Abstract
Intrarectal infusion of butyrate improves colorectal disorders including ulcerative colitis (UC). However, it is not established whether systemically administered butyrate benefits such patients. The current study aimed at exploring and comparing the potential of intraperitoneally, intrarectally, and orally administered butyrate against acetic acid (AA)-induced UC in rats. Intrarectal administration of 2 ml of 50% AA was done after or without prior treatment of rats for 7 consecutive days with 100 mg/kg sodium butyrate (SB) intraperitoneally, intrarectally, or orally. Rats were sacrificed after 48 h of AA-treatment. Subsequently, colon sections were processed routinely for histopathological examination. We clinically observed diarrhea, loose stools, and hemoccult-positive stools, and histologically, epithelial loss and ulceration, crypt damage, goblet cell depletion, hemorrhage, and mucosal infiltration of inflammatory cells. The changes were significantly reduced by intraperitoneal, intrarectal, or oral butyrate, with intraperitoneal butyrate exhibiting the highest potency. It is concluded that intraperitoneal administration of butyrate abrogates the lesions of AA-induced UC and its potency surpasses that of intrarectal or oral butyrate.
Keywords: Butyrate, Oral administration, Intraperitoneal administration, Intrarectal administration, Acetic acid, Ulcerative colitis
1. Introduction
Ulcerative colitis (UC) is a form of inflammatory bowel disease (IBD) whose exact etiology remains enigmatic. Perturbations of the immune system along the gut are implicated in playing a central role. In its active form, UC is characterised by constant diarrhea mixed with blood of gradual onset and weight loss. Blood can also be seen on rectal examination and varying degrees of abdominal pain ranging from mild discomfort to painful bowel movements and/or abdominal cramping during bowel movements may accompany the disease (Torpy et al., 2012). Morphologically, UC is mainly characterized by open sores or ulcers in the colon, loss of epithelium and goblet cells, and infiltration of colonic mucosa by inflammatory cells (Malago and Nondoli, 2008).
Although the exact cause of UC is unknown, it is suggested that over stimulation or inadequate regulation of the mucosal immune system is a major pathophysiologic cause of the disease. Continual activation of the immune system to produce pro-inflammatory chemokines like interleukin (IL)-8 and their subsequent attraction of inflammatory cells that infiltrate the colon mucosa are cascade events that cause the chronic inflammation and ulceration of the colonic epithelium (Malago and Nondoli, 2008; Ke et al., 2013; Liu et al., 2013). The production of these pro-inflammatory cytokines is mediated mainly by transcriptional activation of nuclear factor κB (NF-κB) and mitogen activated protein kinase (MAPK) pathways (Malago et al., 2002; Ke et al., 2013). Several therapeutic interventions such as butyrate enemas modulate the activation of these pathways and suppress the production of pro-inflammatory cytokines to protect the colon against inflammatory injury (Venkatraman et al., 2003; Malago et al., 2005; Ke et al., 2013).
Earlier studies that linked the development of UC and butyrate levels in the colon, observed that deficiency of butyrate leads to disease development and that restoration of butyrate levels by intracolonic infusion treats UC (Roediger, 1980). Since then, butyrate enemas have popularly been used as medicaments stemming from their potential to impart beneficial attributes to the colon. This potential involves an increase in mechanical strength of injured colonic mucosa to hasten the healing process (Bloemen et al., 2010; Mathew et al., 2010), suppression of IL-8 production by intestinal epithelial cells to protect against the inflammatory process (Malago et al., 2005), and clinical remission of UC by protecting against inflammatory and oxidative stress parameters of the disease (Hamer et al., 2010b). Much as butyrate tends to impart a protective effect, several authors have indicated failures or limited success of butyrate to relieve IBD patients (Harig et al., 1989; Sanderson, 1997; Hamer et al., 2010b). One of the proposed reasons includes presence of IL-8 in the intestinal mucosa, which is either activated (Fusunyan et al., 1998; 1999) or inhibited (Huang et al., 1997; Wu et al., 1999) by different doses of butyrate (Malago et al., 2005). Thus high concentrations of butyrate correlate with elevated IL-8 levels in the intestinal mucosa of IBD patients (Dabard et al., 1987; Treem et al., 1994) whereas low levels diminish the disease activity (O'Morain et al., 1984). As the intestinal concentration of butyrate is mainly determined by diet and commensal microbial profile which varies among individuals, the effect of butyrate on chronic IBD remains controversial.
To impart its attributes, butyrate needs to be absorbed by the colonocytes and undergo the subsequent metabolism. Butyrate absorption mainly occurs in the proximal colon whose function is impaired during UC due to damaged epithelial mucosa (Thibault et al., 2010). As a result, there is impairment of butyrate metabolism even when the colon is saturated with butyrate (de Preter et al., 2011; 2012; Kovarik et al., 2011). Furthermore, addition of compounds interfering with the β-oxidation pathway such as carnitine has no effect on butyrate metabolism in active UC (de Preter et al., 2011) and has little effect in mild-to-moderate UC following oral intake (Mikhailova et al., 2011). These observations suggest that absorption of intrarectally administered butyrate can be impaired in active UC and may significantly lower the expected beneficial effects of butyrate. Instead, UC patients could most probably benefit more when butyrate reaches the colon through a systemic route. Unfortunately the effects of parenteral routes of high absorptive properties to the benefit of butyrate in UC have not been studied. In this study we explored the differences in the effect of oral, intraperitoneal, and intrarectal administration of butyrate on a rat model of acetic acid (AA)-induced UC.
2. Materials and methods
2.1. Animals
The experiment used 40 male Wistar rats (Sokoine University of Agriculture, Morogoro, Tanzania) aged 26 weeks and weighing 110 to 130 g. The animals were kept in a restricted access room in which the temperature was controlled and a 12-h light/dark cycle was observed. They were randomly kept in 10 cages (serving as treatment groups) each containing 4 animals (Table 1). The animals were allowed 2 weeks to acclimatize in their groups prior to commencement of the experiment. They were fed standard laboratory diet and given drinking water ad libitum. The study was approved by the Animal Research Committee of the Sokoine University of Agriculture, Tanzania.
Table 1.
Animal groups and treatments
| Group | Treatment | Dose |
|
| Days 1–7 | Day 8 | ||
| 1 | SB | SB: 2 ml, 100 mg/kg, OS | NS: 2 ml, IR |
| 2 | SB | SB: 0.2 ml, 100 mg/kg, IP | NS: 2 ml, IR |
| 3 | SB | SB: 0.2 ml, 100 mg/kg, IR | NS: 2 ml, IR |
| 4 | SB+AA | SB: 2 ml, 100 mg/kg, OS | AA: 2 ml, IR |
| 5 | SB+AA | SB: 0.2 ml, 100 mg/kg, IP | AA: 2 ml, IR |
| 6 | SB+AA | SB: 0.2 ml, 100 mg/kg, IR | AA: 2 ml, IR |
| 7 | AA | NS: 0.2 ml, IR | AA: 2 ml, IR |
| 8 | Control | NS: 2 ml, OS | NS: 2 ml, IR |
| 9 | Control | NS: 0.2 ml, IP | NS: 2 ml, IR |
| 10 | Control | NS: 0.2 ml, IR | NS: 2 ml, IR |
All animals were sacrificed on Day 10. SB: sodium butyrate; AA: acetic acid; NS: normal saline; OS: per oral; IP: intraperitoneal; IR: intrarectal
2.2. Administration of AA to induce UC
In order to induce UC, we intrarectally administered 2 ml of 4% (v/v) AA (Rankem, New Delhi, India) using a soft, 1.5 mm diameter butterfly catheter (Neomedic Limited, 97 High Street, Rickmansworth, Hertfordshire, UK). The catheter was inserted into the anus as far as the proximal colon (about 8 cm long), and then AA was released slowly to ensure that it was in contact with the mucosa for sufficient time. After the chemical was administered, the catheter was withdrawn slowly to avoid any physical trauma to the intestinal mucosa.
2.3. Effect of butyrate on AA colitis
To explore the effect of butyrate on AA-induced UC, sodium butyrate (SB) (Merck Schuchardt OHG, Hohenbrunn, Germany) was given as indicated in Table 1. Briefly, SB was given at 100 mg/kg body weight orally, intraperitoneally, or intrarectally once a day for 7 consecutive days. On Day 8, rats received AA to develop UC for 48 h, during which clinical signs were observed. On Day 10 all animals were sacrificed humanely by chloroform. We provided either SB or physiological saline to matched control groups at a similar route, volume, dose, and time.
2.4. Clinical evaluation of UC
In order to clinically assess AA colitis activity, we examined the rats for stool consistency which included loose stool and diarrhea, occult and/or bleeding which included hemoccult positivity and gross bleeding, and body weight. We expressed the body weight as percentage weight change for each individual rat. It was calculated in comparison to that of Day 8 before administration of AA. From these data, a disease activity index was calculated as done previously (Malago and Nondoli, 2008) (Table 2).
Table 2.
Scoring of AA-induced colitis activity index
| Score | Weight loss (%) | Stool consistency1 | Occult/gross bleeding |
| 0 | None | Normal | Normal |
| 1 | 1–5 | ||
| 2 | 5–10 | Loose stool | Hemoccult positive |
| 3 | 10–20 | ||
| 4 | >20% | Diarrhea | Gross bleeding |
The AA colitis activity index was obtained by combining the scores of weight loss (calculated to the day of, but before, AA administration), stool consistency, and bleeding divided by 3
Normal stools: well formed pellets; Loose stools: pasty and semi-formed stools, which do not stick to the anus; Diarrhea: liquid stools that stick to the anus
2.5. Evaluation of histological changes
Midline laparotomy was done to open the sacrificed rats. Accessed colon was longitudinally opened, fixed with 10% (0.1 g/ml) neutral buffered formalin, and embedded in paraffin. It was then sectioned at 4 μm slices that were deparaffinised on microscopic glass slides before staining with hematoxylin and eosin (H&E). After mounting, the slides were observed under a light microscope (Olympus BX41, Olympus, Japan). Two independent pathologists assessed and quantitated in a blind manner the mucosal integrity for histological evaluation of tissue damage as done previously (Malago and Nondoli, 2008). The evaluation was done on distal colon using a 0–3 scoring scale as shown in Table 3.
Table 3.
Histological score of colitis
| Score | Loss of epithelium (%) | Crypt damage1 (%) | Depletion of goblet cells | Infiltration of inflammatory cells |
| 0 | None | None | None | None |
| 1 | <5 (mild) | <10 (mild) | Mild | Mild |
| 2 | 5–10 (moderate) | 10–20 (moderate) | Moderate | Moderate |
| 3 | >10 (severe) | >20 (severe) | Severe | Severe |
Crypt damage was evaluated as percentage loss of crypt
2.6. Statistical analysis
Numerical values were calculated and presented as mean±standard error of the mean (SEM). One-way analysis of variance (ANOVA) was used to assess statistical significance between the mean values of control and treated rats with comparison of means. Differences were considered significant at 95% confidence interval using Student’s t-test.
3. Results
3.1. Clinical AA colitis and effect of butyrate
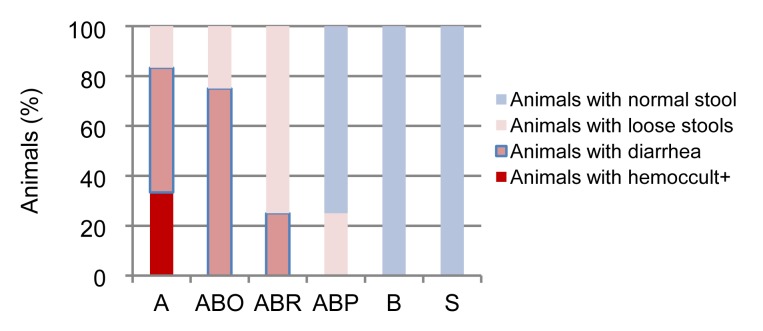
Fig. 1 shows the clinical signs of AA colitis in rats with or without prior treatment with butyrate. As shown in this figure, AA induced hemoccult-positive stool, diarrhea, and loose stool. The hemoccult-positive stool was the severest sign observed in one third (33%) of the animals. This was followed by diarrhea in 50% of AA colitis animals. The least severe sign was loose stool encountered in 17% of these animals. None of the animals receiving AA had a normal stool.
Fig. 1.
Effect of sodium butyrate on clinical indices of colitis
Rats were treated intrarectally with 4% acetic acid alone (A) or after 7 consecutive days of treatment with 100 mg/kg sodium butyrate orally (ABO), intrarectally (ABR), or intraperitoneally (ABP). Values for control rats receiving butyrate alone (B) or physiological saline (S) are also indicated. Results are expressed as the percentage of animals. Eight animals were used for each experimental group
Butyrate effect on AA colitis was exhibited by improvement of colitis signs in a route-dependent manner. According to Fig. 1, all tested butyrate routes (oral, intraperitoneal, and intrarectal) ameliorated the hemoccult-positive stools in all animals. It is apparent from this figure that the potency of butyrate to suppress the severity of AA colitis was route-dependent. Oral butyrate was the least potent as it had a high number of animals (75%) with diarrhea and few (25%) with loose stools. This potency increased with intrarectal butyrate where diarrhea was reduced further by 50%, leading to 75% of animals showing loose stools and only 25% having diarrhea. The strongest butyrate effect was observed in animals receiving intraperitoneal butyrate. In this case, diarrhea had disappeared, 25% of animals showed loose stools, and the majority (75%) recovered from AA colitis signs as manifested by normal stools. Rats receiving butyrate alone or normal saline in any of the three routes did not show any of the clinical signs and had normal stools.
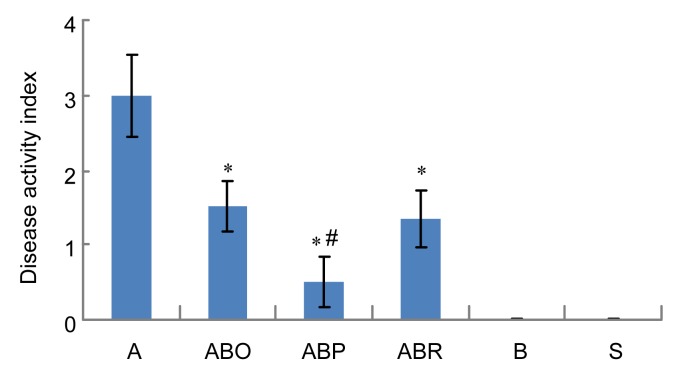
3.2. AA colitis activity index
Fig. 2 indicates that AA induced a disease activity index of about 3. This activity was reduced significantly by all routes of butyrate administration. Oral or intrarectal butyrate reduced the AA activity index about 2.5-fold whereas intraperitoneal butyrate reduced it 5-fold. Comparison among butyrate administration routes indicated a significant difference between intraperitoneal butyrate and either oral or intrarectal butyrate on reducing the AA activity index. In that case, intraperitoneal butyrate reduced the disease activity by 2-fold more than either oral or intrarectal butyrate. No disease activity was observed in animals receiving butyrate or normal saline alone.
Fig. 2.
Effect of sodium butyrate administration on acetic acid (AA) colitis disease activity index
Rats were treated intrarectally with 4% acetic acid alone (A) or after 7 consecutive days of treatment with 100 mg/kg sodium butyrate orally (ABO), intrarectally (ABR) or intraperitoneally (ABP). Values for control rats receiving butyrate alone (B) or physiological saline (S) are also indicated. Disease activity index is obtained by combining scores of weight loss, stool consistency, and bleeding divided by 3. Results are expressed as mean±SEM (n=8). * Significantly different from AA colitis group at P<0.05. # Significantly different from another route of butyrate administration at P<0.05
3.3. Gross pathology
Gross lesions of colon mucosa from rats treated with AA were edematous with hemorrhagic erosions scattered throughout the colon. In some animals, less severe lesions with edema of the colon, multiple mucosal erosions and marked congestion of mesenteric blood vessels were observed. The lesions were more severe in the distal colon than in the middle and proximal colons. Rats treated with butyrate orally or intrarectally prior to AA had less congestion of the colon without edema. Those given butyrate intraperitoneally had mild congestion and some appeared normal. The butyrate-treated and control rats had no gross pathological lesions (data not shown).
3.4. Histopathological findings
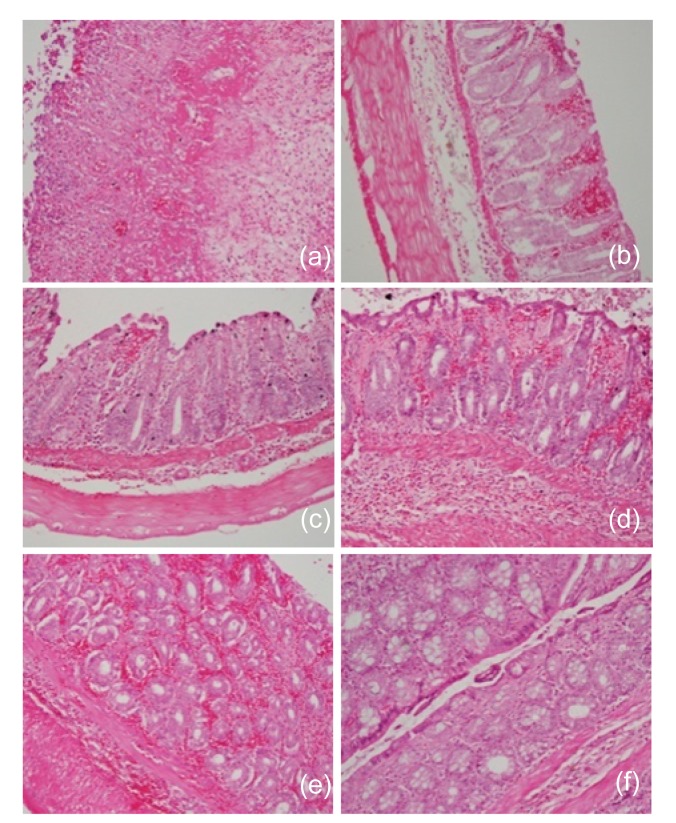
The observed histopathological lesions due to AA following or without prior treatment with SB are shown in Fig. 3. According to Fig. 3a, the histological changes due to AA were vivid. They included massive haemorrhage, complete absence of epithelial lining on the mucosal surface, severely damaged villi and crypts with subsequent loss of their architecture except in a few crypts, depletion of goblet cells, and severe infiltration of inflammatory cells. Compared with rats receiving butyrate prior to AA (Figs. 3b–3e), these rats were the most severely affected.
Fig. 3.
Histological sections of rat colon
Rats were treated intrarectally with 4% acetic acid alone (a), or following 100 mg/kg sodium butyrate orally (b), intraperitoneally (c), or intrarectally (d and e). (a) Massive hemorrhage, loss of epithelial lining, loss of crypt and villi, and massive infiltration of inflammatory cells due to acetic acid; (b) Considerable intact epithelium under which there is moderate hemorrhage between crypts and villi, loss of some goblet cells, and moderate infiltration of inflammatory cells in the submucosa; (c) A more intact epithelium, mild hemorrhage, and mild infiltration of inflammatory cells; (d) A considerable intact epithelium with severe hemorrhage underneath and destroyed villi and some crypts, and severe loss of goblet cells in villi, severe cellular infiltration in the submucosa; (e) Intercryptal hemorrhage and retention of crypt architecture; (f) The photomicrographs also include those of control rats treated with sodium butyrate alone with no significant changes. Each photo represents 8 animals (4 animals per treatment in duplicate experiments). Hematoxylin and eosin, original magnification ×200
Figs. 3b–3e show the effect of prior treatment of rats with butyrate on AA-induced UC. Rats receiving butyrate intrarectally had a considerably distorted but intact epithelial lining on the mucosal surface underneath which there was haemorrhage that occupied mainly the intervillous and intercryptal spaces. The villi were moderately damaged, their architecture could be traced with difficulty in some areas, and most of their goblet cells were lost. There was mild damage to the crypts and retention of most of their goblet cells. In addition, there was a vivid infiltration of inflammatory cells in the mucosa. There was little difference between these animals and those exposed to AA after oral butyrate. In the latter, villous damage was mild, the architecture of most villi was easily traceable and the loss of goblet cells was mild. Although the histology of the colon remained distorted in both treatments, when compared to sections of rats exposed to AA alone (Fig. 3a), it is obvious that intrarectal (Fig. 3d) or oral (Fig. 3b) butyrate significantly benefited rats against the AA-induced epithelial damage.
Rats receiving butyrate intraperitoneally prior to AA were least severely affected when compared to those given intrarectally or orally. Their colon histology looked almost normal with quite a good epithelial lining, intact villi and crypts, undamaged goblet cells, mild haemorrhage, and little mucosa infiltration by inflammatory cells (Fig. 3c). Indeed, these rats had highly improved from the UC lesions when compared to those not receiving butyrate (Fig. 3a).
The histological results of rats exposed to butyrate alone are shown in Fig. 3f. According to this figure, the epithelial lining is intact, the crypts are not damaged, goblet cell depletion is lacking or very low, and there is very mild inflammatory cells infiltrating the mucosa. These results are more or less the same as those from the normal control animals receiving normal saline (data not shown).
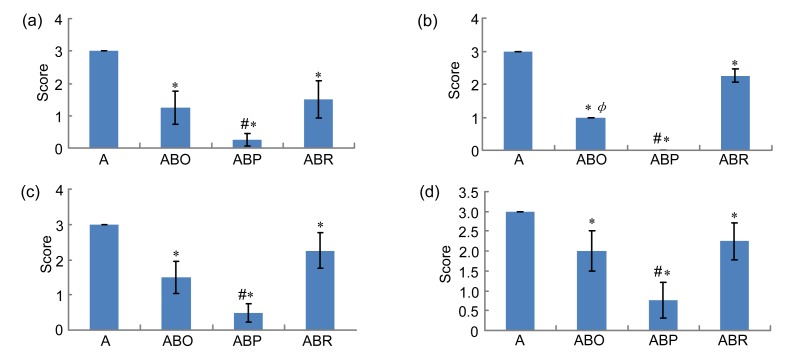
3.5. Scores of histopathological lesions
Fig. 4 presents scores of histopathological lesions. It is clear that exposure to AA led to a severe (score 3) loss of epithelium (Fig. 4a), crypt damage (Fig. 4b), depletion of goblet cells (Fig. 4c), and infiltration of inflammatory cells (Fig. 4d). Pre-treatment with butyrate significantly reduced the severity of these changes. The reduction of epithelial loss was to about half following oral or intrarectal butyrate and to almost complete abrogation when butyrate was given intraperitoneally (Fig. 4a). Crypt damage was reduced by one third (score 2, moderate damage), two thirds (score 1, mild damage), or complete abrogation (score 0, no damage) when butyrate was given intrarectally, orally, or intraperitoneally, respectively (Fig. 4b). The preventive effect of butyrate on goblet cell depletion varied from about 1-fold (moderate depletion) with intrarectal administration through 2-fold (moderate depletion) with oral butyrate to 6-fold (mild depletion) after intraperitoneal butyrate (Fig. 4c). Fig. 4d indicates that butyrate reduced the severe infiltration of inflammatory cells to moderate (score 2) when given orally or intrarectally and to mild (score 1) by intraperitoneal butyrate.
Fig. 4.
Histological scores of loss of epithelium (a), crypt damage (b), depletion of goblet cells (c), and infiltration of inflammatory cells (d)
Rats were treated intrarectally with 4% acetic acid (AA) alone (A) or after 7 consecutive days of treatment with 100 mg/kg sodium butyrate orally (ABO), intrarectally (ABR), or intraperitoneally (ABP). Results are expressed as mean±SEM of at least 8 individual rats. * Significantly different from AA exposed rats (P<0.05); # Significantly different from another routes of butyrate administration at P<0.05; Φ Significantly different between oral and intrarectal butyrate at P<0.05
Fig. 4 further indicates that the effects of intraperitoneal butyrate in preventing epithelial loss (Fig. 4a), crypt damage (Fig. 4b), depletion of goblet cells (Fig. 4c), and infiltration of inflammatory cells (Fig. 4d) were significantly different from those of oral or intrarectal administration of butyrate. When the effects of oral and intrarectal butyrate were compared, oral administration of butyrate tended to have a higher suppressive effect to these lesions, which was significant only for crypt damage (Fig. 4b). Butyrate alone did not cause any significant changes in the epithelium, crypt, goblet cells or infiltration of inflammatory cells into the colon mucosa.
4. Discussion
Our results on the AA-induced UC rat model have harmonized with those of other researchers. Previous studies have shown that intrarectal administration of AA to create a rat model of UC results in inflammation and ulceration in the lining of the colon. This can be seen histologically as distortion of crypt architecture, loss of epithelial cells, ulceration, haemorrhage, and neutrophil infiltration (Sotnikova et al., 2013; Minaiyan et al., 2014). In such a condition, the colon cannot absorb liquid from the stools, resulting in a larger volume of watery stools and subsequent weight loss (Zhao et al., 2014). The hemorrhagic and ulcerative colon mucosa may also bleed to produce bloody faeces (Sotnikova et al., 2013). The rat model of AA was thoroughly studied and established to correlate with human UC by several scientists including Fabia et al. (1992) who explored the effect of 4%, 6%, and 8% AA to the rat colon. They found that exposure of the colon to 4% AA for 15 s produced a uniform colitis in all treated rats with morphological similarities to human UC. Subsequent studies have reproduced this model with close resemblance to human UC in terms of pathogenesis, histopathological features and inflammatory mediator profile. Consistent noteworthy features of human colitis observed in these models include mucosal hemorrhage, loss of epithelial cells and ulceration, damage of crypts and distortion of crypt architecture, depletion of goblet cells, infiltration of inflammatory cells, particularly neutrophils in lamina propria, necrosis of mucosal and submucosal layers, vascular dilation, and edematous submucosa (La et al., 2003; Cetinkaya et al., 2005; Randhawa et al., 2014). Indeed, the morphological changes observed in our study were consistent with previous findings in rat models of colitis, which correlate well with human UC.
Topical administration of butyrate to cure colitis has been fairly well demonstrated (Scheppach et al., 1992; Hamer et al., 2010a; 2010b). This is done mainly through intrarectal administration of enemas that contain butyrate. The procedure is one of the earliest approaches to treat UC even in patients who had been unresponsive to or intolerant of standard therapy (Scheppach et al., 1992). The intrarectally administered butyrate needs to be absorbed before it works. Normally butyrate absorption mainly occurs in proximal colon whose function is impaired during UC. This hinders absorption of topically administered butyrate and may not benefit UC patients. However, butyrate absorption in the colon can be increased by manipulating electrolyte composition in the rectal lumen (Holtug et al., 1995) since rectal butyrate absorption remains normal during UC (Hove et al., 1995). Thus, topical butyrate, given intrarectally in form of SB, plays a double role; firstly by employing sodium ions, it accelerates rectal absorption of SB and secondly, the absorbed butyrate imparts healing to the colonocytes. The end result is epithelial proliferation to restore the damaged epithelium, especially the lost colonic epithelial continuity.
Contrary to the obviously evident successful use of topically administered butyrate enemas against UC, studies using the oral route are rare in literature (Vieira et al., 2012) and those for intraperitoneal route are not found. Oral administration of butyrate has been shown to significantly improve trophism and reduce leukocyte infiltration in the colon mucosa and revert to normal the UC-associated alterations of transforming growth factor-β (TGF-β), IL-10, T lymphocytes and dendritic cells (Vieira et al., 2012). In doing so, oral butyrate attenuates the inflammatory profile of colonic mucosa. The effect of oral butyrate on UC has further been studied in combination with standard drugs. A randomized, double-blind, placebo-controlled study by Vernia et al. (2000) demonstrated that a combined oral SB and mesalazine treatment results in a more favourable UC trend compared to oral mesalazine alone. The trend includes decreased disease activity index as well as endoscopic and histologic scores. Apart from oral intake of butyrate, other studies have demonstrated that oral administration of dietary fibers that are subsequently fermented in the colon to produce butyrate is as effective as standard therapeutic drugs like mesalamine in maintaining remission of UC (Fernández-Bañares et al., 1999). In addition, several recent studies focusing on the role of orally given butyrate producing bacteria like Butyricicoccus spp. or Clostridium tyrobutyricum, have revealed that the bacteria increase the butyrate levels in the colon and subsequently cure UC (Hudcovic et al., 2012; Eeckhaut et al., 2013). All these facts and our own results in this study consistently indicate that orally given butyrate could be a potential route to treat UC.
We have demonstrated the potential of intraperitoneally administered butyrate to prevent the severity of AA-induced UC lesions. To the best of our knowledge, this finding has not been reported before. However, the systemic effect of butyrate to other body systems and organs has been reported. For instance, intraperitoneal injection of butyrate at 50–200 mg/kg body weight decreases gentamicin-induced nephrotoxicity in rats by enhancing renal antioxidant enzyme activity and expression of prohibitin protein (Sun et al., 2013). When given at 1200 mg/kg, intraperitoneal butyrate ameliorates an aging-associated deficit in object recognition memory in rats (Reolon et al., 2011). Silingardi et al. (2010) further demonstrated that chronic intraperitoneal administration of butyrate to long-term monocularly deprived adult rats causes a complete recovery of visual acuity. A more recent study has also reported that intraperitoneal injections of butyrate for 28 d to adult C57BL/6 mice prevent repressed contextual fear memory caused by isoflurane (Zhong et al., 2014). All these facts and our own study affirm that butyrate has a potential to impart protective roles to various body organs and systems through systemic administration.
We have also assessed the AA-induced UC lesions in two other different orders; firstly, AA was given every 24 h for 2 d followed by SB for 4 consecutive days and secondly, alternating AA and SB each given every fortnight for 6 d. Our results (data not shown) indicate that butyrate given after AA has less protective potency than when given prior to AA and that butyrate does not benefit rats when alternated with AA. Thus in this very study, we extend further the protective potency of systemic butyrate to include UC. We chose a 100 mg/kg dose on the grounds that previous studies using 50, 100, and 200 mg/kg in rats (Sun et al., 2013) and 100 to 10 000 mg/kg in mice (Ferrante et al., 2003) did not have any detrimental effect. The used dose may also have no effect in humans since intravenous SB dose of 500 mg/(kg·d) for 10 consecutive days has shown no toxicity in these subjects (Miller et al., 1987). Nonetheless, establishment of the exact amount of butyrate for intraperitoneal administration against human UC needs to be researched.
Although there is no single study that has established the amount of SB reaching the colonocytes following intraperitoneal administration, it is known from human studies that the normal colon butyrate levels of (5.0±4.2) to (14.7±2.9) mmol/kg chyme correspond to 29 μmol/L of portal blood (Cummings et al., 1987). Furthermore, in vitro studies have indicated that butyrate consumption by colonocytes is 1.5 and 0.5 mmol/L after 4 and 12 h of treatment, respectively, even if the high dose of 50 mmol/L is supplied (Sauer et al., 2007). The 545 mmol/L solution (calculated from 100 mg/kg dose, 120 g average body weight, 0.2 ml SB (110.09 g/mol) per animal) used in our study was far higher than the blood butyrate levels of 29 μmol/L. We assumed that our 100 mg/kg dose will supply enough butyrate to reach the colonocytes at adequate physiological levels despite the fact that butyrate is rapidly used after its absorption in blood. Since diffusion of intraperitoneally injected substances between the fluid in the peritoneal cavity and the blood is limited after reaching equilibrium and the rate of absorption depends on diffusion rate of the substance (Clark, 1921), we suggest that the used SB dose was enough to provide colonocytes a physiological concentration of butyrate for adequate time to impart its effect.
During UC, the inflamed intestinal mucosa impairs butyrate metabolism and predisposes colonocytes to intracellular butyrate deficiency. The impaired butyrate metabolism could be due to luminal compounds interfering with butyrate metabolism, changes in luminal butyrate concentrations or pH, and defects in β-oxidation or butyrate transport (Thibault et al., 2010). Butyrate deficiency develops from decreased uptake of butyrate by the inflamed mucosa through down-regulation of the monocarboxylate transporter 1 (Thibault et al., 2007). The intracellular butyrate deficiency in colonocytes may decrease its protective effects against UC. Under such conditions, intraperitoneal butyrate that reaches the colonocytes from blood at the basolateral side could be more potent in curing UC. This could in part explain our findings that intraperitoneal butyrate was the most potent route in reducing the severity of AA colitis.
The benefits of systemic administration of butyrate could stem from the ability of butyrate to suppress adverse systemic changes occurring during UC that are pivotal to development of the disease. These systemic effects occur even in topically induced colitis models. Recently, Hou et al. (2014) demonstrated that intrarectal AA increases blood lymphocyte counts, creatinine, prostaglandin E2, and malondialdehyde concentrations, and diamine oxidase and inducible nitric oxide synthase (iNOS) activities, and decreases insulin concentrations and glutathione peroxidase activity in a porcine model of colitis. These effects were attenuated by oral administration of tributyrin, a good dietary source of butyrate and led to protection against AA colitis. These observations clearly indicate that UC patients could benefit from approaches that abrogate systemic events pivotal to the development of the disease. There is another advantage in employing systemic administration of butyrate. Patients with IBD have increased production of inflammatory cytokines like IL-12/23p40 and tumour necrosis factor-α (TNF-α) from peripheral blood mononuclear cells. To inhibit the release of these cytokines, higher concentrations of topical butyrate are needed in these patients than in healthy subjects. This is because of impaired sensitivity to the inhibitory action of butyrate in IBD (Kovarik et al., 2011). This insensitivity could be overcome by systemic butyrate. It can be suggested from our findings that the potency of oral and intraperitoneal butyrate could be due to the ability of butyrate to prevent the systemic adverse effects of intrarectal AA. The observed differences in the potency between intraperitoneal and oral butyrate could be due to the variations in uptake and the rate of absorption between the two routes.
AA induces UC through induction of oxidative and inflammatory responses. Oxidative responses include production of oxidative enzymes like superoxide dismutase and glutathione whereas inflammatory responses are manifested by production of inflammatory mediators like TNF-α, IL-1β, IL-6, IL-8, iNOS, cyclo-oxygenase-2, myeloperoxidase, and lactate dehydrogenase (Sakthivel and Chandrasekaran, 2014; Vinod and Guruvayoorappan, 2014). Most agents that have shown potency in reducing the AA colitis lesions and signs have antioxidant and/or anti-inflammatory effects (Sotnikova et al., 2013; Minaiyan et al., 2014; Sakthivel and Chandrasekaran, 2014; Vinod and Guruvayoorappan, 2014; Zhao et al., 2014). The suppressive effect of butyrate on AA induced UC clinical signs and lesions observed in our study could be mediated via the butyrate’s anti-inflammatory and antioxidant effects. We have demonstrated previously that butyrate modulates IL-8 production to prevent inflammatory responses (Malago et al., 2005). Several other researchers have also reported the anti-inflammatory role of butyrate in UC. Zimmerman et al. (2012) observed that butyrate inhibits interferon-γ (IFN-γ) activation resulting in inhibition of iNOS up-regulation in colonocytes to suppress colonic inflammation. Butyrate has also been shown to modulate the function of intestinal macrophages, which are the most abundant immune cell type in the lamina propria. Treatment of these macrophages with butyrate leads to down-regulation of proinflammatory mediators, including nitric oxide, IL-6, and IL-12 (Chang et al., 2014). The anti-oxidative role of butyrate is exemplified by its ability to reduce the activity of pro-oxidative malondialdehyde and increase activities of antioxidant glutathione peroxidase, superoxide dismutase, and catalase in the colonic mucosa. It does this partly by up-regulating the gene expressions of glutathione peroxidase and catalase. In addition, butyrate promotes antioxidative status in the blood by elevating the α-tocopherol level (Wu and Chen, 2011).
5. Conclusions
The results presented here provide knowledge on the potential of systemic butyrate to prevent UC lesions. This potential is higher than that of the popularly used topical butyrate and could be mediated via the ability of butyrate to suppress the inflammatory and oxidative systemic effects that are central to the development of UC. Considering this potential and the challenges in treating UC, further research is needed to explore the exact mechanisms of systemic butyrate and whether this approach would benefit UC patients equally or more than the topically administered butyrate. Findings from such research could provide a milestone towards treating UC.
Footnotes
Compliance with ethics guidelines: Joshua J. MALAGO and Catherine L. SANGU declare that they have no conflict of interest.
All institutional and national guidelines for the care and use of laboratory animals were followed.
References
- 1.Bloemen JG, Schreinemacher MH, de Bruine AP, et al. Butyrate enemas improve intestinal anastomotic strength in rat model. Dis Colon Rectum. 2010;53(7):1069–1075. doi: 10.1007/DCR.0b013e3181d881b7. [DOI] [PubMed] [Google Scholar]
- 2.Cetinkaya A, Bulbuloglu E, Kurutas EB, et al. Beneficial effects of N-acetylcysteine on acetic acid-induced colitis in rats. Tohoku J Exp Med. 2005;206(2):131–139. doi: 10.1620/tjem.206.131. [DOI] [PubMed] [Google Scholar]
- 3.Chang PV, Hao L, Offermanns S, et al. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. PNAS. 2014;111(6):2247–2252. doi: 10.1073/pnas.1322269111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark AJ. Absorption from the peritoneal cavity. J Pharmacol Exp Ther. 1921;16(6):415–433. [Google Scholar]
- 5.Cummings JH, Pomare EW, Branch WJ, et al. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28(10):1221–1227. doi: 10.1136/gut.28.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dabard J, Hudault S, Saby MA. Production of butyric acid in human premature baby suffering from necrotizing enterocolitis. Proceedings of the 9th International Symposium on Gnotobiology; Versailles. 1987. pp. 90–95. [Google Scholar]
- 7.de Preter V, Geboes KP, Bulteel V, et al. Kinetics of butyrate metabolism in the normal colon and in ulcerative colitis: the effects of substrate concentration and carnitine on the β-oxidation pathway. Aliment Pharmacol Ther. 2011;34(5):526–532. doi: 10.1111/j.1365-2036.2011.04757.x. [DOI] [PubMed] [Google Scholar]
- 8.de Preter V, Arijs I, Windey K, et al. Impaired butyrate oxidation in ulcerative colitis is due to decreased butyrate uptake and a defect in the oxidation pathway. Inflamm Bowel Dis. 2012;18(6):1127–1136. doi: 10.1002/ibd.21894. [DOI] [PubMed] [Google Scholar]
- 9.Eeckhaut V, Machiels K, Perrier C, et al. Butyricicoccus pullicaecorum in inflammatory bowel disease. Gut. 2013;62(12):1745–1752. doi: 10.1136/gutjnl-2012-303611. [DOI] [PubMed] [Google Scholar]
- 10.Fabia R, Willén R, Ar'Rajab A, et al. Acetic acid-induced colitis in the rat: a reproducible experimental model for acute ulcerative colitis. Eur Surg Res. 1992;24(4):211–225. doi: 10.1159/000129209. [DOI] [PubMed] [Google Scholar]
- 11.Fernández-Bañares F, Hinojosa J, Sánchez-Lombraña JL, et al. Randomized clinical trial of Plantago ovata seeds (dietary fiber) as compared with mesalamine in maintaining remission in ulcerative colitis. Spanish Group for the Study of Crohn’s Disease and Ulcerative Colitis (GETECCU) Am J Gastroenterol. 1999;94(2):427–433. doi: 10.1016/S0002-9270(98)00753-9. [DOI] [PubMed] [Google Scholar]
- 12.Ferrante RJ, Kubilus JK, Lee J, et al. Histone deacetylase inhibition by sodium butyrate chemotherapy ameliorates the neurodegenerative phenotype in Huntington’s disease mice. J Neurosci. 2003;23(28):9418–9427. doi: 10.1523/JNEUROSCI.23-28-09418.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fusunyan RD, Quinn JJ, Ohno Y, et al. Butyrate enhances interleukin (IL)-8 secretion by intestinal epithelial cells in response to IL-1β and lipopolysaccharide. Pediatr Res. 1998;43(1):84–90. doi: 10.1203/00006450-199801000-00013. [DOI] [PubMed] [Google Scholar]
- 14.Fusunyan RD, Quinn JJ, Fujimoto M, et al. Butyrate switches the pattern of chemokine secretion by intestinal epithelial cells through histone acetylation. Mol Med. 1999;5(9):631–640. [PMC free article] [PubMed] [Google Scholar]
- 15.Hamer HM, Jonkers DM, Renes IB, et al. Butyrate enemas do not affect human colonic MUC2 and TFF3 expression. Eur J Gastroenterol Hepatol. 2010;22(9):1134–1140. doi: 10.1097/MEG.0b013e32833a6ca0. [DOI] [PubMed] [Google Scholar]
- 16.Hamer HM, Jonkers DM, Vanhoutvin SA, et al. Effect of butyrate enemas on inflammation and antioxidant status in the colonic mucosa of patients with ulcerative colitis in remission. Clin Nutr. 2010;29(6):738–744. doi: 10.1016/j.clnu.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Harig JM, Soergel KH, Komorowski RA, et al. Treatment of diversion colitis with short chain fatty acid irrigation. N Engl J Med. 1989;320(1):23–28. doi: 10.1056/NEJM198901053200105. [DOI] [PubMed] [Google Scholar]
- 18.Holtug K, Hove H, Mortensen PB. Stimulation of butyrate absorption in the human rectum in vivo . Scand J Gastroenterol. 1995;30(10):982–988. doi: 10.3109/00365529509096342. [DOI] [PubMed] [Google Scholar]
- 19.Hou Y, Wang L, Yi D, et al. Dietary supplementation with tributyrin alleviates intestinal injury in piglets challenged with intrarectal administration of acetic acid. Br J Nutr. 2014;111(10):1748–1758. doi: 10.1017/S0007114514000038. [DOI] [PubMed] [Google Scholar]
- 20.Hove H, Holtug K, Jeppesen PB, et al. Butyrate absorption and lactate secretion in ulcerative colitis. Dis Colon Rectum. 1995;38(5):519–525. doi: 10.1007/BF02148853. [DOI] [PubMed] [Google Scholar]
- 21.Huang N, Katz JP, Martin DR, et al. Inhibition of IL-8 gene expression in Caco-2 cells by compounds which induce histone hyperacetylation. Cytokine. 1997;9(1):27–36. doi: 10.1006/cyto.1996.0132. [DOI] [PubMed] [Google Scholar]
- 22.Hudcovic T, Kolinska J, Klepetar J, et al. Protective effect of Clostridium tyrobutyricum in acute dextran sodium sulphate-induced colitis: differential regulation of tumour necrosis factor-α and interleukin-18 in BALB/c and severe combined immunodeficiency mice. Clin Exp Immunol. 2012;167(2):356–365. doi: 10.1111/j.1365-2249.2011.04498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ke X, Chen J, Zhang X, et al. Qing Hua Chang Yin attenuates lipopolysaccharide-induced inflammatory response in human intestinal cells by inhibiting NF-κB activation. Exp Ther Med. 2013;6(1):189–193. doi: 10.3892/etm.2013.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kovarik JJ, Tillinger W, Hofer J, et al. Impaired anti-inflammatory efficacy of n-butyrate in patients with IBD. Eur J Clin Invest. 2011;41(3):291–298. doi: 10.1111/j.1365-2362.2010.02407.x. [DOI] [PubMed] [Google Scholar]
- 25.La JH, Kim TW, Sung TS, et al. Visceral hypersensitivity and altered colonic motility after subsidence of inflammation in a rat model of colitis. World J Gastroenterol. 2003;9(12):2791–2795. doi: 10.3748/wjg.v9.i12.2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu H, Patel NR, Walter L, et al. Constitutive expression of MMP9 in intestinal epithelium worsens murine acute colitis and is associated with increased levels of proinflammatory cytokine Kc. Am J Physiol Gastrointest Liver Physiol. 2013;304(9):G793–G803. doi: 10.1152/ajpgi.00249.2012. [DOI] [PubMed] [Google Scholar]
- 27.Malago JJ, Nondoli H. Sodium arsenite reduces severity of dextran sulfate sodium-induced ulcerative colitis in rats. J Zhejiang Univ-Sci B. 2008;9(4):341–350. doi: 10.1631/jzus.B0720198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malago JJ, Koninkx JFJG, van Dijk JE. The heat shock response and cytoprotection of the intestinal epithelium. Cell Stress Chaperones. 2002;7(2):191–199. doi: 10.1379/1466-1268(2002)007<0191:THSRAC>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malago JJ, Koninkx JFJG, Tooten PCJ, et al. Anti-inflammatory properties of heat shock protein 70 and butyrate on Salmonella-induced interleukin-8 secretion in enterocyte-like Caco-2 cells. Clin Exp Immun. 2005;141(1):62–71. doi: 10.1111/j.1365-2249.2005.02810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mathew AJ, Wann VC, Abraham DT, et al. The effect of butyrate on the healing of colonic anastomoses in rats. J Invest Surg. 2010;23(2):101–104. doi: 10.3109/08941930903469367. [DOI] [PubMed] [Google Scholar]
- 31.Mikhailova TL, Sishkova E, Poniewierka E, et al. Randomised clinical trial: the efficacy and safety of propionyl-L-carnitine therapy in patients with ulcerative colitis receiving stable oral treatment. Aliment Pharmacol Ther. 2011;34(9):1088–1097. doi: 10.1111/j.1365-2036.2011.04844.x. [DOI] [PubMed] [Google Scholar]
- 32.Miller AA, Kurschel E, Osieka R, et al. Clinical pharmacology of sodium butyrate in patients with acute leukemia. Eur J Cancer Clin Oncol. 1987;23(9):1283–1287. doi: 10.1016/0277-5379(87)90109-X. [DOI] [PubMed] [Google Scholar]
- 33.Minaiyan M, Ghassemi-Dehkordi N, Mahzouni P, et al. Anti-inflammatory effect of Helichrysum oligocephalum DC extract on acetic acid-induced acute colitis in rats. Adv Biomed Res. 2014;3(1):87. doi: 10.4103/2277-9175.128000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Morain C, Segal AW, Levi AJ. Elemental diet as primary treatment of acute Crohn’s disease: a controlled trial. Br Med J (Clin Res Ed) 1984;288(6434):1859–1862. doi: 10.1136/bmj.288.6434.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Randhawa PK, Singh K, Singh N, et al. A review on chemical-induced inflammatory bowel disease models in rodents. Korean J Physiol Pharmacol. 2014;18(4):279–288. doi: 10.4196/kjpp.2014.18.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reolon GK, Maurmann N, Werenicz A, et al. Posttraining systemic administration of the histone deacetylase inhibitor sodium butyrate ameliorates aging-related memory decline in rats. Behav Brain Res. 2011;221(1):329–332. doi: 10.1016/j.bbr.2011.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roediger WEW. The colonic epithelium in ulcerative colitis: an energy deficiency disease. Lancet. 1980;2(8197):712–715. doi: 10.1016/S0140-6736(80)91934-0. [DOI] [PubMed] [Google Scholar]
- 38.Sakthivel KM, Chandrasekaran G. Protective effect of Acacia ferruginea against ulcerative colitis via modulating inflammatory mediators, cytokine profile and NF-κb signal transduction pathways. J Environ Pathol Toxicol Oncol. 2014;33(2):83–98. doi: 10.1615/JEnvironPatholToxicolOncol.2014008425. [DOI] [PubMed] [Google Scholar]
- 39.Sanderson IR. Diet and gut inflammation. Curr Opin Gastroenterol. 1997;13(6):518–524. doi: 10.1097/00001574-199711000-00013. [DOI] [Google Scholar]
- 40.Sauer J, Richter KK, Pool-Zobel BL. Physiological concentrations of butyrate favorably modulate genes of oxidative and metabolic stress in primary human colon cells. J Nutr Biochem. 2007;18(11):736–745. doi: 10.1016/j.jnutbio.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 41.Scheppach W, Sommer H, Kirchner T, et al. Effect of butyrate enemas on the colonic mucosa in distal ulcerative colitis. Gastroenterology. 1992;103(1):51–56. doi: 10.1016/0016-5085(92)91094-k. [DOI] [PubMed] [Google Scholar]
- 42.Silingardi D, Scali M, Belluomini G, et al. Epigenetic treatments of adult rats promote recovery from visual acuity deficits induced by long-term monocular deprivation. Eur J Neurosci. 2010;31(12):2185–2192. doi: 10.1111/j.1460-9568.2010.07261.x. [DOI] [PubMed] [Google Scholar]
- 43.Sotnikova R, Nosalova V, Navarova J. Efficacy of quercetin derivatives in prevention of ulcerative colitis in rats. Interdiscip Toxicol. 2013;6(1):9–12. doi: 10.2478/intox-2013-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun X, Zhang B, Hong X, et al. Histone deacetylase inhibitor, sodium butyrate, attenuates gentamicin-induced nephrotoxicity by increasing prohibitin protein expression in rats. Eur J Pharmacol. 2013;707(1-3):147–154. doi: 10.1016/j.ejphar.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 45.Thibault R, de Coppet P, Daly K, et al. Down-regulation of the monocarboxylate transporter 1 is involved in butyrate deficiency during intestinal inflammation. Gastroenterology. 2007;133(6):1916–1927. doi: 10.1053/j.gastro.2007.08.041. [DOI] [PubMed] [Google Scholar]
- 46.Thibault R, Blachier F, Darcy-Vrillon B, et al. Butyrate utilization by the colonic mucosa in inflammatory bowel diseases: a transport deficiency. Inflamm Bowel Dis. 2010;16(4):684–965. doi: 10.1002/ibd.21108. [DOI] [PubMed] [Google Scholar]
- 47.Torpy JM, Lynm C, Golub RM. Ulcerative colitis. JAMA. 2012;307(1):104. doi: 10.1001/jama.2011.1889. [DOI] [PubMed] [Google Scholar]
- 48.Treem WR, Ahsan N, Shoup M, et al. Fecal short-chain fatty acids in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 1994;18(2):159–164. doi: 10.1097/00005176-199402000-00007. [DOI] [PubMed] [Google Scholar]
- 49.Venkatraman A, Ramakrishna BS, Shaji RV, et al. Amelioration of dextran sulfate colitis by butyrate: role of heat shock protein 70 and NF-κB. Am J Physiol Gastrointest Liver Physiol. 2003;285(1):G177–G184. doi: 10.1152/ajpgi.00307.2002. [DOI] [PubMed] [Google Scholar]
- 50.Vernia P, Monteleone G, Grandinetti G, et al. Combined oral sodium butyrate and mesalazine treatment compared to oral mesalazine alone in ulcerative colitis: randomized, double-blind, placebo-controlled pilot study. Dig Dis Sci. 2000;45(5):976–981. doi: 10.1023/A:1005537411244. [DOI] [PubMed] [Google Scholar]
- 51.Vieira EL, Leonel AJ, Sad AP, et al. Oral administration of sodium butyrate attenuates inflammation and mucosal lesion in experimental acute ulcerative colitis. J Nutr Biochem. 2012;23(5):430–436. doi: 10.1016/j.jnutbio.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 52.Vinod PV, Guruvayoorappan C. Protective effect of marine mangrove Rhizophora apiculata on acetic acid induced experimental colitis by regulating anti-oxidant enzymes, inflammatory mediators and nuclear factor-kappa B subunits. Int Immunopharmacol. 2014;18(1):124–134. doi: 10.1016/j.intimp.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 53.Wu GD, Huang N, Wen X, et al. High-level expression of IκB-β in the surface epithelium of the colon: in vitro evidence for an immunomodulatory role. J Leukoc Biol. 1999;66(6):1049–1056. doi: 10.1002/jlb.66.6.1049. [DOI] [PubMed] [Google Scholar]
- 54.Wu WT, Chen HL. Konjac glucomannan and inulin systematically modulate antioxidant defense in rats fed a high-fat fiber-free diet. J Agric Food Chem. 2011;59(17):9194–9200. doi: 10.1021/jf202060p. [DOI] [PubMed] [Google Scholar]
- 55.Zhao L, Wu H, Zhao A, et al. The in vivo and in vitro study of polysaccharides from a two-herb formula on ulcerative colitis and potential mechanism of action. J Ethnopharmacol. 2014;153(1):151–159. doi: 10.1016/j.jep.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 56.Zhong T, Qing QJ, Yang Y, et al. Repression of contexual fear memory induced by isoflurane is accompanied by reduction in histone acetylation and rescued by sodium butyrate. Br J Anaesth. 2014;113(4):634–643. doi: 10.1093/bja/aeu184. [DOI] [PubMed] [Google Scholar]
- 57.Zimmerman MA, Singh N, Martin PM, et al. Butyrate suppresses colonic inflammation through HDAC1-dependent Fas upregulation and Fas-mediated apoptosis of T cells. Am J Physiol Gastrointest Liver Physiol. 2012;302(12):G1405–G1415. doi: 10.1152/ajpgi.00543.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]