In this randomized clinical trial comparing micafungin 100 mg with standard-care antifungal prophylaxis (fluconazole, liposomal amphotericin B, or caspofungin) in high-risk liver transplant patients, micafungin 100 mg was noninferior and had a better kidney safety profile.
Keywords: antifungal therapy, liver transplant, micafungin, prophylaxis, infection
Abstract
Background. Invasive fungal infection (IFI) following liver transplant is associated with significant morbidity and mortality. Antifungal prophylaxis is rational for liver transplant patients at high IFI risk.
Methods. In this open-label, noninferiority study, patients were randomized 1:1 to receive intravenous micafungin 100 mg or center-specific standard care (fluconazole, liposomal amphotericin B, or caspofungin) posttransplant. The primary endpoint was clinical success (absence of a proven/probable IFI and no need for additional antifungals) at end of prophylaxis (EOP). Noninferiority (10% margin) of micafungin vs standard care was assessed in the per protocol and full analysis sets. Safety assessments included adverse events and liver and kidney function tests.
Results. The full analysis set comprised 344 patients (172 micafungin; 172 standard care). Mean age was 51.2 years; 48.0% had a Model for End-Stage Liver Disease score ≥20. At EOP (mean treatment duration, 17 days), clinical success was 98.6% for micafungin and 99.3% for standard care (Δ standard care – micafungin [95% confidence interval], 0.7% [−2.7% to 4.4%]) in the per protocol set and 96.5% and 93.6%, respectively (−2.9% [−8.0% to 1.9%]), in the full analysis set. Incidences of drug-related adverse events for micafungin and standard care were 11.6% and 16.3%, leading to discontinuation in 6.4% and 11.6% of cases, respectively. At EOP, liver function tests were similar but creatinine clearance was higher in micafungin- vs standard care–treated patients.
Conclusions. Micafungin was noninferior to standard care as antifungal prophylaxis in liver transplant patients at high risk for IFI. Adverse event profiles and liver function at EOP were similar, although kidney function was better with micafungin.
Clinical Trials Registration. NCT01058174.
Liver transplant recipients are susceptible to invasive fungal infection (IFI), with infection rates of 8.4%–17.7% reported in contemporary epidemiological studies [1–6]. Most IFIs in solid organ transplant recipients belong to the genera Aspergillus and Candida [7, 8]. Candida species account for the majority (60%–91%) of IFI in liver transplant patients [3, 5, 8, 9], with Candida albicans and Candida glabrata being most commonly observed [8, 10, 11].
Risk factors associated with IFI in liver transplant recipients include pre- and postoperative renal failure, retransplantation, substantial intraoperative transfusion of cellular blood products [9], and high Model for End-Stage Liver Disease (MELD) score [2, 12, 13]. As IFIs are associated with mortality rates of 25%–90% [2, 4, 9, 14, 15], antifungal prophylaxis is a rational approach for liver transplant patients considered at high risk.
Fluconazole and liposomal amphotericin B are US guideline–recommended options for antifungal prophylaxis in liver transplant recipients with multiple IFI risk factors [15–17]. However, some strains of Candida have shown resistance to fluconazole [17–19], and amphotericin B nephrotoxicity causes concern in renally impaired patients [20, 21]. The echinocandins have demonstrated broad efficacy against Candida species, have low toxicity and few drug–drug interactions, and are established first-line treatments for invasive candidiasis [22–24]. Some centers give echinocandins as primary prophylaxis in liver transplant recipients [23], and European recommendations support their use in patients at high IFI risk [25]. The aim of TENPIN (Liver Transplant European Study Into the Prevention of Fungal Infection) was to demonstrate noninferiority of the echinocandin micafungin vs center-specific standard care for IFI prevention in liver transplant recipients deemed at high risk of IFI.
METHODS
Patients and Study Design
TENPIN was a phase 3b, international, multicenter, randomized, open-label, parallel-group, noninferiority study of antifungal prophylaxis in liver transplant recipients (ClinicalTrials.gov identifier: NCT01058174; ClinicalTrialsRegister.eu EudraCT number 2008-005214-49). Patients aged ≥18 years undergoing orthotopic whole or split liver allograft transplant were eligible for inclusion based on the presence of at least 1 of the IFI risk factors described in Supplementary Appendix 1.1. Supplementary Appendix 1.2 details the exclusion criteria.
Eligible patients were randomized 1:1 to receive, posttransplant, either intravenous micafungin 100 mg/day (2.0 mg/kg/day if body weight was <40 kg) or center-specific standard care (a predefined regimen according to local protocol of either intravenous fluconazole 200–400 mg/day, intravenous liposomal amphotericin B 1–3 mg/kg/day, or intravenous caspofungin 70 mg single loading dose followed by 50 mg once daily). Patients were randomized at admission if they fulfilled the high IFI risk criteria or within 5 days posttransplant following intra- or postoperative events. Supplementary Appendix 1.3 and Supplementary Figure 1 describe the randomization procedure.
Prophylaxis lasted 21 days or until hospital discharge (whichever occurred first), or longer in patients with persistent risk factors. End of study (EOS) was 3 months postrandomization, and long-term follow-up was 6 months postrandomization. Patients who developed a proven or probable IFI during prophylaxis were discontinued from the study drug, treated with appropriate antifungal therapy, and required to remain in the study and complete all assessments. A proven or probable IFI, diagnosed by an investigator according to the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group definitions [26], was confirmed by an independent data review board (IDRB).
The study protocol (ISN/protocol 9463-EC-0001) was approved by the independent Ethics committee or institutional review board at each center and was conducted in accordance with the ethical principles that originate in the Declaration of Helsinki. Patients' written informed consent was obtained prior to screening.
The manuscript was written in accordance with the CONSORT (Consolidated Standards for Reporting Trials) statement for randomized controlled trials recommendations [27].
Outcomes
Efficacy
Fungal infection status was evaluated at baseline (methods provided in Supplementary Appendix 1.4), during prophylaxis, at end of prophylaxis (EOP), and at EOS by the investigator and blindly assessed by the IDRB. During prophylaxis, fungal infection status was assessed at a minimum of twice weekly, with assessments conducted at least 72 hours apart.
The primary efficacy endpoint was clinical success (defined as a composite of absence of a proven or probable IFI and no initiation of antifungal treatment at EOP) assessed by the IDRB. Antifungal treatment was defined as either additional antifungal medication or increased study drug dose due to apparent inadequate efficacy.
Secondary prespecified efficacy endpoints included absence of a proven or probable IFI at EOP and EOS or EOS-month 3 (ie, later than postrandomization day 76) as assessed by the IDRB and investigator; absence of initiation of antifungal therapy at EOP as assessed by the IDRB; time to proven or probable IFI from randomization according to the IDRB; fungal-free survival at EOS; and end of long-term follow-up according to the investigator.
Safety
Treatment-emergent adverse events (AEs) were recorded up to EOS, and serious AEs, including death, until 30 days after EOS. Overall mortality was considered a safety parameter. Routine laboratory assessments of biochemistry, hematology, and urinalysis were performed at baseline and throughout the study. Hepatic and renal function was assessed using standard laboratory biochemical tests.
Statistical Analysis
Efficacy data were analyzed for the full analysis set (FAS), that is, all randomized patients who received at least 1 dose of study medication and without proven or possible IFI at baseline (according to the IDRB); and the per protocol set (PPS), that is, all FAS patients who completed the study without major protocol violations. The PPS was used as the primary analysis set and the FAS as the confirmatory analysis set for the primary efficacy endpoint and all secondary endpoints. The safety analysis set (SAF) included all randomized patients who received at least 1 dose of study medication.
The study sample size was calculated using a noninferiority margin of 10% for the absolute difference in clinical success rates based on clinical judgment by an expert panel. Based on this noninferiority margin, assuming a true success rate of 94% in the standard care group, 135 patients per group were necessary to demonstrate noninferiority of micafungin vs standard care with a power of at least 90% using a 1-sided type I risk error of 2.5%. Assuming that 80% of the randomized subjects were included in the PPS, 169 patients were required to be randomized into each treatment group. Clinically, a noninferiority margin of 10% was also deemed applicable to the FAS.
For the composite primary efficacy endpoint of clinical success, rates were compared between the standard care and micafungin groups at EOP, and 2-sided 95% confidence intervals (CIs) for the difference in success rates were calculated using the Newcombe–Wilson method. If the upper limit of the 95% CI for the difference in success rates was <10%, then the noninferiority of micafungin to standard care was declared. The analysis assumed that the infection rate among the individual treatment regimens was homogeneous. The secondary efficacy endpoints of absence of proven or probable IFI (EOP, EOS, and EOS-month 3) and absence of antifungal therapy (EOP), and fungal-free survival were similarly analyzed, although as the study was powered for the primary endpoint, statistical noninferiority was tested on this endpoint only.
Time to proven or probable IFI was modeled using Cox regression, with covariates including baseline MELD score (≤29 or ≥30) and treatment group. Patients with no proven or probable IFI during prophylaxis were censored at the assessment visit. Nonparametric analysis was undertaken, Kaplan–Meier curves are provided, and the log-rank test was utilized to compare treatment groups for the endpoints of time to proven or probable IFI and fungal-free survival.
Baseline characteristics (FAS) and safety variables (SAF) were summarized by descriptive statistics.
In a post hoc analysis to further explore renal safety, estimates of glomerular filtration rate (GFR) and creatinine clearance (CrCl) were derived for the SAF and also for the SAF patients who did not require renal replacement therapy (RRT) at baseline, using the 4-variable Modification of Diet in Renal Disease formula and Cockcroft–Gault formula, respectively. Differences in GFR and CrCl between treatment groups were compared at weekly time points using repeated measures analysis of variance and at EOP by analysis of covariance.
Data analysis for this paper was generated using SAS/STAT software, version 9.3 (SAS Institute Inc, Cary, North Carolina).
RESULTS
Patients
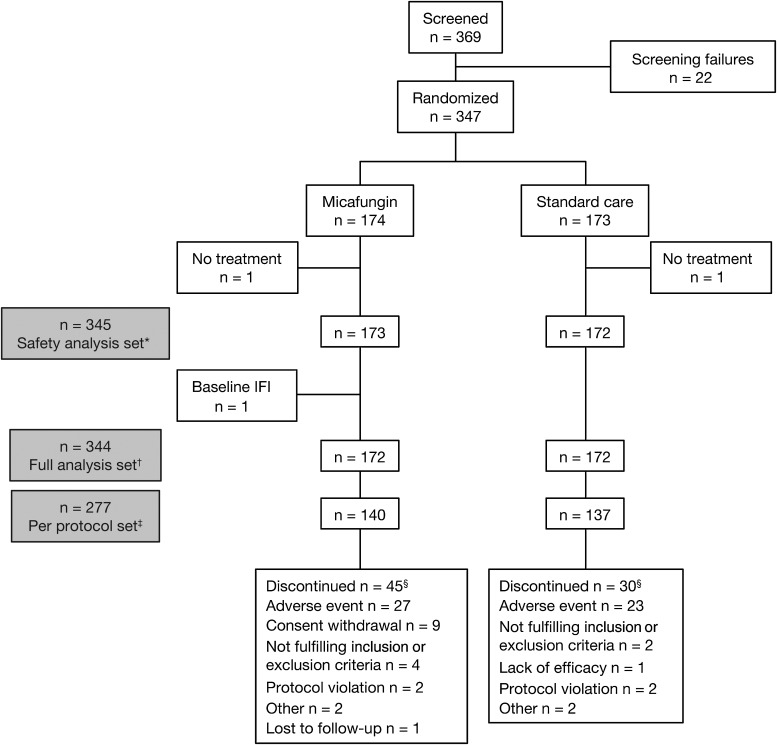
The study ran from 15 December 2009 to 3 May 2012 at 37 European centers. Figure 1 shows participant flow. Forty-five micafungin-treated patients were excluded from the study, mainly due to AEs (n = 27) or withdrawal of consent (n = 9), and 30 patients treated with standard care were excluded, mainly due to AEs (n = 23). Excluding MELD score, demographic and baseline characteristics were balanced between treatment groups (Table 1). The most common indications for transplantation were cirrhosis (67.5%) and hepatocellular carcinoma (16.2%). Supplementary Appendix 2.1 and Supplementary Table 1 detail the proportion of patients in each high-risk IFI category. The mean duration of exposure was 16.7 (standard deviation [SD], 7.0) days in the micafungin group and 17.1 (SD, 8.0) days in the standard care group.
Figure 1.
Disposition of patients. *All randomized patients with at least 1 dose of study drug. †All randomized patients with at least 1 dose of study drug and without an invasive fungal infection (IFI) at baseline. ‡All patients who completed the study without major protocol deviations or violations. §Patients from the full analysis set who discontinued; for every patient, only the primary reason for discontinuation was collected.
Table 1.
Demographic and Baseline Characteristics
| Characteristic | Micafungin 100 mg (n = 172) | Standard Care (n = 172) | Total (n = 344) |
|---|---|---|---|
| Male sex, No. (%) | 118 (68.6) | 114 (66.3) | 232 (67.4) |
| Mean ± SD age, y | 51.9 ± 10.5 | 50.5 ± 11.8 | 51.2 ± 11.2 |
| Mean ± SD BMI, kg/m2 | 25.8 ± 4.3 | 25.3 ± 5.0 | 25.6 ± 4.6 |
| Ethnicity, No. (%) | |||
| White | 165 (95.9) | 159 (92.4) | 324 (94.2) |
| Black | 3 (1.7) | 7 (4.1) | 10 (2.9) |
| Asian | 3 (1.7) | 5 (2.9) | 8 (2.3) |
| Other | 1 (0.6) | 1 (0.6) | 2 (0.6) |
| Region, No. (%) | |||
| Western Europe | 71 (41.3) | 72 (41.9) | 143 (41.6) |
| Eastern Europe | 17 (9.9) | 14 (8.1) | 31 (9.0) |
| Southern Europe | 84 (48.8) | 86 (50.0) | 170 (49.4) |
| Mean ± SD MELD score | 19.9 ± 10.0 | 21.1 ± 10.0 | 20.5 ± 10.0 |
| MELD score, No. (%) | |||
| <20 | 98 (57.0) | 81 (47.1) | 179 (52.0) |
| 20–29 | 43 (25.0) | 54 (31.4) | 97 (28.2) |
| ≥30 | 31 (18.0) | 37 (21.5) | 68 (19.8) |
| CMV mismatch (recipient/donor), No. (%) | |||
| Negative/negative | 26 (15.2) | 20 (11.8) | 46 (13.5) |
| Negative/positive | 27 (15.8) | 23 (13.5) | 50 (14.7) |
| Positive/negative | 34 (19.9) | 46 (27.1) | 80 (23.5) |
| Positive/positive | 70 (40.9) | 67 (39.4) | 137 (40.2) |
Percentages are based on all patients with available data in the respective treatment regimen. For CMV mismatch, the number of patients with available data is equal to the number of patients with both assessments available.
Abbreviations: BMI, body mass index; CMV, cytomegalovirus; MELD, Model for End-Stage Liver Disease; SD, standard deviation.
Efficacy
Primary Efficacy Endpoint
The clinical success rate of micafungin 100 mg was noninferior to standard care at EOP as assessed by the IDRB in the primary analysis PPS: 98.6% (138/140) for micafungin and 99.3% (136/137) for standard care (Δ standard care – micafungin, 0.7% [95% CI, −2.7% to 4.4%]). This was confirmed in the FAS (Table 2). Of the 67 FAS patients excluded from PPS, 67.1% were excluded because their EOP assessment was >3 days after last treatment administration of study drug.
Table 2.
Clinical Success Rate for Micafungin and Standard Care at the End of the Prophylaxis Period and as Assessed by the Independent Data Review Board (Per Protocol and Full Analysis Sets)
| Per Protocol Set (Primary Analysis) | Micafungin, No. (%) (n = 140) | Standard Care, No. (%) (n = 137) | Difference, % (95% CI) Standard Care – Micafungin |
|---|---|---|---|
| Clinical success | 138 (98.6) | 136 (99.3) | 0.7 (−2.7 to 4.4) |
| No clinical success | 2 (1.4) | 1 (0.7) | |
| Invasive fungal infectiona | 2 (1.4) | 1 (0.7) | |
| Antifungal treatmenta | 0 | 0 | |
| No assessments available | 0 | 0 | |
| Full Analysis Set (Confirmatory Analysis) | Micafungin, (n = 172) No. (%) | Standard Care, (n = 172) No. (%) | Difference, % (95% CI) Standard Care – Micafungin |
| Clinical success | 166 (96.5) | 161 (93.6) | −2.9 (−8.0 to 1.9) |
| No clinical success | 6 (3.5) | 11 (6.4) | |
| Invasive fungal infectiona | 4 (2.3) | 8 (4.7) | |
| Antifungal treatmenta | 2 (1.2) | 7 (4.1) | |
| No assessments available | 2 (1.2) | 3 (1.7) |
Abbreviation: CI, confidence interval.
a One patient may have proven or probable invasive fungal infection and may have started antifungal treatment.
Secondary Efficacy Endpoints in the Full Analysis Set
Micafungin was similar to standard care with regard to absence of proven or probable IFI at EOP and EOS, according to both IDRB and investigator assessments (Supplementary Appendix 2.2 and Supplementary Table 2). At EOP, there were 4 (2.3%) IDRB-confirmed IFIs in micafungin-treated patients and 8 (4.7%) in standard care–treated patients. At EOS, there were 3 new infections in each group. Supplementary Appendix 2.3 and Supplementary Table 3 detail the infecting species. With regard to absence of initiation of systemic antifungal treatment, the success rate of micafungin (98.8%) was also similar to standard care (95.9%) at EOP (Δ standard care – micafungin, −3.0% [95% CI, −7.2% to .7%]).
Cox regression modeling of time to proven or probable IFI gave a hazard ratio of 0.72 (95% CI, .27–1.90) indicating no significant difference between the groups. Nonparametric analysis was consistent with this finding (log-rank test P = .498; Supplementary Appendix 2.4 and Supplementary Figure 2).
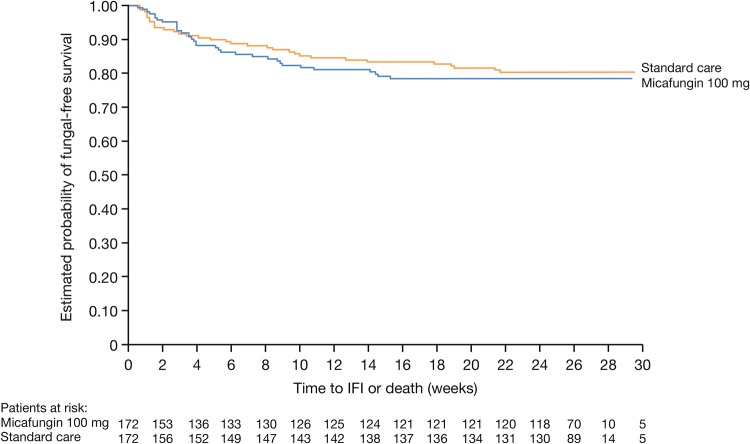
Investigator-assessed fungal-free survival at EOS was 80.8% (126/156) and 83.2% (139/167) in the micafungin- and standard care–treated patients, respectively (Δ 2.5% [95% CI, −5.9% to 10.9%]) and at the end of long-term follow-up was 78.1% (121/155) and 80.0% (132/165), respectively (Δ 1.9% [95% CI, −7.0% to 10.9%]). Kaplan–Meier analyses showed no significant difference in fungal-free survival at long-term follow-up (log-rank P = .679) (Figure 2).
Figure 2.
Fungal-free survival in micafungin and standard care treatment groups during long-term follow-up (full analysis set). Abbreviation: IFI, invasive fungal infection.
Safety
Hepatic and Renal Functions
There were no clinically relevant differences in hepatic function tests over time between micafungin- and standard care–treated patients (Supplementary Appendix 2.5 and Supplementary Figure 3).
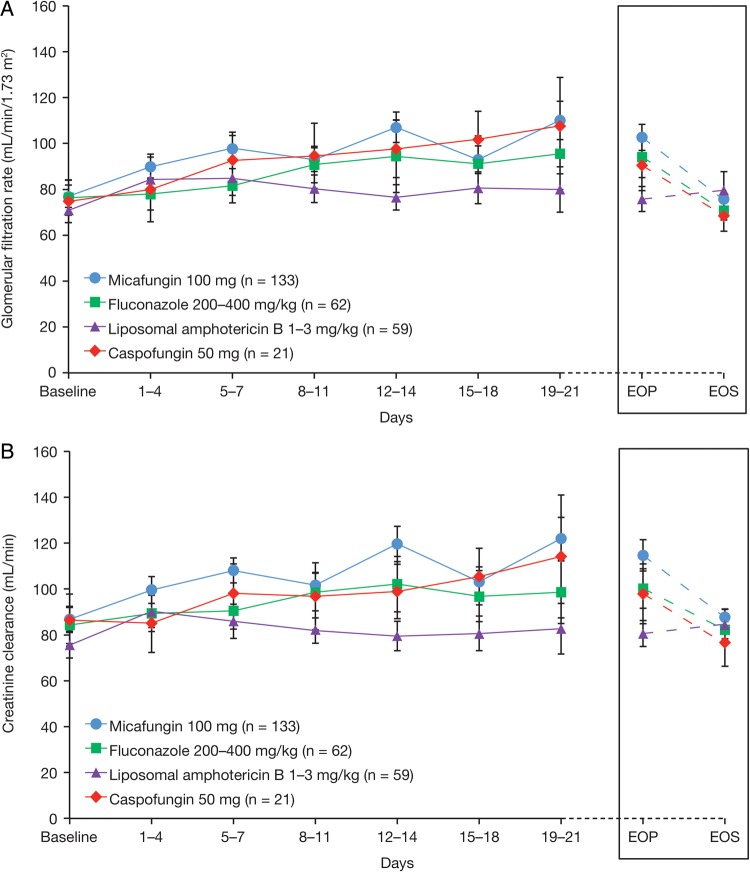
Serum creatinine and urea levels were lower in the micafungin group than standard care at most weekly time points and at EOP, suggesting better renal function in these patients. Post hoc analysis showed that mean GFR and mean CrCl were higher for micafungin vs standard care at most time points during prophylaxis, although differences were not statistically significant (Supplementary Appendix 2.5 and Supplementary Figure 4). However, at EOP when data from all patients at any time were considered, mean GFR was significantly higher in micafungin- vs standard care–treated patients (P = .049), as was mean CrCl (P = .012). For patients not requiring RRT at baseline, significantly higher mean GFR (P = .033) and mean CrCl (P = .013) were observed for micafungin (n = 133) vs standard care (n = 142) at EOP, although not at weekly time points. Liposomal amphotericin B was associated with the lowest GFR and CrCl in standard care patients (Figure 3A and 3B).
Figure 3.
Mean glomerular filtration rate (A) and mean creatinine clearance (B) in patients not requiring renal replacement therapy, according to specific study drug. Abbreviations: EOP, end of prophylaxis; EOS, end of study.
Treatment-Emergent Adverse Events
There were fewer AEs leading to study drug discontinuation in micafungin- than standard care–treated patients (6.4% vs 11.6%, respectively, for treatment-related AEs; Supplementary Appendix 2.6 and Supplementary Table 4). Table 3 shows AEs reported in >10% of patients in either treatment group. Although a higher overall rate of graft rejection was reported for micafungin (19.1%) than standard care (11%), the rates of biopsy-confirmed, treated acute rejection were similar between the 2 groups (9.8% and 8.1%, respectively). Most graft rejections were mild to moderate in severity, and the rate of severe rejections was the same in each group (2.3%). The most common treatment-related AEs are detailed in Supplementary Appendix 2.6.
Table 3.
Treatment-Emergent Adverse Events in >10% of Patients in the Micafungin Treatment or Standard Care Group (Safety Analysis Set)
| Treatment-Emergent Adverse Events | Micafungin (n = 173), No. (%) | Standard Care (n = 172), No. (%) |
|---|---|---|
| Abdominal pain | 21 (12.1) | 15 (8.7) |
| Diarrhea | 19 (11.0) | 19 (11.0) |
| Pleural effusion | 26 (15.0) | 38 (22.1) |
| Anemia | 20 (11.6) | 23 (13.4) |
| Liver transplant rejection (clinical) | 29 (16.8) | 14 (8.1) |
| Pyrexia | 16 (9.2) | 19 (11.0) |
| Hypertension | 26 (15.0) | 18 (10.5) |
| Cholestasis | 12 (6.9) | 19 (11.0) |
Deaths
In the micafungin group, 14 patients died during prophylaxis and 15 during long-term follow-up; in the standard care group, 11 died during prophylaxis and 12 during long-term follow-up. Two deaths (1 in each group) were considered to be possibly study drug related by the investigator. Cause of death was mostly septic shock with multiorgan failure and/or worsening of the underlying disease.
DISCUSSION
TENPIN is the first randomized controlled trial of an echinocandin as prophylaxis in liver transplant patients and the largest antifungal study in this patient population to date. Micafungin demonstrated a clinical success rate that was statistically noninferior to standard care, confirming that micafungin 100 mg is as effective as standard care for antifungal prophylaxis in liver transplant recipients at high risk of IFI. In addition, micafungin had similar efficacy to standard care across all secondary efficacy outcomes assessed. Having an IDRB confirm the investigator's decision based on a standard definition [26] strengthens the study results and was necessary, given that the definition of IFI can vary between centers and physicians.
IFI rates of up to 17.7% [1–6] and 3-month cumulative IFI rates of approximately 4% [10] have been reported in liver transplant recipients without risk factors. The overall low rate of IFI (≤2%) in our study provides evidence supporting antifungal prophylaxis in high-risk liver transplant recipients. Micafungin is indicated for prophylaxis against Candida infection in other high-risk patient groups [28, 29]; low rates of breakthrough IFI were observed for micafungin 50 mg (1.6%) and fluconazole (2.4%) in a trial comparing these agents in hematopoietic stem cell transplant recipients [30]. In that study [30], 1 patient on micafungin had a breakthrough Aspergillus infection (7 on fluconazole), whereas 2 patients on micafungin in the current study were infected with Aspergillus species and 2 on standard care (1 each with fluconazole and liposomal amphotericin B).
The overall safety profile of micafungin was comparable to that of standard care, with a low incidence of hepatic and renal AEs. The pattern of liver function tests was similar between micafungin and standard care; however, renal function with micafungin may be better than with established standard care regimens, such as liposomal amphotericin B. This is consistent with a prophylaxis study in high-risk liver transplant recipients where micafungin was similarly efficacious to amphotericin B lipid complex, with lower early renal dysfunction and no additional risk of hepatic dysfunction [21]. In our study, more graft rejection episodes were reported in micafungin patients than standard care. However, further investigation found similar rates of biopsy-confirmed treated acute rejection—an objective measure of rejection—and there was no evidence that micafungin differed from standard care in this regard.
Alternatives to fluconazole and liposomal amphotericin B, the current guideline-recommended options for antifungal prophylaxis in liver transplant recipients, are needed. Candida species such as C. glabrata and C. krusei are less susceptible, or even resistant in the case of fluconazole, to these agents [17–19, 31]. Drug–drug interactions with fluconazole complicate dosing with the immunosuppressants ciclosporin, tacrolimus, and sirolimus [32]. Fluconazole requires dose adjustment in patients receiving RRT, as the procedure results in a significant clearance of fluconazole, which varies depending on the technique used [33, 34]. Although nephrotoxicity risk is reduced with liposomal amphotericin B compared with amphotericin B deoxycholate [15, 17], it may still limit its use [20, 35], especially in recipients with renal dysfunction.
Echinocandins may be useful alternatives for simplifying antifungal prophylaxis in liver transplant, exhibiting excellent fungicidal activity against Candida species, with low minimum inhibitory concentrations against the majority of isolates [36, 37]. Resistance to echinocandins is a relatively rare event [37–39]. However, cases of resistance due to therapy-acquired FKS mutations have been reported [37, 38], and evidence of increasing resistance among strains of C. glabrata has emerged [40]. The probability of resistance may increase as echinocandin use escalates, and this needs to be considered when employing these agents as primary prophylaxis with prolonged exposure. As echinocandins do not interact with the P450 cytochrome or P-glycoprotein systems, they have a low potential for drug–drug interactions [36, 37]. However, caspofungin [41], but not micafungin [42–45], does interact with ciclosporin and tacrolimus, and micafungin interacts with sirolimus [28].
This study's main limitations include those inherently associated with open-label trials, such as potential bias, which can, for example, lead to greater withdrawal of consent in 1 treatment arm. This was observed in this study, as 9 patients on micafungin withdrew consent compared with none on standard care. In addition, standard care therapy was decided as per local guidelines and standard care dosing was not uniform (although it was within a preagreed range and consistent with each center), which may have impacted results. As the choice of standard care was center specific, and therefore could be based on local epidemiology or other clinical considerations relevant to the local patient population, this could be considered as a positive bias in favor of standard care in the TENPIN clinical trial setting. For the post hoc safety analysis on renal function, it was only possible to exclude patients who had RRT at baseline, because information on whether they received RRT during prophylaxis or thereafter was not collected. In addition, it was not possible to adjust for known nephrotoxic concomitant medications.
In summary, micafungin was demonstrated to be noninferior to standard care for antifungal prophylaxis in liver transplant patients at high risk of IFI, with a similar overall safety profile but associated with better renal function throughout prophylaxis than standard care. Micafungin therefore provides an additional prophylactic option, especially in patients with a high risk of infection by species resistant to current standard care, patients at risk of drug–drug interactions, or patients with renal impairment or receiving RRT.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. Medical writing assistance was provided by Lisa Thomas, PhD, Connect2CME Ltd, Westerham, United Kingdom.
Author contributions. The study sponsor, together with lead investigators, designed the study and the sponsor conducted all the statistical analyses. The members of the steering committee were involved in the study design, reviewed the protocol, and provided guidance on the study conduct. All the authors had access to the data and contributed to data interpretation. All authors participated in the preparation of the manuscript with support from a professional medical writer, funded by Astellas Pharma Europe Ltd. All authors reviewed and approved the final version, and made the decision to submit the manuscript for publication and vouch for the completeness and accuracy of the data and analyses.
Financial support. The TENPIN study was funded by Astellas Pharma Europe Ltd, Chertsey, United Kingdom.
Potential conflicts of interest. F. S. has received speaker fees and/or research funding from, and/or has participated at advisory boards for, Novartis, Astellas, Roche, Genzyme, Merck Sharp & Dohme, Gilead, Gambro, and Vital Therapies. A. P. has received research funding and speaker fees from Astellas, Novartis, Roche, Nycomed, TEVA, Genzyme, and has participated at advisory boards for Novartis. C. C. has received research grants, travel support, and/or lecture fees from Pfizer, Gilead, Merck, Astellas, Novartis, and Genzyme and has participated at advisory boards for Novartis and Genzyme. M. L. has received research grants from Merck Sharp & Dohme and Pfizer. B. G.-E. has received speaker fees from Pfizer and speaker fees and research grants from Astellas and Novartis. L. F. is a member of an advisory committee for Novartis and has received grant support from Novartis and Astellas and speaker fees from Gilead Sciences. S. P. is an independent contractor employed by Astellas Pharma. L. T., A. K., and M. B. are full-time employees of Astellas Pharma. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
APPENDIX
Independent Data Review Board. Dr Claudio Viscoli, University of Genoa, Genoa, Italy; Dr Jean-Pierre Gangneux, Hôpital Pontchaillou, Rennes, France; Professor Manuel Cuenca-Estrella, Instituto de Salud Carlos III, Madrid, Spain.
TENPIN Study Investigators. Johann Pratschke, Universitätsklinik Innsbruck, Austria; Johan Decruyenaere and Jan J. De Waele, Ghent University Hospital, Belgium; Pierre-François Laterre, St Luc University Hospital, Université catholique de Louvain, Belgium; Christophe Moreno, Hôpital Universitaire Erasme, Belgium; Peter Michielsen, Universitair Ziekenhuis, Belgium; Olivier Cointault, Hôpital de Rangueil, France; Lutz Fischer, University Medical Center Hamburg-Eppendorf, Germany; Peter Neuhaus and Andreas Pascher, Charité Universitätsmedizin, Germany; Peter Schemmer, Universitätsklinikum Heidelberg, Germany; Carlos Cervera, Hospital Clinic i Provincial, Spain; Evaristo Varo, Hospital Clínico Universitario de Santiago, Spain; Miguel Montejo, Hospital de Cruces, Spain; Emilio Bouza, Hospital Universitario Gregorio Maranon, Spain; Marino Blanes, Hospital Universitari I Politecnic La Fe de Valencia, Spain; Juan Carlos Pozo, Hospital Universitario Reina Sofia, Spain; Jesus Fortun, Hospital Ramon y Cajal, Spain; Faouzi Saliba, Hôpital Paul Brousse, France; Lionel Rostaing, Hopital de Rangueil, France; Catherine Paugam-Burtz, Hôpital Beaujon, France; Daniel Eyraud, Hopital Pitie-Salpetriere, France; Tahir Shah, Queen Elizabeth Hospital, United Kingdom; Nigel Heaton, Kings College Hospital, United Kingdom; Róbert M. Langer, Semmelweis University, Hungary; Aiden McCormick, St Vincent's University Hospital, Ireland; Umberto Cillo, Azienda Ospedaliera-Università di Padova, Italy; Mauro Salizzoni, Azienda Ospedaliero Univ S. Giovanni Battista di Torino, Italy; Manuela Lugano, Azienda Ospedaliero-Universitaria di Udine, Italy; Andrea De Gasperi, Azienda Ospedaliera Ospedale Niguarda Ca’ Granda, Italy; Luís Tomé, Hospitais Universidade Coimbra, Portugal; Jorge Daniel, Hospital Geral de Santo Antonio, Portugal; Irinel Popescu, Fundeni Clinical Institute, Romania; Yan G. Moysyuk, Sc. Research Institute of Transplantology and Artificial Organs, Russian Federation; Alexey V. Chzhao, Sc. Research Institute of Emergency Care, Russian Federation; Vladimir E. Zagaynov, Privolzhsky District Medical Centre, Russian Federation; Bo-Göran Ericzon, Karolinska University Hospital, Sweden.
References
- 1.Vera A, Contreras F, Guevara F. Incidence and risk factors for infections after liver transplant: single-center experience at the University Hospital Fundacion Santa Fe de Bogota, Colombia. Transpl Infect Dis. 2011;13:608–15. doi: 10.1111/j.1399-3062.2011.00640.x. [DOI] [PubMed] [Google Scholar]
- 2.Raghuram A, Restrepo A, Safadjou S, et al. Invasive fungal infections following liver transplantation: incidence, risk factors, survival, and impact of fluconazole-resistant Candida parapsilosis (2003–2007) Liver Transpl. 2012;18:1100–9. doi: 10.1002/lt.23467. [DOI] [PubMed] [Google Scholar]
- 3.Zicker M, Colombo AL, Ferraz-Neto BH, Camargo LF. Epidemiology of fungal infections in liver transplant recipients: a six-year study of a large Brazilian liver transplantation centre. Mem Inst Oswaldo Cruz. 2011;106:339–45. doi: 10.1590/s0074-02762011000300014. [DOI] [PubMed] [Google Scholar]
- 4.Yang CH, He XS, Chen J, et al. Fungal infection in patients after liver transplantation in years 2003 to 2012. Ann Transplant. 2012;17:59–63. doi: 10.12659/aot.883695. [DOI] [PubMed] [Google Scholar]
- 5.Shi XJ, Lu SC, He L, et al. Diagnosis and treatment of fungal infection after liver transplantation. Chin Med J (Engl) 2011;124:1015–7. [PubMed] [Google Scholar]
- 6.Pacholczyk M, Lagiewska B, Lisik W, Wasiak D, Chmura A. Invasive fungal infections following liver transplantation—risk factors, incidence and outcome. Ann Transplant. 2011;16:14–6. doi: 10.12659/aot.881989. [DOI] [PubMed] [Google Scholar]
- 7.Singh N. Fungal infections in the recipients of solid organ transplantation. Infect Dis Clin North Am. 2003;17:113–34. doi: 10.1016/s0891-5520(02)00067-3. viii. [DOI] [PubMed] [Google Scholar]
- 8.Grossi PA. Clinical aspects of invasive candidiasis in solid organ transplant recipients. Drugs. 2009;69(suppl 1):15–20. doi: 10.2165/11315510-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 9.Eschenauer GA, Lam SW, Carver PL. Antifungal prophylaxis in liver transplant recipients. Liver Transpl. 2009;15:842–58. doi: 10.1002/lt.21826. [DOI] [PubMed] [Google Scholar]
- 10.Pappas PG, Alexander BD, Andes DR, et al. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET) Clin Infect Dis. 2010;50:1101–11. doi: 10.1086/651262. [DOI] [PubMed] [Google Scholar]
- 11.Neofytos D, Fishman JA, Horn D, et al. Epidemiology and outcome of invasive fungal infections in solid organ transplant recipients. Transpl Infect Dis. 2010;12:220–9. doi: 10.1111/j.1399-3062.2010.00492.x. [DOI] [PubMed] [Google Scholar]
- 12.Saliba F, Delvart V, Ichai P, et al. Fungal infections after liver transplantation: outcomes and risk factors revisited in the MELD era. Clin Transplant. 2013;27:E454–61. doi: 10.1111/ctr.12129. [DOI] [PubMed] [Google Scholar]
- 13.Lichtenstern C, Hochreiter M, Zehnter VD, et al. Pretransplant model for end stage liver disease score predicts posttransplant incidence of fungal infections after liver transplantation. Mycoses. 2013;56:350–7. doi: 10.1111/myc.12041. [DOI] [PubMed] [Google Scholar]
- 14.Singh N, Paterson DL. Aspergillus infections in transplant recipients. Clin Microbiol Rev. 2005;18:44–69. doi: 10.1128/CMR.18.1.44-69.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh N, Husain S the AST Infectious Diseases Community of Practice. Invasive aspergillosis in solid organ transplant recipients. Am J Transplant. 2009;9(suppl 4):S180–91. doi: 10.1111/j.1600-6143.2009.02910.x. [DOI] [PubMed] [Google Scholar]
- 16.Pappas PG, Kauffman CA, Andes D, et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:503–35. doi: 10.1086/596757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pappas PG, Silveirab FP the AST Infectious Diseases Community of Practice. Candida in solid organ transplant recipients. Am J Transplant. 2009;9(suppl 4):S173–9. doi: 10.1111/j.1600-6143.2009.02909.x. [DOI] [PubMed] [Google Scholar]
- 18.Arendrup MC. Epidemiology of invasive candidiasis. Curr Opin Crit Care. 2010;16:445–52. doi: 10.1097/MCC.0b013e32833e84d2. [DOI] [PubMed] [Google Scholar]
- 19.Krcmery V, Barnes AJ. Non-albicans Candida spp. causing fungaemia: pathogenicity and antifungal resistance. J Hosp Infect. 2002;50:243–60. doi: 10.1053/jhin.2001.1151. [DOI] [PubMed] [Google Scholar]
- 20.Deray G. Amphotericin B nephrotoxicity. J Antimicrob Chemother. 2002;49(suppl 1):37–41. doi: 10.1093/jac/49.suppl_1.37. [DOI] [PubMed] [Google Scholar]
- 21.Sun HY, Cacciarelli TV, Singh N. Micafungin versus amphotericin B lipid complex for the prevention of invasive fungal infections in high-risk liver transplant recipients. Transplantation. 2013;96:573–8. doi: 10.1097/TP.0b013e31829d674f. [DOI] [PubMed] [Google Scholar]
- 22.Glockner A. Treatment and prophylaxis of invasive candidiasis with anidulafungin, caspofungin and micafungin: review of the literature. Eur J Med Res. 2011;16:167–79. doi: 10.1186/2047-783X-16-4-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh N, Wagener MM, Cacciarelli TV, Levitsky J. Antifungal management practices in liver transplant recipients. Am J Transplant. 2008;8:426–31. doi: 10.1111/j.1600-6143.2007.02089.x. [DOI] [PubMed] [Google Scholar]
- 24.Cornely OA, Bassetti M, Calandra T, et al. ESCMID guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect. 2012;18(suppl 7):19–37. doi: 10.1111/1469-0691.12039. [DOI] [PubMed] [Google Scholar]
- 25.Gavalda J, Meije Y, Fortun J, et al. Invasive fungal infections in SOT recipients. Clin Microbiol Infect. 2014;20(suppl 7):27–48. doi: 10.1111/1469-0691.12660. [DOI] [PubMed] [Google Scholar]
- 26.De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813–21. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010;152:726–32. doi: 10.7326/0003-4819-152-11-201006010-00232. [DOI] [PubMed] [Google Scholar]
- 28.Astellas Pharma Europe B.V. Mycamine: summary of product characteristics. Available at: https://www.medicines.org.uk/emc/medicine/20997/SPC/Mycamine+50mg+and+100mg+powder+for+solution+for+infusion/ . Accessed 26 February 2014.
- 29.Astellas Pharma US Inc. Mycamine prescribing information. Available at: https://www.mycamine.com/PrescribingInformation.aspx . Accessed 26 February 2014.
- 30.van Burik JA, Ratanatharathorn V, Stepan DE, et al. Micafungin versus fluconazole for prophylaxis against invasive fungal infections during neutropenia in patients undergoing hematopoietic stem cell transplantation. Clin Infect Dis. 2004;39:1407–16. doi: 10.1086/422312. [DOI] [PubMed] [Google Scholar]
- 31.Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev. 2007;20:133–63. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pfizer Ltd. Diflucan: summary of product characteristics. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Diflucan_30/WC500121908.pdf . Accessed 26 February 2014.
- 33.Pittrow L, Penk A. Dosage adjustment of fluconazole during continuous renal replacement therapy (CAVH, CVVH, CAVHD, CVVHD) Mycoses. 1999;42:17–9. doi: 10.1046/j.1439-0507.1999.00269.x. [DOI] [PubMed] [Google Scholar]
- 34.Muhl E, Martens T, Iven H, Rob P, Bruch HP. Influence of continuous veno-venous haemodiafiltration and continuous veno-venous haemofiltration on the pharmacokinetics of fluconazole. Eur J Clin Pharmacol. 2000;56:671–8. doi: 10.1007/s002280000216. [DOI] [PubMed] [Google Scholar]
- 35.Moen MD, Lyseng-Williamson KA, Scott LJ. Liposomal amphotericin B: a review of its use as empirical therapy in febrile neutropenia and in the treatment of invasive fungal infections. Drugs. 2009;69:361–92. doi: 10.2165/00003495-200969030-00010. [DOI] [PubMed] [Google Scholar]
- 36.Eschenauer GA, DePestel DD, Carver PL. Comparison of echinocandin antifungals. Ther Clin Risk Manag. 2007;3:71–97. doi: 10.2147/tcrm.2007.3.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen SC, Slavin MA, Sorrell TC. Echinocandin antifungal drugs in fungal infections: a comparison. Drugs. 2011;71:11–41. doi: 10.2165/11585270-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 38.Beyda ND, Lewis RE, Garey KW. Echinocandin resistance in Candida species: mechanisms of reduced susceptibility and therapeutic approaches. Ann Pharmacother. 2012;46:1086–96. doi: 10.1345/aph.1R020. [DOI] [PubMed] [Google Scholar]
- 39.Perlin DS. Echinocandin resistance, susceptibility testing and prophylaxis: implications for patient management. Drugs. 2014;74:1573–85. doi: 10.1007/s40265-014-0286-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alexander BD, Johnson MD, Pfeiffer CD, et al. Increasing echinocandin resistance in Candida glabrata: clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin Infect Dis. 2013;56:1724–32. doi: 10.1093/cid/cit136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Merck Sharp & Dohme Ltd. Cancidas: summary of product characteristics. Available at: http://www.medicines.org.uk/emc/medicine/12843#PHARMACOKINETIC_PROPS. Accessed 26 February 2014.
- 42.Hebert MF, Townsend RW, Austin S, et al. Concomitant cyclosporine and micafungin pharmacokinetics in healthy volunteers. J Clin Pharmacol. 2005;45:954–60. doi: 10.1177/0091270005278601. [DOI] [PubMed] [Google Scholar]
- 43.Inoue Y, Saito T, Ogawa K, et al. Drug interactions between micafungin at high doses and cyclosporine A in febrile neutropenia patients after allogeneic hematopoietic stem cell transplantation. Int J Clin Pharmacol Ther. 2012;50:831–7. doi: 10.5414/CP201738. [DOI] [PubMed] [Google Scholar]
- 44.Hebert MF, Blough DK, Townsend RW, et al. Concomitant tacrolimus and micafungin pharmacokinetics in healthy volunteers. J Clin Pharmacol. 2005;45:1018–24. doi: 10.1177/0091270005279274. [DOI] [PubMed] [Google Scholar]
- 45.Fukuoka N, Imataki O, Ohnishi H, et al. Micafungin does not influence the concentration of tacrolimus in patients after allogeneic hematopoietic stem cell transplantation. Transplant Proc. 2010;42:2725–30. doi: 10.1016/j.transproceed.2010.04.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.