Abstract
MicroRNAs (miRNAs) are small noncoding RNA molecules ~22 nucleotides in length that can post-transcriptionally repress gene expression. MiRNAs bind to their target messenger RNAs (mRNAs), leading to mRNA degradation or suppression of translation. miRNAs have recently been shown to play pivotal roles in skin development and are linked to various skin pathologies, cancer, and wound healing. Chronic wounds represent a major health burden and drain on resources and developing more effective treatments is therefore a necessity. Increase in the understanding of the regulation of chronic wound biology is therefore required to develop newer therapies. This review focuses on the role of miRNAs in cutaneous biology, the various methods of miRNA modulation, and the therapeutic opportunities in treatment of skin diseases and wound healing.
Keywords: MicroRNA, Hypoxia, Wound healing, Skin
1. Introduction
1.1. MicroRNAs: The Body's In-Built Gene Expression Regulators
MiRNAs have become important as therapeutic targets as they target a group of functionally similar genes and can be manipulated to target entire pathways as opposed to conventional practice of manipulating individual genes. The miRNAs usually bind to the 3¢ untranslated region of the target messenger RNAs (mRNAs) and can repress protein expression by blocking translation or by degrading the mRNA (1). They are predicted to be regulating almost 30 % of the coding genes (2) and thus differential expression of miRNAs may result in various physiological abnormalities.
1.1.1. MicroRNA Biogenesis
MiRNAs are encoded in the human genome as miRNA genes and are then processed to mature miRNAs. RNA polymerase II transcribes primary miRNAs (pri-miRNAs), which are then capped and polyadenylated. The microprocessor complex, which is composed of the RNase III enzyme drosha and DGCR8, then cleaves the pri-miRNAs into shorter premature miRNAs (pre-miRNA). The resulting pre-miRNAs are then exported to the cytoplasm through the Ran-GTP-dependent nuclear export factor exportin-5. Another RNase III enzyme, dicer, then cleaves the premiRNAs into 18-to 24-nt double-stranded RNAs. The resulting RNA-duplex associates with the miRNA-induced silencing complex (RISC), where one of the strands is degraded while the other becomes the mature miRNA. The mature miRNAs interact with target mRNAs via complementarity binding with a particular region known as “seed sequence.” The resultant complex hinders assembly of ribosome, subsequently suppressing gene expression. An overview of the key processes involved in the biogenesis of miRNA is illustrated in (see Fig 1).
Fig. 1.
miRNA biogenesis pathway.
miRNA research in the fi eld of dermatology is in its early phase, but the early findings are substantial, pointing toward a vast opportunity for developing effective therapies for treatment of skin diseases and wounds.
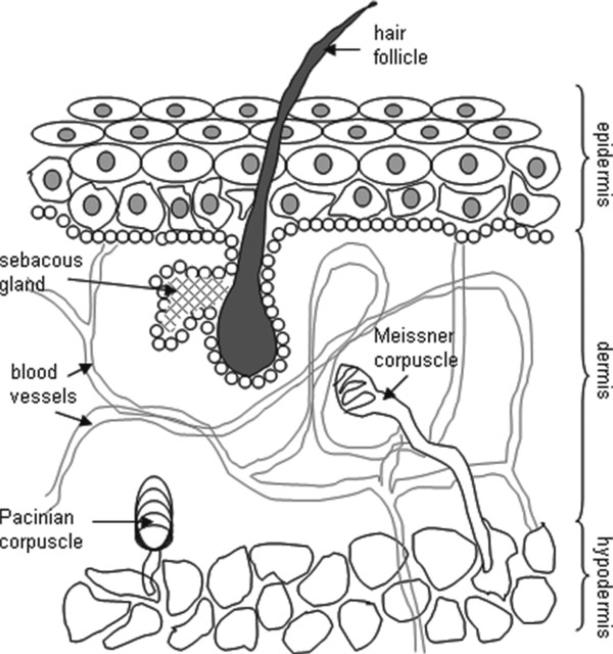
1.2. The Skin: The Largest and the Most Varied Organ of the Body
The skin is made of three distinct layers of tissue (3).
Epidermis
The epidermis is seen on the surface of the skin. The epidermis contains five layers. From bottom to top the layers are named stratum basale, stratum spinosum, stratum granulosum, stratum licidum, and stratum corneum. The epidermis is composed mostly of keratinocytes along with dendritic cells, melanocytes, Langerhans, and Merkel cells. The melanocytes produce a dark pigment called melanin which contributes to skin color and provides UV protection. They are located at the bottom of the epidermis. Basal cells are small cells found at the bottom of the epidermis.
Dermis
The dermis consists of collagenous and elastic fibers populated by fibroblasts, macrophages, mast cells, and lymphocytes and also consist of a glycosaminoglycan/proteoglycan fraction which functions as a supporting matrix or ground substance and makes up its base. The dermis supplies the avascular epidermis with nutrients by means of its vascular network. It contains sense organs for touch, pressure, pain, and temperature (Meissner's corpuscles, Pacinian corpuscles, free nerve endings), as well as blood vessels, nerve fibers, sebaceous, and sweat glands. The dermis also contains hair follicles which are epidermal outgrowths and have a reservoir of stem cells that may regenerate the epidermis.
Subcutaneous layer or Hypodermis
The hypodermis is composed of adipocyte lobules. This layer acts as a protective cushion and helps to insulate the body by monitoring heat gain and heat loss (see Fig. 2).
Fig. 2.
Schematic diagram showing the structure of the skin.
1.2.1. Skin Morphogenesis and the Role of MicroRNAs
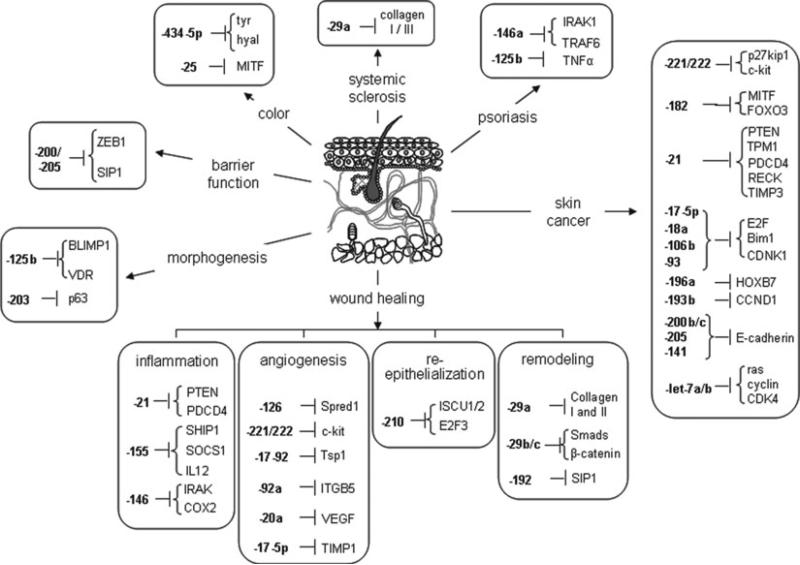
Increasing evidence suggests that miRNAs may play important roles in regulating self-renewal and differentiation in skin stem cells. The human epidermis continuously renews itself by a process which involves generation of transient amplifying cells by basal epidermal stem cells, which move outward from the basal membrane, migrate through the epidermis, and undergo terminal differentiation (4, 5). MiRNAs play an important role during this process, as demonstrated by epithelium-specific depletion of dicer or dgcr8 genes which lead to improper barrier formation, abnormal hair follicle development, and hyper proliferation of basal interfollicular keratinocytes (6–8). Some of the most highly expressed miRNAs in the skin are miR-152, -143, -126, -21, -27a, -214, -16, -203, -125b, -34a, -205, -27b, -30b, -125a, -191, -200 family (-200a, -200b, -200c, -141, -429), -199 family (-199a, -199b), and -19/-20 family (-19b, -20, -17-5p, -93) (3). MiR-125b silences Blimp1 and VDR and represses stem cell differentiation (9). When miR-125b is sustained in stem cell progenies, stemness dominates over epidermal, oil-gland, and hair-follicle differentiation. Another microRNA (miRNA), miR-203 promotes epidermal differentiation by restricting proliferative potential and inducing cell-cycle exit by silencing p63 which is an essential regulator of stem-cell maintenance in stratified epithelial tissues (10). Nine additional miRNAs (miR-23b, miR-95, miR-210, miR-224, miR-26a, miR-200a, miR-27b, miR-328, and miR-376a) have recently been found that are associated with human keratinocyte differentiation in vitro and in vivo (11).
1.3. MicroRNA Regulation of Skin Function: Wound Healing
Chronic wounds represent a major and rising socioeconomic threat affecting over 6.5 million people in the United States costing in excess of the US $25 billion annually (12). Wound healing is a physiological response to injury that ends in wound closure. In humans, wound healing proceeds along a cascade comprising of the following overlapping phases (13): hemostasis and in flammation, proliferative (granulation, vascularization, and wound closure), and remodeling (can continue from weeks to years and encompasses scarring, tensile strength, and turnover of extracellular matrix components).
1.3.1. MicroRNAs in Wound Healing
In flammation
Mounting of a successful in flammatory response as well as resolution of the in flammation at the right time is critical to healing of a wound. Disruption of miRNA biogenesis has a major impact on the overall immune system. The inflammation response to wound is tightly regulated by proinflammatory signals as well as signals that are anti-inflammatory to resolve infl ammation. An imbalance between these signals results in chronic inflammation and derails the healing cascade (12). miRNA have been shown responsive to as well as regulate some of the key mediators of inflammatory response in the course of wound healing. Emerging studies indicate that miRNAs, especially miR-21, miR-146a/b, and miR-155, play a key role in regulating several hubs that orchestrate the in flammatory process (12, 14). miR-146 and miR-155 are induced by TNF-α and IL-1β among others. miR-21 is induced by IL-6. miR-21 silences a number of inflammatory mediators like PTEN and PDCD4; miR-146 silences IRAK and COX2 while miR-155 silences SHIP1, SOCS1, and IL-12 (12).
Angiogenesis
The first series of observations establishing key significance of miRNAs in the regulation of angiogenesis came from experimental studies involved in arresting miRNA biogenesis by Dicer depletion to deplete the miRNA pools of vascular tissues and cells (15). One of the drivers of wound angiogenesis is reactive oxygen species derived from NADPH oxidase which is subject to control by miRNAs (16). Hypoxia drives angiogenesis and hypoxia-sensitive miR-200b is involved in such induction of angiogenesis via directly targeting Ets-1 (17). Various aspects of angiogenesis, such as proliferation, migration, and morphogenesis of endothelial cells, can be regulated by specific miRNAs in an endothelial-specific manner. Some of the most well-characterized miRNA-regulated proteins involved in angiogenesis are Spred1 (miR-126) (18), c-kit (miR-221/222) (19), Tsp-1 (miR-17-92) (20), ITGB5 (miR-92a) (21), VEGF (miR-20a) (22), and TIMP-1 (miR-17-5p) (23).
1.3.2. Re-Epithelialization and Wound Closure
Restoration of an intact epidermal barrier is enabled through wound epithelialization, also known as re-epithelialization (24, 25). A wound that is not epithelialized is not considered “healed” no matter how perfectly restored the underlying dermal structures may be. Thus, wound re-epithelialization is a critical and defining feature of wound repair. Re-epithelialization of the wound occurs as the result of three overlapping keratinocyte functions: migration, proliferation, and differentiation. It begins with dissolution of cell–cell and cell–substratum contacts which is followed by the polarization and initiation of directional migration in basal and a subset of suprabasilar keratinocytes over the provisional wound matrix. A subset of keratinocytes immediately adjacent to, but not within the wound bed, then undergoes mitosis, and finally there is multi-layering of the newly formed epidermis and induction of differentiation-specific gene products to restore the functionality of the epidermis.
Problematic wounds are generally characterized by tissue ischemia (13) and mostly arise due to vascular complications. Hypoxia is a component of ischemia and is defined (13) by a lower tissue partial pressure of oxygen (pO2) compared with the pO2 to which the specific tissue element in question is adjusted to under healthy conditions in vivo, i.e., it represents a reduction in oxygen delivery below tissue demand. Limitations in the ability of the vasculature to deliver O2 -rich blood to the wound tissue leads to, among other consequences, hypoxia. Three major factors may contribute to wound tissue hypoxia (13): (1) peripheral vascular diseases (PVDs) limiting the O2 supply, (2) increased O2 demand of the healing tissue, and (3) generation of reactive oxygen species (ROS) by way of respiratory burst and for redox signaling. Wound hypoxia may range anywhere from near-anoxia to mild–modest hypoxia. It is likely that the magnitude of wound hypoxia is not uniformly distributed throughout the affected tissue especially in large wounds. This is most likely the case in chronic wounds presented clinically as opposed to experimental wounds, which are more controlled and homogenous in nature, where the situation presents towards near-anoxic pockets. Primarily based on the tumor literature, hypoxia is generally viewed as being angiogenic. This is true with the condition that hypoxia be acute and mild to modest in magnitude. Extreme near-anoxic hypoxia, as commonly noted in problem wounds, is not compatible with tissue repair. Adequate wound tissue oxygenation is required but may not be sufficient to favorably influence healing outcomes.
Hypoxia induces specific miRNAs collectively referred to as hypoxamiRs (26). Among the known hypoxamiRs, miRNA-210 has been reported to be the most sensitive to hypoxia. miR-210 is directly under the control of hypoxia regulated genes—hypoxiainducible factor-1 and -2 (Hif-1 α and Hif-2 α) and NF-κB. Hypoxia regulated miRNAs are generally considered to favor angiogenesis (27); however, in ischemic situation, they impair cell proliferation and migration. Keratinocyte proliferation and migration is essential for re-epithelialization and wound closure and miR-210 silences some key proteins like E2F3 which is required for keratinocyte proliferation. Silencing-specific hypoxamiRs may therefore represent a prudent approach to facilitate tissue repair. The iron sulfur cluster assembly proteins ISCU1/2 have also been identified as HIF-dependent and silenced by miR-210 and can potentially affect healing outcomes. ISCU1/2 are essential for iron sulfur cluster biogenesis which are incorporated into wide variety of proteins, many of which like Complex I and Aconitase are involved in mitochondrial metabolism. miR-210 can thus impair mitochondrial activity by repressing ISCU1/2 (26, 28). This will translate into compromised ATP production. In hypoxic condition, this may be compensated due to the Pasteur effect and result in a more optimized energy production. In the short term, therefore, this may result in hypoxic cellular adaptation and may improve survival. In contrast, chronic repression of mitochondrial function during hypoxia has been linked to various pathologic consequences including ischemic diseases (26). These outcomes are not compatible with the higher energy demands associated with tissue repair and so, this may be another possible mechanism for compromised healing upon HIF stabilization in ischemic wounds.
1.3.3. Remodeling Phase
Collagen deposition is an important aspect of the remodeling phase. miR-29a directly regulates collagen expression at the post-transcriptional level (29). Mammalian fetal skin can heal without a scar whereas during the late gestational stage, it transitions to a scarring phenotype (30, 31). Several miRNAs are differentially expressed between the two stages and probably contribute to this transition and miRNA-29b, miRNA-29c, and miRNA-192 have been found to be the key mediators with their levels being highly induced during the late gestation phase (32). MiRNA-29b and miR-29c targets repress several extracellular matrix proteins, anti- fibrotic TGF-β and proteins like Smads, β-catenin which are involved in the signaling pathways important for scarless healing (33, 34) while miR-192 enhanced collagen 1α2 expression by targeting the Smad-interacting protein 1 (SIP1) (35) (see Fig.3) summarizes the miRNA involved in the different phases of wound healing.
Fig. 3.
MicroRNAs involved in skin morphogenesis and function.
1.4. MicroRNA
Regulation of Other Functions of the Skin
The role of miRNAs in regulation of the other functions of the skin is not yet very well characterized and work is in progress to elucidate the mechanisms. A key function of the skin is to serve as a barrier and prevent loss of water from the organism. A key protein involved in the skin's barrier function is E-cadherin which is required for the maintenance of proper localization of key tight junctional proteins and its absence results in permeable tight junctions compromising epidermal barrier function of the skin (36, 37). miR-200 and miR-205 silence the transcriptional repressors of E-cadherin—ZEB1 and SIP1 (also known as ZEB2) (38–40). Thus, miR-200 and miR-205 are expected to positively regulate E-cadherin and seem to be essential in maintaining epithelial stability. These studies were however performed in cell lines and transformed cells and the significance in normal skin biology remains to be elucidated. Another important function of the skin is pigmentation. Human pigmentation involves production and dispersion of melanin by epidermal melanocytes to neighboring keratinocytes. Skin pigments are essential for absorbing the harmful ultraviolet radiations less than 310 nm and also regulate skin vitamin D production (41, 42). Among the miRNAs regulating skin color, miR-25 silenced micropthalmia-associated transcription factor (MITF) in skin melanocytes that regulates genes linked to coat color like tyrosinase (tyr) and tyrosinase-related protein 1 (43). Another miRNA, miR-434-5p has been implicated in skin whitening and lightening by targeting tyr and hyaluronidase (hyal) genes (44), which play an important role in melanin production.
1.5. MicroRNAs Involved in Skin Pathology
1.5.1. Skin Cancer
A number of miRNAs have also been implicated in various skin diseases.
Up-regulation of miR-221/222 silences p27kip1 and c-kit causing increased cell proliferation and melanogenesis respectively.
High miR-182 silences MITF and FOXO3 resulting in increased metastasis. miR-21 silences PTEN, TPM1, PDCD4, RECK, TIMP3 and so elevated miR-21 results in metastasis. miR-17-5p, miR-18a, miR-106b, and miR-93 when high also cause cancer by repressing E2F, CDKN1, and BIm1 causing increased cell proliferation and reduced apoptosis. Release in repression of HOXB7 (miR-196a), CCND1 (miR-193b), E-cadherin (miR-200b, -200c, -141, -205), ras, cyclin, and CDK4 (let-7a and let-7b) increases migration, proliferation, invasion, and metastasis and thus results in skin cancer.
1.5.2. Psoriasis
High miR-146a silences IRAK1/TRAF6 while less miR-125b elevates TNF-α, both of which are important players in psoriasis (3).
1.5.3. Systemic Sclerosis
Elevated miR-29a silences type I and type III collagens which results in increased collagen deposition and fibrosis which are hallmarks of systemic sclerosis (3).
2. Materials
We have developed a murine and a porcine delayed healing model to study the role of miRNAs in wound healing(45, 46). Varied heterogeneity of the wounds as well as ethical challenges in repeated biopsy collections from human patients presents a big challenge in studying the mechanisms of healing. The porcine model provides researchers with a preclinical alternative to understand the basic processes of tissue repair and to develop and validate strategies for clinical treatment. This is particularly useful because pig skin closely resembles human skin anatomically as well as physiologically (46) and this model is highly suitable for testing the clinical relevance for possible wound healing therapies. However, limited availability of genetically modified animals, antibodies, and reagents makes the porcine model less useful for study of molecular mechanisms in detail. To address this concern, a murine bipedicle flap model has been developed and characterized (45). These two models thus provide very strong tools to researchers to study molecular mechanisms of the wound healing process, develop therapies, and test their clinical relevance.
3. Methods
The potential to therapeutically regulate miRNA levels at the wound site make miRNA-based therapies an attractive possibility in wound healing. MiRNA therapeutics provides a unique advantage because by modulating a single miRNA, a group of functionally related genes in a pathway can be targeted as opposed to modulating a single gene at a time in conventional gene therapy.
MiRNAs can be up-regulated using mimics and down-regulated using anti-miRs. The over-expression of an miRNA can be achieved using synthetic short double-stranded oligonucleotide (mimics). Mimics are double-stranded with one strand called guide whose sequence is same as the mature miRNA while the other called passenger is complimentary to the mature sequence and only the guide sequence is incorporated into the RISC complex (47). Efficacy of mimics has not yet been demonstrated in vivo. Another approach, however, has been successfully tested in vivo and involves using expression vector systems (47). Anti-miRNAs essentially act as competitive inhibitors binding to the mature miRNA and also can affect miRNA maturation by binding to the pre-miRNA. Three different approaches have been used to deliver anti-miRs to mammalian tissues (47)—(a) Intravenous injection of antagomiRs (chemically modified cholesterol-conjugated single strand oligos); (b) conjugation of RNA oligos with other lipophilic molecules, i.e., high-density lipoproteins; (c) Using locked nucleic acid (LNA)-modified oligonucleotides.
3.1. In Vivo Delivery Systems
Successful delivery of the synthetic oligonucleotides will depend on their resistance to degradation in tissues, specificity, and high binding affinity to the specific miRNA in question. To achieve these goals, chemical modifications of the oligonucleotides are often necessary. Delivery of anti-miRNAs to mammalian tissues is generally administered by using either of these approaches (47, 48): (a) Intravenous injection of antagomiRs (chemically modified cholesterol-conjugated single strand oligos); or (b) conjugation of RNA oligos with other lipophilic molecules, i.e., high-density lipoproteins.
Three forms of chemically modified oligonucleotides that have been used are (a) 2¢-O-methyl group (OMe)–modified oligonucleotides; (b) 2¢-O-methoxyethyl-modified oligonucleotides, and (c) Locked nucleic acid or LNA (48).
Fig. 4.
Schematic diagram demonstrating the principle behind sponges, erasers, and masks.
Fig. 5.
a–c) Schematic diagram demonstrating principle of laser capture microdissection. (i–iii) Representative example of laser capture microdissection from mouse skin. (a, i) Skin is sectioned onto a membrane slide; (b, ii) Specific region to be dissected is first marked. The laser cuts along the marked lines; (c, iii) Dissected sections are then catapulted into labeled tubes.
Acknowledgments
Wound healing research in the author's laboratory is funded by NIH awards GM 077185 and GM 069589 to CK Sen.
Footnotes
A number of miRNA therapeutic studies are currently going through clinical trials (45). A phase I clinical trial in which an LNA-based anti-miRNA targeting miR-122 was developed as hepatitis C therapy has recently been completed. A number of clinical trials involving skin carcinoma are also under way. This validates the viability of miRNAs as therapeutic targets and miRNA inhibitors and mimics as a new class of drugs. Further development of the technology into a successful therapy involves identifying new miRNA targets and developing ef ficient in vivo delivery system.
A big drawback of miRNA-based therapy lies in the fact that a single miRNA can silence a number of different proteins and so specificity of treatment becomes a problem. In order to address this issue, a number of different approaches have been adopted (45). Sponges are essentially competitive inhibitors which contain multiple, tandem binding sites to an miRNA of interest. Sponges have a bulge at the position cleaved by the Ago2. They can thus stably interact with the miRNA target that cannot be sliced. Sponges therefore have the advantage of being able to block all the miRNAs that recognize the same sequence and thus inhibit all the miRNAs of the same family resulting in a much more effective outcome. miRNA “erasers” are similar to sponges, except that they use only two copies of the perfectly complementary antisense sequence of the miRNA. The mask approach involves an oligonucleotide which is made to bind to the miRNA target sequence of the specific mRNA of interest, thus preventing the miRNA/mRNA association. Thus, using this approach, the miRNA interaction with one speci fic target can be modulated (see Fig. 4a, b, c).
In order to do basic research to understand the role of miRNAs in wound healing, a murine and porcine delayed healing model has now been developed (47). The porcine model provides researchers with a preclinical model to understand the basic processes of tissue repair and to develop and validate strategies for clinical treatment. This is particularly useful because varied heterogeneity of the wounds as well as ethical challenges in repeated biopsy collections from human patients present a big challenge in studying the mechanisms of healing. Since pig skin closely resembles human skin anatomically as well as physiologically, this model is highly suitable for testing the clinical relevance for possible wound healing therapies. On the other hand, while this model is of high translational significance and clinical relevance, limited availability of genetically modified animals, antibodies, and reagents makes them less useful for study of molecular mechanisms in detail. To address this concern, a murine bipedicle flap model has been developed and characterized. These two models thus provide very strong tools to researchers to study molecular mechanisms of the wound healing process, develop therapies, and test their clinical relevance. As mentioned before, the same miRNAs often have been found to have different and contrasting function in different cell types. To solve this problem, a new technology employing laser capture micro dissection can be used to perform cell type-specific miRNA studies from in vivo tissue samples. Recent publications demonstrate the feasibility of using this technique for analysis of genes captured from blood vessels from human tissues (49), prostate cancer epithelial and interstitial stromal cells, and epithelial cells from other regions (see Fig. 5).
References
- 1.Bartel DP. MicroRNAs: genomics, bio-genesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 2.Miranda KC, Huynh T, Tay Y, Ang YS, Tam WL, Thomson AM, Lim B, Rigoutsos I. A pattern-based method for the identification of microRNA binding sites and their corresponding heteroduplexes. Cell. 2006;126(6):1203–1217. doi: 10.1016/j.cell.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 3.Banerjee J, Chan YC, Sen CK. MicroRNAs in skin and wound healing. Physiol Genomics. 2011;43(10):543–556. doi: 10.1152/physiolgenomics.00157.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fuchs E. Skin stem cells: rising to the surface. J Cell Biol. 2008;180(2):273–284. doi: 10.1083/jcb.200708185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blanpain C, Fuchs E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nat Rev Mol Cell Biol. 2009;10(3):207–217. doi: 10.1038/nrm2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andl T, Murchison EP, Liu F, Zhang Y, Yunta-Gonzalez M, Tobias JW, Andl CD, Seykora JT, Hannon GJ, Millar SE. The miRNA-processing enzyme dicer is essential for the morphogenesis and maintenance of hair follicles. Curr Biol. 2006;16(10):1041–1049. doi: 10.1016/j.cub.2006.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yi R, O'Carroll D, Pasolli HA, Zhang Z, Dietrich FS, Tarakhovsky A, Fuchs E. Morphogenesis in skin is governed by discrete sets of differentially expressed microRNAs. Nat Genet. 2006;38(3):356–362. doi: 10.1038/ng1744. [DOI] [PubMed] [Google Scholar]
- 8.Yi R, Pasolli HA, Landthaler M, Hafner M, Ojo T, Sheridan R, Sander C, O'Carroll D, Stoffel M, Tuschl T, Fuchs E. Dgcr8-dependent microRNA biogenesis is essential for skin development. Proc Natl Acad Sci USA. 2009;106(2):498–502. doi: 10.1073/pnas.0810766105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang L, Stokes N, Polak L, Fuchs E. Specific microRNAs are preferentially expressed by skin stem cells to balance self-renewal and early lineage commitment. Cell Stem Cell. 2011;8(3):294–308. doi: 10.1016/j.stem.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yi R, Poy MN, Stoffel M, Fuchs E. A skin microRNA promotes differentiation by repressing ‘stemness’. Nature. 2008;452(7184):225–229. doi: 10.1038/nature06642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hildebrand J, Rutze M, Walz N, Gallinat S, Wenck H, Deppert W, Grundhoff A, Knott A. A comprehensive analysis of microRNA expression during human keratinocyte differentiation in vitro and in vivo. J Invest Dermatol. 2011;131(1):20–29. doi: 10.1038/jid.2010.268. [DOI] [PubMed] [Google Scholar]
- 12.Roy S, Sen CK. MiRNA in innate immune responses: novel players in wound inflammation. Physiol Genomics. 2011;43(10):557–565. doi: 10.1152/physiolgenomics.00160.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sen CK. Wound healing essentials: let there be oxygen. Wound Repair Regen. 2009;17(1):1–18. doi: 10.1111/j.1524-475X.2008.00436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu N, Zhang D, Chen S, Liu X, Lin L, Huang X, Guo Z, Liu J, Wang Y, Yuan W, Qin Y. Endothelial enriched microRNAs regulate angiotensinii-induced endothelial inflammation and migration. Atherosclerosis. 2011;215(2):286–293. doi: 10.1016/j.atherosclerosis.2010.12.024. [DOI] [PubMed] [Google Scholar]
- 15.Sen CK, Gordillo GM, Khanna S, Roy S. Micromanaging vascular biology: tiny microRNAs play big band. J Vasc Res. 2009;46(6):527–540. doi: 10.1159/000226221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shilo S, Roy S, Khanna S, Sen CK. Evidence for the involvement of miRNA in redox regulated angiogenic response of human microvascular endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28(3):471–477. doi: 10.1161/ATVBAHA.107.160655. [DOI] [PubMed] [Google Scholar]
- 17.Chan YC, Khanna S, Roy S, Sen CK. Mir-200b targets ets-1 and is down-regulated by hypoxia to induce angiogenic response of endothelial cells. J Biol Chem. 2011;286(3):2047–2056. doi: 10.1074/jbc.M110.158790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fish JE, Santoro MM, Morton SU, Yu S, Yeh RF, Wythe JD, Ivey KN, Bruneau BG, Stainier DY, Srivastava D. Mir-126 regulates angiogenic signaling and vascular integrity. Dev Cell. 2008;15(2):272–284. doi: 10.1016/j.devcel.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poliseno L, Salmena L, Zhang J, Carver B, Haveman WJ, Pandol fi PP. A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Nature. 2010;465(7301):1033–1038. doi: 10.1038/nature09144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dews M, Homayouni A, Yu D, Murphy D, Sevignani C, Wentzel E, Furth EE, Lee WM, Enders GH, Mendell JT, Thomas-Tikhonenko A. Augmentation of tumor angiogenesis by a Myc-activated microRNA cluster. Nat Genet. 2006;38(9):1060–1065. doi: 10.1038/ng1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonauer A, Carmona G, Iwasaki M, Mione M, Koyanagi M, Fischer A, Burch fi eld J, Fox H, Doebele C, Ohtani K, Chavakis E, Potente M, Tjwa M, Urbich C, Zeiher AM, Dimmeler S. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science. 2009;324(5935):1710–1713. doi: 10.1126/science.1174381. [DOI] [PubMed] [Google Scholar]
- 22.Hua Z, Lv Q, Ye W, Wong CK, Cai G, Gu D, Ji Y, Zhao C, Wang J, Yang BB, Zhang Y. MiRNA-directed regulation of vegf and other angiogenic factors under hypoxia. PLoS One. 2006;1:e116. doi: 10.1371/journal.pone.0000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Otsuka M, Zheng M, Hayashi M, Lee JD, Yoshino O, Lin S, Han J. Impaired microRNA processing causes corpus luteum insufficiency and infertility in mice. J Clin Invest. 2008;118(5):1944–1954. doi: 10.1172/JCI33680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brauchle M, Angermeyer K, Hubner G, Werner S. Large induction of keratinocyte growth factor expression by serum growth factors and pro-inflammatory cytokines in cultured fibroblasts. Oncogene. 1994;9(11):3199–3204. [PubMed] [Google Scholar]
- 25.Takehara K. Growth regulation of skin fibroblasts. J Dermatol Sci. 2000;24(suppl 1):S70–S77. doi: 10.1016/s0923-1811(00)00144-4. [DOI] [PubMed] [Google Scholar]
- 26.Chan SY, Loscalzo J. MicroRNA-210: a unique and pleiotropic hypoxamir. Cell Cycle. 2010;9(6):1072–1083. doi: 10.4161/cc.9.6.11006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guimbellot JS, Erickson SW, Mehta T, Wen H, Page GP, Sorscher EJ, Hong JS. Correlation of microRNA levels during hypoxia with predicted target mRNAs through genome-wide microarray analysis. BMC Med Genomics. 2009;2:15. doi: 10.1186/1755-8794-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan SY, Zhang YY, Hemann C, Mahoney CE, Zweier JL, Loscalzo J. MicroRNA-210 controls mitochondrial metabolism during hypoxia by repressing the iron-sulfur cluster assembly proteins iscu1/2. Cell Metab. 2009;10(4):273–284. doi: 10.1016/j.cmet.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maurer B, Stanczyk J, Jungel A, Akhmetshina A, Trenkmann M, Brock M, Kowal-Bielecka O, Gay RE, Michel BA, Distler JH, Gay S, Distler O. MicroRNA-29, a key regulator of collagen expression in systemic sclerosis. Arthritis Rheum. 2010;62(6):1733–1743. doi: 10.1002/art.27443. [DOI] [PubMed] [Google Scholar]
- 30.Beanes SR, Hu FY, Soo C, Dang CM, Urata M, Ting K, Atkinson JB, Benhaim P, Hedrick MH, Lorenz HP. Confocal microscopic analysis of scarless repair in the fetal rat: defining the transition. Plast Reconstr Surg. 2002;109(1):160–170. doi: 10.1097/00006534-200201000-00026. [DOI] [PubMed] [Google Scholar]
- 31.Beanes SR, Dang C, Soo C, Ting K. Skin repair and scar formation: the central role of tgf-beta. Expert Rev Mol Med. 2003;5(8):1–22. doi: 10.1017/S1462399403005817. [DOI] [PubMed] [Google Scholar]
- 32.Cheng J, Yu H, Deng S, Shen G. MicroRNA pro filing in mid- and late-gestational fetal skin: implication for scarless wound healing. Tohoku J Exp Med. 2010;221(3):203–209. doi: 10.1620/tjem.221.203. [DOI] [PubMed] [Google Scholar]
- 33.Yan HL, Xue G, Mei Q, Wang YZ, Ding FX, Liu MF, Lu MH, Tang Y, Yu HY, Sun SH. Repression of the mir-17-92 cluster by p53 has an important function in hypoxia-induced apoptosis. EMBO J. 2009;28(18):2719–2732. doi: 10.1038/emboj.2009.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Rooij E, Sutherland LB, Thatcher JE, DiMaio JM, Naseem RH, Marshall WS, Hill JA, Olson EN. Dysregulation of microRNAs after myocardial infarction reveals a role of mir-29 in cardiac fibrosis. Proc Natl Acad Sci U S A. 2008;105(35):13027–13032. doi: 10.1073/pnas.0805038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kato M, Zhang J, Wang M, Lanting L, Yuan H, Rossi JJ, Natarajan R. MicroRNA-192 in diabetic kidney glomeruli and its function in tgf-beta-induced collagen expression via inhibition of e-box repressors. Proc Natl Acad Sci USA. 2007;104(9):3432–3437. doi: 10.1073/pnas.0611192104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tinkle CL, Lechler T, Pasolli HA, Fuchs E. Conditional targeting of e-cadherin in skin: insights into hyperproliferative and degenerative responses. Proc Natl Acad Sci USA. 2004;101(2):552–557. doi: 10.1073/pnas.0307437100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tunggal JA, Helfrich I, Schmitz A, Schwarz H, Gunzel D, Fromm M, Kemler R, Krieg T, Niessen CM. E-cadherin is essential for in vivo epidermal barrier function by regulating tight junctions. EMBO J. 2005;24(6):1146–1156. doi: 10.1038/sj.emboj.7600605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park SM, Gaur AB, Lengyel E, Peter ME. The mir-200 family determines the epithelial phenotype of cancer cells by targeting the e-cadherin repressors zeb1 and zeb2. Genes Dev. 2008;22(7):894–907. doi: 10.1101/gad.1640608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Korpal M, Lee ES, Hu G, Kang Y. The mir-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of e-cadherin transcriptional repressors zeb1 and zeb2. J Biol Chem. 2008;283(22):14910–14914. doi: 10.1074/jbc.C800074200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y, Goodall GJ. The mir-200 family and mir-205 regulate epithelial to mesenchymal transition by targeting zeb1 and sip1. Nat Cell Biol. 2008;10(5):593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 41.Kosmadaki MG, Naif A, Hee-Young P. Recent progresses in understanding pigmentation. G Ital Dermatol Venereol. 2010;145(1):47–55. [PubMed] [Google Scholar]
- 42.Neer RM. The evolutionary significance of vitamin d, skin pigment, and ultraviolet light. Am J Phys Anthropol. 1975;43(3):409–416. doi: 10.1002/ajpa.1330430322. [DOI] [PubMed] [Google Scholar]
- 43.Zhu Z, He J, Jia X, Jiang J, Bai R, Yu X, Lv L, Fan R, He X, Geng J, You R, Dong Y, Qiao D, Lee KB, Smith GW, Dong C. MicroRNA-25 functions in regulation of pigmentation by targeting the transcription factor MITF in alpaca (lama pacos) skin melanocytes. Domest Anim Endocrinol. 2010;38(3):200–209. doi: 10.1016/j.domaniend.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 44.Wu D, Chen JS, Chang DC, Lin SL. Mir-434-5p mediates skin whitening and lightening. Clin Cosmet Investig Dermatol. 2008;1:19–35. doi: 10.2147/ccid.s4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Biswas S, Roy S, Banerjee J, Hussain SR, Khanna S, Meenakshisundaram G, Kuppusamy P, Friedman A, Sen CK. Hypoxia inducible microRNA 210 attenuates keratinocyte proliferation and impairs closure in a murine model of ischemic wounds. Proc Natl Acad Sci USA. 2010;107(15):6976–6981. doi: 10.1073/pnas.1001653107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roy S, Biswas S, Khanna S, Gordillo G, Bergdall V, Green J, Marsh CB, Gould LJ, Sen CK. Characterization of a preclinical model of chronic ischemic wound. Physiol Genomics. 2009;37(3):211–224. doi: 10.1152/physiolgenomics.90362.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fasanaro P, Greco S, Ivan M, Capogrossi MC, Martelli F. MicroRNA: emerging therapeutic targets in acute ischemic diseases. Pharmacol Ther. 2010;125(1):92–104. doi: 10.1016/j.pharmthera.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 48.Weiler J, Hunziker J, Hall J. Anti-miRNA oligonucleotides (amos): ammunition to target miRNAs implicated in human disease? Gene Ther. 2006;13(6):496–502. doi: 10.1038/sj.gt.3302654. [DOI] [PubMed] [Google Scholar]
- 49.Roy S, Patel D, Khanna S, Gordillo GM, Biswas S, Friedman A, Sen CK. Transcriptome-wide analysis of blood vessels laser captured from human skin and chronic wound-edge tissue. Proc Natl Acad Sci USA. 2007;104(36):14472–14477. doi: 10.1073/pnas.0706793104. [DOI] [PMC free article] [PubMed] [Google Scholar]