Abstract
Two concepts are gaining increasing acceptance in GPCR pharmacology: (1) pre-coupling of GPCRs with their preferred signaling molecules, and (2) GPCR oligomerization. This is begging the introduction of new models, such as GPCR oligomer-containing signaling complexes with GPCR homodimers as functional building blocks. The model favors the formation of GPCR heterotetramers, heteromers of homodimers coupled to their cognate G-protein. The GPCR heterotetramer offers the optimal frame for a canonical antagonistic interaction between activated Gs and Gi proteins, which can simultaneously bind to their respective preferred receptors and adenylyl cyclase catalytic units. This review addresses the current evidence for pre-coupling of the various specific components that provide the very elaborate signaling machinery exemplified by the Gs-Gi-adenylyl cyclase-coupled GPCR heterotetramer.
Keywords: GPCR, oligomerization, signalosome, adenylyl cyclase
Introduction: The classical view of GPCR signal transduction
G protein-coupled receptors (GPCRs), G proteins, and adenylyl cyclases (ACs) are the three elements of the most studied transmembrane cell-signaling pathway [1]. Figure 1 depicts the classical and still generally accepted sequential model of GPCR-mediated signal transduction involving AC activation: first, binding of one agonist molecule to one GPCR molecule leads to G protein recruitment in its GDP-bound heterotrimeric Gαβγ form; G protein coupling triggers guanylyl nucleotide exchange, GDP for GTP, which induces rapid dissociation of Gα and Gβγ into free active subunits; in its “active” GTP-bound state Gα binds and activates AC to produce cyclic adenosine monophosphate (cAMP); the Gα binding site for AC overlaps with the Gα binding site for Gβγ, supporting that Gβγ should dissociate from Gα to allow effector activation [2]; finally, Gα GTPase activity induces hydrolysis of GTP to GDP, which terminates AC signaling and promotes dissociation of Gα from AC and reconstitution of the free heterotrimeric Gαβγ protein. The rate of GTP hydrolysis is much slower than the rate of cAMP production, which provides a catalytic mechanism by which one agonist-receptor complex can generate multiple cAMP molecules per minute [3].
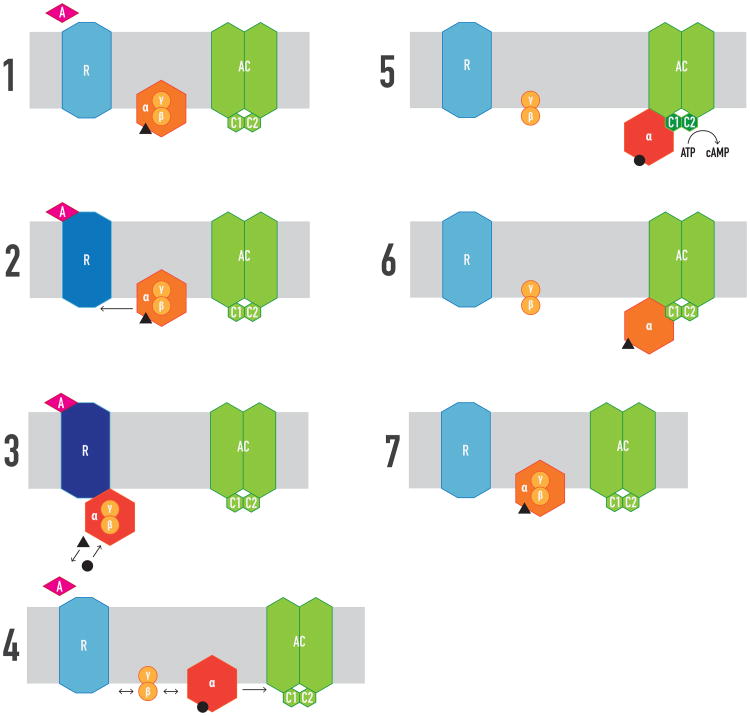
Figure 1. Classical view of ligand-GPCR-G protein-AC interactions.
1. There is no pre-coupling of GPCR (R), G proteins and AC, which bind by random collision. 2. G protein couples to R upon agonist (A) binding. 3. R-G coupling increases the affinity of A for R and activates G protein, inducing exchange of GTP (black circle) for GDP (black triangle). 4. GTP binding induces dissociation of Gα and Gβγ into free active subunits and a reduction of the affinity of A. 5. Gα-GTP binds and activates AC. 6. GTP is hydrolyzed to GDP, which terminates AC signaling. 7. Gα-GTP promotes Gαβγ reconstitution. C1 and C2: catalytic domains of AC. Changes in colors indicate changes in conformation, which imply changes in activity or affinity for their cognate ligands or proteins. Black triangle and circle indicate GD
This scheme is related to the stimulatory Gs proteins, which include Gsα or Golfα subunits (here referred as Gsα for simplicity). Gs proteins stimulate all isoforms of transmembrane AC. By contrast, members of the Gi/o family (which include different Gα subunits, here referred as Giα for simplicity) inhibit AC activity (more precisely, AC1, AC5 and AC6 isoforms) [4]. The same sequential model of Gs protein activation would apply to Gi activation, but in this case, GTP-bound Giα binds and inhibits AC [1]. The key to the regulation of AC activity is the conformational state of the interface between the two cytoplasmic domains C1 and C2, which constitute the catalytic domain core. Gsα binds to C2 and increases the affinity of C1 and C2, promoting catalysis, while Giα, by binding to C1, works in the opposite direction [4]. The pseudosymmetrical arrangement of interacting C1 and C2 domains allows, at least in theory, the simultaneous binding of Giα and Gsα [5], which provides a structural frame for the canonical antagonistic interaction between Gs- and Gi-coupled receptors at the AC level (Figure 2). However, the regulation of AC activity upon simultaneous binding of Giα and Gsα to C1 and C2 is an oversimplification and simultaneous intermolecular interactions of Gsα and Gβγ subunits with the cytoplasmic N-terminal domain of AC are also involved [6].
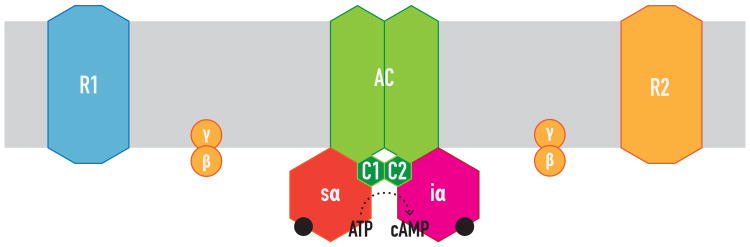
Figure 2. Classical view of canonical Gs-Gi canonical interaction at the level of AC.
Gsα activated by a specific GPCR (R1) binds to the C2 catalytic domain of AC and promotes catalysis. This is counteracted by the simultaneous binding of Giα activated by another GPCR (R2) to the C1 catalytic domain of AC, all by random collision.
Individual cells express a large number of different GPCRs, G protein subunits and G protein-linked effectors other than AC [7]. How these components are selected and organized in the cell to provide the most adaptive signaling response constitutes a challenge for classical pharmacology and a matter of intense debate. This review addresses the current evidence for pre-coupling of the various components that provide the elaborate signaling machinery exemplified by the Gs-Gi-AC-coupled GPCR heterotetramer.
Setting the stage for the GPCR heterotetramer: Pre-coupling versus random collision coupling
Explicitly, the classical model implies that the four components, GPCR, Gα, Gβγ and AC, are freely mobile molecules able to interact by random ‘collision coupling’. Tolkovsky and Levitzki initially put forward the collision-coupling mode of GPCR signaling in 1978 from results of β-adrenergic receptor-mediated AC activation obtained in turkey erythrocytes [8]. But concomitantly, Levitzki and coworkers also reported that in the same cell system activation of adenosine receptors (now identified as the A2A receptor subtype) did not fit with collision coupling but with a ‘pre-coupling’ mode of transmission, a pre-coupled GPCR-AC mechanism [9], “…where the receptor and the enzyme are permanently coupled to each other and adenosine binds to the receptor and induces the first-order process of cyclase activation” [10]. This mode of AC activation by the A2A receptor was afterwards named ‘restricted collision-coupling’ [11] and it was suggested to indicate a tight complex formation between the A2A receptor and its G protein [12].
Collision coupling would be in line with the ‘fluid mosaic’ model of the plasma membrane, which considers the lipid bilayer as an isotropic milieu where membrane-embedded proteins diffuse in two dimensions and collide at random with each other [13]. Collision coupling was supported by early studies on the signaling of the GPCR rhodopsin and its G protein transducin (Gt) and on purified β-adrenergic receptors, G proteins and AC in reconstituted phospholipid vesicles (reviewed in refs. 3 and 7). However, the outer segment disks of photoreceptors provide a unique lipid composition that allows rhodopsin and Gt a much higher lateral mobility than that of most mammalian membrane proteins. Similarly, receptors and G proteins are likely to be quite mobile in reconstituted lipid vesicles. However, there is now abundant experimental evidence indicating that the lateral motion of membrane components is very much constrained by various mechanisms that include interactions with the cytoskeleton and membrane microdomains, such as ‘lipid rafts’. These are small microdomains (25-100 nm) that have decreased fluidity compared to other portions of the plasma membrane based upon their high cholesterol and sphingolipid content [14]. In fact, many GPCRs and their cognate signaling proteins (most G protein subunits and AC isoforms) have been localized in the lipid rafts [15-17]. Enrichment, compartmentalization, of GPCR signaling components in lipid rafts could then be a universal mechanism for increasing the effective concentration of these proteins by restricting their movement and favoring their specific intermolecular interactions and, consequently, the transmission of the sequence of conformational changes initiated by the agonist and leading to the enzymatic response. Compartmentalization favors pre-coupling of the optimal ‘signalosome’, a combination of optimal receptor subtypes, G protein subunits and effector isoforms that can lead to the most adaptive cellular signal transduction.
Accumulating experimental evidence suggests that GPCR activation commonly occurs without dissociation of the receptor from its G protein, without G-protein subunit dissociation and also with pre-coupling of the heterotrimeric G protein to AC. Dissociation of rod Gt upon activation of rhodopsin has served for many years as a general model of G-protein activation [1]. Yet, it now seems to represent a specific case that in fact just applies to the rod but not to the cone Gt. Activation of rod photoreceptors by light induces a massive cellular Gt redistribution from the outer segments containing the rhodopsin-harboring discs, where it is concentrated during darkness, throughout the entire neuron, which represents a process of adaptation to different levels of illumination [18]. The experiments by Rosenzweig et al. [19] indicate that dissociation and diffusion of Gt subunits are all that is required for Gt redistribution in rods. By contrast, cone Gt does not dissociate during activation, and the heterotrimeric complex remains sequestered within the outer segment [19]. The differences between rod and cone Gt were explained by a stronger affinity of Gβ3γ8 for Gtα and for the plasma membrane in the cone Gt protein, as compared with Gβ1γ1 for Gtα in the rode Gt protein [19]. As pointed out by Neubig [7], the existence of a preferred combination of the many different available Gβ and βγ subunits for particularly effective receptor-effector coupling already calls for pre-assembled receptor-heterotrimeric G protein-effector complexes.
Experiments with resonance energy transfer (RET) techniques (Box 1) have provided clear evidence indicating that, as for cone Gt, G protein subunits remain associated and pre-coupled during activation of many other GPCRs [20-22]. The readout of experiments with RET donor and acceptor molecules fused to Gα and Gγ subunits indicates that G protein activation upon ligand binding to the receptors does not lead to dissociation, but implies a conformational change with rearrangement, reorientation, of its subunits [21,22]. Levitzki and collaborators already advanced this interpretation after finding successful signal transduction of a yeast pheromone receptor with fused G protein subunits [23]. Levitzki was also first to suggest that G proteins form stable complexes with AC being independent of the activation state of G proteins, mostly based upon co-purification of AC from turkey erythrocyte membranes (reviewed in ref. 3). RET experiments have also supported these interactions [6,24]. The structural model of G protein activation proposed by Michel Bouvier's research group [21] implies a significant modification of the quaternary structure of the heterotrimeric G protein, a reorientation without dissociation of the subunits still compatible with the existence of overlapping binding sites of Gα for AC and Gβγ [2]. Their findings suggest that during G protein activation Gβγ is maintained in the heterotrimeric complex while being displaced away from its original site of association with Gα [21].
Box 1. Resonance energy transfer techniques in the study of GPCR functional units.
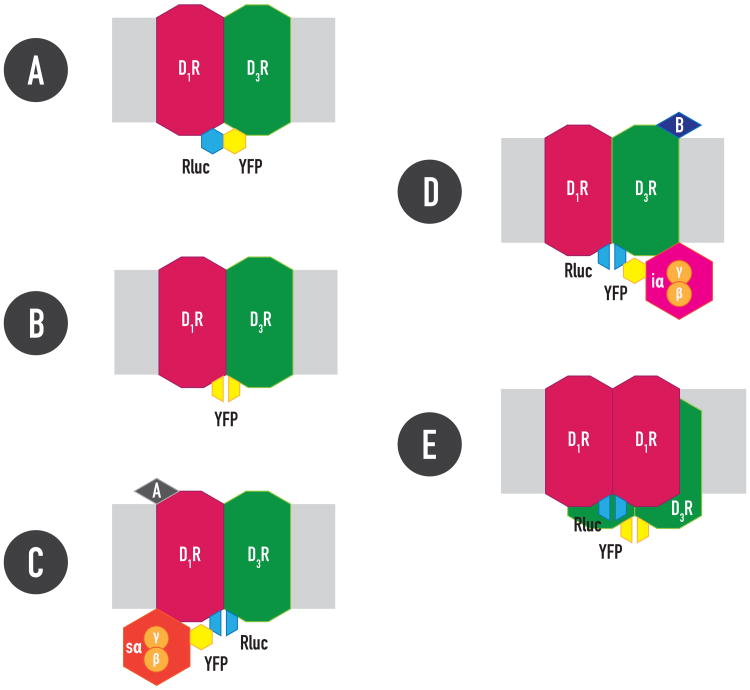
Over the last decade an explosion of optical techniques have developed that allow visualization of GPCRs and their signaling machinery as well as their modifications and protein interactions by resonance energy transfer techniques (RET) in intact cells. These include fluorescence resonance energy transfer (FRET) and bioluminescence resonance energy transfer (BRET) (see ref. 57 for recent review). RET consists of non-radiative energy transfer between a donor and an acceptor (sensors). Excitation of the donor results in emission of light by the acceptor only if the two molecules are in close proximity (< 10 nm). Donor and acceptor (two fluorophores in FRET and a luminescent enzyme and a fluorophore in BRET) are fused to the putative interacting proteins and relative RET values are used to demonstrate the existence of very close proximity between both proteins. Ligand-dependent changes in RET values can be used to determine dynamic intermolecular protein-protein interactions (or intramolecular interactions when both sensors are located in the same protein). BRET usually relies on the use of Renilla luciferase (Rluc) as RET donor, which in the presence of its substrate, coelenterazine, transfers energy to a green fluorescence protein (GFP) variant, such as yellow fluorescence protein (YFP) (Figure IA).
Bimolecular complementation of RET sensors, such as Rluc or YFP, is another strategy used to study protein interactions. Complementary hemi-moieties of the sensor are fused to the putative interacting proteins. A very close proximity allows reconstitution of Rluc or YFP and bioluminescence or fluorescence can be measured (Figures IB-ID). Combining RET and complementation of RET sensors can provide invaluable methods to study interactions of more than two proteins in a GPCR-containing signalosome. The recently introduced CODA-RET (complemented donor-accepted resonance energy transfer) assay [58], allows evaluating ligand-induced changes in G protein interactions by a GPCR oligomer. For instance, significant BRET values were obtained with complementation of Rluc hemi-moieties fused to dopamine D1 and D3 receptors and FYP fused to Giα or Gsα. Furthermore, those BRET values were selectively and dose-dependently modified by agonist binding to the D1 or the D3 receptor, respectively [54], indicating that agonist binding to the D1 or the D3 receptor in the D1-D3 receptor heteromer engages Gs or Gi proteins, respectively (Box 1, Figures IC and ID). BRET with complementation of Rluc hemi-moieties fused to D1 and YFP hemi-moieties fused to D3 receptors was then used to show a tetrameric structure of the D1-D3 receptor heteromer [54] (Figure IE).
Setting the stage for the GPCR heterotetramer: Dimers versus monomers
It has been long recognized that agonists show ‘binding heterogeneity’, a dispersion of affinities for GPCRs in membrane preparations from transfected mammalian cell lines or from native tissues. The typical illustration is a competition experiment between a moderate concentration of a radiolabelled antagonist and increasing concentrations of the agonist, which results in a non-steep or even biphasic curve, apparently indicating the existence of two populations of GPCRs, with high and low affinities for the agonist. In the presence of a G protein, guanylyl nucleotides promote an apparent inter-conversion from the high to the low affinity sites, with steepening and shifting to the right of the competition curve.
In line with the classical view of GPCR signal transduction, a still common view of GPCR signaling holds that agonists promote the formation of a transient complex between the monomeric receptor and the G protein, with conformational changes in both molecules that lead to an increased affinity of the receptor for the agonist and for the heterotrimeric Gαβγ protein, which leads to G protein activation (Figure 1). The deep-rooted ‘ternary complex’ model of ligand-GPCR binding [25] proposes that the receptor-G protein complex accounts for the high affinity site for the agonist, while the G protein-uncoupled receptor accounts for the low affinity site. Guanylyl nucleotides would promote uncoupling of the receptor-G protein complex, converting all receptors into low affinity sites. According to the model, a non-steep or biphasic antagonist-agonist competitive-inhibition curve implies the existence of two populations of receptors, coupled and non-coupled to the G protein. Implicitly, this assumes a limited pool of G proteins, an assumption difficult to reconcile with the fact that the expression of G proteins in native cell systems outnumbers that of GPCRs [7]. An alternative model that could explain complex radioligand curves is the ‘dimer-cooperativity’ model, which considers GPCR oligomerization, or at least GPCR dimers [26]. Allosteric communication through the two GPCR units (protomers) allows negative (or positive) cooperativity, meaning that the binding of a ligand to the first protomer decreases (or increases) the affinity of the ligand for the second protomer. This mechanism does not depend on a limited pool of G proteins, which can always be coupled, acting as an additional allosteric modulator that increases the affinity of the agonist and provides the conformation of the dimer that allows cooperativity of a ligand through the protomers [26].
In apparent support for the monomer-ternary complex model, biphasic and guanylyl nucleotide-sensitive antagonist/agonist competitive inhibition curves were obtained with GPCR monomers (of β2-adrenergic receptors, μ opioid receptors and rhodopsin) reconstituted in nanodisks, where they also activated G proteins [27-30]. In these studies, the proportion of receptor with high affinity for agonists increased from zero to 100% by increasing the G protein pool, indicating that the agonist binding heterogeneity was totally G protein-dependent. However, recent studies by Redka et al. [31,32] have shown that G proteins have a modulatory effect and impart sensitivity to guanylyl nucleotides, but they are not required for the agonist binding heterogeneity, implying cooperativity and dependence to GPCR oligomerization. These seminal studies were obtained by comparing the effect of G proteins and guanylyl nucleotides on antagonist/agonist competition experiments from monomeric muscarinic M2 receptors (in solution or reconstituted nanodisks) and oligomeric M2 receptors (preferentially in tetrameric form, in reconstituted vesicles). Significantly, these studies also showed that GPCR oligomers but not monomers resemble M2 receptors in myocardial tissue in their qualitative response to guanylyl nucleotides [32]. As reviewed recently, dissociation kinetic experiments performed in membrane preparations from tissues and in living cells, cast no doubt that cooperativity is an allosteric property of some ligands that bind to GPCR dimers or oligomers [26].
The analysis of the GPCR agonist binding heterogeneity therefore reveals that at least two GPCR protomers form part of a ‘minimal signalosome unit’ (Figure 3). Additional evidence has been provided by application of RET (Box 1) and other biophysical techniques in living cells, such as fluorescence correlation spectroscopy and analysis of single fluorescence-labeled receptor molecules by total internal reflection fluorescence microscopy [33,34]. Interestingly, although oligomerization of family C GPCR is well established, there is still resistance to accept homo- and heteromerization for family A and B GPCRs. However, recent crystallographic evidence has been reported for family A GPCR homodimers, including the chemokine CXCR4 receptor, the μ-opioid and κ-opioid receptor, the β1-adrenergic receptors and rhodopsin (reviewed in refs. 26 and 35). Irrespective of the GPCR family, homodimers seem to be a predominant species [33,34] with potential dynamic formation of higher-order oligomers, particularly tetramers [31,32,36]. Biochemical evidence implicates at least two distinct G protein regions in their interactions with the GPCR, localized in the Gα and Gγ subunits [37,38]. The distance between these two regions in the Gαβγ heterotrimer is larger than the width of one GPCR protomer, indicating that for both contacts to take place simultaneously, one heterotrimeric G protein must contact two receptor protomers [37]. Then, the pentameric structure constituted by one GPCR homodimer and one heterotrimeric G protein provides a main functional unit and oligomeric entities can be viewed as multimers of dimers [26]. Recently, a pentameric structure with two rhodopsin protomers and one heterotrimeric Gt has also been identified [39].
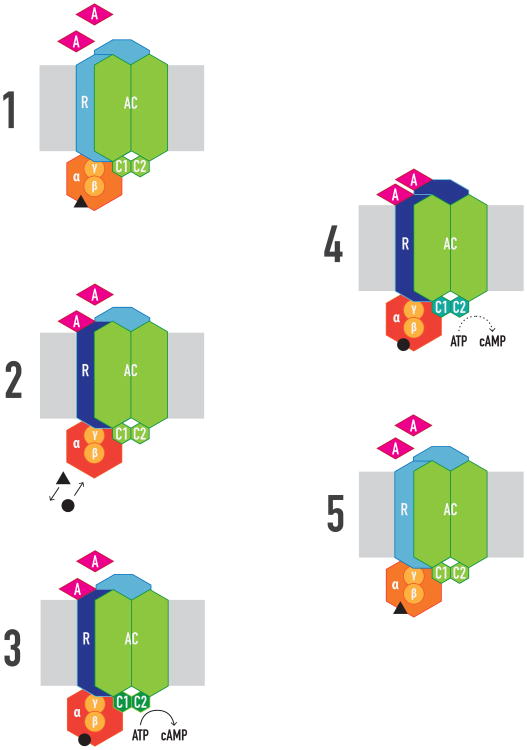
Figure 3. The minimal signalosome unit.
Constituted by a GPCR (R) homodimer precoupled to one heterotrimeric Gαβγ protein and one effector molecule like AC. 1. The agonist (A) has the same initial affinity for both R protomers. 2. Binding of A to the first protomer is sufficient to activate G protein, inducing exchange of GTP (black circle) for GDP (black triangle), but no R-G dissociation or of Gα and Gβγ takes place; A does not change its affinity for the first, but for the second protomer (negative or positive cooperativity). 3. Activated G protein activates AC. 4. Binding of A to the second protomer allosterically modulates (increases or decreases) the efficacy of A binding to the first protomer. 5. GTP is hydrolyzed to GDP, which terminates AC signaling.
In addition to ligand-binding properties, unique allosteric properties for each GPCR homodimer emerge in relation to intrinsic or signaling efficacy. Most experimental data agree with the model that proposes that ligand occupancy to the first protomer is enough to produce a significant G protein activation and functional response. When the ligand binds to the second protomer in the homodimer, it will often act as an allosteric modulator of the intrinsic efficacy of the ligand when bound to the first protomer, by potentiating or reducing the functional response, irrespective of the allosteric modulations at the binding level (reviewed in ref. 26) (Figure 3). Furthermore, in addition to considering the allosteric modulations of an orthosteric agonist binding to the first promoter on the same ligand binding to the orthosteric site in the second protomer, a significant number of possible pharmacological allosteric modulations conduced by the homodimer appear when considering other ligands, not only orthosteric agonists and antagonists [26], but also positive and negative allosteric modulators and even bitopic (allosteric and orthosteric) ligands [40].
GPCR heteromers and the Gs-Gi-coupled GPCR heterotetramer
Although still fought with skepticism, the list of putative functional and pharmacologically significant GPCR heteromers keeps increasing (stored in the GPCR Oligomerization Knowledge Base; http://www.gpcr-okb.org) [41]. It must however be acknowledged that many candidates do not follow the consensus criteria recently established for their identification in native tissues [42]. Heteromerization opens a new dimension of possible molecular and functional protein interactions within a signalosome. The extensively studied adenosine A2A-dopamine D2 receptor heteromer provides a frame for the realization of the scope of these interactions. When considering GPCR heteromers as conduits of allosteric interactions, two possible scenarios should be considered [43]. In the first scenario, a ligand binding to one of the receptors in the heteromer leads to changes in the properties (affinity or intrinsic efficacy) of a ligand binding to the second molecularly different receptor. A good example is the allosteric antagonistic interaction between A2A receptor agonists on D2 receptor agonists in the A2A-D2 receptor heteromer, by which A2A receptor agonists decrease the affinity and intrinsic efficacy of D2 agonists [44,45]. The A2A-D2 receptor heteromer is selectively localized in a main type of neuron in the striatum, the GABAergic striato-pallidal neuron, where it plays a seminal role controlling basal ganglia function and dysfunction [45-47]. It has been hypothesized that these allosteric interactions between A2A and D2 receptor agonists within the A2A-D2 receptor heteromer provide a mechanism responsible for the behavioral depressant effects of adenosine analogues and for the psychostimulant effects of selective A2A receptor antagonists and the non-selective adenosine receptor antagonist caffeine, with implications for several neuropsychiatric disorders [47]. In fact, the same mechanism provided the main rationale for the use of A2A receptor antagonists, such as KW 6002, in Parkinson's disease [48,49].
The second scenario of allosteric modulations within GPCR heteromers suggests a ligand-independent modulation in which one of the protomers acts as modulator of a ligand binding to the other molecularly different protomer [43]. Again, the A2A-D2 receptor heteromer provides a valuable example. Screening with various in vitro and in vivo techniques led to the finding of very different qualitative properties of several selective A2A receptor antagonists. The most striking finding was a selective decrease in the affinity of SCH 442416 when A2A receptor forms heteromers with D2 receptor, compared to when it does not heteromerize or when it forms heteromers with the adenosine A1 receptor [50]. In competitive experiments with the also orthosteric A2A receptor antagonist [3H]ZM 241385, binding of SCH 442416 demonstrated a strong negative cooperativity when A2A receptor was co-expressed with the D2 receptor [50]. The implications of these results are several: first, not only agonists, but also GPCR antagonists can manifest binding heterogeneity compatible with negative cooperativity, which in this case would indicate A2A receptor oligomerization; secondly, evidence for a D2 receptor-dependent allosteric modulation that determines negative cooperativity of a ligand binding to an A2A receptor oligomer strongly suggests that the A2AR-D2R receptor heteromer comprises at least two A2AR protomers; finally, these results constitute a proof of concept for the possibility of selectively targeting GPCR heteromer [26]. The weak efficacy of SCH 442416 at the A2A-D2 receptors heteromer does not make it a potentially useful antiparkinsonian drug, but makes it a preferential presynaptic A2AR antagonist that potently reduces cortico-striatal glutamatergic neurotransmission [50]. This property has been recently shown to be successful in a non-human primate model of marijuana abuse [51].
A2A and D2 receptors couple preferentially to Gs and Gi proteins, respectively. In some cell systems, including striatal cells, D2 receptor agonists potently inhibit A2A receptor agonist-mediated AC activation [52,53]. Such a canonical interaction is difficult to explain in the frame of the A2A-D2 receptor heteromer, since it would imply simultaneous reciprocal antagonistic interactions. It was then hypothesized that it depends on a population of A2A and D2 receptors not forming heteromers, which would not be subjected to the A2A receptor-mediated allosteric modulation that would prevent D2 receptor agonist-mediated Gi activation [47]. However, a recent study strongly suggests that, in fact, the canonical Gs-Gi interaction at the AC level is a property of A2A-D2 receptor heteromers [54]. In transfected HEK-293 cells, a concentration of a D2 receptor agonist that effectively counteracted forskolin-induced AC did not significantly modify AC activation induced by an A2A receptor agonist, but the canonical Gs-Gi interaction appeared upon molecular interactions with specific neuronal calcium-binding proteins that counteracted the allosteric modulation in the A2A-D2 receptor heteromer [54]. Significantly, the same mechanisms were identified in striatal cells in culture [54].
Altogether, the studies on A2A-D2 receptor heteromers can be better explained by a model that considers heteromers of homodimers coupled to their preferred G protein, the heterotetramer (Figure 4). This structure provides the possibility of simultaneous binding of Gs and Gi proteins to their respective preferred receptors and AC catalytic units, giving the optimal frame for a canonical Gs-Gi interaction. This could be a general scheme that should apply to other, if not all, GPCR heteromers containing both preferred Gs- and Gi-coupled receptors. The same scheme has been recently reported for dopamine D1-D3 receptor heteromers [55]. D1 and D3 receptors couple preferentially to Gs/olf and Gi proteins, respectively, and they are also localized in striatal cells (in the striato-nigral neuron). Combining RET techniques with complementation of one of the RET sensors allowed demonstrating that, in the D1-D3 receptor heteromer, agonist binding to the D1 or the D3 receptor engages Gs or Gi proteins, respectively (Box 1, Figure I) [55]. RET with double complementation of RET sensors was then used to uncover a tetrameric structure of the D1-D3 receptor heteromer [54] (Figure I), and the same approach has been recently used to identify A2A-D2 receptor heterotetramers [56].
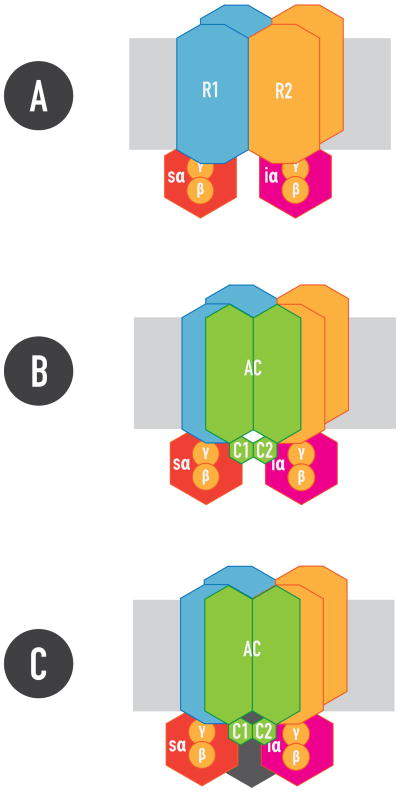
Figure 4. The GPCR heterotetramer.
1. Constituted by two GPCR homodimers, R1 and R2, pre-coupled to one Gs and one Gi protein, respectively. 2. Gs and Gi coupled the GPCR heterotetramer can simultaneously couple to the C2 and C1 catalytic domains of AC, respectively, providing the frame for the canonical Gs-Gi interaction at the AC level. 3. Additional proteins, such as neuronal calcium binding proteins (in black), bind and modulate the Gs-Gi interaction in the heterotetramer (see text).
Figure I. (Box 1) RET-complementation techniques.
RLuc: Renilla luciferase; YFP: Yellow Fluorescence protein; A and B: dopamine D1 and D3 receptor agonists, respectively.
Concluding remarks
A fast-growing body of experimental evidence calls for revisiting classical views of GPCR function and signaling. The collision-coupling mode of GPCR signaling, dependent on the obsolete fluid-mosaic plasma membrane model, and the ternary-complex model of ligand-GPCR binding, dependent on a monomeric GPCR model, should be substituted by new models that integrate a pre-coupling of GPCRs with their preferred signaling molecules and GPCR oligomerization [26]. GPCR oligomer-containing signalosomes with GPCR homodimers as functional building blocks provide such a model. The model favors the formation of GPCR heterotetramers, heteromers of homodimers coupled to their preferred G protein, which provide the frame for the canonical Gs-Gi interaction at the AC level. We put forward the hypothesis that GPCR heteromerization is a prerequisite for a canonical Gs-Gi interaction at AC. However, many other structures of GPCR oligomer-containing signalosomes should be possible, which would include receptor heteromers coupled to different combinations of the same or different G proteins and different effectors. Signalosomes constitute a very adaptive signaling device for the cell, providing the optimal interacting subtypes of receptors, of G protein subunits and effectors already in place. Acceptance of such a concept will shift our interest to the study of functional and pharmacological properties of GPCR oligomers and GPCR-containing signalosomes, which should provide better targets for drug development.
Highlights.
Pre-coupling and oligomerization are rising key concepts in GPCR pharmacology
Merging of both concepts can offer better models to understand GPCR function
Signaling complexes with GPCR homodimers as functional units provide such a model
The model favors the formation of heteromers of homodimers, the GPCR heterotetramer
Acknowledgments
The author is in debt with the countless scientific discussions with Prof. Carme Lluis, to whom this article is dedicated. Work supported by the intramural funds of the National Institute on Drug Abuse.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gilman AG. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- 2.Cabrera-Vera TM, et al. Insights into G protein structure, function and regulation. Endocr Rev. 2003;23:765–781. doi: 10.1210/er.2000-0026. [DOI] [PubMed] [Google Scholar]
- 3.Levitzki A, Klein S. G-protein subunit dissociation is not an integral part of G-protein action. Chembiochem. 2002;3:815–818. doi: 10.1002/1439-7633(20020902)3:9<815::AID-CBIC815>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 4.Sadana R, Dessauer CW. Physiological roles for G protein-regulated adenylyl cyclase isoforms: insights from knockout and overexpression studies. Neurosignals. 2009;17:5–22. doi: 10.1159/000166277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dessauer CW, et al. Identification of a Gialpha binding site on type V adenylyl cyclase. J Biol Chem. 1998;273:25831–25839. doi: 10.1074/jbc.273.40.25831. [DOI] [PubMed] [Google Scholar]
- 6.Sadana R, et al. N terminus of type 5 adenylyl cyclase scaffolds Gs heterotrimer. Mol Pharmacol. 2009;76:1256–1264. doi: 10.1124/mol.109.058370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neubig RR. Membrane organization in G-protein mechanisms. FASEB J. 1994;8:939–946. doi: 10.1096/fasebj.8.12.8088459. [DOI] [PubMed] [Google Scholar]
- 8.Tolkovsky AM, Levitzki A. Mode of coupling between the beta-adrenergic receptor and adenylate cyclase in turkey erythrocytes. Biochemistry. 1978;17:3795–3810. doi: 10.1021/bi00611a020. [DOI] [PubMed] [Google Scholar]
- 9.Tolkovsky AM, Levitzki A. Coupling of a single adenylate cyclase to two receptors: adenosine and catecholamine. Biochemistry. 1979;17:3811–3817. doi: 10.1021/bi00611a021. [DOI] [PubMed] [Google Scholar]
- 10.Braun S, Levitzki A. Adenosine receptor permanently coupled to turkey erythrocyte adenylate cyclase. Biochemistry. 1979;18:2134–2138. doi: 10.1021/bi00577a045. [DOI] [PubMed] [Google Scholar]
- 11.Gross W, Lohse MJ. Mechanism of activation of A2 adenosine receptors. II. A restricted collision-coupling model of receptor-effector interaction. Mol Pharmacol. 1991;39:524–530. [PubMed] [Google Scholar]
- 12.Nanoff C, et al. The A2 adenosine receptor: guanine nucleotide modulation of agonist binding is enhanced by proteolysis. Mol Pharmacol. 1991;39:130–135. [PMC free article] [PubMed] [Google Scholar]
- 13.Singer SJ, Nicolson GL. The fluid mosaic model of the structure of cell membranes. Science. 1972;175:720–131. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- 14.Pike LJ. The challenge of lipid rafts. J Lipid Res. 2009;50(Suppl 1):S323–328. doi: 10.1194/jlr.R800040-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cooper DM, Crossthwaite AJ. Higher-order organization and regulation of adenylyl cyclases. Trends Pharmacol Sci. 2006;27:426–431. doi: 10.1016/j.tips.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Allen JA, et al. Halverson-Tamboli RA, Rasenick MM. Lipid raft microdomains and neurotransmitter signalling. Nat Rev Neurosci. 2007;8:128–140. doi: 10.1038/nrn2059. [DOI] [PubMed] [Google Scholar]
- 17.Patel HH, et al. G-protein-coupled receptor-signaling components in membrane raft and caveolae microdomains. Handb Exp Pharmacol. 2008;186:167–184. doi: 10.1007/978-3-540-72843-6_7. [DOI] [PubMed] [Google Scholar]
- 18.Slepak VZ, Hurley JB. Mechanism of light-induced translocation of arrestin and transducin in photoreceptors: interaction-restricted diffusion. IUBMB Life. 2008;60:2–9. doi: 10.1002/iub.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenzweig DH, et al. Subunit dissociation and diffusion determine the subcellular localization of rod and cone transducins. J Neurosci. 2007;27:5484–5494. doi: 10.1523/JNEUROSCI.1421-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nobles M, et al. Heterotrimeric G proteins precouple with G protein-coupled receptors in living cells. Proc Natl Acad Sci USA. 2005;102:18706–18711. doi: 10.1073/pnas.0504778102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galés C, et al. Probing the activation-promoted structural rearrangements in preassembled receptor-G protein complexes. Nat Struct Mol Biol. 2006;13:778–786. doi: 10.1038/nsmb1134. [DOI] [PubMed] [Google Scholar]
- 22.Hein P, et al. Gs activation is time-limiting in initiating receptor-mediated signaling. J Biol Chem. 2006;281:33345–33351. doi: 10.1074/jbc.M606713200. [DOI] [PubMed] [Google Scholar]
- 23.Klein S, et al. Signal transduction by a nondissociable heterotrimeric yeast G protein. Proc Natl Acad Sci USA. 2000;97:3219–3223. doi: 10.1073/pnas.050015797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rebois RV, et al. Heterotrimeric G proteins form stable complexes with adenylyl cyclase and Kir3.1 channels in living cells. J Cell Sci. 2006;119:2807–2818. doi: 10.1242/jcs.03021. [DOI] [PubMed] [Google Scholar]
- 25.De Lean A, et al. A ternary complex model explains the agonist-specific binding properties of the adenylate cyclase-coupled beta-adrenergic receptor. J Biol Chem. 1980;255:7108–7117. [PubMed] [Google Scholar]
- 26.Ferré S, et al. G protein-coupled receptor oligomerization revisited: functional and pharmacological perspectives. Pharmacol Rev. 2014;66:413–434. doi: 10.1124/pr.113.008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whorton MR, et al. A monomeric G protein-coupled receptor isolated in a high-density lipoprotein particle efficiently activates its G protein. Proc Natl Acad Sci USA. 2007;104:7682–7687. doi: 10.1073/pnas.0611448104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bayburt TH, et al. Transducin activation by nanoscale lipid bilayers containing one and two rhodopsins. J Biol Chem. 2007;282:14875–14881. doi: 10.1074/jbc.M701433200. [DOI] [PubMed] [Google Scholar]
- 29.Ernst OP, et al. Monomeric G protein-coupled receptor rhodopsin in solution activates its G protein transducin at the diffusion limit. Proc Natl Acad Sci USA. 2007;104:10859–10864. doi: 10.1073/pnas.0701967104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuszak AJ, et al. Purification and functional reconstitution of monomeric mu-opioid receptors: allosteric modulation of agonist binding by Gi2. J Biol Chem. 2009;284:26732–26741. doi: 10.1074/jbc.M109.026922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Redka DS, et al. Efficacy as an intrinsic property of the M(2) muscarinic receptor in its tetrameric state. Biochemistry. 2013;52:7405–7427. doi: 10.1021/bi4003869. [DOI] [PubMed] [Google Scholar]
- 32.Redka DS, et al. Coupling of g proteins to reconstituted monomers and tetramers of the M2 muscarinic receptor. J Biol Chem. 2014;289:24347–24365. doi: 10.1074/jbc.M114.559294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calebiro D, et al. Single-molecule analysis of fluorescently labeled G-protein-coupled receptors reveals complexes with distinct dynamics and organization. Proc Natl Acad Sci USA. 2013;110:743–748. doi: 10.1073/pnas.1205798110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herrick-Davis K, et al. Fluorescence correlation spectroscopy analysis of serotonin, adrenergic, muscarinic, and dopamine receptor dimerization: the oligomer number puzzle. Mol Pharmacol. 2013;84:630–642. doi: 10.1124/mol.113.087072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jastrzebska B. GPCR: G protein complexes--the fundamental signaling assembly. Amino Acids. 2013;45:1303–1314. doi: 10.1007/s00726-013-1593-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patowary S, et al. The muscarinic M3 acetylcholine receptor exists as two differently sized complexes at the plasma membrane. Biochem J. 2013;452:303–312. doi: 10.1042/BJ20121902. [DOI] [PubMed] [Google Scholar]
- 37.Oldham WM, Hamm HE. Heterotrimeric G protein activation by G-protein-coupled receptors. Nat Rev Mol Cell Biol. 2008;9:60–71. doi: 10.1038/nrm2299. [DOI] [PubMed] [Google Scholar]
- 38.Rasmussen SG, et al. Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature. 2011;477:549–555. doi: 10.1038/nature10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jastrzebska B, et al. Asymmetry of the rhodopsin dimer in complex with transducin. FASEB J. 2013;27:1572–1584. doi: 10.1096/fj.12-225383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lane JR, et al. A new mechanism of allostery in a G protein-coupled receptor dimer. Nat Chem Biol. 2014;10:745–752. doi: 10.1038/nchembio.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khelashvili G, et al. CR-OKB: the G Protein Coupled Receptor Oligomer Knowledge Base. Bioinformatics. 2010;26:1804–1805. doi: 10.1093/bioinformatics/btq264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferré S, et al. Building a new conceptual framework for receptor heteromers. Nat Chem Biol. 2009;5:131–134. doi: 10.1038/nchembio0309-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kenakin T, Miller LJ. Seven transmembrane receptors as shapeshifting proteins: the impact of allosteric modulation and functional selectivity on new drug discovery. Pharmacol Rev. 2010;62:265–304. doi: 10.1124/pr.108.000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ferré S, et al. Stimulation of high-affinity adenosine A2 receptors decreases the affinity of dopamine D2 receptors in rat striatal membranes. Proc Natl Acad Sci USA. 1991;88:7238–7241. doi: 10.1073/pnas.88.16.7238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Azdad K, et al. Dopamine D2 and adenosine A2A receptors regulate NMDA-mediated excitation in accumbens neurons through A2A-D2 receptor heteromerization. Neuropsychopharmacology. 2009;34:972–986. doi: 10.1038/npp.2008.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trifilieff P, et al. Detection of antigen interactions ex vivo by proximity ligation assay: endogenous dopamine D2-adenosine A2 receptor complexes in the striatum. Biotechniques. 2011;51:111–118. doi: 10.2144/000113719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ferré S, et al. Adenosine A(2A) Receptors and A(2A) Receptor Heteromers as Key Players in Striatal Function. Front Neuroanat. 2011;5:36. doi: 10.3389/fnana.2011.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Armentero MT, et al. Past,present and future of A(2A) adenosine receptor antagonists in the therapy of Parkinson's disease. Pharmacol Ther. 2011;132:280–299. doi: 10.1016/j.pharmthera.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jorg M, et al. The dopamine D2 and adenosine A2A receptors: past, present and future trends for the treatment of Parkinson's disease. Curr Med Chem. 2014;21:3188–3210. doi: 10.2174/1389200215666140217110716. [DOI] [PubMed] [Google Scholar]
- 50.Orru M, et al. Striatal pre- and postsynaptic profile of adenosine A(2A) receptor antagonists. PLoS One. 2011;6:e16088. doi: 10.1371/journal.pone.0016088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Justinová Z, et al. Differential effects ofpresynaptic versus postsynaptic adenosine A2A receptor blockade on Δ9-tetrahydrocannabinol (THC) self-administration in squirrel monkeys. J Neurosci. 2014;34:6480–6484. doi: 10.1523/JNEUROSCI.5073-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kull B, et al. Reciprocal interactions between adenosine A2A and dopamine D2 receptors in Chinese hamster ovary cells co-transfected with the two receptors. Biochem Pharmacol. 1999;58:1035–1045. doi: 10.1016/s0006-2952(99)00184-7. [DOI] [PubMed] [Google Scholar]
- 53.Hillion J, et al. Coaggregation, cointernalization, and codesensitization of adenosine A2A receptors and dopamine D2 receptors. J Biol Chem. 2002;277:18091–18097. doi: 10.1074/jbc.M107731200. [DOI] [PubMed] [Google Scholar]
- 54.Navarro G, et al. Intracellular calcium levels determine differential modulation of allosteric interactions within G prtein-coupled receptor heteromers. Chem Biol. 2014;21:1546–1556. doi: 10.1016/j.chembiol.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guitart X, et al. Functional selectivity of allosteric interactions within G protein-coupled receptor oligomers: the dopamine D1-D3 receptor heterotetramer. Mol Pharmacol. 2014;86:417–429. doi: 10.1124/mol.114.093096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bonaventura J, et al. Allosteric mechanisms of caffeine: The adenosine A2A-dopamine D2 receptor heterotetramer (submitted) doi: 10.1016/j.neuropharm.2015.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lohse MJ, et al. Fluorescence/bioluminescence resonance energy transfer techniques to study G-protein-coupled receptor activation and signaling. Pharmacol Rev. 2012;64:299–336. doi: 10.1124/pr.110.004309. [DOI] [PubMed] [Google Scholar]
- 58.Urizar E, et al. CODA-RET reveals functional selectivity as a result of GPCR heteromerization. Nat Chem Biol. 2011;7:624–630. doi: 10.1038/nchembio.623. [DOI] [PMC free article] [PubMed] [Google Scholar]