(See the HIV/AIDS Major Article by Siedner et al on pages 1120–7.)
The CD4 T-cell count at the start of antiretroviral therapy (ART) is a critical indicator in measuring how well programs are responding to human immunodeficiency virus (HIV). CD4 cell count measures at initiation of ART are strongly associated with morbidity, mortality, life expectancy, and program costs [1–5].
The first programs to start providing ART in sub-Saharan Africa were initially confronted with very sick populations: the median CD4 cell count at start of ART in these early programs was <50 cells/µL [6]. As HIV testing and program reach expanded, the CD4 count at initiation of ART increased to around 150 cells/µL by 2006–2007 [6, 7]. Since then, guidelines have evolved toward recommending ART initiation at higher CD4 counts [8], and this has been associated with further increases in CD4 at start of ART [9]. The expectation is that as guidelines change and program coverage improves, most patients will present to care and start ART earlier, and this will result in reductions in mortality, morbidity, and costs. Put simply, the job will be become progressively simpler as initial efforts to expand access are rewarded by a patient population that is increasingly asymptomatic, requiring fewer clinic resources and fewer clinical visits.
The findings of a systematic review and meta-analysis of trends in CD4 at presentation and ART initiation in sub-Saharan Africa, by Siedner et al, published in this issue of Clinical Infectious Diseases may therefore come as a disappointment. This systematic review assembled a large meta-analytic dataset of studies reporting CD4 at presentation or at start of ART in sub-Saharan Africa and, surprisingly, found no evidence of change between 2002 and 2013 [10]. However, it would be wrong to take the findings as meaning that no progress has been made. Meta-analysis of published, aggregate data is not the ideal approach to analyzing trends in CD4 cell counts. In particular, such analyses will be prone to ecological bias, where misleading conclusions about individuals are derived from aggregate-level data [11]. For example, the authors included published data from 2 ART programs in Côte d'Ivoire and Malawi, which participate in the International Epidemiological Databases to Evaluate AIDS (IeDEA) [12]. They used the median CD4 count of 128 cells/µL reported in the publication [3] and assigned this value to the median of the study period (2005). These aggregated data were then included in the meta-regression analysis, which found no change in the CD4 count at start of ART. However, the data from these programs show that CD4 count in fact increased by about 10 cells per year from 2004 to 2007.
The way in which ecologic bias (also known as the ecological fallacy, aggregation bias, or cross-level bias) can influence analyses of CD4 cell count at initiation is illustrated in Figure 1. The black bubbles and the broken line represent the meta-regression of the data aggregated at the program level: no trend over time in CD4 counts is seen. The red lines show that the aggregated data hide the fact that in most ART programs the CD4 cell count increased, with the rate of increase differing between sites.
Figure 1.
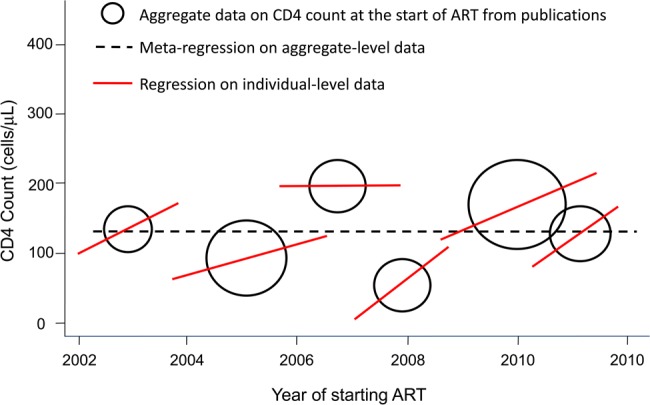
Ecological bias: hypothetical example of aggregate and individual level CD4 cell count data at the start of antiretroviral therapy (ART). In 5 of 6 programs, the CD4 cell count at the start of ART increased over calendar time (solid regression lines), yet the regression line fit to the aggregated data indicates that there was no change (broken regression line).
In support of this interpretation, among the largest cohort studies (>10 000 patients) included in the meta-analysis that provide information on CD4 change over time, all but 1 [13] report either calendar year increases in median CD4 cell count [14–17], reductions in the proportion of patients presenting with low CD4 cell counts [18], or both [19, 20]; these 7 studies account for around two-thirds (65%) of the data included in the meta-analysis. Furthermore, 2 recent analyses of large cohort collaborations reported improvements in immune status at the start of ART, for both adults [21] and children [22]. The analysis in adults was based on individual-level data from almost 400 000 patients and showed that in low- and middle-income countries the increase was from about 90 cells/µL in 2002 to about 150 cells/µL in 2009 [21]. The annual increase varied within country income groups and was greater among women than men. For example, among lower middle-income countries, the annual change in median CD4 cell counts ranged from –2 cells/µL among Nigerian men to 13 cells/µL among women in Côte d'Ivoire [21]. More recent data from the IeDEA collaborative cohort from 2013 show that among the 19 countries from sub-Saharan Africa, the median CD4 count at the start of ART was >200 cells/µL in 17 countries, >250 cells/µL, in 9 countries, and >300 cells/µL in 2 countries [23].
In light of these data, an appropriate conclusion to draw from the available data is that there have been program-level increases in CD4 at start of ART over time in most but not all programs, and that the magnitude of this increase has been variable. This finding is consistent with operational research demonstrating that some clinics and programs function better than others. Reasons for this include human resource constraints, geographic differences in distance to services, and differing engagement strategies [21].
Thus, although progress has been made, the findings of all the studies draw attention to the fact that the CD4 cell count at start of ART initiation in sub-Saharan Africa remains far too low. The current consensus definitions of late presenters in Europe—a CD4 cell count of <350 cells/µL or an AIDS diagnosis within 6 months of HIV diagnosis—is associated with a >13-fold increased risk of AIDS or death [24]. According to this definition, even the latest estimates for 2013 indicate that the majority of patients starting ART in sub-Saharan Africa are late presenters.
The meta-analysis [10] also highlights important declines in CD4 status around 100 cells/mm3 between engagement in care and initiation of treatment, despite the fact that average CD4 at engagement in care warrants immediate initiation of ART. A 100 cell/mm3 difference represents a period of approximately one year, a period of depletion linked to both decreased future life expectancy and increased risk of transmission. There are important issues that need to be evaluated between engagement in care and initiation of ART, such as management of coinfections to reduce the likelihood of immune response inflammatory syndrome and patient readiness.
In conclusion, to further reduce HIV-associated illness and death, a major focus of attention is needed on increasing CD4 at entry to care. As the authors of the systematic review and meta-analysis rightly conclude, renewed efforts are needed to improve the timeliness of ART initiation, including through earlier HIV testing and referral. Community-based testing approaches that include house-to-house testing [25], point-of-care CD4 testing, and peer support [26] are among the interventions that could help raise the baseline. With almost 12 million people now on ART in low- and middle-income settings, emphasis is shifting toward adapting service delivery models to support the management of HIV as a lifelong chronic disease. This requires decentralizing care such that healthier patients require less clinical investment. Although this is certainly needed and appropriate for those on ART, programs need to retain clinical capacity to respond to late presenters as a critical component of the AIDS response. Finally, monitoring of CD4 cell counts at presentation and start of ART in sub-Saharan Africa and elsewhere should be based on individual-level data, rather than aggregate data.
Note
Potential conflicts of interest. All authors: No potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Meyer-Rath G, Brennan AT, Fox MP, et al. Rates and cost of hospitalization before and after initiation of antiretroviral therapy in urban and rural settings in South Africa. J Acquir Immune Defic Syndr. 2013;62:322–8. doi: 10.1097/QAI.0b013e31827e8785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hogg RS, Yip B, Chan KJ, et al. Rates of disease progression by baseline CD4 cell count and viral load after initiating triple-drug therapy. JAMA. 2001;286:2568–77. doi: 10.1001/jama.286.20.2568. [DOI] [PubMed] [Google Scholar]
- 3.May M, Boulle A, Phiri S, et al. Prognosis of patients with HIV-1 infection starting antiretroviral therapy in sub-Saharan Africa: a collaborative analysis of scale-up programmes. Lancet. 2010;376:449–57. doi: 10.1016/S0140-6736(10)60666-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson LF, Mossong J, Dorrington RE, et al. Life expectancies of South African adults starting antiretroviral treatment: collaborative analysis of cohort studies. PLoS Med. 2013;10:e1001418. doi: 10.1371/journal.pmed.1001418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mills EJ, Bakanda C, Birungi J, et al. Life expectancy of persons receiving combination antiretroviral therapy in low-income countries: a cohort analysis from Uganda. Ann Intern Med. 2011;155:209–16. doi: 10.7326/0003-4819-155-4-201108160-00358. [DOI] [PubMed] [Google Scholar]
- 6.Boulle A, Van Cutsem G, Hilderbrand K, et al. Seven-year experience of a primary care antiretroviral treatment programme in Khayelitsha, South Africa. AIDS. 2010;24:563–72. doi: 10.1097/QAD.0b013e328333bfb7. [DOI] [PubMed] [Google Scholar]
- 7.Keiser O, Anastos K, Schechter M, et al. Art-Link Collaboration of International Databases to Evaluate AIDS; Antiretroviral therapy in resource-limited settings 1996 to 2006: patient characteristics, treatment regimens and monitoring in sub-Saharan Africa, Asia and Latin America. Trop Med Int Health. 2008;13:870–9. doi: 10.1111/j.1365-3156.2008.02078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vitoria M, Vella S, Ford N. Scaling up antiretroviral therapy in resource-limited settings: adapting guidance to meet the challenges. Curr Opin HIV AIDS. 2013;8:12–8. doi: 10.1097/COH.0b013e32835b8123. [DOI] [PubMed] [Google Scholar]
- 9.Mutimura E, Addison D, Anastos K, et al. Trends in and correlates of CD4+ cell count at antiretroviral therapy initiation after changes in national ART guidelines in Rwanda. AIDS. 2015;29:67–76. doi: 10.1097/QAD.0000000000000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siedner MJ, Ng CK, Bassett IV, Katz IT, Bangsberg DR, Tsai AC. Trends in CD4 count at presentation to care and treatment initiation in sub-Saharan Africa, 2002–2013. Clin Infect Dis. 2015;60:1120–7. doi: 10.1093/cid/ciu1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenland S, Morgenstern H. Ecological bias, confounding, and effect modification. Int J Epidemiol. 1989;18:269–74. doi: 10.1093/ije/18.1.269. [DOI] [PubMed] [Google Scholar]
- 12.Egger M, Ekouevi DK, Williams C, et al. Cohort Profile: the international epidemiological databases to evaluate AIDS (IeDEA) in sub-Saharan Africa. Int J Epidemiol. 2012;41:1256–64. doi: 10.1093/ije/dyr080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lessells RJ, Mutevedzi PC, Iwuji CC, Newell ML. Reduction in early mortality on antiretroviral therapy for adults in rural South Africa since change in CD4+ cell count eligibility criteria. J Acquir Immune Defic Syndr. 2014;65:e17–24. doi: 10.1097/QAI.0b013e31829ceb14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cornell M, Grimsrud A, Fairall L, et al. Temporal changes in programme outcomes among adult patients initiating antiretroviral therapy across South Africa, 2002–2007. AIDS. 2010;24:2263–70. doi: 10.1097/QAD.0b013e32833d45c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geng EH, Hunt PW, Diero LO, et al. Trends in the clinical characteristics of HIV-infected patients initiating antiretroviral therapy in Kenya, Uganda and Tanzania between 2002 and 2009. J Int AIDS Soc. 2011;14:46. doi: 10.1186/1758-2652-14-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mills EJ, Bakanda C, Birungi J, et al. Male gender predicts mortality in a large cohort of patients receiving antiretroviral therapy in Uganda. J Int AIDS Soc. 2011;14:52. doi: 10.1186/1758-2652-14-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGuire M, Ben Farhat J, Pedrono G, et al. Task-sharing of HIV care and ART initiation: evaluation of a mixed-care non-physician provider model for ART delivery in rural Malawi. PLoS One. 2013;8:e74090. doi: 10.1371/journal.pone.0074090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahonkhai AA, Noubary F, Munro A, et al. Not all are lost: interrupted laboratory monitoring, early death, and loss to follow-up (LTFU) in a large South African treatment program. PLoS One. 2012;7:e32993. doi: 10.1371/journal.pone.0032993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lahuerta M, Wu Y, Hoffman S, et al. Advanced HIV disease at entry into HIV care and initiation of antiretroviral therapy during 2006–2011: findings from four sub-Saharan African countries. Clin Infect Dis. 2014;58:432–41. doi: 10.1093/cid/cit724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Somi G, Keogh SC, Todd J, et al. Low mortality risk but high loss to follow-up among patients in the Tanzanian national HIV care and treatment programme. Trop Med Int Health. 2012;17:497–506. doi: 10.1111/j.1365-3156.2011.02952.x. [DOI] [PubMed] [Google Scholar]
- 21.Avila D, Althoff KN, Mugglin C, et al. IeDEA, ART Cohort Collaborations; Immunodeficiency at the start of combination antiretroviral therapy in low-, middle-, and high-income countries. J Acquir Immune Defic Syndr. 2014;65:e8–16. doi: 10.1097/QAI.0b013e3182a39979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koller M, Patel K, Chi B, et al. Immunodeficiency in children starting antiretroviral therapy in low-, middle-, and high-income countries. J Acquir Immune Defic Syndr. 2015;68:62–72. doi: 10.1097/QAI.0000000000000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooper D. Where are we headed with ART—beyond an undetectable viral load. 20th International AIDS Conference,; Melbourne, 20–25 July 2014; AIDS 2014 Daily Plenary Session. [Google Scholar]
- 24.Mocroft A, Lundgren JD, Sabin ML, et al. Risk factors and outcomes for late presentation for HIV-positive persons in Europe: results from the Collaboration of Observational HIV Epidemiological Research Europe Study (COHERE) PLoS Med. 2013;10:e1001510. doi: 10.1371/journal.pmed.1001510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suthar AB, Ford N, Bachanas PJ, et al. Towards universal voluntary HIV testing and counselling: a systematic review and meta-analysis of community-based approaches. PLoS Med. 2013;10:e1001496. doi: 10.1371/journal.pmed.1001496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Govindasamy D, Meghij J, Kebede Negussi E, Clare Baggaley R, Ford N, Kranzer K. Interventions to improve or facilitate linkage to or retention in pre-ART (HIV) care and initiation of ART in low- and middle-income settings—a systematic review. J Int AIDS Soc. 2014;17:19032. doi: 10.7448/IAS.17.1.19032. [DOI] [PMC free article] [PubMed] [Google Scholar]


