Abstract
The Duffy Ag expressed on RBCs, capillaries, and postcapillary venular endothelial cells binds selective CXC and CC chemokines with high affinity. Cells transfected with the Duffy Ag internalize but do not degrade chemokine ligand. It has been proposed that Duffy Ag transports chemokines across the endothelium. We hypothesized that Duffy Ag participates in the movement of chemokines across the endothelium and, by doing so, modifies neutrophil transmigration. We found that the Duffy Ag transfected into human endothelial cells facilitates movement of the radiolabeled CXC chemokine, growth related oncogene-α/CXC chemokine ligand 1 (GRO-α/CXCL1), across an endothelial monolayer. In addition, neutrophil migration toward GRO-α/CXCL1 and IL-8 (IL-8/CXCL8) was enhanced across an endothelial monolayer expressing the Duffy Ag. Furthermore, GRO-α/CXCL1 stimulation of endothelial cells expressing the Duffy Ag did not affect gene expression by oligonucleotide microarray analysis. These in vitro observations are supported by the finding that IL-8/CXCL8-driven neutrophil recruitment into the lungs was markedly attenuated in transgenic mice lacking the Duffy Ag. We conclude that Duffy Ag has a role in enhancing leukocyte recruitment to sites of inflammation by facilitating movement of chemokines across the endothelium.
Duffy Ag is a minor blood group Ag and the erythrocyte receptor for the malarial parasite Plasmodium vivax (1). The Duffy Ag on erythrocytes binds selective CXC and CC chemokines, and is proposed to function as a chemokine sink in the circulation (2–5). Postcapillary venular endothelial cells, the site of leukocyte emigration in most organs, also express the Duffy Ag, even in individuals who do not express Duffy Ag on their erythrocytes (6, 7). The expression of Duffy Ag is up-regulated on endothelial cells during inflammatory states in the kidney (8, 9). Its high affinity binding to several CXC and CC chemokines, focal expression at the site of leukocyte emigration, up-regulation during tissue inflammation, and conserved endothelial expression even in individuals whose erythrocytes lack Duffy Ag all suggest a biological role for Duffy Ag in regulating inflammatory cell recruitment to sites of inflammation. Some have postulated that endothe lial Duffy Ag could have a proinflammatory role by facilitating the access of chemokines to circulating leukocytes and enhancing leukocyte migration (7, 9). Alternatively, endothelial Duffy Ag could reduce the intensity of inflammation by sequestering chemokines at sites of inflammation (10).
We have recently shown that there is enhanced Duffy Ag expression on parenchymal vascular beds and the alveolar septa of human lung tissue during suppurative pneumonia, a process defined by neutrophilic infiltration of the airspaces (11). The goals of this study were to determine whether Duffy Ag enhances movement of chemokines across the endothelium in vitro and whether Duffy Ag alters neutrophil migration toward chemokines in vitro and in vivo. Herein, we report that Duffy Ag, stably transfected into an immortalized human endothelial cell line and displaying high affinity binding to growth-related oncogene (GRO)3-α/CXC chemokine ligand (CXCL) 1 (GRO-α/CXCL1), facilitates the movement of radiolabeled 125I-labeled (125I) GRO-α/CXCL1 across an endothelial monolayer. We show that the presence of Duffy Ag enhances neutrophil transendothelial migration toward GRO-α/CXCL1 and IL-8/CXCL8 in vitro. Stimulation of Duffy transfectants with GRO-α/CXCL1 does not result in adhesion molecule gene up-regulation or other alterations in the endothelial cell gene profile. Finally, we show that the absence of Duffy Ag in vivo markedly attenuates IL-8/CXCL8-mediated neutrophil recruitment into the airspaces of mice lacking the Duffy Ag. These findings suggest that endothelial Duffy Ag has an active role in chemokine-mediated neutrophil recruitment by promoting the transendothelial movement of chemokines.
Materials and Methods
Endothelial cell culture
HUVECs were immortalized by transformation with human papilloma virus-16 E6-E7 genes (12). The immortalized HUVEC line (IVEC), clone 4-5-2G, has an indefinite lifespan in culture but is nontumorigenic. The cell line demonstrates cobblestone morphology, expresses Factor VIII-related Ag, takes up Dil-Ac-LDL, and expresses the integrin subunits αvβ3, αvβ5, β1, α2, α3, β4, and α6, consistent with its endothelial origin. IVEC were maintained in endothelial growth medium-2 containing 2% FCS (BioWhit-taker, Walkersville, MD).
Cloning of human Duffy cDNA
The human Duffy cDNA was cloned by the PCR using primers designed from GenBank AF055992, flanking the open reading frame and containing the native start site (13). A human pancreas cDNA library served as the PCR template. PCR was conducted for 30 cycles according to the manufacturer's protocol (PerkinElmer/Cetus, Norwalk, CT) using Taq polymer-ase. PCR products were blunt-end ligated into a mammalian cell expression plasmid vector, pcDNA3.1/hygromycin B+ (Invitrogen, Carlsbad, CA) containing a hygromycin resistance gene and sequenced. PCR clones were selected for the correct sequence and proper orientation in the expression vector.
Transfection and stable expression of Duffy Ag
IVEC were transfected with the pcDNA3.1/hygromycin B+ vector containing the Duffy PCR insert. The transfectants were selected by resistance to hygromycin B (Boehringer Mannheim, Indianapolis, IN). Individual clones were isolated using cloning cylinders and subcloned by the limiting dilution technique. Individual clones were then tested for Duffy Ag surface expression by flow cytometry (FACScan; BD Biosciences, San Jose, CA) using the mAb αFy6, a generous gift from Dr. P. Rubinstein of the New York Blood Center (New York, NY). Clones that were used for subsequent studies showed greater than one log10 increase in mean fluorescence intensity as compared with mock-transfected cells incubated with αFy6. This shift in mean fluorescence intensity displayed by Duffy-transfected cells labeled with αFy6 was specific, because cells incubated with an isotype mouse IgG1 control Ab did not show a significant shift.
Receptor binding assay
125I-GRO-α/CXCL1 (specific activity 2200 Ci/mmol) was obtained from Amersham Life Science (Piscataway, NJ) and New England Nuclear (Boston, MA). Unlabeled IL-8/CXCL8 and GRO-α/CXCL1 were purchased from PeproTech (Rocky Hill, NJ). Unlabeled RANTES/CC chemokine ligand (CCL) 5, monocyte chemotactic protein-1 (MCP-1/CCL2), and macrophage inflammatory protein-1α (MIP-1α/CCL3) were obtained from R&D Systems (Minneapolis, MN). Stable transfectants were seeded into 96-well break-apart plates (Dynatech Laboratories, Chantilly, VA) at 60,000 cells/well.
To determine the Kd of GRO-α/CXCL1 to the Duffy Ag, each well was incubated with 0.2 nM 125I-GRO-α/CXCL1 and increasing concentrations of unlabeled GRO-α/CXCL1 for 90 min. Nonspecific binding was defined as the amount of radioactivity measured in the well containing 1 μM unlabeled ligand. The total number of cells was counted in parallel wells. All samples were performed in triplicates or quadruplicates. Data were analyzed using the LIGAND program (Biosoft, Cambridge, U.K.). The Kd was obtained by Scatchard analysis.
To determine the chemokine binding properties of the expressed Duffy Ag, each well was incubated with 0.2 nM 125I-GRO-α/CXCL1 and varying concentrations of unlabeled ligand at 37°C for 90 min. Wells were washed twice with binding buffer (RPMI 1640, 0.2% BSA), and dried. The radioactivity in each well was measured by scintillation counting (ICN Biomedicals, Costa Mesa, CA). Nonspecific binding was defined as the amount of radioactivity measured in the well containing 1 μM unlabeled ligand.
Endothelial gene expression profile
Duffy transfected endothelial cells were incubated at 1 × 107 in the presence or absence of 20 nM GRO-α/CXCL1 for 4 h at 37°C. Duffy transfectants stimulated with TNF-α at 10 ng/ml served as the positive control. At the end of the incubation, cells were harvested and RNA was isolated using the Qiagen RNeasy Midi kit (Valencia, CA). Biotin-labeled cRNA was generated using the Enzo BioArray HighYield RNA Transcript Labeling kit (Enzo Biochem, Farmingdale, NY) and hybridized with the Affymetrix U95Av2 oligonucleotide array (Affymetrix, Santa Clara, CA) then washed and stained per the manufacturer's protocol. Data from each array was analyzed using the software package Microarray Suite 5.0 (Affymetrix, Santa Clara, CA). After normalization by average intensity, the expression level of each gene was compared in the presence or absence of GRO-α/CXCL1 or TNF-α. The data were then filtered to remove all genes with a <2-fold increase or decrease in expression at a significance level of p < 0.001 (Wilcoxon rank-sum test).
125I-GRO-α transit assay
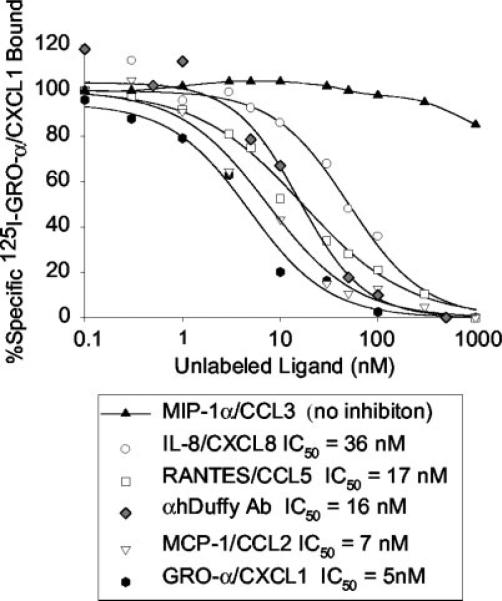
A peptide sequence from the N terminus of human Duffy Ag (STENSSQLDFEDVWNSS) was used to generate goat anti-human Duffy polyclonal Ab in collaboration with Invitrogen (Huntsville, AL). Serum from the immunized goat was affinity purified and the goat anti-Duffy Ig was characterized by its ability to displace 125I-GRO-α/CXCL1 from Duffy transfectants. We determined that the inhibitory concentration of the anti-Duffy Ab required to displace 50% of 125I-GRO-α/CXCL1 from Duffy binding sites (IC50) was 16 nM (see Fig. 3). Nonimmune goat IgG was used as the control Ab (Zymed Laboratories, San Francisco, CA).
FIGURE 3.
Competition binding studies using 125I-GRO-α/CXCL1 and various unlabeled ligands. Data are presented as the percentage of total bound 125I-GRO-α/CXCL1 as a function of increasing concentrations of unlabeled ligand. A curve fitting program was used to determine the IC50 total binding of 125I-GRO-α/CXCL1 to unlabeled GRO-α/CXCL1, IL-8/CXCL8, MCP-1/CCL2, αhDuffy Ab, RANTES/CCL5, MIP-1α/CCL3, and are shown in the symbol legend. Each condition was performed in triplicate or quadruplicate.
Costar 24-well plates containing polycarbonate transwell filters with a pore size of 3 μm were used for the transit experiments (Corning Costar, Cambridge, MA). The transwell filters were precoated with human placental type IV collagen at 10 μg/cm2 (Sigma-Aldrich, St. Louis, MO). Duffy expressing endothelial cells were seeded at 2 × 105/well onto the collagen-coated membrane, allowed to adhere, and grown to confluence at least 7 days before each experiment.
On the day of the experiment, cells were pretreated with the respective Ab (0.49 μM) for 30 min. Following pretreatment with Ab, medium was changed to contain 0.1% human serum albumin (HSA), 2.5 μM 10,000 m.w. Texas Red dextran, 20 nM GRO-α/CXCL1, 0.2 nM 125I-GRO-α/CXCL1, 0.49 μM of either anti-Duffy Ab or control Ig in the bottom well. Cells were incubated for 4 h at 37°C in 5% CO2. At the end of the incubation, the amounts of 125I-GRO-α/CXCL1 and Texas Red dextran were measured from a 50-μl aliquot obtained from the top well using a gamma counter (1470 Wizard; Wallac Oy, Turku, Finland) and cytofluorometer set at excitation wavelength 590 nm and emission wavelength 645 nm (Cyto-Fluor II; PerSeptive Biosystems, Foster City, CA), respectively. The percent of 125I-GRO-α/CXCL1 crossing the monolayer was calculated using the following equation: 125I-GRO-α/CXCL1 crossing = 100% × (cpm of sample wells – cpm of medium alone)/equilibrium cpm, where equilibrium cpm was defined as the radioactivity in a 50-μl aliquot after mixing the contents of the top and bottom wells.
In all wells of each experiment, Texas Red dextran, a fluorophore-conjugated dextran of 10,000 m.w. (Molecular Probe, Eugene, OR), was placed on the same side as the chemokine and served as a marker to determine the diffusion of a low m.w. compound similar in size to chemokines. The percent dextran crossing was used to control for any differences in the integrity of the monolayer from well to well and was calculated using the following equation: % dextran crossing = 100% × (fluorescence of sample wells – fluorescence of medium alone/equilibrium fluorescence), where equilibrium fluorescence was defined as radioactivity in a 50-μl aliquot after manually mixing the contents of a top and bottom well.
Neutrophil transendothelial migration assay
Polymorphonuclear neutrophils (PMN) were isolated from a healthy volunteer, and loaded with calcein-AM as previously reported (14). PMN at 1 × 106 in a 200-μl volume of RPMI 1640 without phenol red (BioWhit-taker, Walkersville, MD) containing 0.1% HSA (Centeon, Kankakee, IL) were placed in the top well of the transwell. IL-8/CXCL8 at 1 nM or GRO-α/CXCL1 at 20 nM were placed in the bottom chamber in a total volume of 1.0 ml of RPMI 1640 containing 0.1% HSA. The transwell chambers were incubated for 3 h at 37°C in 5% CO2. The transwell insert was removed from each well, and 1.0 ml of 2% Triton-X was added to the bottom chamber contents, mixed, and the fluorescence of each well was measured using emission and excitation wavelengths of 485 and 530 nm, respectively. To calculate the percentage of neutrophils migrating from the top to the bottom chamber, a maximal fluorescence for neutrophils was derived. At time 0, 200 μl of 1 × 106 PMN were added to 800 μl of medium. The mixture was added to 1.0 ml of 2% Triton-X, and calcein fluorescence (fl.) was measured. The percent of PMN migration was calculated using the following formula:
mDuffy knockout and wild-type mice
Mice with targeted deletion of the Duffy gene have been previously described (10). Briefly, a 1.1-kb neo gene eliminating a segment of the intron and 90 bp of exon II was inserted into the genomic Duffy locus by homologous recombination. Embryonic stem cells containing the targeted mutation were injected into C57BL/6 blastocysts. Duffy−/− mice and their wild-type littermates were bred and housed in specific pathogen-free conditions at the Seattle Veteran's Affairs vivarium until the day of the experiment. Equal numbers of male and female mice, age- and weight-matched, in the N2F2 generation were included in each group. Homozygous Duffy knockout and wild-type mice were generated by breeding Duffy heterozygote breeding pairs. Mice were weaned at 3 wk of age, at which time they were genotyped using tail snip DNA.
Intratracheal instillation of IL-8
Mice were weighed and anesthetized using a ketamine/xylazine solution (70 mg/kg ketamine, 10 mg/kg xylazine). The mice were placed on a 45° incline board, a fiber optic light source was placed just above the thoracic inlet (IntraLux 6000-1; Volpi Manufacturing, Auburn, NY), and the vocal cords were directly visualized using a small catheter introducer (BD Biosciences, Rutherford, NJ). The trachea was cannulated with a 22-gauge gavage feeding needle (Kent Scientific, Litchfield, CT) connected to a tuberculin syringe. The plunger was removed from the tuberculin syringe and 100 μl of saline was placed at the distal end before its use. The correct position of the gavage needle in the trachea was verified by movement of the saline column in the tuberculin syringe. Recombinant human IL-8/CXCL8 (1 × 10−6 M) (Peprotech, Piscataway, NJ) was diluted in PBS containing 0.1% HSA or PBS + 0.1% HSA alone (Centeon) and a volume of 0.15 ml/100 g was instilled directly into the trachea through the gavage needle.
At 6 h, mice were euthanized with 120 mg/kg pentobarbital. Whole blood was obtained by direct cardiac puncture and the plasma was collected, aliquoted, and stored in −70°C until use. The thoracic cavity was opened by midline incision. The trachea was exposed and cannulated with a 24-gauge catheter, which was secured with a 2-0 silk suture. Whole lung lavage was performed using 0.9% NaCl containing 0.6 mM EDTA instilled in one aliquot of 1.2 ml, followed by three aliquots of 1.0 ml.
BAL processing
Total cell counts in the BAL fluid were performed using a Neubauer chamber. Cell differentials were performed by counting 100 consecutive cells on cytospin preparations stained with DiffQuik.
Results
Cloning of human Duffy cDNA
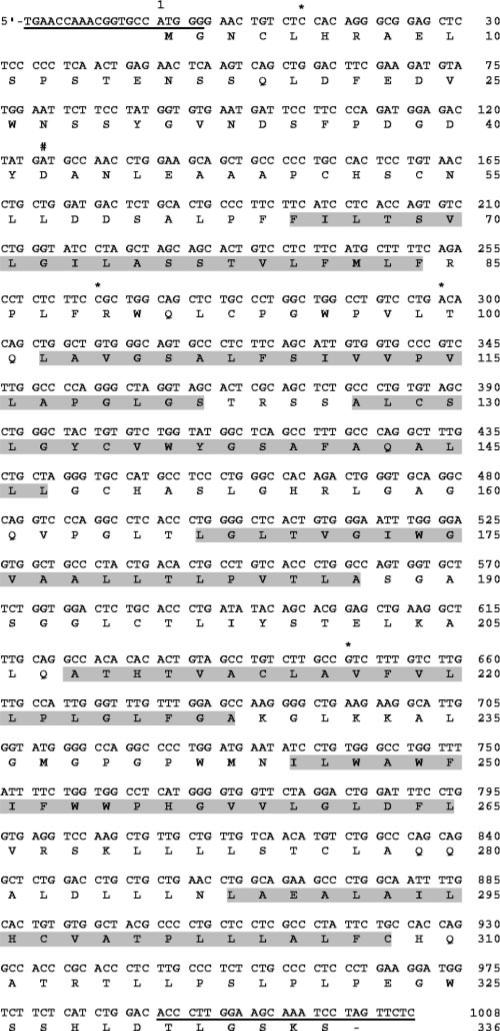
Sequence analysis confirmed that the Duffy cDNA isolated was the spliced isoform derived from two exons (GenBank AY167991). This isoform is the predominant transcript in vivo (15, 16) (Fig. 1). The Duffy cDNA encoded the Fyb epitope (A125). It also contained a silent mutation (G15C), and a polymorphism (G298A: Ala100Thr) that has no observable effect on surface protein expression in vivo or 125I-IL-8/CXCL8-specific binding in vitro (4, 17). The cloned Duffy cDNA also contained either a new polymorphism (A649G:Ile217Val) or a PCR-induced mutation. This Ile217Val substitution occurs within the transmembrane region of the protein and is a conservative mutation.
FIGURE 1.
Human Duffy Ag sequence cloned by PCR (GenBank AY167991). Base sequence numbers reference the start codon. GenBank AF055992 sequence was used to design primers (primer targets are underlined). Predicted transmembrane amino acid sequences are highlighted. Three sequence variances from GenBank AF055992, which encodes the Fyx mutation, are indicated with an asterisk. The Duffy Ag cDNA possesses the following: 1) G15C, a silent mutation; 2) A125 which codes the Fyb epitope; 3) C265 that renders full surface expression of Fyb, in contrast to C265T: Arg89Cys which codes the Fyx mutation; 4) G298A:Ala100Thr, a previously reported polymorphism that does not alter surface expression of Fyb; 5) A649G:Ile217Val, a chemically conserved new polymorphism or PCR mutation.
Duffy expressing endothelial cells bind selective CXC and CC chemokines
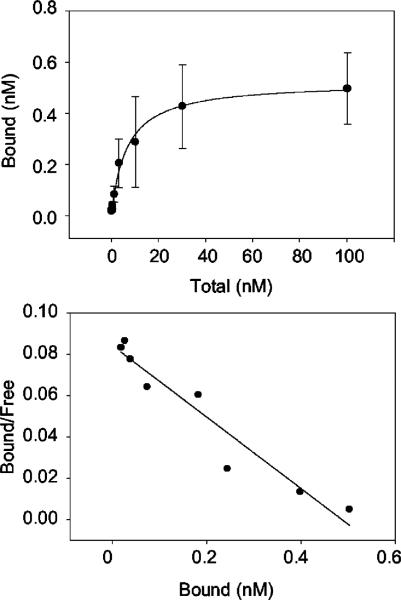
Binding analysis was undertaken to calculate the Kd of GRO-α/CXCL1 using 0.2 nM 125I-GRO-α/CXCL1 and increasing concentrations of unlabeled GRO-α/CXCL1 (Fig. 2). Nonspecific binding was defined as the amount of bound 125I-GRO-α/CXCL1 in the presence of 1 μM unlabeled GRO-α/CXCL1. The binding of 125IGRO-α/CXCL1 was saturable as the amount of total GRO-α/CXCL1 increased. Scatchard analysis indicated a Kd value of 5.6 nM, similar to what has been observed on erythrocytes and endothelial cells (3, 6, 7).
FIGURE 2.
Saturation curve and Scatchard plot of GRO-α/CXCL1 binding. Duffy transfected endothelial cells show saturable binding of 125IGRO-α/CXCL1 (top panel) and a calculated Kd for GRO-α/CXCL1 of 5.6 nM (bottom panel). Duffy transfectants were incubated with 0.2 nM 125IGRO-α/CXCL1 and increasing concentrations of unlabeled GRO-α/CXCL1. Each condition was performed in triplicate or quadruplicate; n = 2 independent experiments.
The ability of various chemokines to compete with 125I-GRO-α/CXCL1 for binding to Duffy expressing cells was tested using 0.2 nM 125I-GRO-α/CXCL1 incubated in the presence of increasing concentrations of unlabeled chemokines (Fig. 3). In addition, we tested the ability of a goat anti-human Duffy polyclonal Ab (αhDuffy Ab) to block binding of 125I-GRO-α/CXCL1 on the Duffy transfectants. The inhibitory concentration of unlabeled ligand required to displace 50% of radiolabeled GRO-α/CXCL1 (IC50) was measured for GRO-α/CXCL1, MCP-1/CCL2, RAN-TES/CCL5, αhDuffy Ab, IL-8/CXCL8, and MIP1-α/CCL3 using Equilibrium Binding Data Analysis software (Biosoft, Cambridge, U.K.). The relative IC50 values observed were: GROα < MCP-1 < αhDuffy Ab < RANTES < IL-8 <<< MIP-1α. Thus, the binding profile of the endothelial Duffy transfectants (Duffy+ cells) was similar to the findings observed in mouse erythrocytes, and K562 cells stably transfected with the Duffy cDNA (18, 19).
Interestingly, the endothelial Duffy transfectants showed a higher than anticipated IC50 of IL-8. IL-8/CXCL8 was a less effective antagonist of 125I-GRO-α/CXCL1 in the endothelial Duffy transfectants. This may be due to differences in the membrane environment on transfected cells vs erythrocytes, as previously suggested for the slightly higher Kd values observed in K562 Duffy transfectants than native erythrocytes (18). Alternatively, the lower affinity of IL-8/CXCL8 for the Duffy transfectants may be the result of a previously unreported polymorphism (A649G:Ile217Val).
Endothelial gene expression following binding of ligand to the Duffy Ag
The Duffy Ag is a member of the family of 7-transmembrane domain receptors, but it lacks the DRY amino acid motif that allows coupling to G proteins (1). The Duffy Ag lacks the ability to transduce a signal given the lack of the DRY motif. Direct evidence for this is based upon the inability to show increases in intracellular calcium ion concentrations following Duffy Ag-ligand binding in transfected 293 cells (4) and the inability of Duffy Ag to stimulate GTPase activity (20). To determine whether the binding of GRO-α/CXCL1 to the Duffy Ag produces a signal that alters gene transcription in endothelial cells, the gene expression pattern of Duffy transfectants was examined using oligonucleotide microarrays (Table I). A 2-fold or higher change in gene expression was chosen as significant.
Table I.
Oligonucleotide microarray analysisa
| Endothelial gene expression profile of Duffy transfectants |
|---|
| Following GRO-α/CXCL1 stimulation; no significant changes |
| Following TNF-α stimulation |
| Up-regulated genes: first 10 of 42 up-regulated genes |
| 1. VCAM-1 |
| 2. Human IFN-γ treatment inducible mRNA |
| 3. Human JE gene encoding a monocyte secretory protein (MCP-1) |
| 4. Diubiquitin |
| 5. Human endothelial leukocyte adhesion molecule-1 (E-selectin) |
| 6. ICAM-1 |
| 7. Human TNF-inducible early response gene |
| 8. Homo sapiens mRNA for KIAA106 protein |
| 9. Human adhesion molecule ninjurin mRNA |
| 10. Human B94 protein mRNA |
| Down-regulated genes: first 10 of 142 down-regulated genes |
| 1. Human signal transducing adaptor molecule 2A (STAM2) mRNA |
| 2. Human SNF-1-like protein kinase mRNAb |
| 3. Human homolog of yeast mutL (hPMS1) gene |
| 4. H. sapiens mRNA for vascular Rab-GAP/TBC-containing proteinb |
| 5. H. sapiens RP58 gene |
| 6. AI961743:wt66f12.x1 H. sapiens cDNA |
| 7. Human mRNA for brain-derived neurotrophic factor |
| 8. Human TRF-1-interacting ankyrin-related ADP-ribose polymerase mRNAb |
| 9. Human myosin H chain 12 (MYO5A) mRNA |
| 10. Human msg1-related gene mRNA |
The gene expression profile was examined in endothelial Duffy transfectants stimulated with 20 nM GRO-α/CXCL1 or 10 ng/mL TNF-α for 4 h using oligonucleotide microarrays. No genes were found to be significantly up-regulated or down-regulated in the Duffy+ cells following GRO-α stimulation. In Duffy+ cells stimulated with TNF-α, 42 genes were up-regulated and 142 genes were down-regulated compared with unstimulated cells (the first 10 in each group are shown for simplicity). The results were similar in two independent experiments.
SNF, sucrose nonfermenting protein; GAP/TBC, GTPase-activating protein/Tre/Bub2/Cdc16 domain; TRF, telomeric repeat binding factor; msg, missing.
Of the 12,000 genes examined, there were no significant changes in the gene expression profile following stimulation of Duffy+ cells with GRO-α/CXCL1 as compared with unstimulated Duffy+ cells (Table I). In addition, neither CXCR1 nor CXCR2 mRNA expression were noted in Duffy+ cells with or without stimulation. To show that the Duffy transfected cells could be stimulated to produce changes in their gene expression profile compared with baseline, transfected cells were also challenged with TNF-α. TNF-α served as the positive control stimulus after preliminary studies showed that incubation with TNF-α at 10 ng/ml increased the expression of ICAM-1 on the surface of Duffy trans fectants (Duffy+ cells) by flow cytometry (data not shown). The gene expression profile in TNF-α-stimulated Duffy+ cells showed that ICAM-1 mRNA expression was 7.5-fold higher than in un-stimulated Duffy+ cells. Moreover, TNF-α stimulation resulted in up-regulation of 42 genes including VCAM-1, endothelial-selectin (E-selectin), ICAM-1, MCP-1/CCL2, IL-8/CXCL8, GRO-β/CXCL2, GRO-γ/CXCL3, and down-regulation of ~142 genes, similar to published findings (Table I) (21). Thus, GRO-α/CXCL1 stimulation of Duffy transfected endothelial cells does not alter gene transcription. Moreover, genes up-regulated following TNF-α stimulation of Duffy transfectants help to characterize and validate the transfected cells as possessing an endothelial phenotype.
125I-GRO-α movement across an endothelial monolayer expressing Duffy Ag
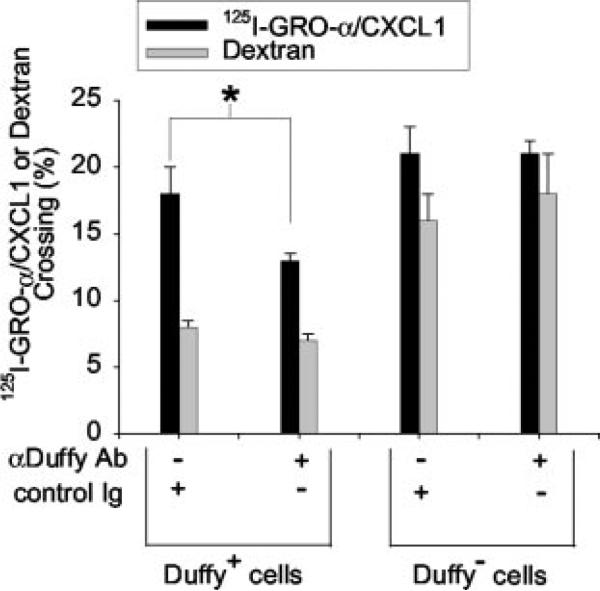
A transwell system was used to test the hypothesis that Duffy Ag participates in chemokine movement across the endothelium (Fig. 4). GRO-α/CXCL1 and tracer amounts of 125I-GRO-α/CXCL1 were placed in the bottom well below either a Duffy+ and Duffy− endothelial monolayer, in the presence or absence of blocking Ab against the Duffy Ag. The concentration of Ab used was 30-fold greater than its IC50 required to displace 125I-GRO-α/CXCL1 bound on Duffy transfected cells. Chemokine movement from the bottom to top well was determined by measuring the amount of 125I-GRO-α/CXCL1 recovered from the top well following a 4-h incubation at 37°C.
FIGURE 4.
Recovery of 125I-GRO-α across Duffy+ and Duffy− endothelial cell monolayer in the presence of either anti-Duffy or control Ab. Duffy+ and Duffy− endothelial cell monolayers were preincubated in the presence of anti-Duffy Ab or control IgG. Twenty nanomolar concentrations of unlabeled GRO-α/CXCL1, 0.2 nM 125I-GRO-α/CXCL1, and 2.5 μM dextran (10,000 m.w. Texas Red dextran) were placed in the bottom wells with 0.5 μM Ab. The amount of 125I-GRO-α/CXCL1 and dextran recovered from the top well were measured following a 4-h incubation at 37°C. Recovery of 125I-GRO-α/CXCL1 is significantly reduced across a Duffy expressing endothelial monolayer in the presence of anti-Duffy Ab (*, p = 0.002 by Mann-Whitney U test, p = 0.007 by ANOVA). Data are mean ± SEM of three independent experiments performed in triplicate or quadruplicate. Wells with [H11022]10% dextran crossing were excluded from statistical analysis.
The data showed that inhibition of Duffy Ag with blocking Ab results in a significant reduction in the translocation of 125I-GRO-α/CXCL1 across a Duffy+ endothelial monolayer (Fig. 4). To demonstrate that the Duffy-specific Ab did not alter the integrity of Duffy+ endothelial monolayers, we also measured the movement of 10,000 m.w. dextran (an inert molecule the size of GRO-α/CXCL1) across each well tested and showed that the translocation of dextran was not significantly different across Duffy+ endothelial monolayers in the presence of either anti-Duffy or control Ab. As expected, anti-Duffy Ab had no effect on either GRO-α/CXCL1 and dextran translocation across Duffy− endothelial monolayers. Notably, translocation of GRO-α/CXCL1 across Duffy+ monolayers cannot be directly compared with Duffy− monolayers given the relative “leakiness” of Duffy− monolayers, as evidenced by the greater translocation of dextran across Duffy− monolayers. Nevertheless, the blocking Ab studies support the conclusion that Duffy Ag facilitates the movement of GRO-α/CXCL1 across an endothelial monolayer.
In vitro neutrophil migration assay
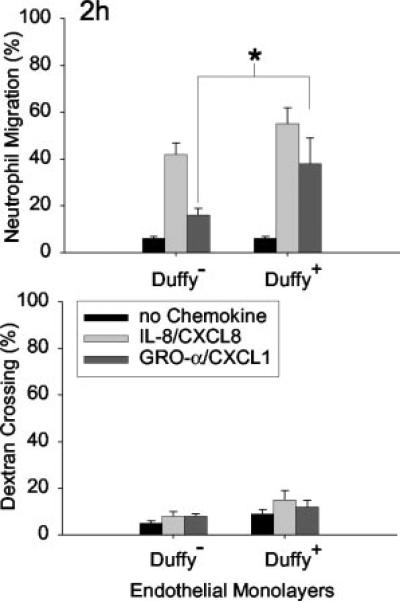
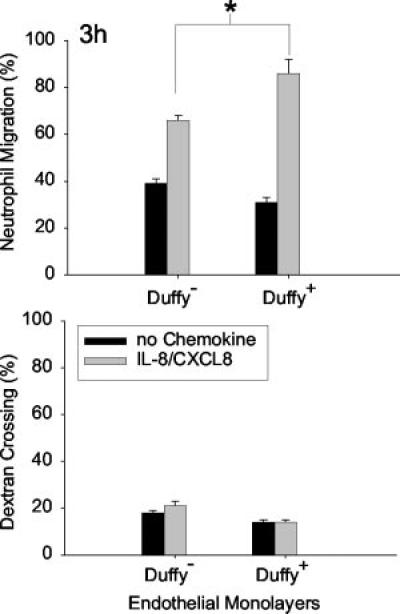
To test whether the presence of Duffy on endothelial cells modifies neutrophil migration toward either GRO-α/CXCL1 or IL-8/CXCL8, Duffy expressing cells (Duffy+) and untransfected cells (Duffy−) were seeded onto transwells as previously described (Figs. 5 and 6) (22). We determined that 1 nM IL-8/CXCL8 and 20 nM of GRO-α/CXCL1 produced equivalent chemotactic activity for neutrophils and, thus, these concentrations were selected for study (23). In four separate experiments performed either in trip-licate or quadruplicate, we showed that GRO-α/CXCL1-mediated neutrophil migration is enhanced across a Duffy+ monolayer following a 2-h incubation (*, p < 0.05; Fig. 5). There were no significant differences in random migration or dextran crossing be tween Duffy+ and Duffy− monolayers. Although, IL-8-mediated neutrophil migration appeared greater across Duffy+ monolayers, it was not statistically significant at 2 h. However, following a 3-h incubation, the differences in IL-8-mediated neutrophil migration across Duffy+ monolayer compared with Duffy− monolayers reached statistical significance (*, p < 0.05; Fig. 6). Although Duffy Ag is not essential for neutrophil migration, the in vitro data showed that Duffy Ag can facilitate both GRO-α/CXCL1 and IL-8/CXCL8-mediated neutrophil transendothelial migration.
FIGURE 5.
Effect of endothelial Duffy Ag expression on GRO-α/CXCL1-mediated neutrophil migration in vitro. GRO-α/CXCL1-mediated neutrophil migration is enhanced across Duffy+ monolayers in vitro following a 2-h incubation (*, p < 0.05 by Mann-Whitney U test). IL-8-mediated neutrophil migration is slightly greater across Duffy+ monolayers, but this is not statistically significant. No significant differences in the percent dextran crossing were noted between Duffy+ and Duffy− mono-layers to account for differences in neutrophil migration. Data are mean ± SEM of four independent experiments performed in either triplicate or quadruplicate.
FIGURE 6.
Effect of endothelial Duffy Ag expression on IL-8/CXCL8-mediated neutrophil migration in vitro. IL-8/CXCL8-mediated neutrophil migration is enhanced across Duffy+ monolayers in vitro following a 3-h incubation (*, p < 0.05 by Mann-Whitney U test). No significant differences in the percent dextran crossing were noted between Duffy+ and Duffy− monolayers to account for differences in neutrophil migration. Data are mean ± SEM of six independent experiments performed in either trip-licate or quadruplicate.
Neutrophil recruitment into the airspaces of mice lacking the Duffy Ag
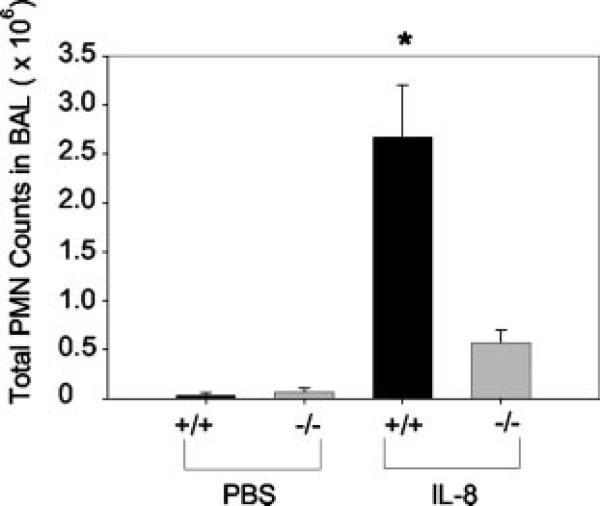
We tested whether Duffy Ag modifies neutrophil migration in vivo by examining neutrophil recruitment into the pulmonary airspaces following intratracheal instillation of human IL-8/CXCL8 in mice lacking the Duffy Ag (Fig. 7). Direct instillation of chemokine into the distal airways provides a direct test of whether Duffy Ag has an effect on chemokine-mediated inflammatory cell recruitment into local tissue beds. IL-8/CXCL8 was chosen as the stimulus because others have shown that murine Duffy Ag also binds angiogenic CXC chemokines, including human IL-8/CXCL8 (19). Initial dose response studies using 0.01, 0.1, and 1 μM IL-8/CXCL8 yielded peak neutrophil recruitment into the airspaces in mice with 1 μM IL-8/CXCL8 (data not shown); thus, 1 μM IL-8/CXCL8 was used for the in vivo studies. Compared with wild-type littermates, there was significantly less neutrophil recruitment into the airspaces following intratracheal instillation of IL-8/CXCL8 in Duffy−/− mice, suggesting that Duffy Ag contributes to chemokine-mediated neutrophil migration (Fig. 7). We also examined plasma levels of IL-8/CXCL8 to determine whether there were measurable differences in plasma IL-8/CXCL8 levels in Duffy−/− vs Duffy+/+ litter-mates. Plasma IL-8/CXCL8 levels were undetectable in all mice studied using a standard ELISA with a detection limit of 31 pg/ml. Lung homogenates showed no significant differences in IL-8/CXCL8 concentrations between Duffy−/− and Duffy+/+ animals, indicating that the amounts instilled were similar in both groups of animals. To address the possibility that the deletion of the Duffy Ag in the knockout mice resulted in reduced peripheral neutrophil counts or functional defects in neutrophil chemotaxis, we measured total peripheral neutrophil counts and in vitro neutrophil chemotaxis in Duffy−/− animals and found no differences as compared with Duffy+/+ animals.
FIGURE 7.
Total neutrophil counts in the BAL of Duffy+/+ and Duffy−/− mice 6 h following intratracheal instillation of 1 μM IL-8/CXCL8. There was minimal neutrophil recruitment into the pulmonary airspaces following instillation of PBS. Alveolar neutrophil recruitment to IL-8/CXCL8 was impaired in Duffy−/− mice. Data are mean ± SEM combined from two independent experiments performed with n = 8 or 9 in each of the PBS groups and n = 16 in each of the IL-8/CXCL8 groups. (*, p < 0.0001 by Mann-Whitney U test).
Discussion
We have previously shown that Duffy Ag expression is enhanced in human lungs with a histologic diagnosis of suppurative pneumonia, a process defined by neutrophilic infiltration of the airspaces (11). The enhanced Duffy Ag expression occurs along microvessels and alveolar septa, particularly in regions of neutrophil accumulation (11). This is in contrast to normal lungs and lungs with acute lung injury, which showed only low level Duffy Ag expression. These observations suggest that expression of Duffy Ag is regulated in the lung microvasculature by the inflammatory process, and that Duffy Ag may have a functional role in the lung parenchyma during inflammation. Because of the up-regulation of Duffy Ag at tissue sites of inflammation, its colocalization to areas of inflammatory cell accumulation, and its multiple chemokine binding properties (9, 11), the main focus of this study was to test whether Duffy Ag plays a role in modifying chemokine-mediated neutrophil recruitment in vitro and in vivo. We showed enhanced neutrophil transmigration across a Duffy+ monolayer toward two different chemokines, GRO-α/CXCL1 and IL-8/CXCL8. In addition, we found that Duffy knockout mice have markedly reduced neutrophil counts in the bronchoalveolar lavage following intratracheal instillation of IL-8/CXCL8 as compared with wild-type littermates. These findings support the interpretation that Duffy Ag is important in facilitating chemokine-mediated neutrophil migration. The in vitro and in vivo approaches are complementary and support the conclusion that Duffy Ag has an important biological function in tissue sites of inflammation.
To gain a better understanding of how Duffy Ag contributes to neutrophil migration, we examined whether GRO-α/CXCL1-Duffy Ag interactions change the endothelial gene expression profile. We showed that stimulation of Duffy transfectants with GRO-α/CXCL1 had no observable effect in altering endothelial gene transcription. This is the first direct evidence that GRO-α/CXCL1 binding to the Duffy Ag does not produce intracellular signaling events resulting in changes in gene transcription. Despite the ability of the Duffy endothelial transfectants to express molecules that might affect neutrophil transmigration when stimulated with TNF-α (e.g., VCAM-1, E-selectin, ICAM-1), this is not a mechanism that would explain the differences we observed in the neutrophil transmigration experiments with GRO-α/CXCL1, because GRO-α/CXCL1 binding in these cells does not up-regulate the expression of adhesion molecules or other genes that might affect neutrophil migration.
It has been previously postulated that the Duffy Ag can participate in chemokine transport across endothelial cells (7, 24). We determined whether Duffy Ag contributes to chemokine movement across the endothelium in vitro, and showed that the presence of Duffy Ag facilitates movement of 125I-GRO-α/CXCL1 across a Duffy+ monolayer. Thus, our in vitro data are in keeping with the hypothesis that Duffy Ag on endothelial cells can function as a shuttling molecule for chemokines.
Others also have shown lines of evidence to suggest that the function of the Duffy Ag is different in endothelial cells than in erythrocytes (7, 24, 25). In situ binding studies performed in rabbit skin show that 125I-IL-8 originating from the abluminal side is internalized by venular endothelial cells, localizes to caveolae, and is subsequently presented on the luminal surface (24). The molecule participating in the transport and presentation of IL-8 in endothelial cells is unknown. However, it binds IL-8 and RANTES but not MIP-1α (24), similar to the binding profile of the Duffy Ag (24, 26). In addition, K562 erythroleukemic cells stably transfected with Duffy cDNA rapidly internalize radiolabeled GRO-α/CXCL1, but this GRO-α/CXCR1 is not degraded following internalization (7). Furthermore, immunoelectron microscopy and immunohistochemistry studies have localized Duffy Ag to both apical and basal membrane domains of endothelial cells as well as within caveolae (25). These independent findings support the concept that the potential biological role of Duffy includes internalization and transport of chemokines across endothelial cells (7).
Animal studies have produced discrepant results about the function of Duffy Ag in vivo (10, 27); however, differences in dose and time intervals make direct comparisons of the studies difficult. Luo et al. (27) found that Duffy−/− mice had reduced neutrophil accumulation in the lungs and intestines 24 h following i.p. injection of 10 mg/kg LPS or 3% thioglycolate. These findings suggest that Duffy Ag may facilitate neutrophil accumulation in tissue following specific inflammatory stimuli (27). Our in vitro and in vivo data are in keeping with these findings and provide direct support for the role of the Duffy Ag in chemokine-mediated neutrophil recruitment into the lungs.
In contrast, Dawson et al. (10) found increased neutrophil accumulation in the lungs and livers of Duffy−/− mice 2 h following i.p. injection of 30 mg/kg LPS, and they concluded that Duffy Ag functions as a chemokine sink. However, with an overwhelming dose of LPS in circulation at the early time, the exaggerated systemic inflammatory response observed in the Duffy−/− mice could be explained by the absence of Duffy Ag on erythrocytes that normally bind excess chemokines in circulation. This interpretation is supported by the findings of Olszyna et al. (28), who showed that in humans with experimental endotoxemia, erythrocyte-associated IL-8 peaked 2 h following LPS injection, then fell as neutrophil-associated IL-8/CXCL8 began to rise at 4 and 6 h. The findings of Dawson et al. (10) and those of Luo et al. (27) highlight the complex roles of the Duffy Ag in two different locations: one expressed on erythrocytes in the circulation and the other expressed predominantly on endothelial cells in tissue.
Endothelial Duffy Ag is genetically conserved, even in individuals who do not express Duffy on their RBCs (6, 7). It is expressed on endothelial cells of the postcapillary venules where leukocytes emigrate (6). Duffy Ag is also up-regulated during inflammation and binds CXC and CC chemokines (4, 6, 8, 9). The data presented herein are the first direct evidence that Duffy Ag can promote neutrophil recruitment into tissue such as the lungs, when IL-8/CXCL8 is the stimulus. The in vitro data suggest a potential mechanism whereby Duffy Ag on endothelial cells contributes to transendothelial movement of chemokine. We conclude that endothelial Duffy Ag is a biologically relevant molecule that participates in regulating inflammatory cell recruitment to tissue sites of inflammation.
Acknowledgments
We gratefully acknowledge Amy Selk for excellent technical assistance.
Footnotes
This work was supported in part by the Medical Research Service of the Department of Veteran's Affairs and National Institutes of Health Grants HL70178, HL69955, and HL30542.
Abbreviations used in this paper: GRO, growth-related oncogene; CXCL, CXC chemokine ligand; 125I, 125I-labeled; IVEC, immortalized HUVEC; CCL, CC chemokine ligand; MCP, monocyte chemotactic protein; MIP, macrophage inflammatory protein; HSA, human serum albumin; PMN, polymorphonuclear neutrophil; fl., calcein fluorescence; Duffy+ cells, endothelial Duffy transfectants.
References
- 1.Hadley TJ, Peiper SC. From malaria to chemokine receptor: the emerging physiologic role of the Duffy blood group antigen. Blood. 1997;89:3077. [PubMed] [Google Scholar]
- 2.Darbonne WC, Rice GC, Mohler MA, Apple T, Hebert CA, Valente AJ, Baker JB. Red blood cells are a sink for interleukin 8, a leukocyte chemotaxin. J. Clin. Invest. 1991;88:1362. doi: 10.1172/JCI115442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neote K, Darbonne W, Ogez J, Horuk R, Schall TJ. Identification of a promiscuous inflammatory peptide receptor on the surface of red blood cells. J. Biol. Chem. 1993;268:12247. [PubMed] [Google Scholar]
- 4.Neote K, Mak JY, Kolakowski LF, Schall TJ. Functional and biochemical analysis of the cloned Duffy antigen: identity with the red blood cell chemokine receptor. Blood. 1994;84:44. [PubMed] [Google Scholar]
- 5.Horuk R, Chitnis CE, Darbonne WC, Colby TJ, Rybicki A, Hadley TJ, Miller LH. A receptor for the malarial parasite Plasmodium vivax: the erythrocyte chemokine receptor. Science. 1993;261:1182. doi: 10.1126/science.7689250. [DOI] [PubMed] [Google Scholar]
- 6.Hadley TJ, Lu ZH, Wasniowska K, Martin AW, Peiper SC, Hesselgesser J, Horuk R. Postcapillary venule endothelial cells in kidney express a multispecific chemokine receptor that is structurally and functionally identical to the erythroid isoform, which is the Duffy blood group antigen. J. Clin. Invest. 1994;94:985. doi: 10.1172/JCI117465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peiper SC, Wang ZX, Neote K, Martin AW, Showell HJ, Conklyn MJ, Ogborne K, Hadley TJ, Lu ZH, Hesselgesser J, et al. The Duffy antigen/receptor for chemokines (DARC) is expressed in endothelial cells of Duffy negative individuals who lack the erythrocyte receptor. J. Exp. Med. 1995;181:1311. doi: 10.1084/jem.181.4.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu XH, Hadley TJ, Xu L, Peiper SC, Ray PE. Up-regulation of Duffy antigen receptor expression in children with renal disease. Kidney Int. 1999;55:1491. doi: 10.1046/j.1523-1755.1999.00385.x. [DOI] [PubMed] [Google Scholar]
- 9.Segerer S, Regele H, Mac KM, Kain R, Cartron JP, Colin Y, Kerjaschki D, Schlondorff D. The Duffy antigen receptor for chemokines is up-regulated during acute renal transplant rejection and crescentic glomerulonephritis. Kidney Int. 2000;58:1546. doi: 10.1046/j.1523-1755.2000.00316.x. [DOI] [PubMed] [Google Scholar]
- 10.Dawson TC, Lentsch AB, Wang Z, Cowhig JE, Rot A, Maeda N, Peiper SC. Exaggerated response to endotoxin in mice lacking the Duffy antigen/receptor for chemokines (DARC). Blood. 2000;96:1681. [PubMed] [Google Scholar]
- 11.Lee JS, Frevert CW, Thorning DR, Segerer S, Alpers CE, Cartron JP, Colin Y, Wong VA, Martin TR, Goodman RB. Enhanced expression of Duffy antigen in the lungs during suppurative pneumonia. J. Histochem. Cytochem. 2003;51:159. doi: 10.1177/002215540305100204. [DOI] [PubMed] [Google Scholar]
- 12.Rhim JS, Tsai WP, Chen ZQ, Chen Z, Van Waes C, Burger AM, Lautenberger JA. A human vascular endothelial cell model to study angiogenesis and tumorigenesis. Carcinogenesis. 1998;19:673. doi: 10.1093/carcin/19.4.673. [DOI] [PubMed] [Google Scholar]
- 13.Kozak M. An analysis of 5′-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987;15:8125. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frevert CW, Wong VA, Goodman RB, Goodwin R, Martin TR. Rapid fluorescence-based measurement of neutrophil migration in vitro. J. Immunol. Methods. 1998;213:41. doi: 10.1016/s0022-1759(98)00016-7. [DOI] [PubMed] [Google Scholar]
- 15.Iwamoto S, Li J, Omi T, Ikemoto S, Kajii E. Identification of a novel exon and spliced form of Duffy mRNA that is the predominant transcript in both erythroid and postcapillary venule endothelium. Blood. 1996;87:378. [PubMed] [Google Scholar]
- 16.Rios M, Chaudhuri A, Mallinson G, Sausais L, Gomensoro-Garcia AE, Hannon J, Rosenberger S, Poole J, Burgess G, Pogo O, Reid M. New genotypes in Fy(a−b−) individuals: nonsense mutations (Trp to stop) in the coding sequence of either FY A or FY B. Br. J. Haematol. 2000;108:448. doi: 10.1046/j.1365-2141.2000.01882.x. [DOI] [PubMed] [Google Scholar]
- 17.Yazdanbakhsh K, Rios M, Storry JR, Kosower N, Parasol N, Chaudhuri A, Reid ME. Molecular mechanisms that lead to reduced expression of Duffy antigens. Transfusion. 2000;40:310. doi: 10.1046/j.1537-2995.2000.40030310.x. [DOI] [PubMed] [Google Scholar]
- 18.Chaudhuri A, Zbrzezna V, Polyakova J, Pogo AO, Hesselgesser J, Horuk R. Expression of the Duffy antigen in K562 cells: evidence that it is the human erythrocyte chemokine receptor. J. Biol. Chem. 1994;269:7835. [PubMed] [Google Scholar]
- 19.Luo H, Chaudhuri A, Johnson KR, Neote K, Zbrzezna V, He Y, Pogo AO. Cloning, characterization, and mapping of a murine promiscuous chemokine receptor gene: homolog of the human Duffy gene. Genome Res. 1997;7:932. doi: 10.1101/gr.7.9.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horuk R, Colby TJ, Darbonne WC, Schall TJ, Neote K. The human erythrocyte inflammatory peptide (chemokine) receptor: biochemical characterization, solubilization, and development of a binding assay for the soluble receptor. Biochemistry. 1993;32:5733. doi: 10.1021/bi00073a002. [DOI] [PubMed] [Google Scholar]
- 21.Zhou J, Jin Y, Gao Y, Wang H, Hu G, Huang Y, Chen Q, Feng M, Wu C. Genomic-scale analysis of gene expression profiles in TNF-α treated human umbilical vein endothelial cells. Inflamm. Res. 2002;51:332. doi: 10.1007/pl00000312. [DOI] [PubMed] [Google Scholar]
- 22.Smart SJ, Casale TB. TNF-α-induced transendothelial neutrophil migration is IL-8 dependent. Am. J. Physiol. 1994;266:L238. doi: 10.1152/ajplung.1994.266.3.L238. [DOI] [PubMed] [Google Scholar]
- 23.Goodman RB, Strieter RM, Frevert CW, Cummings CJ, Tekamp-Olson P, Kunkel SL, Walz A, Martin TR. Quantitative comparison of C-X-C chemokines produced by endotoxin-stimulated human alveolar macrophages. Am. J. Physiol. 1998;275:L87. doi: 10.1152/ajplung.1998.275.1.L87. [DOI] [PubMed] [Google Scholar]
- 24.Middleton J, Neil S, Wintle J, Clark-Lewis I, Moore H, Lam C, Auer M, Hub E, Rot A. Transcytosis and surface presentation of IL-8 by venular endothelial cells. Cell. 1997;91:385. doi: 10.1016/s0092-8674(00)80422-5. [DOI] [PubMed] [Google Scholar]
- 25.Chaudhuri A, Nielsen S, Elkjaer ML, Zbrzezna V, Fang F, Pogo AO. Detection of Duffy antigen in the plasma membranes and caveolae of vascular endothelial and epithelial cells of nonerythroid organs. Blood. 1997;89:701. [PubMed] [Google Scholar]
- 26.Rot A, Hub E, Middleton J, Pons F, Rabeck C, Thierer K, Wintle J, Wolff B, Zsak M, Dukor P. Some aspects of IL-8 pathophysiology. III. Chemokine interaction with endothelial cells. J. Leukocyte Biol. 1996;59:39. doi: 10.1002/jlb.59.1.39. [DOI] [PubMed] [Google Scholar]
- 27.Luo H, Chaudhuri A, Zbrzezna V, He Y, Pogo AO. Deletion of the murine Duffy gene (Dfy) reveals that the Duffy receptor is functionally redundant. Mol. Cell. Biol. 2000;20:3097. doi: 10.1128/mcb.20.9.3097-3101.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olszyna DP, De Jonge E, Dekkers PE, van Deventer SJ, van Der Poll T. Induction of cell-associated chemokines after endotoxin administration to healthy humans. Infect. Immun. 2001;69:2736. doi: 10.1128/IAI.69.4.2736-2738.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]