Abstract
Objective
To demonstrate the feasibility of integrated screening for cryptococcal antigenemia and tuberculosis (TB) prior to antiretroviral therapy (ART) initiation and to assess disease specific and all-cause mortality in the first 6 months of follow-up.
Methods
We enrolled a cohort of HIV-infected, ART-naïve adults with CD4 counts ≤ 250 cells/µL in rural Uganda who were followed for 6 months after ART initiation. All subjects underwent screening for TB; those with CD4 ≤ 100 cells/µL also had cryptococcal antigen (CrAg) screening. For those who screened positive, standard treatment for TB or preemptive treatment for cryptococcal infection was initiated, followed by ART two weeks later.
Results
Of 540 participants enrolled, pre-ART screening detected 10.6% (57/540) with prevalent TB and 6.8% (12/177 with CD4 count ≤ 100 cells/µL) with positive serum CrAg. After ART initiation, 13 (2.4%) patients were diagnosed with TB and one patient developed cryptococcal meningitis. Overall 7.2% of participants died (incidence rate 15.6 per 100 person years at risk). Death rates were significantly higher among subjects with TB and cryptococcal antigenemia compared to subjects without these diagnoses. In multivariate analysis, significant risk factors for mortality were male sex, baseline anemia of hemoglobin ≤ 10 mg/dL, wasting defined as body mass index ≤ 15.5 kg/m2, and opportunistic infections (TB, positive serum CrAg).
Conclusion
Pre-ART screening for opportunistic infections detects many prevalent cases of TB and cryptococcal infection. However, severely immunosuppressed and symptomatic HIV patients continue to experience high mortality after ART initiation.
Keywords: cryptococcal antigen, screening, antiretroviral therapy, early mortality, unmasking tuberculosis, tuberculosis, anemia
Introduction
Efforts to address the high burden of HIV disease in sub-Saharan Africa (SSA) have led to a rapid increase in the number of patients receiving antiretroviral therapy (ART). With 4.2 million fewer HIV-related deaths in low and middle income countries between 2002 and 2012, and 9 million sub-Saharan Africans projected to be receiving ART by the end of 2014, the expansion in ART access has been dramatic and life-saving [1, 2]. However, in a meta-analysis of sub-Saharan Africans initiating ART, 17% (range 11–24%) died within the first year, largely due to opportunistic pathogens such as Mycobacterium tuberculosis and Cryptococcus neoformans [3]. Mortality in the first year on ART was as high as 40%, with the majority of deaths occurring in the first three months after ART initiation [3–5]. Patients with the lowest CD4 T-cell counts have the highest mortality risk [5–11], and despite expanded voluntary HIV counseling and testing efforts, many patients still present with advanced immunosuppression [12–15].
Cryptococcus neoformans is a ubiquitous soil fungus that causes an estimated 720,000 cases of cryptococcal meningitis (CM) annually in SSA [16]. In HIV-infected Africans, CM is responsible for up to 20% of deaths in the first year on ART [4]. Serum cryptococcal antigen (CrAg) positivity is an independent predictor of mortality [17–21] and is prevalent in 2–21% of HIV-infected patients with CD4 count ≤ 100 cells/µL [17–19, 22–25]. CrAg screening and preemptive fluconazole treatment has been shown to reduce CM-related mortality and is cost-effective [24, 26].
There is a significant burden of undiagnosed TB in high prevalence HIV populations [27–30]. Prospective cohort studies in South Africa have demonstrated that 17–19% of HIV-infected, ART-eligible patients have positive sputum cultures for TB [31–34]. It is also known that patients with active TB at the time of ART initiation have a high mortality rate in the first few years after starting ART [35–37]. This suggests that more intensive pre-ART TB screening and treatment may avert mortality.
As national programs work toward the United Nations goal of 15 million persons on ART by 2015 [1, 38], the ability of ART programs to effectively diagnose and treat opportunistic infections (OIs) in the early ART period will be essential to reduce mortality. In rural areas, ART programs face the additional challenges of limited diagnostic and treatment services and widely dispersed patient populations [39, 40]. While WHO guidelines recommend screening for TB in HIV patients [41], few studies have assessed the operational performance of TB diagnostic tests in rural populations. Therefore, we sought to describe the performance of integrated OI screening in a cohort of HIV-positive, ART-naïve, rural Ugandans initiating ART in the second phase of the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR).
Methods
Study setting
We recruited patients at the Kiboga District Hospital HIV clinic, a rural government hospital 130 km northwest of Kampala, Uganda which serves a multi-district population of over 300,000 [42]. In a 2011 Ugandan sero-survey, adult HIV prevalence in the region was 11.1% for women and 8.2% for men [43]. First-line ART was comprised of either zidovudine (AZT) or tenofovir (TDF), lamivudine (3TC), and either nevirapine (NVP) or efavirenz (EFV). Co-trimoxazole prophylaxis was initiated at the time of HIV diagnosis.
Study design and participants
Between 23rd September 2010 and 19th November 2012, we prospectively enrolled HIV-positive, ART-eligible (CD4+ T-cell count < 250 cells/µL), and ART-naïve adults > 18 years of age after informed consent. Participants with ALT or AST greater than five times the upper limit of normal or creatinine clearance < 25 mL/min were excluded from the study due to increased risk of medication-related adverse events. Those already receiving treatment for TB or cryptococcal disease were also excluded.
Screening and treatment for OIs
Prior to ART initiation, participants with CD4 counts ≤ 100 cells/µL were screened for cryptococcal infection using a latex agglutination assay for serum cryptococcal antigen (CrAg) (Immuno-Mycologics, Norman, USA) and clinical evaluation for signs and symptoms of CM [44]. Serum CrAg-positive subjects were treated with a fungicidal dose of oral fluconazole (Diflucan, Pfizer), 800 mg daily for four weeks. If clinically stable after two weeks of treatment, these patients then began ART. Participants diagnosed with CM at screening were referred to Mulago National Referral Hospital for amphotericin treatment.
All participants underwent clinical and laboratory screening for TB, including: (1) four intensified case finding (ICF) screening questions developed from the WHO STOP-TB guidelines (current cough, fever, weight loss, or night sweats) [41], (2) Ziehl-Neelsen staining and/or fluorescence microscopy for acid-fast bacilli (AFB) on two sputum smears, and (3) sputum culture on the first specimen. Participants unable to expectorate sputum underwent sputum induction with hypertonic saline. TB sputum samples were packaged with refrigerant and sent by courier to the Mycobacteriology laboratory (BSL-3) at Makerere University in Kampala, Uganda for TB culture analysis. Inoculated Mycobacterial Growth Indicator Tubes (MGIT) and Lowenstein-Jensen (LJ) media were incubated at 37°C and monitored for growth for up to 6 and 8 weeks, respectively, as previously described [45]. TB status was defined using WHO criteria [47]. Smear-positive TB was defined as one or more AFB-positive sputum samples. Smear-negative TB was defined as two AFB-negative sputum samples and evidence of pulmonary disease on chest radiograph and/or a positive sputum culture. Extra-pulmonary TB (EPTB) was defined [47] as one culture or smear positive specimen from an extra-pulmonary site or strong clinical evidence of extra-pulmonary disease (on lymph node aspirate, lymph node biopsy, and/or abdominal ultrasound), followed by a decision to begin full treatment. TB treatment was comprised of a 2-month induction phase with isoniazid, rifampin, pyrazinamide, and ethambutol (RHZE), followed by a 6-month continuation phase with isoniazid and ethambutol (EH) [46]. ART was initiated after 2 weeks of TB treatment.
Follow-up and Outcomes
We followed individuals for 6 months after ART initiation for the primary study outcomes of death and occurrence of major OIs (TB or CM). Censoring occurred upon one of the following events: death, transfer to another clinic, withdrawal from the study, loss to follow-up, administrative closure, or completion of 6 months of follow-up. Cause of death was determined by review of laboratory and clinical information for patients who died in the hospital. When death occurred at home, verbal autopsy questions were administered to family members of the deceased by a trained study assistant. A panel of at least three physicians reviewed all deaths and adjudicated the most likely cause of death. Participants were deemed lost to follow-up when they had missed an appointment for 4 weeks and could not be contacted or traced by the outreach team.
Data Collection
We collected data through interviews about demographics, past history of HIV disease, and clinical complaints, physical examination, and laboratory measurements. Participants were seen at enrollment, at 2 weeks, and then monthly for 6 months. Laboratory measurements included CD4+ T-lymphocyte (CD4) count (BD Facs Count, Becton Dickinson, San Jose, CA), complete blood count (Celltac E MEK-7222, Nihon Kohden, Tokyo, Japan); alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (Flexor Junior, Vital Scientific, Dieren, The Netherlands), and serum creatinine (Cobas Integra 400 plus, Roche, Basel, Switzerland). Data were recorded on standardized clinical research forms at the study site and transmitted via the DataFax system to a secure server at the National Institutes of Health in Bethesda, Maryland.
Statistical analysis
We used the Student’s t-test to compare means of continuous variables. We used the chi-square (χ2) test to compare proportions of dichotomous and polytomous data. Kaplan Meier survival curves and stratified log-rank test were used to describe mortality per 100 person years at risk (PYAR) in the first 6 months on ART. Finally, using the Cox proportional hazards regression, we evaluated risk factors for death, both in the entire cohort and the subset of participants with CD4 count ≤ 100. Covariates for inclusion in the multivariate regression model were considered based on an unadjusted association with mortality (p<0.25) and other previously published risk factors. A p-value <0.05 was considered significant, and all reported p-values were 2-sided. Analyses were carried out using STATA (StataCorp. Version 11, College Station, TX).
Ethical considerations
Informed consent discussions were conducted in the participant’s primary language, either English or Luganda, and written consent forms were signed. For subjects with limited literacy, a witness not associated with the study was present to ensure full understanding. The study was approved by the relevant institutional review boards in Uganda (#HS740) and the Johns Hopkins University.
Results
Patient Characteristics
Five hundred forty participants were enrolled between September 2010 and November 2012, and follow-up was completed in May 2013 (Figure 1). Sixty percent (324/540) of the participants were female. At enrollment, median age was 36 years (IQR, 30–44 years), median CD4 count, 155 cells/µL (IQR, 72–206 cells/µL), mean hemoglobin (Hb), 11.7 g/dL (S.D. 2.2 g/dL), and mean body mass index (BMI), 20.6 kg/m2 (S.D. 3.4 kg/m2) (Table 1). Over a median follow-up period of 6.1 months (IQR, 5.7–6.3 months), 535 (99.1%) initiated ART and 22 (4.1%) were lost to follow-up.
Figure 1. Flow of study participants.
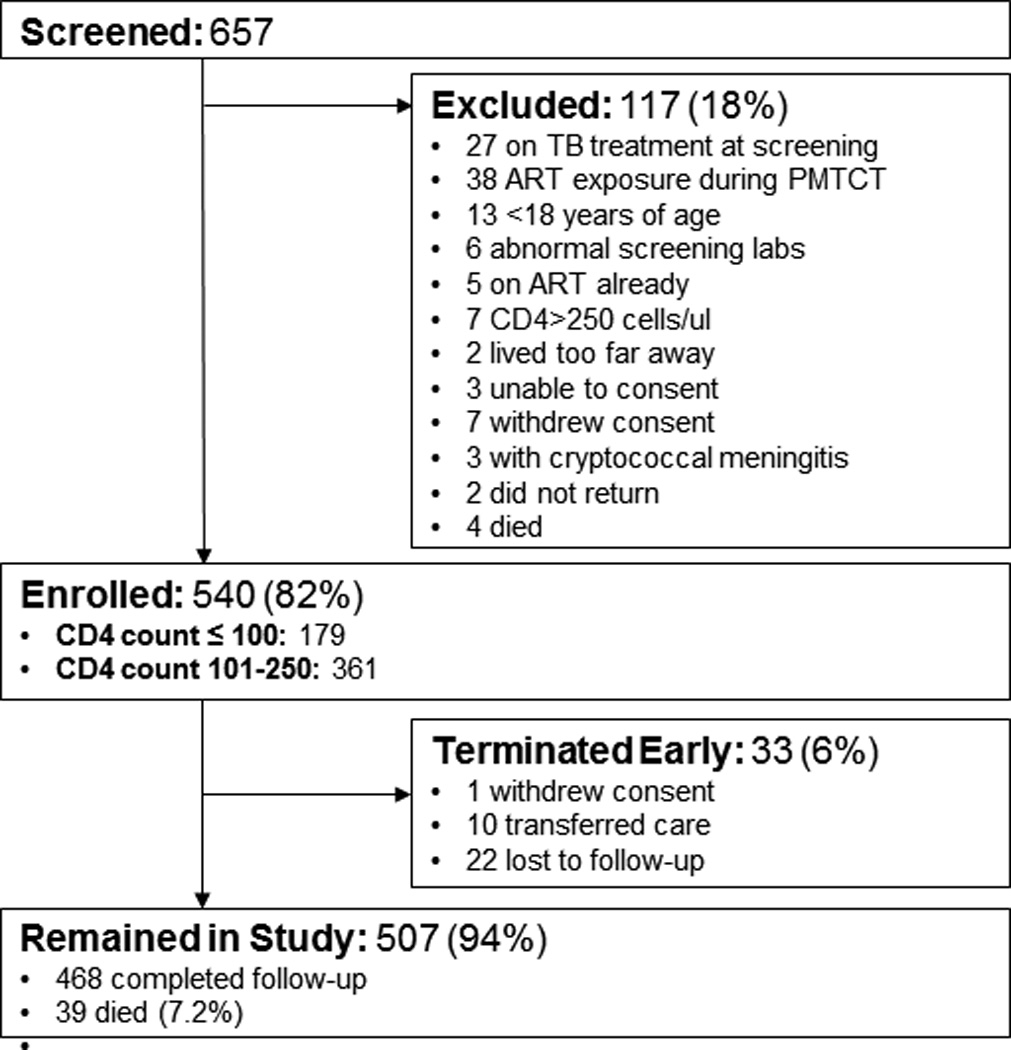
Table 1.
Demographic characteristics of the cohort
| Total (N=540) |
Alive (N=468) |
Dead (N=39) |
Lost to follow up, Transferred, or Withdrew from study (N=33) |
||
|---|---|---|---|---|---|
| Female, N (%) | 324 (60%) | 294 (63%) | 15 (38%) | 15 (45%) | |
| Age, years, median (IQR) | 36 (30–44) | 36 (30–44) | 36 (29–48) | 33 (28–40) | |
| CD4 T cell count, cells/µL median, (IQR) | 155 (72–206) | 158 (79–210) | 66 (18–159) | 106 (61–196) | |
| Hemoglobin, mg/dL mean (S.D.) | 11.7 (2.2) | 11.8 (2.1) | 8.9 (2.2) | 11.3 (2.4) | |
| WHO Stage, N (%) | |||||
| I | 38 (7%) | 36 (8%) | 1 (3%) | 1 (4%) | |
| II | 183 (35%) | 174 (37%) | 2 (6%) | 7 (26%) | |
| III | 214 (40%) | 188 (40%) | 12 (34%) | 14 (52%) | |
| IV | 95 (18%) | 70 (15%) | 20 (57%) | 5 (19%) | |
| missing | 10 | 0 | 4 | 6 | |
| TB at baseline (N, %) | 59 (10.9%) | 41 (8.8%) | 12 (30.8%) | 6 (18.2%) | |
| CrAg positive at baseline (N, %) | 12 (2.2%) | 5 (10.7%) | 3 (7.7%) | 4 (12.1%) | |
N=Number; IQR= interquartile range; SD=standard deviation;
Cryptococcal antigen screening
Among 179 subjects with baseline CD4 count ≤ 100 cells/µL, 177 (97.9%) were screened for serum CrAg. Pre-ART screening detected 12/177 (6.8%) cases of cryptococcal antigenemia. Of these, 1 had symptoms of CM, was diagnosed with CM with CrAg-positive cerebrospinal fluid (CSF), and was referred for treatment. Of the 11 who were asymptomatic, 9 underwent lumbar puncture (2 declined) that showed CrAg-negative CSF. All 11 were treated preemptively with high dose fluconazole, reported perfect adherence, had no adverse events related to the treatment, and did not develop CM during follow-up. One subject (baseline CD4 count, 16 cells/µL) screened negative at enrollment, but after 2 weeks on ART developed symptoms of CM, was found to have CrAg-positive serum and CSF, and subsequently died during treatment for CM.
Tuberculosis screening
All 540 participants were evaluated for TB in pre-ART screening and follow-up visits. In total, 72 (13.3%) were diagnosed with TB. Of these, pre-ART screening detected 79.2% (57/72). Two additional patients were lost to follow-up and diagnosed posthumously with pre-ART TB (Table 2). Follow-up after ART initiation detected 13 additional cases, of which 69.2% were extrapulmonary infections (Supplemental Figure 1).
Table 2.
Prevalent and incident tuberculosis
| Screened for TB (N=540) |
TB culture results available (N=343) |
Positive TB culture (N=25) |
||
|---|---|---|---|---|
| Total Cases N (%) | Total | 72 (13.3%) | 49 (14.3%) | 25 (100%) |
| S+ PTB | 18 (3.3%) | 14 (4.1%) | 10 (40.0%) | |
| S− PTB | 29 (5.4%) | 23 (6.7%) | 14 (56.0%) | |
| EPTB | 25 (4.6%) | 12 (3.5%) | 1 (4.0%) | |
| Pre-ART (Baseline) N (%) | Total | 59 (81.9%) | 43 (87.8%) | 25 (100%) |
| S+ PTB | 16 (22.2%) | 13 (26.5%) | 10 (40.0%) | |
| S− PTB | 27 (37.5%) | 23 (46.9%) | 14 (56.0%) | |
| EPTB | 16 (22.2%) | 7 (14.3%) | 1 (4.0%) | |
| Post-ART N (%) | Total | 13 (18.1%) | 6 (12.2%) | 0 |
| S+ PTB | 2 (2.8%) | 1 (2.0%) | 0 | |
| S− PTB | 2 (2.8%) | 0 (0.0%) | 0 | |
| EPTB | 9 (12.5%) | 5 (10.2%) | 0 | |
TB=tuberculosis; CrAg=serum cryptococcal antigen; S+ PTB=sputum smear-positive pulmonary TB; S− PTB: sputum smear-negative pulmonary TB; EPTB: extra-pulmonary TB; N=number
Of the participants whose first sputum smear was interpretable (513/540, 95.0%), 2.3% (12/513) were smear-positive. Four hundred participants who were smear negative produced a second sputum sample (74.1% of those enrolled), of which 1.5% (6/400) were positive. Overall, one quarter (18/72) of all TB cases were smear-positive, and every smear-positive case presented with at least one symptom (94.4% cough, 94.4% weight loss, 55.6% night sweats, 50.0% fever, and 0% hemoptysis) on the ICF questionnaire.
Sputum cultures were performed in 64.3% (347/540) and not contaminated in 63.5% (343/540) of the participants enrolled (Table 2). Sputum cultures were positive in 51.0% (25/49) of patients who were clinically diagnosed with TB and started treatment; 71.4% (10/14) of smear-positive, 60.9% (14/23) of smear-negative, and 8.3% (1/12) of extrapulmonary TB (EPTB) cases. As with smear-positive cases, all patients with positive TB cultures were symptomatic.
Extrapulmonary cases were diagnosed by lymph node aspirate or biopsy showing AFBs (8%, 2/25), chest radiograph with evidence of pleural effusion (32%, 8/25), abdominal ultrasound showing lymphadenopathy (20%, 5/25), or clinical impression leading to decision to begin anti-TB treatment (40%, 10/25). Locations of EPTB were pleural (32%, 8/25), lymph nodes (24%, 6/25), and disseminated (44%, 11/25).
Comparing patients with and without TB who screened positive on ICF symptoms, we found that weight loss (97.2% v. 58.8%, p<0.001), current fever (66.7% v. 17.3%, p<0.001), night sweats (63.9% v. 20.1%, p<0.001), and hemoptysis (9.7% v. 2.6%, p=0.002) were significantly more likely to occur in patients diagnosed with TB. Presence of cough was not significantly different between those with and without TB (79.2% and 78.4%, respectively, p=0.89). Sensitivity and specificity of various screening tests are listed in Supplemental Table 1.
Mortality
There were 39 deaths in this cohort (7.2%) during 6.5 months of follow-up, one before ART initiation and 38 on ART, occurring at a rate of 15.6 (95% CI, 11.4–21.3) deaths/100 person years at risk (PYAR) overall. Mortality after ART initiation markedly decreased in the second 3 months of follow-up; only 3 deaths occurred in this period compared to 36 deaths in the first 3 months (99.4% v. 93.3% survival). Causes of death were: 9 TB-related (23.1%), 1 possible CM immune reconstitution inflammatory syndrome (IRIS) (2.6%), 7 KS-related (17.9%), 12 acute infections (30.8%), and 10 other (25.6%). Death rates in the 6-month post-ART follow-up period were significantly higher among subjects with TB compared to those without TB (41.2 deaths/100PYAR; 95% CI, 22.8–74.5 v. 9.55 deaths/100 PYAR; 95% CI, 6.2–14.6; p<0.001) and higher among those with a CD4 count ≤ 100 cells/µL who screened positive for serum CrAg compared to those who were CrAg negative (114.9/100 PYAR; 95% CI, 43.1–306.3 v. 25.1/100 PYAR; 95% CI, 15.8–39.9; p=0.018) (Figure 2). During the study period, 25% (3/12) of the baseline CrAg-positive participants and 20.3% (12/59) of the participants diagnosed with TB at pre-ART screening died.
Figure 2. Post-ART mortality outcomes stratified by opportunistic infection and CD4.
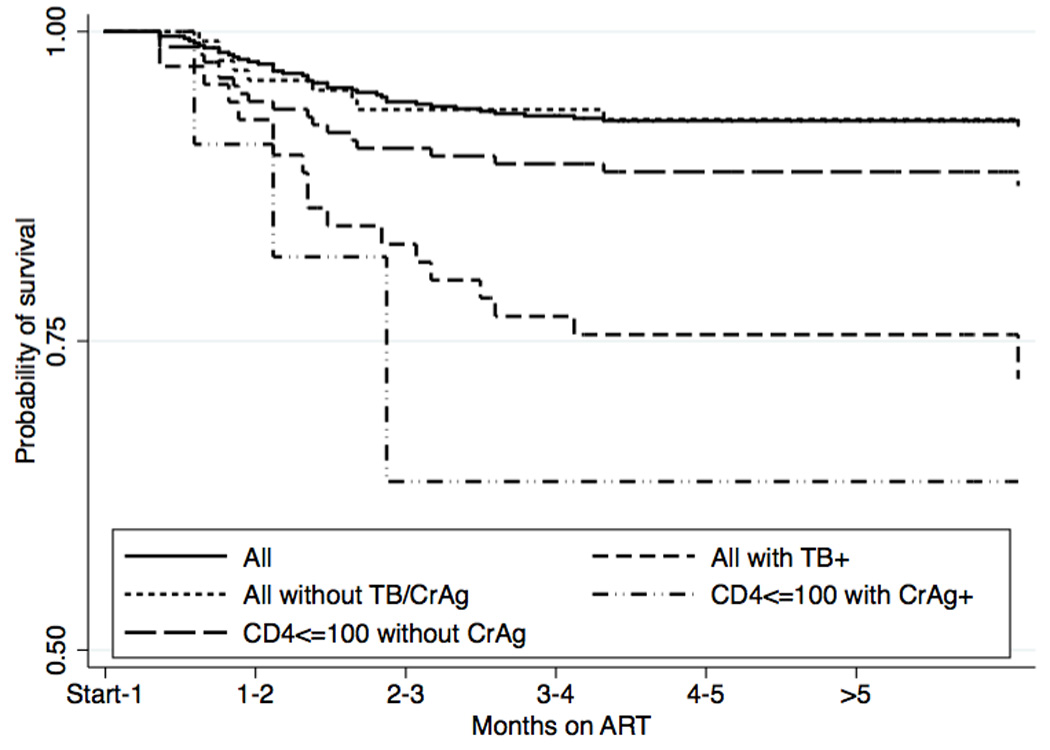
Kaplan Meier survival plot showing significantly decreased survival for participants with TB and those with CD4 count ≤ 100 cells/µL with positive serum CrAg, compared to the entire cohort, patients without TB or serum CrAg, and patients with CD4 count ≤ 100 cells/µL and negative serum CrAg.
In multivariate analysis, significant risk factors for mortality were male sex (HR, 2.36; 95% CI, 1.13–4.93; p=0.022), baseline Hb ≤ 10 mg/dL (HR, 12.84; 95% CI, 2.15-76-71; p=0.005), BMI ≤ 15.5 kg/m2 (HR, 3.99; 95% CI, 1.44–11.00; p=0.0018), and TB infection (HR, 3.39; 95% CI, 1.54–7.48; p=0.002) (Table 3). BMI 15.6–18.5 kg/m2 was associated with mortality but did not reach statistical significance (HR, 2.22; 95% CI, 0.98–5.02; p=0.056). In multivariate analysis of participants with CD4<100 cells/µL, after serum CrAg screening results were added to the model, positive serum CrAg and BMI ≤ 15.5 kg/m2 were the only significant risk factors (HR, 4.50; 95% CI, 1.18–17.16; p=0.028 and HR, 5.69; 95% CI, 1.44–22.48; p=0.013, respectively) while TB infection trended toward significance (HR, 2.77; 95% CI, 0.97–7.94; p=0.057).
Table 3.
Multivariate logistic regression of factors associated with mortality
| Adjusted hazard ratio (95% CI) (N=523) * |
p-value | Adjusted hazard ratioin subset with CD4<100 cells/µL (95% CI) (N=170) * |
p-value | |
|---|---|---|---|---|
| Male sex (ref: female) | 2.36 (1.13 – 4.93) | 0.022 | 1.91 (0.69 – 5.32) | 0.215 |
| Age, years | 1.00 (0.97 – 1.04) | 0.866 | 1.02 (0.97 – 1.07) | 0.407 |
| Baseline CD4 count, | ||||
| ≥200 cells/µL | Reference | - | - | |
| 150–199 cells/µL | 0.51 (0.14 – 1.84) | 0.301 | - | - |
| 100–149 cells/µL | 1.08 (0.32 – 3.58) | 0.901 | - | - |
| 50–99 cells/µL | 0.93 (0.27 – 3.20) | 0.902 | Reference | - |
| <50 cells/µL | 1.53 (0.54 – 4.30) | 0.420 | 1.65 (0.57 – 4.77) | 0.358 |
| Baseline Hb, mg/dL (ref: >10) | Reference | - | Reference | - |
| ≤10 | 12.84 (2.15 – 76.71) | 0.005 | 1.13 (0.08 – 15.76) | 0.929 |
| Baseline BMI, kg/m2 (ref: >18.5) | Reference | - | Reference | - |
| 15.6–18.5 | 2.22 (0.98 – 5.02) | 0.056 | 2.28 (0.73 – 7.10) | 0.157 |
| ≤15.5 | 3.99 (1.44 – 11.00) | 0.008 | 5.69 (1.44– 22.48) | 0.013 |
| Tuberculosis infection | 3.39 (1.54 – 7.48) | 0.002 | 2.77 (0.97 – 7.94) | 0.057 |
| Positive serum CrAg | - | - | 4.50 (1.18 – 17.16) | 0.028 |
Number of subjects reduced from 540 to 523, and in the smaller cohort of participants with CD4≤ 100 cells/µL from 177 to 170, due to missing BMI data
CI=confidence interval; CrAg=serum cryptococcal antigen; Hb=hemoglobin; BMI=body mass index, kg=kilogram, m=meter, N=number
Discussion
In our cohort, serum CrAg screening and preemptive treatment with 4 weeks of high-dose fluconazole resulted in only one subject being diagnosed with CM in the first 6 months of ART treatment. This rate (2.6%) is significantly lower than the 12–20% of deaths reported in other sub-Saharan cohorts [4, 5, 10] and corroborates the modeled benefits of CrAg screening and preemptive fluconazole treatment [21, 45].
Despite a marked reduction in CM deaths, one quarter of subjects with positive serum CrAg at baseline died during the study period – a significantly higher mortality rate when compared to the rest of the study participants. The adjusted hazard ratio of positive serum CrAg for all-cause mortality was 4.50 (p=0.028), similar to other African cohorts [16–18]. Therefore, serum CrAg positivity, even when detected and treated early to prevent progression to CM, confers a poor prognosis [17–19, 48]. One limitation of our study is that we did not use the new point-of-care CrAg lateral flow assay (LFA) [49], which is more sensitive than latex agglutination and may have diagnosed the participant who screened negative and then developed CM 2 weeks after starting ART. Until more sensitive methods are operationalized, a negative CrAg screening result should not preclude re-testing if signs or symptoms of CM develop after ART initiation. The impact of pre-ART CrAg screening using the LFA on post-ART mortality is currently being evaluated in Uganda.
Our pre- and early ART screening also identified a significant number of patients (13%) with subclinical TB infection that likely would not otherwise have been recognized at baseline, and demonstrated that the incidence of TB in the first 3 months of ART decreased after effective baseline screening [50]. The intensive case finding tool was highly non-specific; sputum smear analysis had low sensitivity; sputum culture was unreliable due to long sample transport times, slow to process, and too expensive to be a cost-effective screening tool; and individualized clinical assessment required advanced training and was highly provider-dependent. Chest radiographs were not routinely available due to issues surrounding lack of electricity and equipment disrepair, even in a district hospital. More reliable and convenient tests are needed for effective and widespread TB screening as no one test was sufficiently sensitive and specific (Supplemental Figure 1 and Table 1). In addition to Xpert MTB/RIF, which was unavailable in Kiboga during the study period, a promising new TB test is urine lipoarabinomannan lateral flow assay (LAM LFA); when combined with sputum smear, LAM LFA can improve overall sensitivity particularly in immunosuppressed HIV-positive populations [32, 45, 51].
The large number of TB cases present at baseline (10.9% of the cohort, of which 72.9% were pulmonary infections) increases the risk of nosocomial transmission to other HIV-infected clinic patients. The level of prevalent TB in this rural population (nearly 11%) was greater than the 6.5% reported in the urban Infectious Diseases Institute cohort in Kampala, Uganda [52]. This difference could be explained by decreased access to health care facilities, less diagnostic capacity to diagnose smear-negative and extrapulmonary cases (with low rates of empiric treatment), and lack of integrated HIV/TB care in the rural setting leading to a large pool of undiagnosed cases prior to our intensified screening intervention. We support the integration of TB and HIV care, which has been shown to improve outcomes [53] and is recommended by the WHO.
Extrapulmonary and smear-negative cases comprised a majority (85%) of the TB cases diagnosed after ART initiation and occurred throughout the follow-up period. Overall, 35% of the TB cases were extrapulmonary, much higher than the national proportion in Uganda of 11% [47], perhaps due to modulation of the disease after ART and unrecognized IRIS or unmasked TB [50].
Patients with TB at baseline had a significantly higher mortality rate than those without TB. The 16.7% of subjects with TB who died in the first 6.5 months on ART is slightly higher than previously reported data (13.7% at 8 months and 12–15% at 6 months, respectively) [36, 54], and suggests that screening did not decrease TB-specific mortality. TB was the single most common cause of death, underlying 23.1% of cohort deaths. While this percentage closely matches previous meta-analyses of post-ART mortality [3, 4], the contribution of CM to cohort mortality was dramatically lower than expected. Although mortality in our cohort at 6 months post-ART initiation was also lower than other sub-Saharan cohorts [6, 37, 55–61], the average baseline CD4 count was higher, possibly due to late roll-out of ART and patients accessing care earlier, high pre-ART mortality, and barriers to care (e.g. transport) for the sickest individuals in Kiboga District.
Our findings agree with previous studies where male sex, anemia, low BMI, and TB infection were independent predictors of death, but differ in that low CD4 count was not an independent predictor of death [3–5, 11, 59, 61, 62]. Many of the published risk factors for death in HIV patients starting ART come from urban sites early in the ART roll-out in SSA. The difference in our risk factor analysis could be attributed to factors unique to rural populations. Anemia, which was strongly predictive of mortality, is a risk factor that is likely impacted by rural behaviors and environments. Late presentation to HIV care due to distance and difficulty accessing clinic services increases the burden of opportunistic infections, many of which cause or coincide with anemia; this overlap may explain why anemia dropped out as a risk factor for mortality when serum CrAg was added to the multivariate regression (Table 3). Subsistence farming frequently involves limited access to meat and other densely nutritious foods. Reduced consumption of vitamin B12, iron, and folate could further contribute to low hemoglobin levels.
Five years after the renewal of PEPFAR, opportunistic infections continue to contribute to significant ART-associated early mortality in SSA. Baseline screening was successful at identifying active TB and asymptomatic cryptococcal antigenemia. Although TB-related mortality did not change, post-ART CM incidence was low in the setting of preemptive fluconazole treatment for serum CrAg-positive subjects. Given the continued burden of TB co-infection and the difficulty of diagnosis, improved rapid point-of-care TB diagnostics will be needed to scale-up pre-ART TB screening and improve early ART outcomes.
Supplementary Material
Acknowledgements
We thank the dedicated members of our study team: Stella Namirembe Magara, Justine Bukirwa, Samuel Kabanda, Abdulatif Agaba, Robert Mutumba, and Grace Menya. We also thank the Kiboga District Hospital Medical superintendents for partnering with the Infectious Diseases Institute to develop Kiboga as a rural research site. We thank Richard Mwesiga of the Infectious Diseases Institute extended Kibaale Kiboga project, and Alex Coutinho, Agnes Kiragga, Allan Etonu, Henry Onen, and Doreen Kizza of the Infectious Diseases Institute for their support. This work is supported by the United States National Institutes of Health Office of the Director, Fogarty International Center, Office of AIDS Research, National Cancer Center, National Eye Institute, National Heart, Blood, and Lung Institute, National Institute of Dental & Craniofacial Research, National Institute On Drug Abuse, National Institute of Mental Health, National Institute of Allergy and Infectious Diseases Health, and NIH Office of Women’s Health and Research through the International Clinical Research Scholars and Fellows Program at Vanderbilt University (R24 TW007988). LP, MH, BA, AS, MS, AM, and TN were all Fogarty Scholars. Funding for this study is also provided by a Pfizer Investigator-Initiated Research Grant (YCM, DBM). Diflucan (fluconazole) was kindly donated by Pfizer. YCM also receives salary support from the Fogarty International Center, NIH (1R25TW009340, R24TW008861, R24TW007988, and D43TW009771) and Johns Hopkins University Center for AIDS Research (Grant Number 1P30AI094189 from the National Institute of Allergy And Infectious Diseases).
Footnotes
Conflicts of Interest
All authors declare no conflicts of interest.
Authors' contributions
MS, TND, and YCM conceived the study, designed the study, and wrote the study protocol. BJA and ASS edited the study protocol and coordinated the study. LP, AMN, MH coordinated and completed the study. DBM contributed to the study design in the area of cryptococcal disease. AM contributed to the conception of the study at Kiboga District Hospital. All authors contributed to the writing and editing of this manuscript. All authors read and approved the final manuscript.
References
- 1.Global Update on HIV Treatment 2013: Results, Impact and Opportunities. Kuala Lumpur: WHO; 2013. [Google Scholar]
- 2.Antiretroviral Medicines in Low-and Middle-Income Countries: Forcasts of Global and Regional Demand for 2013–2016. The World Health Organization. 2014 [Google Scholar]
- 3.Gupta A, Nadkarni G, Yang W-T, Chandrasekhar A, Gupte N, Bisson GP, et al. Early Mortality in Adults Initiating Antiretroviral Therapy (ART) in Low- and Middle-Income Countries (LMIC): A Systematic Review and Meta-Analysis. PLoS ONE. 2011;6:e28691. doi: 10.1371/journal.pone.0028691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lawn SD, Harries AD, Anglaret X, Myer L, Wood R. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS. 2008;22:1897–1908. doi: 10.1097/QAD.0b013e32830007cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castelnuovo B, Manabe Y, Kiragga A, Kamya M, Easterbrook P, Kambugu A. Cause-specific mortality and the contribution of immune reconstitution inflammatory syndrome in the first 3 years after antiretroviral therapy initiation in an urban African cohort. Clin Infect Dis. 2009;49:965–972. doi: 10.1086/605500. [DOI] [PubMed] [Google Scholar]
- 6.Zachariah R, Fitzgerald M, Massaquoi M, Pasulani O, Arnould L, Makombe S, et al. Risk factors for high early mortality in patients on antiretroviral treatment in a rural district of Malawi. AIDS. 2006;20:2355–2360. doi: 10.1097/QAD.0b013e32801086b0. [DOI] [PubMed] [Google Scholar]
- 7.Kassa A, Tekab A, Shewaamarec A, Jerened D. Incidence of tuberculosis and early mortality in a large cohort of HIV infected patients receiving antiretroviral therapy in a tertiary hospital in Addis Ababa, Ethiopia. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2012;106:363–370. doi: 10.1016/j.trstmh.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Tadesse K, Haile F, Hiruy N. Predictors of Mortality among Patients Enrolled on Antiretroviral Therapy in Aksum Hospital, Northern Ethiopia: A Retrospective Cohort Study. PLoS ONE. 2014;9:e87392. doi: 10.1371/journal.pone.0087392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Russell E, Charalambous S, Pemba L, Churchyard G, Grant A, Fielding K. Low haemoglobin predicts early mortality among adults starting antiretroviral therapy in an HIV care programme in South Africa: a cohort study. BMC Public Health. 2010;10 doi: 10.1186/1471-2458-10-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore D, Yiannoutsos C, Musick B, Tappero J, Degerman R, Campbell J, et al. Determinants of early and late mortality among HIV-infected individuals receiving home-based antiretroviral therapy in rural Uganda. J Acquir Immune Defic Syndr. 2011;58:289–296. doi: 10.1097/QAI.0b013e3182303716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toure S, Kouadio B, Seyler C, Traore M, Dakoury-Dogbo N, Duvignac J, et al. Rapid scaling-up of antiretroviral therapy in 10,000 adults in Côte d'Ivoire: 2-year outcomes and determinants. AIDS. 2008;22:873–882. doi: 10.1097/QAD.0b013e3282f768f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nash D, Wua Y, Elula B, Hoosa D, El Sadra W. Program-level and contextual-level determinants of low-median CD4+ cell count in cohorts of persons initiating ART in eight sub-Saharan African countries. AIDS. 2011;25:1523–1533. doi: 10.1097/QAD.0b013e32834811b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lahuerta M, Lima J, Nuwagaba-Biribonwoha H, Okamura M, Fernanda Alvim M, Fernandes R, et al. Factors Associated with Late Antiretroviral Therapy Initiation among Adults in Mozambique. PLoS ONE. 2012;7:e37125. doi: 10.1371/journal.pone.0037125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ndawinz J, Chaix B, Koulla-Shiro S, Delaporte E, Okouda B, Abanda A, et al. Factors associated with late antiretroviral therapy initiation in Cameroon: a representative multilevel analysis. J Antimicrob Chemother. 2013;68:1388–1399. doi: 10.1093/jac/dkt011. [DOI] [PubMed] [Google Scholar]
- 15.IeDea, Collaborations ARTC. Avila D, Althoff KN, Mugglin C, Wools-Kaloustian K, et al. Immunodeficiency at the start of combination antiretroviral therapy in low-, middle-, and high-income countries. J Acquir Immune Defic Syndr. 2014;65:e8–e16. doi: 10.1097/QAI.0b013e3182a39979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park B, Wannemuehler K, Marston B, Govender N, Pappas P, Chiller T. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23:525–530. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 17.Liechty C, Solberg P, Were W, Ekwaru J, Ransom R, Weidle P, et al. Asymptomatic serum cryptococcal antigenemia and early mortality during antiretroviral therapy in rural Uganda. Trop Med Int Health. 2007;12:929–935. doi: 10.1111/j.1365-3156.2007.01874.x. [DOI] [PubMed] [Google Scholar]
- 18.Jarvis J, Lawn S, Vogt M, Bangani N, Wood R, Harrison T. Screening for cryptococcal antigenemia in patients accessing an antiretroviral treatment program in South Africa. Clin Infect Dis. 2009;48:856–862. doi: 10.1086/597262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meyer A, Kendi C, Penner J, Odhiambo N, Otieno B, Omondi E, et al. The impact of routine cryptococcal antigen screening on survival among HIV-infected individuals with advanced immunosuppression in Kenya. Trop Med Int Health. 2013;18:495–503. doi: 10.1111/tmi.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ganiem AR, Indrati AR, Wisaksana R, Meijerink H, van der Ven A, Alisjahbana B, et al. Asymptomatic cryptococcal antigenemia is associated with mortality among HIV-positive patients in Indonesia. J Int AIDS Soc. 2014;17:18821. doi: 10.7448/IAS.17.1.18821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Worodria W, Massinga-Loembe M, Mayanja-Kizza H, Namaganda J, Kambugu A, Manabe YC, et al. Antiretroviral treatment-associated tuberculosis in a prospective cohort of HIV-infected patients starting ART. Clin Dev Immunol. 2011;2011:758350. doi: 10.1155/2011/758350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mamoojee Y, Shakoor S, Gorton R, Sarfo S, Appiah L, Norman B, et al. Short Communication: Low seroprevalence of cryptococcal antigenaemia in patients with advanced HIV infection enrolling in an antiretroviral programme in Ghana. Trop Med Int Health. 2011;16:53–56. doi: 10.1111/j.1365-3156.2010.02683.x. [DOI] [PubMed] [Google Scholar]
- 23.Oyella J, Meya D, Bajunirwe F, Kamya M. Prevalence and factors associated with cryptococcal antigenemia among severely immunosuppressed HIV-infected adults in Uganda: a cross-sectional study. J Int AIDS Soc. 2012;15:15. doi: 10.1186/1758-2652-15-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meya D, Manabe Y, Castelnuovo B, Cook B, Elbireer A, Kambugu A, et al. Cost-effectiveness of serum cryptococcal antigen screening to prevent deaths among HIV-infected persons with a CD4+ cell count < or = 100 cells/microL who start HIV therapy in resource-limited settings. Clin Infect Dis. 2010;51:448–455. doi: 10.1086/655143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Osazuwa O, Dirisu O, Okuonghae E. Cryptococcal antigenemia in anti-retroviral naïve AIDS patients: prevalence and its association with CD4 cell count. Acta Med Iran. 2012;50:344–347. [PubMed] [Google Scholar]
- 26.Jarvis JN, Harrison TS, Lawn SD, Meintjes G, Wood R, Cleary S. Cost effectiveness of cryptococcal antigen screening as a strategy to prevent HIV-associated cryptococcal meningitis in South Africa. PLoS One. 2013;8:e69288. doi: 10.1371/journal.pone.0069288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lawn S, Myer L, Bekker L, Wood R. Burden of tuberculosis in an antiretroviral treatment programme in sub-Saharan Africa: impact on treatment outcomes and implications for tuberculosis control. AIDS. 2006;20:1605–1612. doi: 10.1097/01.aids.0000238406.93249.cd. [DOI] [PubMed] [Google Scholar]
- 28.Lawn S, Harries A, Wood R. Strategies to reduce early morbidity and mortality in adults receiving antiretroviral therapy in resource-limited settings. Curr Opin HIV AIDS. 2010;5:18–26. doi: 10.1097/COH.0b013e328333850f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wood R, Middelkoop K, Myer L, Grant A, Whitelaw A, Lawn S, et al. Undiagnosed tuberculosis in a community with high HIV prevalence: implications for tuberculosis control. Am J Respir Crit Care Med. 2007;175:87–93. doi: 10.1164/rccm.200606-759OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moore D, Liechty C, Ekwaru P, Were W, Mwima G, Solberg P, et al. Prevalence, incidence and mortality associated with tuberculosis in HIV-infected patients initiating antiretroviral therapy in rural Uganda. AIDS. 2007;21:713–719. doi: 10.1097/QAD.0b013e328013f632. [DOI] [PubMed] [Google Scholar]
- 31.Bassett I, Wang B, Chetty S, Giddy J, Losina E, Mazibuko M, et al. Intensive tuberculosis screening for HIV-infected patients starting antiretroviral therapy in Durban, South Africa. Clin Infect Dis. 2010;51:823–829. doi: 10.1086/656282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lawn S, Kerkhoff A, Vogt M, Wood R. Diagnostic accuracy of a low-cost, urine antigen, point-of-care screening assay for HIV-associated pulmonary tuberculosis before antiretroviral therapy: a descriptive study. Lancet Infect Dis. 2012;12:201–209. doi: 10.1016/S1473-3099(11)70251-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hanifa Y, Fielding K, Charalambous S, Variava E, Luke B, Churchyard G, et al. Tuberculosis among adults starting antiretroviral therapy in South Africa: the need for routine case finding. Int J Tuberc Lung Dis. 2012;16:1252–1259. doi: 10.5588/ijtld.11.0733. [DOI] [PubMed] [Google Scholar]
- 34.Lawn S, Brooks S, Kranzer K, Nicol M, Whitelaw A, Vogt M, et al. Screening for HIV-associated tuberculosis and rifampicin resistance before antiretroviral therapy using the Xpert MTB/RIF assay: a prospective study. PLoS Med. 2011;8:e1001067. doi: 10.1371/journal.pmed.1001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lawn SD, Harries AD, Meintjes G, Getahunf H, Havlirg DV, Wood R. Reducing deaths from tuberculosis in antiretroviral treatment programmes in sub-Saharan Africa. AIDS. 2012;26:2121–2133. doi: 10.1097/QAD.0b013e3283565dd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nansera D, Bajunirwe F, Elyanu P, Asiimwe C, Amanyire G, Graziano F. Mortality and loss to follow-up among tuberculosis and HIV co-infected patients in rural southwestern Uganda. Int J Tuberc Lung Dis. 2012;16:1371–1376. doi: 10.5588/ijtld.11.0589. [DOI] [PubMed] [Google Scholar]
- 37.Gupta A, Wood R, Kaplan R, Bekker L, Lawn S. Prevalent and incident tuberculosis are independent risk factors for mortality among patients accessing antiretroviral therapy in South Africa. PLoS One. 2013;8:e55824. doi: 10.1371/journal.pone.0055824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Granich R, Williams B, Montanerc J. Fifteen million people on antiretroviral treatment by 2015: treatment as prevention. Curr Opin HIV AIDS. 2013;8:41–49. doi: 10.1097/COH.0b013e32835b80dd. [DOI] [PubMed] [Google Scholar]
- 39.Dube C, Nozaki I, Hayakawa T, Kakimoto K, Yamadad N, Simpungwee JB. Expansion of antiretroviral treatment to rural health centre level by a mobile service in Mumbwa district, Zambia. Bull World Health Organ. 2010;88:788–791. doi: 10.2471/BLT.09.063982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mutevedzi PC, Lessells RJ, Heller T, Bärnighausen T, Cooke GS, Newella M-L. Scale-up of a decentralized HIV treatment programme in rural KwaZulu-Natal, South Africa: does rapid expansion affect patient outcomes? Bull World Health Organ. 2010;88:593–600. doi: 10.2471/BLT.09.069419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guidelines for intensified tuberculosis case-finding and isoniazid preventive therapy for people living with HIV in resource-constrained settings. The World Health Organization. 2011
- 42.The State of Uganda Population Report. UNFPA Uganda. 2008:1–115. [Google Scholar]
- 43.Kampala, Uganda: Uganda AIDS Commission; 2014. 2013 Uganda HIV and AIDS Country Progress Report. [Google Scholar]
- 44.Boulware DR, Meya DB, Muzoora C, Rolfes MA, Huppler Hullsiek K, Musubire A, et al. Timing of antiretroviral therapy after diagnosis of cryptococcal meningitis. N Engl J Med. 2014;370:2487–2498. doi: 10.1056/NEJMoa1312884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakiyingi L, Moodley VM, Manabe YC, Nicol MP, Holshouser M, Armstrong DT, et al. Diagnostic accuracy of a rapid urine lipoarabinomannan test for tuberculosis in HIV-infected adults. J Acquir Immune Defic Syndr. 2014 doi: 10.1097/QAI.0000000000000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Manual of the National Tuberculosis and Leprosy Programme. Republic of Uganda Ministry of Health. 2010 [Google Scholar]
- 47.World Health Organization; 2012. Global Tuberculosis Report. [Google Scholar]
- 48.Manabe YC, Nonyane BA, Nakiyingi L, Mbabazi O, Lubega G, Shah M, et al. Point-of-Care Lateral Flow Assays for Tuberculosis and Cryptococcal Antigenuria Predict Death in HIV Infected Adults in Uganda. PLoS One. 2014;9:e101459. doi: 10.1371/journal.pone.0101459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jarvis J, Percival A, Bauman S, Pelfrey J, Meintjes G, Williams G, et al. Evaluation of a novel point-of-care cryptococcal antigen test on serum, plasma, and urinefrom patients with HIV-associated cryptococcal meningitis. Clin. Infect. Dis. 2011;53:1019–1023. doi: 10.1093/cid/cir613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Manabe Y, Breen R, Perti T, Girardi E, Sterling T. Unmasked tuberculosis and tuberculosis immune reconstitution inflammatory disease: a disease spectrum after initiation of antiretroviral therapy. J Infect Dis. 2009;199:437–444. doi: 10.1086/595985. [DOI] [PubMed] [Google Scholar]
- 51.Shah M, Ssengooba W, Armstrong D, Nakiyingi L, Holshouser M, Ellner J, et al. Comparative performance of urinary lipoarabinomannan assays and Xpert MTB/RIF in HIV-infected individuals with suspected tuberculosis in Uganda. AIDS. 2014 doi: 10.1097/QAD.0000000000000264. epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hermans S, van Leth F, Manabe Y, Hoepelman A, Lange J, Kambugu A. Earlier initiation of antiretroviral therapy, increased tuberculosis case finding and reduced mortality in a setting of improved HIV care: a retrospective cohort study. HIV Med. 2012;13:337–344. doi: 10.1111/j.1468-1293.2011.00980.x. [DOI] [PubMed] [Google Scholar]
- 53.Hermans SM, Castelnuovo B, Katabira C, Mbidde P, Lange JM, Hoepelman AI, et al. Integration of HIV and TB Services Results in Improved TB Treatment Outcomes and Earlier Prioritized ART Initiation in a Large Urban HIV Clinic in Uganda. J Acquir Immune Defic Syndr. 2012;60 doi: 10.1097/QAI.0b013e318251aeb4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lawn S, Myer L, Bekker L, Wood R. Burden of tuberculosis in an antiretroviral treatment programme in sub-Saharan Africa: impact on treatment outcomes and implications for tuberculosis control. AIDS. 2006;20:1605–1612. doi: 10.1097/01.aids.0000238406.93249.cd. [DOI] [PubMed] [Google Scholar]
- 55.Weidle P, Malamba S, Mwebaze R, Sozi C, Rukundo G, Downing R, et al. Assessment of a pilot antiretroviral drug therapy programme in Uganda: patients' response, survival, and drug resistance. Lancet. 2002;360:34–40. doi: 10.1016/S0140-6736(02)09330-3. [DOI] [PubMed] [Google Scholar]
- 56.Coetzee D, Hildebrand K, Boulle A, Maartens G, Louis F, Labatala V, et al. Outcomes after two years of providing antiretroviral treatment in Khayelitsha, South Africa. AIDS. 2004;18:887–895. doi: 10.1097/00002030-200404090-00006. [DOI] [PubMed] [Google Scholar]
- 57.Wester C, Kim S, Bussmann H, Avalos A, Ndwapi N, Peter T, et al. Initial response to highly active antiretroviral therapy in HIV-1C-infected adults in a public sector treatment program in Botswana. J Acquir Immune Defic Syndr. 2005;40:336–343. doi: 10.1097/01.qai.0000159668.80207.5b. [DOI] [PubMed] [Google Scholar]
- 58.Ferradini L, Jeannin A, Pinoges L, Izopet J, Odhiambo D, Mankhambo L, et al. Scaling up of highly active antiretroviral therapy in a rural district of Malawi: an effectiveness assessment. Lancet. 2006;367:1335–1342. doi: 10.1016/S0140-6736(06)68580-2. [DOI] [PubMed] [Google Scholar]
- 59.Etard J, Ndiaye I, Thierry-Mieg M, Guèye N, Guèye P, Lanièce I, et al. Mortality and causes of death in adults receiving highly active antiretroviral therapy in Senegal: a 7-year cohort study. AIDS. 2006;20:1181–1189. doi: 10.1097/01.aids.0000226959.87471.01. [DOI] [PubMed] [Google Scholar]
- 60.Makombe S, Harries A, Yu J, Hochgesang M, Mhango E, Weigel R, et al. Outcomes of tuberculosis patients who start antiretroviral therapy under routine programme conditions in Malawi. Int J Tuberc Lung Dis. 2007;11:412–416. [PubMed] [Google Scholar]
- 61.Johannessen A, Naman E, Ngowi B, Sandvik L, Matee M, Aglen H, et al. Predictors of mortality in HIV-infected patients starting antiretroviral therapy in a rural hospital in Tanzania. BMC Infect Dis. 2008;8 doi: 10.1186/1471-2334-8-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stringer J, Zulu I, Levy J, Stringer E, Mwango A, Chi B, et al. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: feasibility and early outcomes. JAMA. 2006;296:782–793. doi: 10.1001/jama.296.7.782. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


