Abstract
The somatosensory whisker pathway has been a useful system for increasing our understanding of experience-induced plasticity. However, precisely timed whisker activation in the awake freely moving mouse has been very difficult. This manuscript describes a method for construction of a whisker stimulator that can be attached to a freely moving mouse. The stimulator was used to activate the whiskers in a time-sensitive forebrain-dependent task, trace eyeblink conditioning. After repeatedly pairing whisker stimulation with delivery of a mild periorbital shock following a stimulus-free trace interval, trace-conditioned mice were able to learn the association. This study demonstrates the potential for using the whisker stimulator in time-sensitive behavioral tasks, such as trace eyeblink conditioning, thus enhancing our ability to examine experience-induced neuronal plasticity in the somatosensory whisker pathway in awake behaving rodents.
Keywords: Trace conditioning, Eyeblink, Somatosensory, Barrel cortex, PMBSF, Vibrissae
Well characterized neuronal pathways have played a vital role in determining how experience modulates neuronal processing in the brain. One such neuronal pathway is the whisker-barrel projection. The whisker-barrel projection is a very complex yet highly organized neuronal pathway. Each whisker on the rodent’s face transmits angular displacement to primary somatosensory barrel cortex via a tri-synaptic pathway (trigeminal nerve to medullary barrelets: thalamic barreloids: somatosensory barrel cortex; Woolsey and Van der Loos, 1970). Axons from each large facial whisker on the whisker pad project to a corresponding barrel in layer IV of the barrel cortex, so that the whisker-barrel cortex is a topographically organized map of the whisker pad on the face. Each barrel in layer IV of primary somatosensory cortex consists of a cell sparse, dendrite rich hollow surrounded by a cell dense, dendrite sparse wall surrounded and separated by an inter-barrel region called the septa (Woolsey et al., 1975; Simons and Woolsey, 1984; Rice et al., 1985; Greenough and Chang, 1988).
This highly organized, yet very plastic neuronal system has been extensively utilized in rodents to increase our understanding of experience-induced plasticity. For example, studies utilizing a whisker texture discrimination task have shown that whisker tactile input is encoded at the level of the neocortex, not as individual sensory features such as amplitude, velocity, or frequency, but as the product of both the frequency and amplitude of the stimulus (Guic-Robles et al., 1992; Arabzadeh et al., 2003, 2004). These studies have greatly assisted our understanding of how the neocortex processes sensory information. Using the whisker-barrel system in conditioning paradigms, such as whisker–whisker, whisker–shock, and whisker–air puff pairing, has also increased our understanding of intra- and inter-layer neocortical processing of sensory information. For example, whisker–whisker pairing studies have demonstrated an experience-induced increase in neocortical inhibition in layer II/III (Goldreich et al., 1998) and rapid reorganization of sensory receptive fields that follows a different time course of activation depending upon the neocortical layer for the paired and unpaired whiskers (Diamond et al., 1993, 1994; Armstrong-James et al., 1994). Likewise, whisker–shock and trace whisker–air puff conditioning have also been shown to result in an increase in the receptive field size in layer IV of the neocortex for the conditioned whiskers (Siucinska and Kossut, 1996; Galvez et al., 2006). These studies and many others utilizing the whisker-barrel system have played a key role in assisting our understanding of how the somatosensory neocortex processes tactile information.
Unfortunately, when working with the rodent whisker system, unless one is utilizing an anesthetized preparation, stimulation of individual whiskers in a well-controlled manner required with many learning paradigms, is very difficult. The studies mentioned above relied on anesthetized preparations in the case of the whisker texture discrimination studies, passive whisker stimulation in the case of the whisker-pairing studies, or careful stimulation by hand with a paint brush in the case of the whisker-shock studies. However, for learning paradigms that examine well-timed behavioral responses, such as eyeblink conditioning, it is necessary to utilize precisely timed stimuli. Modulation of the timing between the conditioned and unconditioned stimulus in eyeblink conditioning by as little as a few hundred milliseconds can dramatically alter not only the forebrain dependency of the task (McLaughlin et al, 2002; Moyer et al., 1990; Tseng et al., 2004; Weiss et al., 1999), but also the onset and peak latency of the conditioned response (Schneiderman and Gormezano, 1964; Smith, 1968). Utilizing the whisker-barrel system to further our understanding of experience-induced plasticity would be greatly facilitated with better control over the timing of whisker stimulation in conscious, freely moving rodents, thus allowing one to time lock whisker stimulation to another stimulus while studying possible changes in neuronal properties such as neurotransmitter release or neuronal firing.
We describe a technique for precisely timed stimulation of mouse facial whiskers, although the whisker stimulator should also work in rats and other small animals. This technique enables us to examine not only activity dependent plasticity, as is routinely conducted in many whisker learning tasks, but also to examine associative learning induced plasticity in the somatosensory barrel neocortex. Using this method mice were trained on a time-sensitive forebrain-dependent task, trace eyeblink conditioning, with whisker vibration as the conditioned stimulus.
1. Methods
A “headbolt” connector affixed to the mouse’s skull where a tether and the stimulator can be attached is required for whisker stimulation. We used a design routinely employed in our laboratory for eyeblink conditioning (Tseng et al., 2004; Weiss and Disterhoft, 2008). Briefly, the headbolt connector consists of a segment from a 221 series nylon strip connector (Cooper Interconnect) containing seven holes. The first hole was threaded to help anchor the tether. This was then followed by three empty holes, and three with gold plated pins, two soldered to teflon-coated stainless steel wires (45 μm), and one soldered to a bare stainless steel wire (30 μm). The two coated wires (shock electrodes) were subcutaneously passed through the periorbital region caudal to the eye to deliver a periorbital shock, the unconditioned stimulus (US). The bare wire was secured to a stainless steel skull screw (00–90 × 1/16 in.) inserted 1/4 of a turn into the skull to serve as an electrical ground. The connector was then cemented to the skull with dental acrylic (Dentsply, grip cement, 675570) to form the headbolt. For a detailed description of the surgical procedure, headbolt, and tether assembly for eyeblink conditioning see Weiss and Disterhoft (2008).
The whisker stimulator is affixed to the base of a tether that can be attached to the headbolt. This design allows removal of the whisker stimulator while the mouse is not being trained, thus minimizing possible damage to the stimulator and allowing the mouse free movement in its home cage. The tether that attaches to the headbolt connector used for eyeblink conditioning is also constructed from a seven hole segment of a 221 series nylon strip connector (Cooper Interconnect). From rostral to caudal the seven holes on the tether are used for a locking screw, three spacers, two for delivery of a periorbital shock, and one for the ground connector.
2. Whisker stimulator
The whisker stimulator is based upon activation of a piezo strip (Piezo Systems, Cambridge, MA, Piezo strip T220-A4-303) that then deflects the whiskers (Figs. 1d and 2d). To construct the whisker stimulator a 1 cm segment of a 221 series nylon strip connector (Cooper Interconnect) was attached to the base of the tether adjacent to the three spacer holes with quick drying epoxy to act as an anchor for the stimulator (Fig. 1a black). Two 6 cm long 17 gauge stainless steel tubes (0.058 OD 0.042 ID, Small parts Inc., 304-RW 17GA) were inserted into two adjacent holes on the 1 cm nylon strip and tether. The stainless steel tubes were then immobilized using quick drying epoxy. Moving the whisker stimulator 6 cm above the rodent’s head served two purposes (Figs. 1b and 2b). First, this distance allowed room for the piezo strip assembly to sit above the rodent’s whiskers. Second, it helped to minimize any vibration of the rodent’s head by the piezo strip.
Fig. 1.
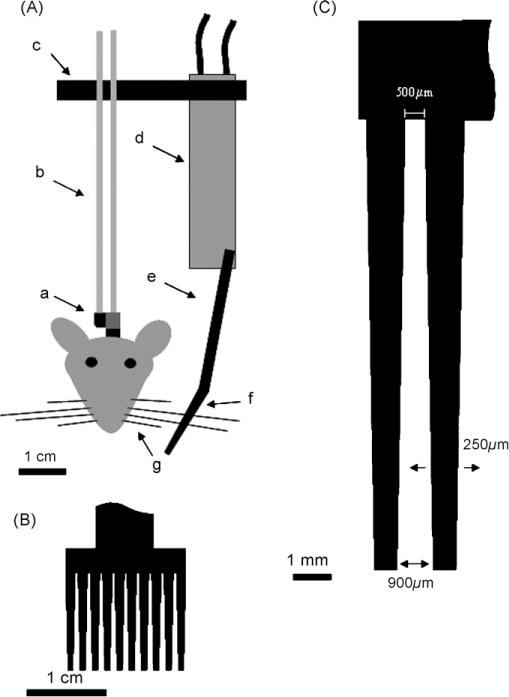
Schematic of whisker stimulator with magnified illustrations of the comb. (A) Illustration of the entire whisker stimulator. (a) Tether (grey) with 1 cm nylon strip for whisker stimulator (black) attached to the headbolt assembly that was surgically affixed to the skull; (b) Seventeen gauge 6 cm long stainless steel tubes; (c) 4 cm long nylon strip; (d) Piezo strip with quick-mount; (e) 3 cm long nylon strip; (f) 1.5 cm × 1.5 cm comb; (g) Undesired whiskers trimmed <1 cm so they do not come into contact with the whisker stimulator comb. Scale bar=1 cm; (B) magnified view of the comb that sits over the whiskers. Scale bar=1 cm; (C) magnified view of two of the ten teeth on the whisker stimulator comb. Each tooth is separated by 500 μm at the base and 900 μm at the tip of the comb. When the piezo strip is activated the comb is displaced 250 μm in each direction. Scale bar=1 mm.
Fig. 2.
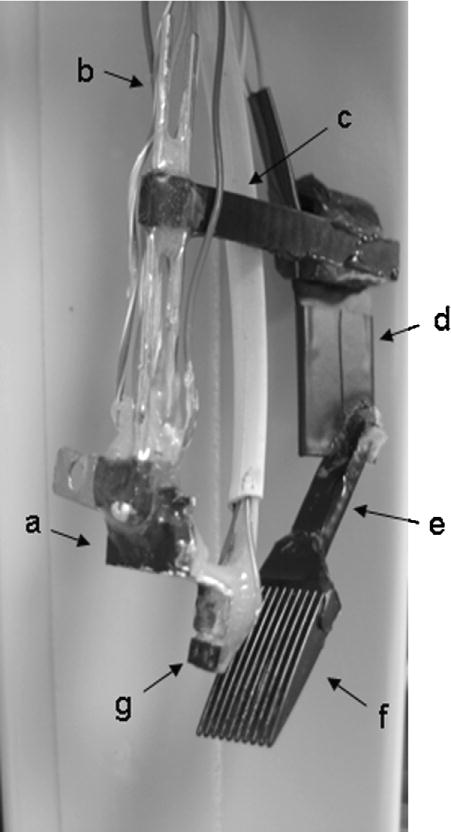
Whisker stimulator attached to the tether used for eyeblink conditioning in mice. (a) Tether that will be attached to headbolt assembly on mouse’s skull; (b) seventeen gauge 6 cm long stainless steel tubes; (c) 4 cm long nylon strip; (d) piezo strip covered in black electrical tape; (e) 3 cm long nylon strip; (f) 1.5 cm ×1.5 cm comb (g) optic sensor used to monitor eyeblinks; necessary for eyeblink conditioning, not necessary for whisker stimulation alone.
To bring the piezo strip above the whiskers the last two holes on a 4 cm nylon strip segment (Figs. 1c and 2c) were inserted 1 cm from the top of the stainless steel tubes and then anchored in place with quick drying epoxy. A “quick-mount” with piezo strip assembly (Piezo Systems, Cambridge, MA, Piezo strip T220-A4-303; Quick-mount Q220-A4-303YB) was then attached to the other end of the 4 cm nylon strip. The quick mount greatly simplifies attaching electrical leads to the piezo strip and offers a stable structure for attaching the nylon strip; however, it is not necessary for construction of the whisker stimulator. With skill and special care electrical leads can be soldered to each side of the piezo strip. However, for simplicity of construction this description will use the quick-mount. The piezo is then covered with electrical tape. To the opposite end of the piezo strip a 3 cm long nylon strip with a 1.5 cm × 1.5 cm comb at the opposite end, was attached. The comb contained 10 teeth. Each tooth was 1000 μm wide at the base and tapered to 600 μm at the tip. The distance between each tooth was 500 μm at the base and increased to 900 μm at the tip (Fig. 1). Great care was taken to insure that the comb was positioned approximately 1 cm from the whisker pad. This allowed enough room for the rodent to groom their whiskers during training.
For activation of the piezo strip 120 V from a United States electrical outlet was reduced to 22 V with a rheostat and gated using a 5V DC miniature solid state relay (Magnecraft; W226R-7-5A1) controlled with a custom made behavioral program on LabView Ver 5.0 (Fig. 3). Activation of the whisker stimulator generated a rostral-caudal 60 Hz 250 μm deflection of the comb (Fig. 1C). Since the whiskers are much thinner than the spacing between each tooth on the comb (whisker thickness is approximately 40 μm) and since the whiskers are not physically attached to the comb, this whisker stimulator is not useful if extremely precise whisker deflections are required. Rather, the whisker stimulator offers a much needed tool for time controlled natural stimulation of the whiskers. Activation of specific whiskers can be achieved by trimming the undesired whiskers less than 1 cm from the whisker pad so they will not come into contact with the stimulator comb. The comb can be positioned farther from the face to allow for longer undesired whiskers; however, by increasing the distance between the comb and the whisker pad one decreases the probability that the shorter whiskers closer to the rodent’s snout would be stimulated.
Fig. 3.
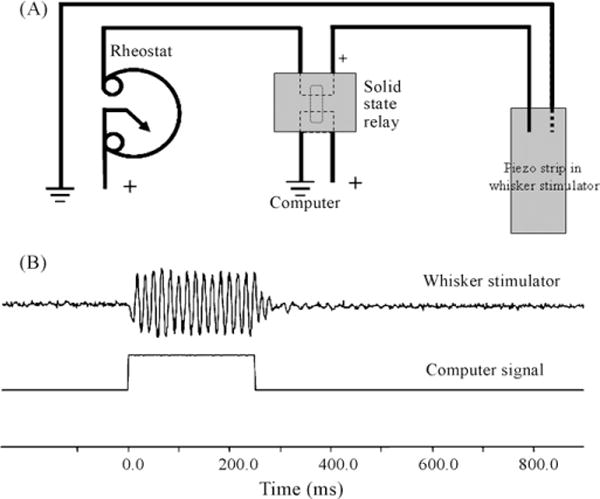
Activation of whisker stimulator can be controlled via a computer switch interface with millisecond precision. (A) Circuit diagram for activation of piezo strip in the whisker stimulator. For a schematic of the solid state relay refer to the manufacture Magnecraft part number W226R-7-5A1 and (B) illustration of activation of the whisker stimulator with the corresponding computer signal. To monitor displacement of the whisker stimulator an optic sensor used for eyeblink conditioning was focused on a single tooth on the comb during activation of the whisker stimulator. Note there is no lag between the computer signal and initiation of whisker stimulation.
3. Conditioning
To illustrate the effectiveness of the whisker stimulator 3-month old C57Bl6 male mice kept on a standard 14:10 light/dark cycle and given food and water ad libitum were trained on a trace-250 eyeblink conditioning paradigm with whisker stimulation as the conditioned stimulus. We have previously demonstrated in rabbits that whisker stimulation can be used as an effective conditioned stimulus in delay and trace eyeblink conditioning (Das et al., 2001; Galvez et al., 2006). Nine mice were trained with a trace- and 4 with a pseudo-conditioning paradigm. For trace-eyeblink conditioning, freely moving, tethered mice were placed into a circular training chamber (diameter=12.5 cm) and conditioned with a 250 ms conditioned stimulus (CS) via the described whisker stimulator (Figs. 1 and 2). The CS was followed by a 250ms trace interval and then a 100ms unconditioned stimulus sufficient to cause reliable eye blinks (US; 0.25–2 mA periorbital square wave shock, 60 Hz, 0.5ms pulses; Tseng et al., 2004). A custom made optic sensor attached to the tether positioned directly in front of the mouse’s eye as described in Weiss and Disterhoft (2008) was used to monitor eyeblinks. Mice were given 30 trials per session with a mean inter-trial interval (ITI) of 45s (randomly varied within 30–60s) per day. Pseudo-conditioned mice randomly received 30 whisker deflections and 30 periorbital shocks with a mean ITI of 22 s (randomly varied within 15–30s) per day. Four training sessions were given. A CR, closure of the eyelid monitored via change in voltage from an infrared reflective sensor, was defined as at least a four standard deviation change in voltage above baseline persisting throughout the 15ms immediately prior to US onset (Fig. 4). An alpha response was defined as at least a four standard deviation change in voltage above baseline that occurred within 35 ms of CS onset. The two types of responses were not mutually exclusive.
Fig. 4.
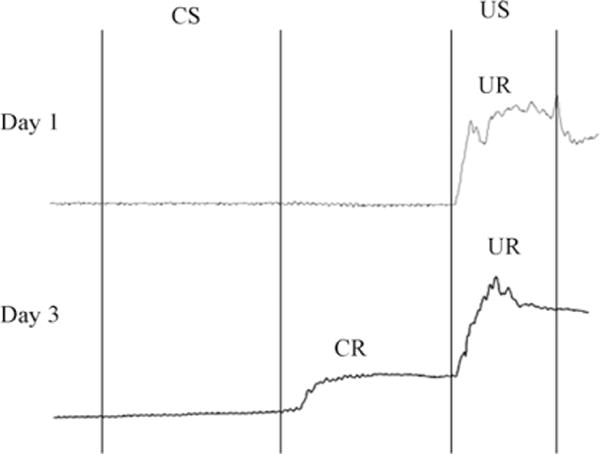
Illustration of eyelid movement during a single trial on day 1 and 3 during trace eyeblink conditioning. CS=Conditioned stimulus; US=unconditioned stimulus; CR=conditioned response; UR=unconditioned response. (A) A single trial on day 1. Note the mouse does not blink in response to whisker stimulation (CS) or during the trace interval. The mouse does exhibit a blink (UR) in response to the peri-orbital shock (US). (B) A single training trial on day 3. Note the mouse uses the whisker stimulation as a signal to blink during the trace interval in anticipation of the US.
Training was done in the presence of 70db white noise. We determined that activation of the piezo strip in the whisker stimulator produces a <55db auditory stimulus. Seventy db white noise has proven sufficient for masking the mechanical noise produced by the piezo strip in rabbits (Galvez et al., 2007). During training the mice appeared comfortable inside the conditioning chamber and were able to support the whisker stimulator. They tended to settle into one portion of the chamber and did not struggle against the tether. Following conditioning the comb on the whisker stimulator was detached and the trace-conditioned mice were given one additional session of conditioning. This test was to determine if the mice were using whisker deflections as the CS or if they were using an external cue that had not been controlled for, such as a noise or vibration from the piezoelectric device, to learn the association.
4. Results
The following analysis demonstrates that we were able to control deflection of mouse facial whiskers with millisecond precision in an awake freely moving learning paradigm with the described whisker stimulator (Fig. 3B). Utilizing the whisker stimulator in a trace-250 eyeblink paradigm, we found that mice exhibited a significant increase in the number of conditioned responses (Fig. 5; F(3,33) = 7.09; p<0.001), demonstrating that they were able to learn that whisker stimulation was associated with, and predicted a mild periorbital shock. The conditioned response on the last day of training had a mean onset of 140 ± 14ms and peak time of 377 ± 36ms after CS onset. Only one of the mice tested exhibited blinks with an onset latency less than 35ms from CS onset (mean onset=15 ± 0.5ms; mean duration=24 ± 1ms), consistent with previously described alpha responses (Solomon et al., 1986). Analysis of the unconditioned response (UR) properties on the first day of training revealed a mean UR peak time of 727 ± 13ms and 733 ± 39ms and the UR duration of 221 ± 10ms and 213 ± 10 ms for trace and pseudo conditioned animals, respectively. Removal of the comb from the stimulator resulted in a drop in performance to pseudo-conditioning levels (Fig. 5; Auditory Test). This analysis demonstrates that the mice learned to associate whisker stimulation with the periorbital shock and not another uncontrolled external cue such as noise or head vibrations.
Fig. 5.
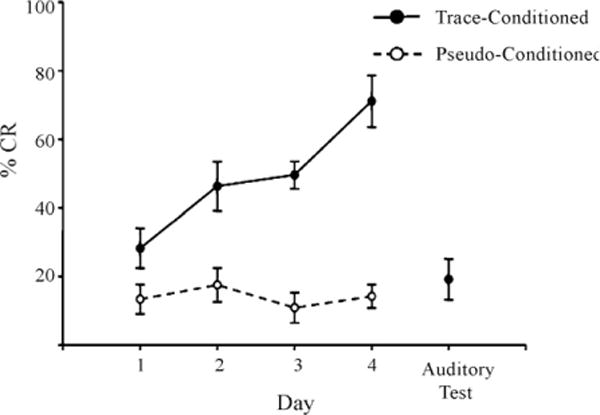
Using the described whisker stimulator to deflect whiskers as a conditioned stimulus, mice were able to learn forebrain-dependent, trace eyeblink conditioning. The percent number of conditioned responses (%CR) across the four days of training significantly increased for the trace-conditioned (filled circles, solid line) compared to the pseudo-conditioned mice (open circles, dotted line; F(3,33) = 7.09; p< 0.001). For the last day of training (Auditory Test) the comb was detached from the whisker stimulator and the trace-conditioned mice were given one additional day of training. Note the drop in performance to pseudo-conditioned levels during the auditory test indicating that the mice utilized whisker stimulation to learn the association. Error bars = standard error of the mean.
5. Discussion
The somatosensory barrel pathway has proven to be a very useful sensory system for studying experience-induced changes in neuronal processing. However, it has been difficult to mechanically stimulate the facial whiskers in a time-sensitive manner in the unanesthetized animal. We have described a technique for precisely timed stimulation of facial whiskers in freely moving mice. We then utilized this method for stimulating the whiskers in a time-sensitive, forebrain-dependent task, trace eyeblink conditioning. This behavioral analysis clearly demonstrates the ability of the stimulator to activate whiskers on a millisecond time scale, an important feature in many learning paradigms.
The following study is not the first demonstration of whisker stimulation in an awake learning paradigm; however, the method we have described has many advantages over previously published methods. One of the first attempts to stimulate whiskers in a time-sensitive paradigm utilized an ingenious technique in which small metal wires were glued to the whiskers (Melzer et al., 1985). The mouse was then placed into an electromagnet that could oscillate the magnetic field and stimulate the whiskers. One major advantage of this paradigm is the experimenter’s ability to control exactly what whiskers were deflected; however, this paradigm has the disadvantage of requiring that a foreign object be permanently attached to the rodent’s whiskers. Rodents often spend an inordinate amount of time trying to remove any foreign objects attached to their whiskers. This would undoubtedly increase the total stimulation to those whiskers and often result in the mouse either chewing the wire off or physically removing their whisker entirely when returned to the home cage between training sessions. With our stimulator one can control which whiskers are stimulated by trimming the undesired whiskers less than 1cm (the distance between the rodent’s face and the stimulator). Furthermore, our whisker stimulator has the added advantage that nothing needs to be physically attached to the whiskers. After the experimental session the stimulator is removed from the headbolt and the mouse is allowed to return to its home cage, decreasing the probability of increased stimulation of the target whiskers while the mouse is not being trained. Furthermore, not having anything physically attached to the whiskers dramatically decreases the probability that the rodent would lose its whiskers over several days of training, increasing the likelihood of the mouse completing the experiment.
To stimulate rodent whiskers many investigators have also utilized experimenter-applied hand stimulation and rodent self-stimulation of the desired whiskers. With hand stimulation the investigator manually deflects the rodent’s whiskers during training (Siucinska and Kossut, 1996, 2004; Musial et al., 1998; Jablonska et al., 1999). Although these studies report precise timing in whisker stimulation, one can imagine the difficulty with keeping the timing of whisker stimulation consistent or time locked to another event. Furthermore, the total time of whisker simulation using manual stimulation would need to be on the time scale of seconds to minutes, the described stimulator is precise to a time scale of milliseconds. With self-stimulation the investigator relies on the individual rodent to stimulate its own whiskers when exploring an environment. Behavioral tasks that have used this method either remove all undesired whiskers and allow the rodent to explore its environment normally (Diamond et al., 1993, 1994; Armstrong-James et al., 1994; Goldreich et al., 1998) or train the rodent to discriminate different textured surfaces (Arabzadeh et al., 2003, 2004) or object orientations (Polley et al., 2004). Although these studies have the advantage of employing a more naturalistic whisker stimulation, they again lack the ability for precisely timed stimulation of the facial whiskers. Furthermore, with self-stimulation one cannot control for the amount or duration of whisker stimulation. The currently described whisker stimulator elegantly deals with all of these issues. Activating the whisker stimulator via a computer controlled switch allows the investigator to achieve not only precision in the onset time of whisker stimulation but also in its duration (Fig. 3). Furthermore, by having the whisker stimulator activated via a computer controlled switch one can also time lock whisker stimulation to delivery of another stimulus, often vital for behavioral training.
Although the whisker stimulator has many advantages over the currently published methods for stimulating whiskers in an awake freely moving mouse, it does have some disadvantages. The largest disadvantage with this system is the need to have a head-bolt surgically attached to the mouse to anchor the stimulator and keep it positioned over the whiskers. Although this is a minor surgery, anesthesia does increase the risk of mortality. Another difficulty with the described stimulator is determining precisely which whisker is being vibrated every time the stimulator is activated. Some precision can be achieved by trimming the whiskers one does not want stimulated less then 1 cm (the distance from the whisker pad to the whisker stimulator). This would insure that the undesired whiskers would not be stimulated. Unfortunately by trimming the undesired whiskers one also runs the risk of inducing plasticity in the trigeminal-whisker pathway. Trimming whiskers has been shown to induce rapid changes in the size of the receptive fields for the trimmed whiskers (Diamond et al., 1993, 1994; Armstrong-James et al., 1994). However, it is important to emphasize that unlike our suggestion of trimming the whiskers to 1 cm, these studies trimmed the whiskers as close to the face as possible. Although it is feasible that even trimming the whiskers 1 cm long would induce some plasticity, to date there have not been any studies directly addressing this issue. Any trimming induced plasticity can be controlled for by trimming the contralateral whiskers and then making within animal comparisons. Alternatively, pseudoconditioning control procedures can be used in animals with comparably trimmed whiskers to define behavioral and neural changes attributable to the training procedure as opposed to those due to the whisker trimming itself.
The current manuscript describes a method for precisely timed stimulation of rodent whiskers in a freely moving awake preparation. To demonstrate the potential of the stimulator, mice were conditioned on a time-sensitive forebrain-dependent task, trace eyeblink conditioning, using whisker stimulation as the conditioned stimulus. Previous work from our laboratory has demonstrated that whisker stimulation can be used as a conditioned stimulus in eyeblink conditioning with rabbits (Das et al., 2001; Galvez et al., 2006). Our study illustrates the usefulness of the whisker stimulator in time-sensitive freely moving behavioral paradigms in awake rodents; thus expanding our potential for examining integration of sensory information to produce experience-induced neuronal plasticity in these species.
References
- Arabzadeh E, Petersen RS, Diamond ME. Encoding of whisker vibration by rat barrel cortex neurons: implications for texture discrimination. J Neurosci. 2003;23:9146–54. doi: 10.1523/JNEUROSCI.23-27-09146.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arabzadeh E, Panzeri S, Diamond ME, Erchova IA, Krupa DJ, Wiest MC, et al. Whisker vibration information carried by rat barrel cortex neurons rapid fluctuations in rat barrel cortex plasticity layer-specific somatosensory cortical activation during active tactile discrimination synaptic responses to whisker deflections in rat barrel cortex as a function of cortical layer and stimulus intensity. J Neurosci. 2004;24:6011–20. [Google Scholar]
- Armstrong-James M, Diamond ME, Ebner FF. An innocuous bias in whisker use in adult rats modifies receptive fields of barrel cortex neurons. J Neurosci. 1994;14:6978–91. doi: 10.1523/JNEUROSCI.14-11-06978.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Weiss C, Disterhoft JF. Eyeblink conditioning in the rabbit (oryctolagus cuniculus) with stimulation of the mystacial vibrissae as a conditioned stimulus. Behav Neurosci. 2001;115:731–6. doi: 10.1037//0735-7044.115.3.731. [DOI] [PubMed] [Google Scholar]
- Diamond ME, Armstrong-James M, Ebner FF. Experience-dependent plasticity in adult rat barrel cortex. Proc Natl Acad Sci USA. 1993;90:2082–6. doi: 10.1073/pnas.90.5.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond ME, Huang W, Ebner FF. Laminar comparison of somatosensory cortical plasticity. Science. 1994;265:1885–8. doi: 10.1126/science.8091215. [DOI] [PubMed] [Google Scholar]
- Galvez R, Weible AP, Disterhoft JF. Cortical barrel lesions impair whisker-CS trace eyeblink conditioning. Learn Mem. 2007;14:94–100. doi: 10.1101/lm.418407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez R, Weiss C, Weible AP, Disterhoft JF. Vibrissae-signaled eyeblink conditioning induces somatosensory cortical plasticity. J Neurosci. 2006;26:6062–8. doi: 10.1523/JNEUROSCI.5582-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldreich D, Peterson BE, Merzenich MM. Optical imaging and electrophysiology of rat barrel cortex. II. Responses to paired-vibrissa deflections. Cerebral Cortex. 1998;8 doi: 10.1093/cercor/8.2.184. [DOI] [PubMed] [Google Scholar]
- Greenough WT, Chang FL. Dendritic pattern formation involves both oriented regression and oriented growth in the barrels of mouse somatosensory cortex. Brain Res. 1988;471:148–52. doi: 10.1016/0165-3806(88)90160-5. [DOI] [PubMed] [Google Scholar]
- Guic-Robles E, Jenkins WM, Bravo H. Vibrissal roughness discrimination is barrelcortex-dependent. Behav Brain Res. 1992;48:145–52. doi: 10.1016/s0166-4328(05)80150-0. [DOI] [PubMed] [Google Scholar]
- Jablonska B, Gierdalski M, Kossut M, Skangiel-Kramska J. Partial blocking of NMDA receptors reduces plastic changes induced by short-lasting classical conditioning in the SI barrel cortex of adult mice. Cereb Cortex. 1999;9:222–31. doi: 10.1093/cercor/9.3.222. [DOI] [PubMed] [Google Scholar]
- McLaughlin J, Skaggs H, Churchwell J, Powell DA. Medial Prefrontal cortex and pavlovian conditioning: trace versus delay conditioning. Behav Neurosci. 2002;116:37–47. [PubMed] [Google Scholar]
- Melzer P, Van der Loos H, Dorfl J, Welker E, Robert P, Emery D, et al. A magnetic device to stimulate selected whiskers of freely moving or restrained small rodents: its application in a deoxyglucose study. Brain Res. 1985;348:229–40. doi: 10.1016/0006-8993(85)90441-x. [DOI] [PubMed] [Google Scholar]
- Moyer JR, Jr, Deyo RA, Disterhoft JF. Hippocampectomy disrupts trace eye-blink conditioning in rabbits. Behav Neurosci. 1990;104:243–52. doi: 10.1037//0735-7044.104.2.243. [DOI] [PubMed] [Google Scholar]
- Musial P, Kublik E, Panecki SJ, Wrobel A. Transient changes of electrical activity in the rat barrel cortex during conditioning. Brain Res. 1998;786:1–10. doi: 10.1016/s0006-8993(97)01290-0. [DOI] [PubMed] [Google Scholar]
- Polley DB, Kvasnak E, Frostig RD. Naturalistic experience transforms sensory maps in the adult cortex of caged animals. Nature. 2004;429:67–71. doi: 10.1038/nature02469. [DOI] [PubMed] [Google Scholar]
- Rice FL, Gomez C, Barstow C, Burnet A, Sands P. A comparative analysis of the development of the primary somatosensory cortex: interspecies similarities during barrel and laminar development. J Comp Neurol. 1985;236:477–95. doi: 10.1002/cne.902360405. [DOI] [PubMed] [Google Scholar]
- Schneiderman N, Gormezano I. Conditioning of the nictitating membrane of the rabbit as a function of CS-US interval. J Comp Physiol Psychol. 1964;57:188–95. doi: 10.1037/h0043419. [DOI] [PubMed] [Google Scholar]
- Smith MC. CS-US interval and US intensity in classical conditioning of the rabbit’s nictitating membrane response. J Comp Physiol Psychol. 1968;66:679–87. doi: 10.1037/h0026550. [DOI] [PubMed] [Google Scholar]
- Simons DJ, Woolsey TA. Morphology of Golgi-Cox-impregnated barrel neurons in rat SmI cortex. J Comp Neurol. 1984;230:119–32. doi: 10.1002/cne.902300111. [DOI] [PubMed] [Google Scholar]
- Siucinska E, Kossut M. Short-lasting classical conditioning induces reversible changes of representational maps of vibrissae in mouse SI cortex—a 2DG study. Cereb Cortex. 1996;6:506–13. doi: 10.1093/cercor/6.3.506. [DOI] [PubMed] [Google Scholar]
- Siucinska E, Kossut M. Experience-dependent changes in cortical whisker representation in the adult mouse: a 2-deoxyglucose study. Neuroscience. 2004;127:961–71. doi: 10.1016/j.neuroscience.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Solomon PR, Vander Schaaf ER, Thompson RF, Weisz DJ. Hippocampus and trace conditioning of the rabbit’s classically conditioned nictitating membrane response. Behav Neurosci. 1986;100:729–44. doi: 10.1037//0735-7044.100.5.729. [DOI] [PubMed] [Google Scholar]
- Tseng W, Guan R, Disterhoft JF, Weiss C. Trace eyeblink conditioning is hippocampally dependent in mice. Hippocampus. 2004;14:58–65. doi: 10.1002/hipo.10157. [DOI] [PubMed] [Google Scholar]
- Weiss C, Bouwmeester H, Power JM, Disterhoft JF. Hippocampal lesions prevent trace eyeblink conditioning in the freely moving rat. Behav Brain Res. 1999;99:123–32. doi: 10.1016/s0166-4328(98)00096-5. [DOI] [PubMed] [Google Scholar]
- Weiss C, Disterhoft J. Evoking blinks with natural stimulation and detecting then with a noninvasive optical device: a simple, inexpensive method for use with freely moving animals. J Neurosci Methods. 2008;173:108–13. doi: 10.1016/j.jneumeth.2008.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolsey TA, Van der Loos H. The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. The description of a cortical field composed of discrete cytoarchitectonic units. Brain Res. 1970;17:205–42. doi: 10.1016/0006-8993(70)90079-x. [DOI] [PubMed] [Google Scholar]
- Woolsey TA, Dierker ML, Wann DF. Mouse SmI cortex: qualitative and quantitative classification of golgi-impregnated barrel neurons. Proc Natl Acad Sci USA. 1975;72:2165–9. doi: 10.1073/pnas.72.6.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]


