities recorded; nonetheless hematologic adverse events are not included in this secondary analysis. Severe or life-threatening metabolic and renal laboratory abnormalities were rare.18
Grade 3 or higher serum creatinine levels were detected in 10 participants, whereas CrCl < 50 mL/min was observed in 16 individuals, making a total of 21/1,045 participants meeting our definition of renal abnormality though study follow-up (5 participants experienced both components of the renal abnormality outcome but not necessarily simultaneously). Table 1 shows a comparison of baseline characteristics between patients with and without a renal abnormality outcome. Although 15 of the 21 participants with renal abnormality outcome had been assigned to the TDF/FTC + EFV treatment arm compared to 6 in the ZDV/3TC +EFV treatment arm, this difference was not statistically significant at the nominal .05 level (P = .08). The majorities of patients with renal abnormalities were men (57%), recruited from Malawi (38%) or India (24%), and were significantly older than those not experiencing such an event (median age of 41 vs 34 years). Significantly lower baseline CrCl was observed in the renal abnormality group (median 77.4 mL/min) compared to those without renal abnormality (median 99.0 mL/min).
Table 1.
Pretreatment characteristics of study sample by renal abnormalities during study follow-up
| Characteristics | Renal abnormality
|
Total (N = 1,045) | P | |
|---|---|---|---|---|
| No (n = 1,024) | Yes (n = 21) | |||
| Randomized treatment arm, n (%)a | ||||
| ZDV/3TC + EFV | 513 (50) | 6 (29) | 519 (50) | .08b |
| TDF/FTC + EFV | 511 (50) | 15 (71) | 526 (50) | |
| Age, years, median (IQR) | 34 (29–40) | 41 (32–46) | 34 (29–41) | .006c |
| Sex, n (%) | ||||
| Female | 474 (46) | 9 (43) | 483 (46) | .83b |
| Male | 550 (54) | 12 (57) | 562 (54) | |
| Country strata, n (%) | ||||
| Brazil | 154 (15) | 1 (5) | 155 (15) | .07d |
| Haiti | 67 (7) | 1 (5) | 68 (7) | |
| India | 164 (16) | 5 (24) | 169 (16) | |
| Malawi | 139 (14) | 8 (38) | 147 (14) | |
| Peru | 86 (8) | 0 (0) | 86 (8) | |
| South Africa | 139 (14) | 1 (5) | 140 (13) | |
| Thailand | 66 (6) | 1 (5) | 67 (6) | |
| United States | 138 (13) | 2 (10) | 140 (13) | |
| Zimbabwe | 71 (7) | 2 (10) | 73 (7) | |
| Body mass index, kg/m2, median (IQR) | 22.5 (20.2–25.2) | 21.6 (18.9–24.4) | 22.4 (20.2–25.2) | .28c |
| Screening CD4 cell count, cells/μL, median (IQR) | 167 (89–229) | 145 (75–215) | 167 (89–228) | .46 c |
| Plasma HIV-1 viral load, log10 copies/mL, median (IQR) | 5.0 (4.6–5.4) | 5.3 (4.7–5.5) | 5.0 (4.6–5.4) | .24 c |
| History of AIDS related diagnoses, n (%) | ||||
| No | 916 (89) | 16 (76) | 932 (89) | .07b |
| Yes | 108 (11) | 5 (24) | 113 (11) | |
| History of tuberculosis, n (%) | ||||
| No | 838 (32) | 15 (71) | 853 (82) | .25 b |
| Yes | 186 (18) | 6 (29) | 192 (18) | |
| History of hypertension, n (%) | ||||
| No | 967 (94) | 19 (90) | 986 (94) | .34 b |
| Yes | 57 (6) | 2 (10) | 59 (6) | |
| History of diabetes, n (%) | ||||
| No | 1014 (99) | 19 (91) | 1,033 (99) | <.001d |
| Yes | 10 (1) | 2 (9) | 12 (1) | |
| Creatinine clearance by CG, mL/min, median (IQR)e | 99.0 (81.6–121.1) | 77.4 (67.5–92.0) | 98.3 (80.9–120.9) | <.001c |
| Abnormal urine dipstick, n (%)f | ||||
| No | 935 (91) | 16 (76) | 951 (91) | .17b |
| Yes | 39 (4) | 2 (10) | 41 (4) | |
| Missing | 50 (5) | 3 (14) | 53 (5) | |
Note: CG = Crockoft-Gault; ZDV = zidovudine; 3TC = lamivudine; EFV = efavirenz; TDF = tenofovir; FTC = emtricitabine; IQR = interquartile range.
Percentages are column percentages.
Fisher’s exact test.
Exact Wilcoxon test.
Chi-square test.
A total of 11 missing values (all in the “no” renal abnormality group).
Urine dipsticks positive for protein ≥ 2+or glucose ≥ 1+.
Logistic regression models of renal abnormality outcome exploring association with covariates such as treatment arm, baseline age, body mass index (BMI), HIV-1 viral load and CD4 cell count, CrCl, history of an AIDS event, history of tuberculosis, history of hypertension, history of diabetes, and abnormal baseline urine dipstick result are shown in Table 2. Older age was associated with higher odds of developing a renal abnormality outcome, particularly among those 41 years or older (odds ratio [OR], 3.35; 95% CI, 1.1–13.1) compared to less than 29 years as reference group. A decline in CrCl of 25 mL/min from baseline CrCl (OR, 3.1; 95% CI, 1.7–5.8) and a history of diabetes were also associated with higher odds of developing a renal abnormality (OR, 10.7; 95% CI, 2.1–55).
Table 2.
Logistic regression analysis of the association between pretreatment covariates and renal abnormality during follow-up (n = 21)
| Variable | Univariate analyses
|
|
|---|---|---|
| Odds ratio (95% CI) | P | |
| Age (vs <29 years) | .008 | |
| 29 to <34 years | 0.97 (0.2–4.6) | |
| 34 to <41 years | 0.81 (0.2–3.8) | |
| ≥41 years | 3.35 (1.1–13.1) | |
| Assigned treatment arm (C vs A)a | 2.51 (0.91–8) | .08 |
| Body mass index, m/kg2 | 0.94 (0.84–1.05) | .25 |
| Country (vs USA) | .072 | |
| Brazil | 0.45 (0.04–5) | |
| Haiti | 1.03 (0.09–11.6) | |
| India | 2.1 (0.4–11) | |
| Malawi | 3.97 (0.83–19) | |
| Peru | Noneb | |
| South Africa | 0.5 (0.04–5.5) | |
| Thailand | 1.05 (0.09–11.7) | |
| Zimbabwe | 1.94 (0.27–14.1) | |
| Plasma HIV-1 viral load, log10 copies/mL | 1.68 (0.79–3.57) | .15 |
| CD4 cell count, per 50 cells/μL | 1 (0.99–1) | .46 |
| Creatinine clearance, per 25 mL/min decrease from baseline | 3.1 (1.7–5.8) | <.001 |
| History of hypertension | 1.05 (0.03–6.9) | .93 |
| History of diabetes | 10.7 (2.1–55) | .045 |
| History of AIDS event | 2.7 (0.95–57.4) | .13 |
| History of tuberculosis | 1.8 (0.57–5.0) | .25 |
| Abnormal urine dipstickc | 3.0 (0.32–13.5) | .17 |
Assigned treatment arm A: zidovudine/lamivudine + efavirenz; arm C: tenofovir/emtricitabine + efavirenz.
No estimate. Peru had no events; model excluding 86 participants from Peru resulted in very similar odds ratio.
Urine dipstick positive for protein (≥ 2+) or glucose (≥ 1+); 3 missing values.
Regarding SNADEs, previously reported data had shown that bacterial infections (12%) were the most common, followed by neuropsychiatric disorders (9%) and renal diagnoses (4%). A total of 42 participants had renal SNADEs recorded and they are summarized in Table 3. Thirty-three (79%) of recorded renal SNADEs were urinary tract infections. Two diagnoses of renal failure and 2 diagnoses of acute renal insufficiency were included in the renal SNADEs category after chair review; 3 of the cases had severe or life-threatening serum creatinine levels and met our definition of renal abnormality. The remaining case of acute renal insufficiency was due to sepsis as a complication of underlying lymphoma resulting in patient’s death and was not included in the renal abnormality analysis. Only one case of acute renal insufficiency was attributed to TDF at 197 weeks of treatment and withholding the drug resulted in improvement. Metabolic SNADEs were rare, occurring in only 23 participants (Table 4). Participants assigned to the TDF/FTC + EFV arm had fewer serious metabolic diagnoses compared to participants assigned to ZDV/3TC + EFV (3 vs 20 participants; P < .001). Eight diagnoses of lipoatrophy (40%) were recorded in the ZDV/3TC + EFV arm compared to none in the TDF/FTC + EFV arm. Six of the cases were women, and the majority of the events were captured after the second year of treatment. Pancreatitis was the second most common recorded SNADE, with 5/6 cases randomized to the ZDV-based treatment arm; however 2 of these cases were attributed to gallstones. Other metabolic diagnoses of interest such as lactic acidosis, glucose intolerance, hyperthyroidism, and fat accumulation were rare.
Table 3.
Serious renal non-AIDS-defining events by randomized treatment arma
| ZDV/3TC/EFV (n = 19) | TDF/FTC/EFV (n = 23) | All events (N = 42) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Renal diagnoses | Male | Female | Subtotal | Male | Female | Subtotal | Male | Female | Subtotal |
| Overall | 7 (37) | 12 (63) | 19 (100) | 6 (26) | 17 (74) | 23 (100) | 13 (31) | 29 (69) | 42 (100) |
| Renal system disease disorder, other | 5 (26) | 11 (58) | 16 (84) | 5 (22) | 17 (74) | 22 (96) | 10 (24) | 28 (67) | 38 (91) |
| Urinary tract infection | 4 (21) | 11 (58) | 15 (79) | 5 (22) | 13 (57) | 18 (78) | 9 (21) | 24 (57) | 33 (79) |
| Renal failureb | 1 (5) | 0 (0) | 1 (5) | 0 (0) | 1 (4) | 1 (4) | 1 (2) | 1 (2) | 2 (5) |
| Cystitis | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (4) | 1 (4) | 0 (0) | 1 (2) | 1 (2) |
| Acute glomerulonephritis | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (4) | 1 (4) | 0 (0) | 1 (2) | 1 (2) |
| Calculus ureteric | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (4) | 1 (4) | 0 (0) | 1 (2) | 1 (2) |
| Nephrolithiasis | 1 (5) | 0 (0) | 1 (5) | 1 (4) | 1 (4) | 2 (9) | 2 (5) | 1 (2) | 3 (7) |
| Acute renal insufficiencyb | 1 (5) | 1 (5) | 2 (11) | 0 (0) | 0 (0) | 0 (0) | 1 (2) | 1 (2) | 2 (5) |
Note: Values given as n (%). 3TC= lamivudine; EFV = efavirenz; FTC = emtricitabine; TDF = tenofovir; ZDV = zidovudine.
An individual could have more than one renal serious non-AIDS-defining events diagnosis resulting in a greater number of diagnoses than individuals.
Two cases of renal failure and one case of acute renal insufficiency had grade 3 or 4 serum creatinine levels and were also included under renal abnormality outcome.
Table 4.
Serious metabolic non-AIDS-defining events by randomized treatment arm
| Metabolic diagnoses | ZDV/3TC/EFV (n = 20) | TDF/FTC/EFV (n = 3) | All events (N = 23) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Subtotal | Male | Female | Subtotal | Male | Female | Subtotal | |
| Overall | 9 (45) | 11 (55) | 20 (100) | 1 (33) | 2 (67) | 3 (100) | 10 (44) | 13 (57) | 23 (100) |
| Lipoatrophy/fat loss (lipodystrophy) | 2 (10) | 6 (30) | 8 (40) | 0 (0) | 0 (0) | 0 (0) | 2 (9) | 6 (26) | 8 (35) |
| Lipoatrophy | 1 (5) | 4 (20) | 5 (25) | 0 (0) | 0 (0) | 0 (0) | 1 (4) | 4 (17) | 5 (22) |
| Lipodystrophy acquired | 1 (5) | 2 (10) | 3 (15) | 0 (0) | 0 (0) | 0 (0) | 1 (4) | 2 (9) | 3 (13) |
| Pancreatitis - clinical or symptomatic | 3 (15) | 2 (10) | 5 (25) | 0 (0) | 1 (33) | 1 (33) | 3 (13) | 3 (13) | 6 (26) |
| Pancreatitis | 2 (10) | 1(5) | 3 (15) | 0 (0) | 1 (33) | 1 (33) | 2 (9) | 2 (9) | 4 (17) |
| Pancreatitis chronic | 1 (5) | 0 (0) | 1 (5) | 0 (0) | 0 (0) | 0 (0) | 1 (4) | 0 (0) | 1 (4) |
| Pancreatitis acute | 0 (0) | 1 (5) | 1 (5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (4) | 1 (4) |
| Diabetes mellitus/glucose intolerance | 2 (10) | 1 (5) | 3 (15) | 1 (33) | 0 (0) | 1 (33) | 3 (13) | 1 (4) | 4 (17) |
| Diabetes mellitus | 2 (10) | 1 (5) | 3 (15) | 1 (33) | 0 (0) | 1 (33) | 3 (13) | 1 (4) | 4 (17) |
| Lactic acidemia/acidosis | 1 (5) | 2 (10) | 3 (15) | 0 (0) | 0 (0) | 0 (0) | 1 (4) | 2 (9) | 3 (13) |
| Lactic acidosis | 1 (5) | 2 (10) | 3 (15) | 0 (0) | 0 (0) | 0 (0) | 1 (4) | 2 (9) | 3 (13) |
| Hyperthyroidism | 1 (5) | 0 (0) | 1 (5) | 0 (0) | 1 (33) | 1 (33) | 1 (4) | 1 (4) | 2 (9) |
| Hyperthyroidism | 1 (5) | 0 (0) | 1 (5) | 0 (0) | 0 (0) | 0 (0) | 1 (4) | 0 (0) | 1 (4) |
| Hypothyroidism | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (33) | 1 (33) | 0 (0) | 1 (4) | 1 (4) |
| Fat accumulation | 1 (5) | 1 (5) | 2 (10) | 0 (0) | 0 (0) | 0 (0) | 1 (4) | 1 (4) | 2 (9) |
| Body fat disorder | 1 (5) | 1 (5) | 2 (10) | 0 (0) | 0 (0) | 0 (0) | 1 (4) | 1 (4) | 2 (9) |
Note: Values given as n (%). 3TC = lamivudine; EFV = efavirenz; FTC = emtricitabine; TDF = tenofovir; ZDV = zidovudine.
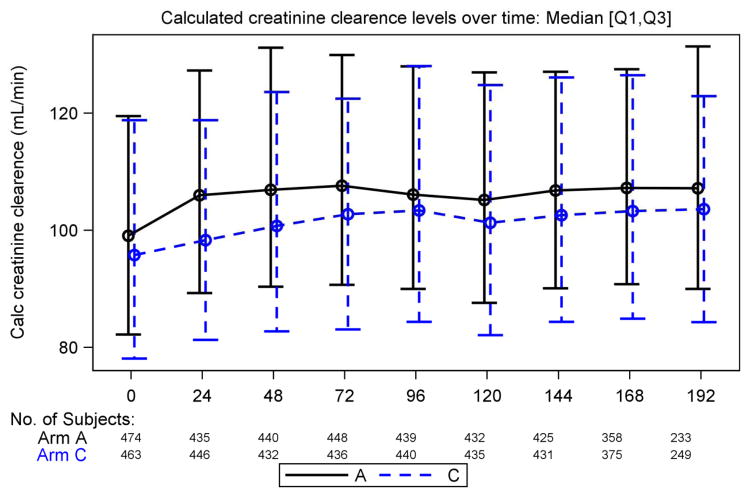
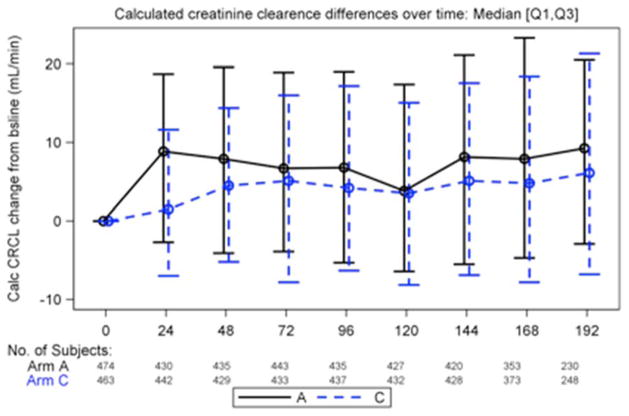
Rates of proteinuria detected by urine dipstick were also comparable between both treatment arms; except at week 144 and 168 when higher rates (mostly trace proteinuria) were reported among those in the TDF/FTC + EFV arm compared to the ZDV/3TC + EFV arm (19% vs 13%, P = .008, and 18% vs 12.4%, P = .043, respectively) as shown in Table 5. Association of serum creatinine levels and significant urine dipstick (protein ≥ 2+ or glucose ≥ 1+) were not statistically significant at any time point. When analyzing the trend of CrCl over time by treatment arm, we observe a statistically significant lower value in the TDF-based arm as compared to the ZDV-based arm. However the magnitude of this difference was small, as shown in Figure 1 (medians ranging from 98 vs 106 mL/min at 24 weeks, P <.001, to 103 vs 106 mL/min at 96 weeks, p = .07; repeated measures analysis, P = .05). Nonetheless, we did not observe significant changes in calculated changes in CrCl from baseline over time in the TDF-based treatment arm compared to ZDV (Figure 2).
Table 5.
Protein urine dipstick results over time by treatment arm
| Protein by urine dipstick | AZT/3TC/EFV | TDF/FTC/EFV | Total | P valuea |
|---|---|---|---|---|
| Week 0b | ||||
| No. of subjects on Step 1c | 519 | 526 | 1,045 | |
| No. of urine specimens collected | 497 | 495 | 992 | |
| Protein - Negative, n (%)d | 377 (75.9) | 353 (71.3) | 730 (73.6) | .10 |
| Trace | 52 (10.5) | 81 (16.4) | 133 (13.4) | |
| 1+ | 46 (9.3) | 44 (8.9) | 90 (9.1) | |
| 2+ | 14 (2.8) | 14 (2.8) | 28 (2.8) | |
| 3+ | 6 (1.2) | 3 (0.6) | 9 (0.9) | |
| 4+ | 1 (0.2) | 0 (0) | 1 (0.1) | |
| Not evaluated or recorded | 1 (0.2) | 0 (0) | 1 (0.1) | |
| Week 24 | ||||
| No. of subjects on Step 1 | 497 | 506 | 1,003 | |
| No. of urine specimens collected | 488 | 490 | 978 | |
| Protein - Negative, n (%) | 427 (87.5) | 422 (86.1) | 849 (86.8) | .28 |
| Trace | 33 (6.8) | 39 (8.0) | 72 (7.4) | |
| 1+ | 20 (4.1) | 23 (4.7) | 43 (4.4) | |
| 2+ | 7 (1.4) | 5 (1.0) | 12 (1.2) | |
| 3+ | 1 (0.2) | 1 (0.2) | 2 (0.2) | |
| 4+ | 0 (0) | 0 (0) | 0 (0) | |
| Not evaluated or recorded | 0 (0) | 0 (0) | 0 (0) | |
| Week 48 | ||||
| No. of subjects on Step 1 | 469 | 474 | 943 | |
| No. of urine specimens collected | 461 | 460 | 921 | |
| Protein - Negative, n (%) | 410 (88.9) | 403 (87.6) | 813 (88.3) | .27 |
| Trace | 31 (6.7) | 33 (7.2) | 64 (6.9) | |
| 1+ | 12 (2.6) | 20 (4.3) | 32 (3.5) | |
| 2+ | 5 (1.1) | 3 (0.7) | 8 (0.9) | |
| 3+ | 3 (0.7) | 1 (0.2) | 4 (0.4) | |
| 4+ | 0 (0) | 0 (0) | 0 (0) | |
| Not evaluated or recorded | 1 (0.2) | 0 (0) | 0 (0) | |
| Week 72 | ||||
| No. of subjects on Step 1 | 457 | 457 | 914 | |
| No. of urine specimens collected | 435 | 443 | 878 | |
| Protein - Negative, n (%) | 383 (88.0) | 382 (86.2) | 765 (87.1) | .28 |
| Trace | 30 (6.9) | 44 (9.9) | 74 (8.4) | |
| 1+ | 16 (3.7) | 14 (3.2) | 30 (3.4) | |
| 2+ | 5 (1.1) | 2 (0.5) | 7 (0.8) | |
| 3+ | 1 (0.2) | 0 (0) | 1 (0.1) | |
| 4+ | 0 (0) | 0 (0) | 0 (0) | |
| Not evaluated or recorded | 0 (0) | 1 (0.2) | 1 (0.1) | |
| Week 96 | ||||
| No. of subjects on Step 1 | 446 | 454 | 900 | |
| No. of urine specimens collected | 423 | 443 | 866 | |
| Protein - Negative, n (%) | 372 (87.9) | 391 (88.3) | 763 (88.1) | .42 |
| Trace | 32 (7.6) | 36 (8.1) | 68 (7.9) | |
| 1+ | 13 (3.1) | 15 (3.4) | 28 (3.2) | |
| 2+ | 4 (0.9) | 1 (0.2) | 5 (0.6) | |
| 3+ | 2 (0.5) | 0 (0) | 2 (0.2) | |
| 4+ | 0 (0) | 0 (0) | 0 (0) | |
| Not evaluated or recorded | 0 (0) | 0 (0) | 0 (0) | |
| Week 120 | ||||
| No. of subjects on Step 1 | 425 | 445 | 870 | |
| No. of urine specimens collected | 405 | 427 | 832 | |
| Protein - Negative, n (%) | 347 (85.7) | 360 (84.3) | 707 (85.0 | .24 |
| Trace | 42 (10.4) | 52 (12.2) | 94 (11.3) | |
| 1+ | 11 (2.7) | 10 (2.3) | 21 (2.5) | |
| 2+ | 2 (0.5) | 3 (0.7) | 5 (0.6) | |
| 3+ | 1 (0.2) | 2 (0.5) | 3 (0.4) | |
| 4+ | 0 (0) | 0 (0) | 0 (0) | |
| Not evaluated or recorded | 2 (0.5) | 0 (0) | 2 (0.2) | |
| Week 144 | ||||
| No. of subjects on Step 1 | 415 | 434 | 849 | |
| No. of urine specimens collected | 394 | 414 | 808 | |
| Protein - Negative, n (%) | 344 (87.3) | 335 (80.9) | 679 (84.0) | .008 |
| Trace | 29 (7.4) | 57 (13.8) | 86 (10.6) | |
| 1+ | 12 (3.0) | 18 (4.3) | 30 (3.7) | |
| 2+ | 6 (1.5) | 4 (1.0) | 10 (1.2) | |
| 3+ | 2 (0.5) | 0 (0) | 2 (0.2) | |
| 4+ | 0 (0) | 0 (0) | 0 (0) | |
| Not evaluated or recorded | 1 (0.3) | 0 (0) | 1 (0.1) | |
| Week 168 | ||||
| No. of subjects on Step 1 | 342 | 365 | 707 | |
| No. of urine specimens collected | 299 | 318 | 617 | |
| Protein - Negative, n (%) | 262 (87.6) | 260 (81.8) | 522 (84.6) | .043 |
| Trace | 19 (6.4) | 37 (11.6) | 56 (9.1) | |
| 1+ | 14 (4.7) | 16 (5.0) | 30 (4.) | |
| 2+ | 2 (0.7) | 3 (0.9) | 5 (0.8) | |
| 3+ | 1 (0.3) | 0 (0) | 1 (0.2) | |
| 4+ | 1 (0.3) | 0 (0) | 1 (0.2) | |
| Not evaluated or recorded | 0 (0) | 2 (0.6) | 2 (0.3) | |
| Week 192 | ||||
| No. of subjects on Step 1 | 216 | 228 | 444 | |
| No. of urine specimens collected | 156 | 163 | 319 | |
| Protein - Negative, n (%) | 138 (88.5) | 136 (83.4) | 274 (85.9) | .10 |
| Trace | 10 (6.4) | 15 (9.2) | 25 (7.8) | |
| 1+ | 5 (3.2) | 10 (6.1) | 15 (4.7) | |
| 2+ | 2 (1.3) | 1 (0.6) | 3 (0.9) | |
| 3+ | 1 (0.6) | 1 (0.6) | 2 (0.6) | |
| 4+ | 0 (0) | 0 (0) | 0 (0) | |
| Not evaluated or recorded | 0 (0) | 0 (0) | 0 (0) |
Note: 3TC = lamivudine; EFV = efavirenz; FTC = emtricitabine; TDF = tenofovir; ZDV = zidovudine.
By Wilcoxon test. The level “not evaluated/not reported” is excluded from treatment comparison.
Week 0 is defined as prior to the randomization date. Other weeks are defined as the scheduled time ±28 days.
At randomization for initial study regimen.
Negative: <10 mg/dL; Trace: ≥10mg/dL and <30 mg/dL; 1+: ≥30 mg/dL and <100 mg/dL; 2+: ≥100 mg/dL and <300 mg/dL; 3+: ≥300 mg/dL and <1,000 mg/dL; 4+: ≥1,000 mg/dL.
Figure 1.
Calculated creatinine clearance (CrCl) over time by treatment group. Continuous line represents treatment arm A (zidovudine/lamivudine + efavirenz [ZDV/3TC + EFV]) dotted line represents treatment arm C (tenofovir/emtricitabine + efavirenz [TDF/FTC + EFV]). Statistically significant differences in CrCl were found at week 24, 48, 72, 144, and 192 by Wilcoxon test.
Figure 2.
Changes in creatinine clearance (CrCl) over time compared to baseline by treatment group. Continuous line represents treatment arm A (zidovudine/lamivudine + efavirenz [ZDV/3TC + EFV]) dotted line represents treatment arm C (tenofovir/emtricitabine + efavirenz [TDF/FTC + EFV]). No statistically significant differences in CrCl changes from baseline over time were observed after week 48 when comparing TDF and ZDV treatment arms.
DISCUSSION
TDF is now the world’s preferred first-line NRTI for HIV treatment.7, 12 Renal safety of the drug has been questioned, although data from developed countries reported rates of serious renal adverse events and graded elevations in serum creatinine in only 0.5% and 2.2% of evaluated patients, respectively.28 This present analysis, comparing the safety of these 2 NRTI regimens in the largest cohort available from a resource-limited setting, did not find a significant difference in decline in creatinine clearance below 50 mL/min, development of grade 3 or 4 serum creatinine levels, or serious renal adverse events associated with TDF use when compared to ZDV. Our findings are consistent with case control studies and clinical trials showing no significant difference in renal toxicity between TDF and other NRTIs as initial ART regimen.29–31 Nonetheless, only a small number of renal events were recorded.
As previously reported, we found that older age and lower baseline CrCl were associated with development of renal abnormalities.28, 32 No significant association was found between race and renal abnormalities despite a predominance of Blacks in this study sample. CD4 cell count, weight, or BMI were not significantly associated with renal abnormality outcome as has been observed in other studies, but the sample sizes were small.32 Diabetes is a known predisposing condition for renal damage and has been associated with an enhanced risk of nephrotoxicity in patients treated with TDF.33 In this study sample, baseline diabetes was associated with increased risk of development a renal abnormality, however only a small number of diabetic cases were recorded in each study group.
Similar to study Gilead 934, occurrence of serious renal adverse events leading to death, drug discontinuation, and serious morbidity outcomes was not significantly higher among TDF versus ZDV recipients.14 TDF-associated renal failure requiring drug discontinuation was reported in only one participant. No cases of Fanconi syndrome were reported, and none were suggested by results of serum phosphorus and urine dipstick for protein and glucose, used as surrogates of tubular dysfunction. However, detection of early or mild cases of proximal tubulopathy might have been missed, as more specific testing to assess this complication (eg, measurements of urine phosphorus, amino acids, and uric acid) was not routinely performed. Rates of proteinuria were similar in both treatment arms, with slightly more abnormalities in TDF treatment arm at week 144, but results at this distant follow-up time may be biased due to missing data based on both drop-out or unrecorded values (only 77% of total participants collected samples).
Although calculated CrCl values were significantly lower in patients receiving TDF compared to ZDV, the magnitude of this difference was small and values were maintained above 90 mL/min, which questions its clinical significance. This finding is likely related to a lower baseline CrCl in the TDF-based treatment arm and results should not be over-interpreted. Nonetheless, we did not observe any significant differences in CrCl changes from baseline over time in the TDF treatment arm compared to ZDV, as suggested in other large cohorts.34–36 In fact, differences in CrCl compared to baseline were smaller in the TDF treatment arm and were maintained through the study period (Figure 2).34–36
Fat redistribution, particularly lipoatrophy, has been associated with NRTI use. This side effect has been linked particularly to stavudine and ZDV use, with evidence suggesting reversibility after switching to abacavir, TDF, or an NRTI-sparing regimen.23 Similarly we found that all cases of lipoatrophy were probably associated with either stavudine or ZDV use (as the protocol allowed single drug substitutions of stavudine with ZDV), and none were associated to TDF use. However, the diagnosis of lipodystrophy was based on physical changes perception and anthropometric measures and was not confirmed by an objective evaluation of fat redistribution such as DEXA, computed tomography (CT), or magnetic resonance (MRI).
This study was limited by being a secondary analysis of already collected data. Appropriate evaluations to assess proximal tubular dysfunction, bone densities, or fat redistribution were not performed in the original study. The small number of renal and metabolic adverse events in our analysis was a result of prescreening eligibility criteria selecting for individuals potentially at lower risk for developing this toxicities, hence these results should be interpreted with caution to avoid effect overestimation. In addition, the use of the Cockroft-Gault formula to estimate the CrCl has not been well validated for HIV-positive individuals and can overestimate CrCl values, however is a common formula used in clinical practice and has been used in similar studies to evaluate renal function.
CONCLUSIONS
This secondary analysis of the PEARLS study suggests that TDF-associated renal toxicity in resource-limited settings is low and not significantly higher than that of ZDV-based regimen, whereas metabolic safety of TDF is superior to ZDV. TDF exposure was not associated with worsening or clinically significant changes in CrCl over time compared to baseline. These data support current WHO recommendations escalating TDF as preferred NRTI in low-income countries. Based upon the data derived from this particular study, routine laboratory monitoring for TDF-associated nephrotoxicity may seldom be required except in select patient populations, such as older individuals (particularly above age 40), with a history of diabetes or with lower baseline CrCl.
Acknowledgments
Financial support/disclosures: The project described was supported by award number UM1AI068636 from the National Institute of Allergy and Infectious Diseases (NIAID), the National Institute of Mental Health (NIMH), the National Institute of Dental and Craniofacial Research (NIDCR), and the National Institutes of Health (NIH). The work was also supported by the Statistical and Data Management Center (SDMC) grant AI68634 from the NIAID. Touzard Romo receives support from the National Institute on Drug Abuse (NIDA) 5T32DA013911, R25MH083620, and Lifespan/Tufts/Brown CFAR P30 AI042853. The pharmaceutical sponsors (Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, and GlaxoSmithKline) provided the study drugs and Gilead Sciences provided funding to purchase a study drug that was not otherwise available. Representatives of the pharmaceutical company sponsors participated as study team members and authors of this manuscript, but did not participate in data collection, data analyses, or interpretation. Bristol-Myers Squibb, Gilead Sciences Inc., GlaxoSmithKline, and Boehringer Ingelheim Pharmaceuticals, Inc. had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflicts of interest: Campbell received National Institutes of Health (NIH) grant support during the conduct of the study and has served as a consultant for Gilead Sciences. Supparatpinyo and Hakim received NIH grant support during the conduct of the study. Smeaton received unrelated grant support from Harvard School of Public Health during the conduct of the study and also received personal fees from Pfizer Inc. outside the submitted work. Flanigan acknowledges grant support from NIH AI ACTG grant 2UMAI 069412-08 and stock ownership of pharmaceutical sponsors (Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, and GlaxoSmithKline) during the conduct of the study. Riviere, Touzard Romo, Kumarasamy, Nyirenda, and Mngqibisa declare no conflicts of interest.
Additional contributions: We acknowledge the valuable contribution of the PEARLS study participants, PEARLS study team, and the following ACTG sites (NIH grant support): 11701 (AI069432), 12101 (AI069476), 30301 (AI069518), 11201 (AI069426), 12001 (AI069423B), 30313 (AI069436), 11101 (AI069463), 11501 (AI069399), 12201 (AI069401), 30022 (AI069421), 11601 (AI069417), 11301 (AI069438), 11302 (AI069438), 3751 (AI046376), 11603 (AI069417), 2401 (AI069513), 3851 (AI68636), 11602 (AI069417), 6101 (AI069450), 2301 (AI069474), 2701 (AI069471), 1501 (AI027661), 2101 (AI69439), 1601 (AI069484), 2851 (AI46370), 2951 (AI069472), 1201 (AI069428), 603 (A1069424; R000124), 3201 (AI069423-; R001111; AI50410), 3652 (AI069439), 6201 (AI069534; AI045008), 30329 (AI069470; RR024156), 2702 (AI069471), 401 (AI069532), 6301 (AI32782), 1101/1108 (AI06951; RR024160), 601 (AI069424), 2705, 3206 (AI069423; AI50410), 5201 (AI034853), 7804 (AI069419; R000457). In addition, we thank the pharmaceutical sponsors (Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, and GlaxoSmithKline) for providing the study drugs.
References
- 1.Palella FJJ, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338(13):853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 2.Mocroft A, Ledergerber B, Katlama C, et al. Decline in the AIDS and death rates in the EuroSIDA study: An observational study. Lancet. 2003;362(9377):22–29. doi: 10.1016/s0140-6736(03)13802-0. [DOI] [PubMed] [Google Scholar]
- 3.Madec Y, Laureillard D, Pinoges L, et al. Response to highly active antiretroviral therapy among severely immuno-compromised HIV-infected patients in Cambodia. AIDS. 2007;21(3):351–359. doi: 10.1097/QAD.0b013e328012c54f. [DOI] [PubMed] [Google Scholar]
- 4.Corey DM, Kim HW, Salazar R, et al. Brief report: Effectiveness of combination antiretroviral therapy on survival and opportunistic infections in a developing world setting: an observational cohort study. J Acquir Immune Defic Syndr. 2007;44(4):451–455. doi: 10.1097/QAI.0b013e31802f8512. [DOI] [PubMed] [Google Scholar]
- 5.Ford N, Calmy A. Improving first-line antiretroviral therapy in resource-limited settings. Curr Opin HIV AIDS. 2010;5(1):38–47. doi: 10.1097/COH.0b013e3283339b41. [DOI] [PubMed] [Google Scholar]
- 6.Menzies NA, Berruti AA, Blandford JM. The determinants of HIV treatment costs in resource limited settings. PLoS One. 2012;7(11):e48726. doi: 10.1371/journal.pone.0048726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akileswaran C, Lurie MN, Flanigan TP, Mayer KH. Lessons learned from use of highly active antiretroviral therapy in Africa. Clin Infect Dis. 2005;41(3):376–385. doi: 10.1086/431482. [DOI] [PubMed] [Google Scholar]
- 8.Amole CD, Brisebois C, Essajee S, et al. Optimizing antiretroviral product selection: A sample approach to improving patient outcomes, saving money, and scaling-up health services in developing countries. J Acquir Immune Defic Syndr. 2011;57:S100–S103. doi: 10.1097/QAI.0b013e318220f016. [DOI] [PubMed] [Google Scholar]
- 9.US Department of Health and Human Services Panel on Antiretroviral Guidelines for Adults and Adolescents. [Accessed December 2013];Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 2009 http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf.
- 10.Thompson MA, Aberg JA, Hoy JF, et al. Antiretroviral treatment of adult HIV infection. 2012 recommendations of the International Antiviral Society–USA Panel. JAMA. 2012;308(4):387–402. doi: 10.1001/jama.2012.7961. [DOI] [PubMed] [Google Scholar]
- 11.Antiretroviral therapy for HIV infection in adults and adolescents: Recommendations for a public health approach. Geneva: World Health Organization; 2010. [Accessed November 2013]. 2010 revision. http://whqlibdoc.who.int/publications/2010/9789241599764_eng.pdf. [PubMed] [Google Scholar]
- 12.Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV Infection. Geneva: World Health Organization; 2013. [Accessed December 2013]. www.who.int/hiv/pub/guidelines/arv2013. [PubMed] [Google Scholar]
- 13.Siegfried N, Uthman OA, Rutherford GW. Optimal time for initiation of antiretroviral therapy in asymptomatic, HIV-infected, treatment-naive adults. Cochrane Database Syst Rev. 2010;3 doi: 10.1002/14651858.CD008272.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallant JE, DeJesus E, Arribas JR, et al. Tenofovir DF, emtricitabine, and efavirenz vs. zidovudine, lamivudine, and efavirenz for HIV. N Engl J Med. 2006;354(3):251–260. doi: 10.1056/NEJMoa051871. [DOI] [PubMed] [Google Scholar]
- 15.Arribas JR, Pozniak AL, Gallant JE, et al. Tenofovir disoproxil fumarate, emtricitabine, and efavirenz compared with zidovudine/lamivudine and efavirenz in treatment-naive patients: 144-week analysis. J Acquir Immune Defic Syndr. 2008;47(1):74–78. doi: 10.1097/QAI.0b013e31815acab8. [DOI] [PubMed] [Google Scholar]
- 16.Rey D, Hoen B, Chavanet P, et al. High rate of early virological failure with the once-daily tenofovir/lamivudine/nevirapine combination in naive HIV-1-infected patients. J Antimicrob Chemother. 2009;63(2):380–388. doi: 10.1093/jac/dkn471. [DOI] [PubMed] [Google Scholar]
- 17.Max B, Sherer R. Management of the adverse effects of antiretroviral therapy and medication adherence. Clin Infect Dis. 2000;30(Suppl 2):S96–116. doi: 10.1086/313859. [DOI] [PubMed] [Google Scholar]
- 18.Campbell TB, Smeaton LM, Kumarasamy N, et al. Efficacy and safety of three antiretroviral regimens for initial treatment of HIV-1: A randomized clinical trial in diverse multinational settings. PLoS Med. 2012;9 (8):e1001290. doi: 10.1371/journal.pmed.1001290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mutimura E, Stewart A, Rheeder P, Crowther NJ. Metabolic function and the prevalence of lipodystrophy in a population of HIV-infected African subjects receiving highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2007;46 (4):451–455. doi: 10.1097/qai.0b013e318158c0a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parakh A, Dubey AP, Kumar A, Maheshwari A. Lipodystrophy and metabolic complications of highly active antiretroviral therapy. Indian J Pediatr. 2009;76(10):1017–1021. doi: 10.1007/s12098-009-0216-9. [DOI] [PubMed] [Google Scholar]
- 21.Hammond E, McKinnon E, Nolan D. Human immunodeficiency virus treatment-induced adipose tissue pathology and lipoatrophy: Prevalence and metabolic consequences. Clin Infect Dis. 2010;51(5):591–599. doi: 10.1086/655765. [DOI] [PubMed] [Google Scholar]
- 22.Haubrich RH, Riddler SA, DiRienzo AG, et al. Metabolic outcomes in a randomized trial of nucleoside, nonnucleoside and protease inhibitor-sparing regimens for initial HIV treatment. AIDS. 2009;23(9):1109–1118. doi: 10.1097/QAD.0b013e32832b4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Vonderen MGA, van Agtmael MA, Hassink EAM, et al. Zidovudine/lamivudine for HIV-1 infection contributes to limb fat loss. PLoS One. 2009;4 (5):e5647. doi: 10.1371/journal.pone.0005647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dlamini J, Ledwaba L, Mokwena N, et al. Lactic acidosis and symptomatic hyperlactataemia in a randomized trial of first-line therapy in HIV-infected adults in South Africa. Antivir Ther. 2011;16(4):605–609. doi: 10.3851/IMP1790. [DOI] [PubMed] [Google Scholar]
- 25.Cooper RD, Wiebe N, Smith N, Keiser P, Naicker S, Tonelli M. Systematic review and meta-analysis: Renal safety of tenofovir disoproxil fumarate in HIV-infected patients. Clin Infect Dis. 2010;51(5):496–505. doi: 10.1086/655681. [DOI] [PubMed] [Google Scholar]
- 26.Smurzynski M, Collier AC, Koletar SL, et al. AIDS clinical trials group longitudinal linked randomized trials (ALLRT): Rationale, design, and baseline characteristics. HIV Clin Trials. 2008;9(4):269–282. doi: 10.1310/hct0904-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albrecht H. Abacavir/3TC vs. tenofovir/FTC: interim results from ACTG 5202. AIDS Clin Care. 2008;20(4):28. [PubMed] [Google Scholar]
- 28.Nelson MR, Katlama C, Montaner JS, et al. The safety of tenofovir disoproxil fumarate for the treatment of HIV infection in adults: the first 4 years. AIDS. 2007;21(10):1273–1281. doi: 10.1097/QAD.0b013e3280b07b33. [DOI] [PubMed] [Google Scholar]
- 29.Gallant JE, Winston JA, DeJesus E, et al. The 3-year renal safety of a tenofovir disoproxil fumarate vs. a thymidine analogue-containing regimen in antiretroviral-naive patients. AIDS. 2008;22(16):2155–2163. doi: 10.1097/QAD.0b013e3283112b8e. [DOI] [PubMed] [Google Scholar]
- 30.Gallant JE, Moore RD. Renal function with use of a tenofovir-containing initial antiretroviral regimen. AIDS. 2009;23(15):1971–1975. doi: 10.1097/QAD.0b013e32832c96e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones R, Stebbing J, Nelson M, et al. Renal dysfunction with tenofovir disoproxil fumarate-containing highly active antiretroviral therapy regimens is not observed more frequently: A cohort and case-control study. J Acquir Immune Defic Syndr. 2004;37(4):1489–1495. doi: 10.1097/01.qai.0000138983.45235.02. [DOI] [PubMed] [Google Scholar]
- 32.Antoniou T, Raboud J, Chirhin S, et al. Incidence of and risk factors for tenofovir-induced nephrotoxicity: A retrospective cohort study. HIV Med. 2005;6(4):284–290. doi: 10.1111/j.1468-1293.2005.00308.x. [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez-Nóvoa S, Alvarez E, Labarga P, Soriano V. Renal toxicity associated with tenofovir use. Expert Opin Drug Saf. 2010;9(4):545–559. doi: 10.1517/14740331003627458. [DOI] [PubMed] [Google Scholar]
- 34.Gallant JE, Parish MA, Keruly JC, Moore RD. Changes in renal function associated with tenofovir disoproxil fumarate treatment, compared with nucleoside reverse-transcriptase inhibitor treatment. Clin Infect Dis. 2005;40(8):1194–1198. doi: 10.1086/428840. [DOI] [PubMed] [Google Scholar]
- 35.Winston J, Deray G, Hawkins T, Szczech L, Wyatt C, Young B. Kidney disease in patients with HIV infection and AIDS. Clin Infect Dis. 2008;47(11):1449–1457. doi: 10.1086/593099. [DOI] [PubMed] [Google Scholar]
- 36.Stohr W, Reid A, Walker AS, et al. Glomerular dysfunction and associated risk factors over 4–5 years following antiretroviral therapy initiation in Africa. Antivir Ther. 2011;16(7):1011–1020. doi: 10.3851/IMP1832. [DOI] [PubMed] [Google Scholar]