Abstract
Decreased bioavailability of nitric oxide (NO) is a major contributor to the pathophysiology of severe falciparum malaria. Tetrahydrobiopterin (BH4) is an enzyme cofactor required for NO synthesis from L-arginine. We hypothesized that systemic levels of BH4 would be decreased in children with cerebral malaria, contributing to low NO bioavailability. In an observational study in Tanzania, we measured urine levels of biopterin in its various redox states (fully reduced [BH4] and the oxidized metabolites, dihydrobiopterin [BH2] and biopterin [B0]) in children with uncomplicated malaria (UM, n = 55), cerebral malaria (CM, n = 45), non-malaria central nervous system conditions (NMC, n = 48), and in 111 healthy controls (HC). Median urine BH4 concentration in CM (1.10 [IQR:0.55–2.18] μmol/mmol creatinine) was significantly lower compared to each of the other three groups — UM (2.10 [IQR:1.32–3.14];p<0.001), NMC (1.52 [IQR:1.01–2.71];p = 0.002), and HC (1.60 [IQR:1.15–2.23];p = 0.005). Oxidized biopterins were increased, and the BH4:BH2 ratio markedly decreased in CM. In a multivariate logistic regression model, each Log10-unit decrease in urine BH4 was independently associated with a 3.85-fold (95% CI:1.89–7.61) increase in odds of CM (p<0.001). Low systemic BH4 levels and increased oxidized biopterins contribute to the low NO bioavailability observed in CM. Adjunctive therapy to regenerate BH4 may have a role in improving NO bioavailability and microvascular perfusion in severe falciparum malaria.
Author Summary
Vascular nitric oxide (NO) bioavailability is decreased in severe falciparum malaria and associated with microvascular dysfunction, increased activation of the cells lining blood vessels (endothelial cells) and increased parasite biomass. Tetrahydrobiopterin (BH4) is an essential cofactor for nitric oxide synthase (NOS) enzymatic conversion of L-arginine to NO and L-citrulline. But when BH4 is low, NOS is “uncoupled” and produces superoxide instead of NO. In oxidative conditions, BH4 is oxidized to dihydrobiopterin (BH2) and biopterin (B0). BH2 competes with remaining BH4 at its NOS binding site, further decreasing NOS-catalyzed NO production. We measured BH4, BH2 and B0 in the urine of children with coma due to falciparum malaria (cerebral malaria), uncomplicated falciparum malaria, children with non-malaria central nervous system conditions and healthy controls. Urine BH4 was significantly decreased and BH2 significantly increased in cerebral malaria compared to uncomplicated malaria, non-malaria central nervous conditions and healthy controls, suggesting increased oxidative stress and insufficient recycling of BH2 back to BH4. Urine BH4 concentration was independently associated with increased risk of cerebral malaria. Given that safe therapies for regenerating BH4 have been studied in chronic vascular disease, this finding of low BH4 in pediatric cerebral malaria offers a new area of investigation for adjunctive therapies aimed at improving NO bioavailability and, consequently, clinical outcomes in severe falciparum malaria.
Introduction
Falciparum malaria causes over 600,000 deaths worldwide each year, with approximately 560,000 fatal cases annually among children in sub-Saharan Africa [1]. Coma in malaria, cerebral malaria (CM), portends a grave outcome among children infected with Plasmodium falciparum and, despite advances in anti-parasitic drug therapies, still has a 10–20% case fatality rate [2–4]. However, the pathogenesis of CM remains poorly understood [5]. CM pathogenesis studies to date show endothelial dysfunction [6], endothelial activation [7–10] and cytoadherence of parasitized red blood cell (pRBC) to endothelial cells in post-capillary venules, resulting in red blood cell sequestration [11–13], microvascular congestion and impaired blood flow to tissues [14–16]. Metabolic derangements and cytotoxic mechanisms contributing to the pathogenesis of CM have also been proposed [5,17–19].
Low nitric oxide (NO) is a key cause and contributor to the microvascular pathophysiology observed in severe malaria [20]. A variety of causes for low bioavailability in malaria have been identified within multiple steps of the NO production pathway [21–23], from low levels of NO synthase (NOS) substrate, arginine [24], to elevated levels of endogenous NOS inhibitors [25,26]. All NOS isoforms require the obligate cofactor tetrahydrobiopterin (BH4) to enzymatically generate NO from L-arginine. The role of this NOS cofactor as a potential contributor to low NO bioavailability in malaria is unknown. In vascular diseases, low BH4 and increased concentrations of its oxidized metabolite, dihydrobiopterin (BH2), are not only associated with impaired NO synthesis, but also linked to generation of reactive oxygen species within the endothelium [27].
In addition to its role in NO synthesis and endothelial function, BH4 is also an essential cofactor for monooxygenase enzymes required for phenylalanine metabolism (phenylalanine hydroxylase [PAH]) as well as biogenic amine neurotransmitter synthesis of catecholamines (tyrosine hydroxylase) and serotonin (tryptophan hydroxylase) [28,29]. (The reader is directed to references 28 and 29 for reviews of BH4 metabolism, which include diagrams of BH4 synthetic and salvage pathways.) We have previously reported elevated plasma phenylalanine in children with CM [18]. This finding is likely attributable to impaired activity of hepatic PAH, which regulates plasma phenylalanine levels within a narrow range by controlling the rate of conversion of phenylalanine to tyrosine [30]. We hypothesized that hyperphenylalaninemia is an indicator of systemic BH4 deficiency and that BH4 deficiency would also contribute to impaired NO bioavailability observed in malaria.
To address this hypothesis we quantified BH4 and its oxidized metabolites in urine as a measure of systemic biopterin availability [28,31–33] in children presenting with CM. Quantifying biopterins in urine requires specific collection methods. With ordinary urine collection and storage, BH4 spontaneously oxidizes to its metabolites, BH2 and, to a lesser extent, fully oxidized biopterin (B0) [34,35]. By collecting urine directly into an anti-oxidant/chelator cocktail in the dark followed by immediate freezing and storage at -80°C until analysis, we overcame this potential artifact of ex vivo spontaneous oxidation. This established collection method for biopterin analysis enabled quantification of BH4 in its reduced in vivo state [35]. In this report, we provide quantitative data on urinary excretion of the active NOS cofactor BH4, as well as BH2 and B0, in children at the time of presentation with CM. We found decreased levels of BH4 and increased levels of oxidized biopterins in children with CM, indicating a marked reduction in BH4 bioavailability in severe malaria. Based on these findings, we propose a pathogenic mechanism wherein low systemic BH4 contributes to low NO bioavailability and endothelial dysfunction in severe malaria.
Results
Clinical variables describing the clinical groups
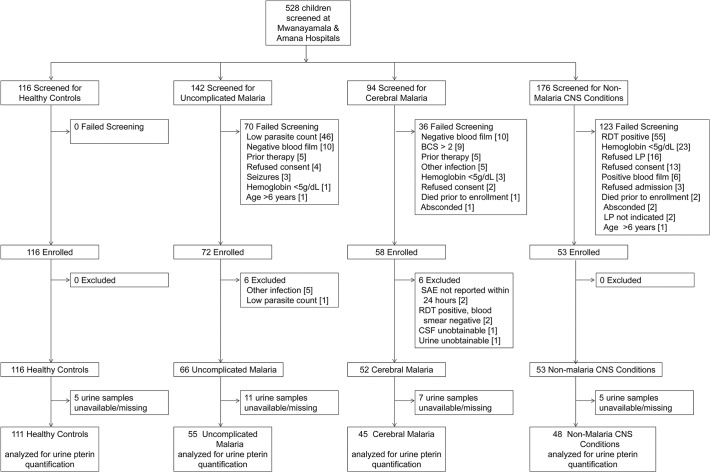
From November 2007 to January 2012 we screened 528 children for enrollment into one of four study groups. After exclusions, 66 children with uncomplicated malaria (UM), 52 with cerebral malaria (CM), 53 with non-malaria central nervous system conditions (NMC), and 116 healthy controls (HC) were enrolled (Fig. 1). Baseline characteristics comparing the four groups are shown in Table 1. Children with CM were significantly older than children from the other groups when compared across all groups and in pairwise comparisons between groups. Two children with CM and one child with NMC had overt renal failure as evidenced by plasma creatinine measurements of 2.7, 5.1, and 2.5 mg/dL, respectively. Children with CM had significantly lower peripheral blood parasitemia, but significantly higher plasma P. falciparum histidine-rich protein-2 (PfHRP-2) concentrations compared to children with UM. To assess biopterin status in CM compared to other infectious and non-infectious central nervous system (CNS) conditions, we enrolled a comparison group comprised of children presenting to the hospital with NMC for which lumbar puncture was clinically indicated. The NMC group proved to be heterogeneous as expected. Clinical and laboratory investigations indicated that at least 10 of the 53 children with NMC suffered from bacterial or fungal meningitis. No evidence for viral encephalitis, trauma, subarachnoid hemorrhage or metabolic encephalopathy was found. Toxic encephalopathy was possible in one child. Idiopathic seizures and aseptic meningitis were other possible diagnostic categories. Seven children with CM and six children with NMC died while hospitalized. All children with UM recovered.
Fig 1. Study flow diagram.
Screening, enrollment and post-hoc exclusions for the four clinical groups are depicted. CNS = central nervous system; BCS = Blantyre Coma Score; RDT = rapid diagnostic test for P. falciparum (ParacheckPf, Omega Diagnostics); LP = lumbar puncture; SAE = serious adverse event; CSF = cerebrospinal fluid.
Table 1. Baseline characteristics of the 4 clinical groups.
| Variable | Healthy Controls (n = 116) | Uncomplicated Malaria (n = 66) | Cerebral Malaria (n = 52) | Non-Malaria CNS Conditions (n = 53) | p-value |
|---|---|---|---|---|---|
| Age, months | 34 (31–38) | 44 (39–49) | 51 (47–55) | 29 (23–34) | <0.001 |
| Male sex, no. (%) | 65 (56.5) | 35 (53.0) | 34 (65.4) | 29 (55.8) | 0.556 A |
| Weight, kg | 12.9 (12.2–13.6) | 14.3 (13.4–15.2) | 15.4 (14.4–16.4) | 11.3 (10.3–12.3) | <0.001 |
| Axillary temperature,°C | 36.7 (36.6–36.7) | 39 (38.8–39.3) | 38.3 (38.0–38.6) | 37.7 (37.4–38.1) | <0.001 |
| Respiratory rate | 32 (31–33) | 36 (34–38) | 43 (40–47) | 37 (34–41) | <0.001 |
| Last meal, hours | 3.2 (2.9–3.6) | 5.9 (4.7–7.1) | 5.9 (4.1–7.7) | 3.8 (2.6–4.9) | <0.001 |
| Hemoglobin, g/dL | 10.8 (10.4–11.1) | 8.4 (7.8–9.0) | 7.0 (6.5–7.5) | 9.8 (9.3–10.3) | <0.001 |
| Blood glucose, mg/dL C | N/D | 119 (96–143) | 119 (105–132) | 119 (93–147) | 0.996 |
| Plasma bicarbonate, mmol/L C | N/D | 19.1 (16.1–22.1) | 20 (18.8–21.3) | 19 (17–21) | 0.678 |
| Plasma creatinine, mg/dL C | N/D | 0.3 (0.3–0.4) | 0.8 (0.5–1.0) | 0.5 (0.4–0.7) | 0.031 |
| Urine leukocyte esterase present, no. (%) D | 3 (5.8) | 7 (19.4) | 4 (8.5) | 2 (4.3) | 0.08 A |
| Plasmodium falciparum parasite density, x 103/ μL; median (interquartile range) | 0 | 87,055 (30,115–209,550) | 45,231 (9396–128,231) | 0 | 0.026 B |
| Plasma PfHRP-2, ng/mL; median (interquartile range) | 0 | 517.4 (39.3–1536.0) | 1,086.5 (379.1–2,585.5) | 0 | 0.011 B |
Data mean (95% confidence interval values) unless otherwise indicated.
ANOVA with Bonferroni post-estimate unless otherwise indicated.
A = Chi-squared,
B = Wilcoxon Rank Sum.
PfHRP-2 = Plasmodium falciparum histidine-rich protein-2.
N/D = Test not done.
C = We were unable to obtain measurements of all analytes for all patients enrolled.
Glucose measurements were obtained in 27, 48 and 43 of the UM, CM and NMC children, respectively.
Bicarbonate results were obtained 31, 48 and 47 of the UM, CM, and NMC children, respectively. Creatinine results were obtained in 16, 39 and 40 of the UM, CM and NMC children, respectively.
D = Three children (1 HC, 1 UM and 1 CM) with bacteriuria remained in the study as the urine was collected by bag collection (i.e., not collected via sterile, invasive procedures), and there was no clinical suspicion of urinary tract infection in any of the three subjects.
Abnormal distribution of urine biopterins in children with cerebral malaria
Urine pterin concentrations differed significantly across the four groups (Table 2). Compared to HC (n = 111), total biopterin levels (BH4 + BH2 + B0) were increased in UM (n = 55) (p<0.001), CM (n = 45) (p<0.001), and NMC (n = 48) (p<0.001) (Fig. 2, panel A). Urine BH4 concentrations were significantly lower in CM than in each of the other three groups (p≤0.005 for all pairwise comparisons) (Fig. 2, panel B). The statistical significance persisted in a linear regression model to control for plasma creatinine (p = 0.021) and in a linear regression model to control for age, weight, gender and plasma creatinine (p = 0.038). Using logistic regression, we found no significant co-variation of urine BH4 by presence or absence of pyuria (OR 1.21 [0.65–2.25]; p = 0.56) or by presence or absence of bacteriuria (OR 0.70 [0.34–1.46]; p = 0.34). Urine BH2 was significantly higher in CM compared to each of the other three groups (p<0.05 for all comparisons). Urine BH4 values differed significantly when compared across UM (2.10 [IQR 1.32–3.14]), CM survivors (1.10 [IQR 0.48–2.25] μmol/mmol creatinine) and CM fatalities (1.02 [IQR 0.77–2.09] μmol/mmol creatinine) (p<0.001).
Table 2. Urine pterins and plasma phenylalanine measurements at enrollment in the 4 clinical groups.
| Variable | Healthy Controls (n = 111) | Uncomplicated Malaria (n = 55) | Cerebral Malaria (n = 45) | Non-Malaria CNS Conditions (n = 48) | p-value |
|---|---|---|---|---|---|
| BH4 A , B , C | 1.60 (1.15–2.23) | 2.10 (1.32–3.14) | 1.10 (0.55–2.18) | 1.52 (1.01–2.71) | <0.001 |
| BH2 A , B , C | 0.96 (0.75–1.33) | 1.60 (0.95–2.98) | 2.24 (1.55–2.89) | 1.83 (1.13–2.30) | <0.001 |
| Biopterin A | 0.09 (0.05–0.16) | 0.24 (0.12–0.52) | 0.22 (0.08–0.37) | 0.13 (0.08–0.30) | <0.001 |
| Total Biopterins A | 2.82 (2.19–3.67) | 4.03 (2.77–5.79) | 3.83 (2.95–4.85) | 3.97 (2.91–5.57) | <0.001 |
| BH4/BH2 Ratio A , B , C | 1.64 (1.21–2.18) | 1.42 (0.75–1.98) | 0.45 (0.21–1.13) | 1.25 (0.63–2.09) | <0.001 |
| BH4/(BH2+B0) A , B , C | 1.52 (1.04–1.97) | 1.17 (0.68–1.85) | 0.39 (0.19–1.06) | 1.12 (0.54–1.93) | <0.001 |
| BH4/Total Biopterins A , B , C | 0.60 (0.51–0.66) | 0.54 (0.40–0.65) | 0.28 (0.16–0.51) | 0.53 (0.35–0.66) | <0.001 |
| NH2 A , B | 3.20 (1.98–4.69) | 18.36 (11.62–24.37) | 19.86 (15.86–23.22) | 6.62 (3.69–11.77) | <0.001 |
| N0 A , B , C | 0.74 (0.52–1.16) | 3.93 (2.26–3.07) | 5.48 (4.11–6.43) | 1.43 (0.88–3.07) | <0.001 |
| Total Neopterin A , B | 3.89 (2.55–5.90) | 22.39 (14.02–32.22) | 25.26 (20.21–30.58) | 7.96 (4.53–14.85) | <0.001 |
| NH2/N0 Ratio A , C | 4.39 (3.26–5.52) | 4.72 (3.52–5.57) | 3.74 (3.22–4.22) | 5.19 (2.83–9.61) | 0.018 |
| Phenylalanine A , B , D | 53 (45–60) | 101 (80–165) | 129.5 (91–208) | 75 (63–114) | <0.001 |
Units: urine pterins μmol/mmol creatinine; amino acid μM.
Median and inter-quartile ranges, p-value Kruskal-Wallis comparison across all 4 groups. BH4 = tetrahydrobiopterin, BH2 = dihydrobiopterin, B0 = biopterin, NH2 = dihydroneopterin, N0 = neopterin.
A = p-value <0.05 by Wilcoxon Rank Sum comparison between cerebral malaria and healthy controls
B = p-value < 0.05 by Wilcoxon Rank Sum comparison between cerebral malaria and non-malaria CNS condition
C = p-value < 0.05 by Wilcoxon Rank Sum comparison between cerebral malaria and uncomplicated malaria
D = Number of plasma samples for healthy controls, uncomplicated malaria, cerebral malaria and non-malaria CNS conditions was 109, 61, 50, and 51, respectively.
Fig 2. Urine biopterin concentrations at enrollment in the 4 clinical groups.
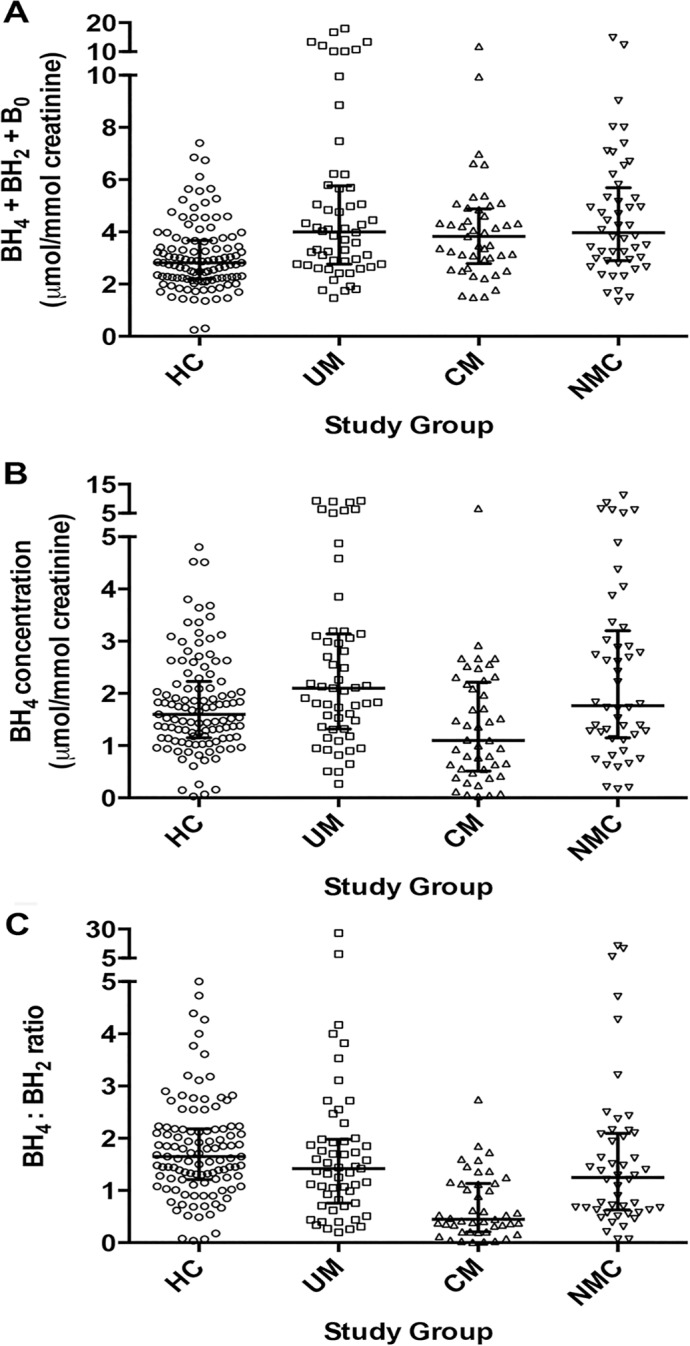
A: Comparison of total urine biopterins (tetrahydrobiopterin [BH4] + dihydrobiopterin [BH2] + biopterin [B0]) concentrations at enrollment for healthy controls (HC, n = 111), uncomplicated malaria (UM, n = 55), cerebral malaria (CM, n = 45) and non-malaria central nervous system conditions (NMC, n = 48) (p<0.001 by Kruskal-Wallis). B: urine tetrahydrobiopterin (BH4) concentrations at enrollment for all 4 groups (p<0.001 by Kruskal-Wallis). Central line indicates median. Upper and lower lines indicate inter-quartile range. C: Comparison of urine tetrahydrobiopterin to dihydrobiopterin (BH4:BH2) ratio at enrollment for all 4 groups (p<0.001 by Kruskal-Wallis). The number of subjects with a sample measured in [B] and [C] is the same as Noted above in [A]. The central line indicates median. Upper and lower lines indicate interquartile range.
NOS activity is affected not only by the availability of the cofactor BH4, but also by the stoichiometric balance of BH4 relative to its major oxidized metabolite BH2. BH2 competes with BH4 at the NOS binding site and can directly inhibit NOS activity [36]. The BH4:BH2 ratio has been proposed as the critical determinant of NO synthesis by endothelial NOS (eNOS) [37]. Accordingly, we analyzed this ratio in the four clinical groups enrolled. The BH4:BH2 ratio was significantly lower in CM compared to each of the other groups (p<0.001 for all comparisons) (Fig. 2, panel C). In our a priori analytical plan, we also compared the ratio of reduced to oxidized biopterins (BH4: BH2+B0) and the proportion of total biopterins as BH4 (BH4/ BH4+ BH2+ B0). Using these alternative renderings to examine reduced to oxidized biopterins, we found the same statistical relationships between CM in pairwise comparison to the other three clinical groups (Table 2).
Compared to children with likely bacterial or cryptococcal meningitis (n = 10), children with cerebral malaria had lower BH4 than this subset of NMC children (1.10 [IQR 0.55–2.18] vs. 1.63 [IQR 1.39–2.90] μmol/mmol creatinine; p = 0.06), significantly higher BH2 than children in this subset (2.24 [IQR 1.55–2.89] vs. 1.50 [IQR 0.93–2.27] μmol/mmol creatinine; p = 0.05), and a significantly lower BH4:BH2 ratio than children with likely bacterial or cryptococcal meningitis (0.45 [0.21–1.13] vs. 1.35 [IQR 0.70–3.21]; p = 0.002).
Collectively, these measurements of urine biopterins demonstrate deficiency in systemic BH4 in CM.
Elevated urine neopterins in cerebral malaria
Guanosine triphosphate cyclohydrolase I (GTPCH-1) is the rate-limiting enzyme in the first step for BH4 de novo synthesis. Mononuclear phagocytes activated by pro-inflammatory cytokines (e.g., INF-γ, TNF-α) show enhanced transcription of the GTPCH-1 gene, GCH1 [38]. However, the activity of the second enzyme for biopterin synthesis, 6-pyruvoyl tetrahydropterin synthase (PTPS), is constitutively very low in these cells and is unresponsive to cytokine-induced transcription activation [28,39,40]. The result is shunting of the GTPCH-1 product, 7,8 dihydroneopterin triphosphate, to neopterin end products—dihydrobiopterin (NH2) and its oxidized metabolite, neopterin (N0) [41]. These neopterin end products accumulate within mononuclear phagocytes and then exit the cells to be excreted in urine. Although the biological function of the neopterins remains obscure, elevated total urine neopterin (NH2 + N0) is established as a marker of the cell-mediated immune response in a variety of inflammatory states [42]. NH2 measurement relies upon oxidation of NH2 to the naturally fluorescent N0 in vitro for quantification [43]. We took advantage of our collection method for preserving urine neopterins in their in vivo reduced (NH2) and oxidized (N0) states to quantify them in CM. In doing so, we sought to determine whether the neopterins, like the biopterins, have a redox ratio skewed towards oxidation in children with CM.
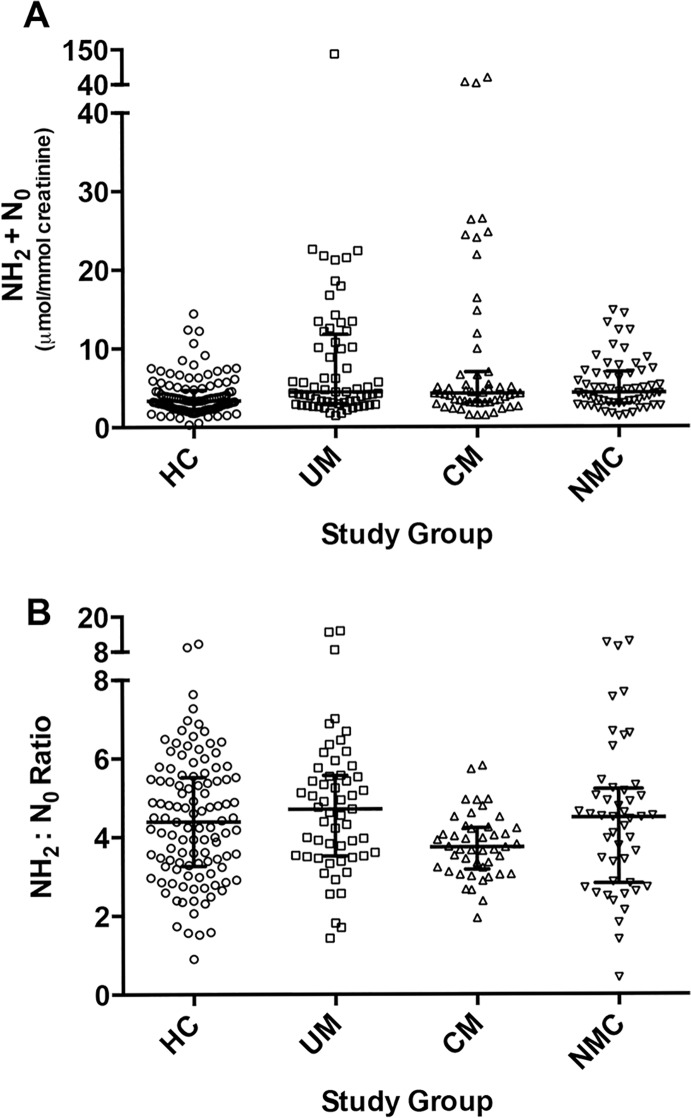
Concentrations of urine neopterin metabolites differed significantly across the four groups (Table 2). Compared to HC, total neopterin (NH2 + N0) levels were increased in UM, CM and NMC (p<0.001 for all comparisons) (Fig. 3, panel A). Total neopterin levels did not differ significantly in UM compared to CM (p = 0.113). The ratio of reduced to oxidized neopterins (NH2:N0) was significantly decreased in CM compared to UM (p = 0.001) and HC (p = 0.017). This ratio did not statistically differ between CM and NMC (p = 0.069) (Fig. 3, panel B). The reduced to oxidized ratio for neopterins correlated with the reduced to oxidized ratio for biopterins (BH4: BH2+B0) (r = 0.28, p<0.001) and oxidized neopterin (N0) correlated with oxidized biopterin (BH2) (r = 0.63, p<0.001). We conclude from these measurements that while total urine neopterin levels did not differ in CM compared to UM, quantifying neopterins in their reduced to oxidized states may indicate oxidative stress in CM compared to UM.
Fig 3. Urine neopterin concentrations at enrollment in the 4 clinical groups.
A: Comparison of total urine neopterins, dihydroneopterin plus neopterin (NH2 + N0), concentrations at enrollment for healthy controls (HC, n = 111), uncomplicated malaria (UM, n = 55), cerebral malaria (CM, n = 45) and non-malaria central nervous system conditions (NMC, n = 48) (p<0.001 by Kruskal-Wallis). The central line indicates median. Upper and lower lines indicate inter-quartile range. B: Comparison of urine dihydroneopterin to neopterin (NH2:N0) at enrollment for all 4 groups (p = 0.018 by Kruskal-Wallis). The number of subjects with a sample measured is the same as noted above in [A]. The central line indicates median. Upper and lower lines indicate inter-quartile range.
Elevated angiopoietin-2 in cerebral malaria
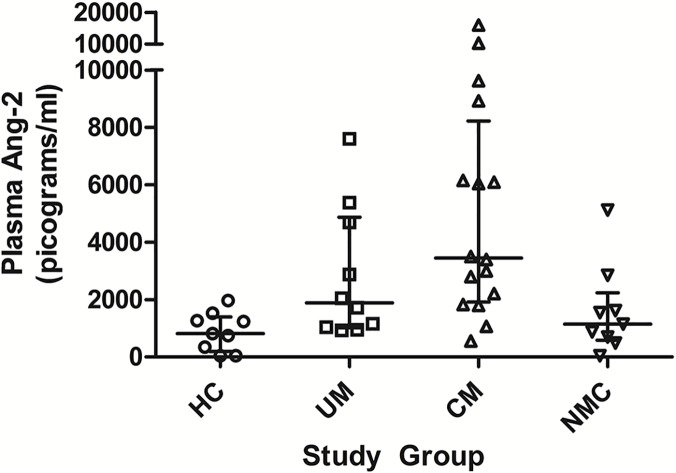
Angiopoietin-2 (Ang-2) is a ligand released from endothelial Weibel-Palade bodies that acts as an autocrine mediator of endothelial activation [44], including up-regulation of intercellular adhesion molecule (ICAM) receptors on endothelial cells [45]. Ang-2 plasma levels correlate with clinical severity in pediatric sepsis [10,46] and are independently associated with mortality in severe malaria [10,46,47]. NO is a known inhibitor of endothelial Weibel-Palade bodies exocytosis [48]. In vitro endothelial cell experiments demonstrate biopterin redox status impacts endothelial dysfunction [37], but it is unknown whether whole body stores of biopterins correlate with in vivo circulating NO-dependent mediators of endothelial activation. To assess for associations between systemic biopterin status and endothelial activation we measured Ang-2 in a subset of randomly selected children enrolled as HC (n = 9), UM (n = 10), CM (n = 16), and NMC (n = 9). Plasma Ang-2 concentration differed significantly across the four groups (Fig. 4)—HC (815 [IQR 345–1264] pg/mL), UM (1882 [IQR 1035–4693] pg/mL), CM (3445 (IQR 2014–7534 pg/mL), and NMC (1149 [691–1616] pg/mL) (p<0.001). Pairwise comparisons among groups showed that median plasma Ang-2 levels were higher in CM compared to HC (p<0.001), UM (p = 0.082) and NMC (p = 0.006). Correlations between plasma Ang-2 and urine biopterin metabolites were assessed within each clinical group and no significant correlations were noted. A correlation scatterplot of plasma Ang-2 and urine BH2 values does visually demonstrate a non-significant correlation (S1 Fig.), indicating that the small sample size may have limited our ability to establish a significant correlation. We conclude that Ang-2 is significantly elevated in severe pediatric falciparum malaria, but we were unable to demonstrate a correlation between Ang-2 and urine biopterin metabolites, possibly due to small sample size.
Fig 4. Plasma angiopoietin-2 concentrations at enrollment in the 4 clinical groups.
Comparison of plasma angiopoietin-2 (Ang-2) concentrations at enrollment in a subset of healthy controls (HC, n = 9), uncomplicated malaria (UM, n = 10), cerebral malaria (CM, n = 16) and non-malaria central nervous system conditions (NMC, n = 9) (p<0.001 by Kruskal-Wallis). The central line indicates median. Upper and lower lines indicate inter-quartile range.
Peripheral blood mononuclear cell mRNA for guanosine triphosphate cyclohydrolase I in children with malaria
To determine if the low urine levels of BH4 observed in CM could in part be explained by decreased GCH1 expression for GTPCH-1, the rate-limiting enzyme in pterin biosynthesis [29], we measured GCH1 mRNA in peripheral blood mononuclear cells (PBMC) isolated from a randomly selected sub-set of children enrolled as HC (n = 5), UM (n = 8) and CM (n = 9). GCH1 mRNA transcription was at least as high in each malaria group [UM (1.35 [IQR 1.11–1.97] 2-ddCt) and CM (1.39 [IQR 0.88–1.77] 2-ddCt)] relative to controls (0.82 [IQR 0.60–0.84] 2-ddCt) (p = 0.082). We also measured GCH1 mRNA in children enrolled into a separate cohort using the same inclusion/exclusion criteria as HC (n = 33) and as CM (n = 9). Combining the measurements from both cohorts [HC n = 38 and CM n = 18], we found no significant difference in GCH1 mRNA between these two groups. We conclude that PBMC GCH1 mRNA and pterin synthesis is not decreased in children with malaria and does not account for the lower BH4.
Hyperphenylalaninemia in children with uncomplicated malaria and cerebral malaria
We measured plasma phenylalanine in all 4 clinical groups to confirm our previous observation of hyperphenylalaninemia in clinical malaria [18] and to assess the relationship between systemic BH4 status and plasma phenylalanine concentration. Hyperphenylalaninemia (upper limit of normal in children age > 12 months, 80uM; upper limit of normal in children age < 12 months, 100 uM) was found in 4 (3.7%) of 109 HC, 44 (72.1%) of 61 UM, 40 (80%) of 50 CM, and 23 (45.1%) of 51 NMC. Plasma phenylalanine concentrations differed significantly across all groups (Table 2). This association remained after using linear regression to control for age, weight, and fasting duration. Using the same linear regression model, plasma phenylalanine concentrations differed significantly in CM compared to UM (p = 0.04), and across UM, CM survivors and CM fatalities (p = 0.015). Plasma phenylalanine concentration correlated with urine BH2 concentration among HC (r = 0.29; p = 0.002), UM (r = 0.43; p = 0.001), and NMC (r = 0.32; p = 0.03), but among CM the correlation was non-significant (r = 0.22; p = 0.15). Similarly, when analyzing UM and CM children jointly, there was a significant correlation between plasma phenylalanine and BH2 was observed (r = 0.38; p<0.001), but we when employed a partial correlation test to control for malaria disease severity, this correlation was non-significant (r = 0.17; p = 0.09). Plasma phenylalanine did not correlate with BH4 or the BH4/BH2 ratio. Despite a non-significant correlation among CM, overall we found a moderate correlation between plasma phenylalanine and urine BH2 levels, a result that is consistent with the body of literature demonstrating the ability of BH2 to inhibit PAH enzymatic conversion of phenylalanine to tyrosine [28].
Bivariate and multivariate regression analysis correlating urine BH4 to severity of malaria
In bivariate logistic regression among all malaria patients (Table 3), a unit decrease in Log10-urine BH4 and Log10-urine BH4/BH2 ratio was associated with 2.94-fold (95% CI 1.65–5.23) increase and 3.29-fold (95% CI 1.87–5.76) increase, respectively, in the odds of CM (p<0.001). A unit increase in log10-urine BH2 was associated with a 1.91-fold increase (95% CI 0.99–3.67) in the odds of CM (p = 0.04). In the backward stepwise multivariate logistic regression model for risk of CM, variables were retained in the model if their p-value was < 0.2. The final model included urine BH4, urine BH2, P. falciparum histidine-rich protein-2 (PfHRP-2), respiratory rate and weight (Table 3). Urine BH4 and urine BH2 levels were independently associated with odds of CM: a unit decrease in Log10-urine BH4 was associated with 3.85-fold (95% CI 1.89–7.61) increase in the odds of CM (p<0.001). Note that as Table 3 models risk of CM for every unit increase in a given variable, the inverse is displayed—i.e., a unit increase in Log10-urine BH4 was associated with 0.26-fold (95% CI 0.13–0.53) decrease in odds of CM [p<0.001]. A unit increase in Log10-urine BH2 was associated with a 2.86-fold (95% CI 1.01–8.07) increase in the odds of CM (p = 0.047).
Table 3. Bivariate and multivariate logistic regression analysis of factors associated with increased odds of cerebral malaria.
| Variable | N | Bivariate OR (95% CI) | p-value | Multivariate OR (95% CI) | p-value |
|---|---|---|---|---|---|
| Urine BH4 | 100 | 0.34 (0.19–0.60) | <0.001 | 0.26 (0.13–0.51) | <0.001 |
| Urine BH2 | 100 | 1.91 (0.99–3.68) | 0.053 | 3.38 (1.24–9.21) | 0.018 |
| Phenylalanine | 111 | 1.78 (0.89–3.55) | 0.101 | — | >0.05 A |
| PfHRP-2 | 106 | 1.26 (1.03–1.53) | 0.024 | — | >0.05 |
| Hemoglobin | 116 | 0.73 (0.60–0.89) | 0.001 | — | >0.05 A |
| Bicarbonate | 79 | 1.03 (0.95–1.11) | 0.480 | — | — B |
| Respiratory Rate | 118 | 1.07 (1.04–1.17) | 0.001 | 1.10 (1.05–1.17) | <0.001 |
| Weight | 118 | 1.09 (0.98–1.21) | 0.106 | 1.19 (1.02–1.39) | 0.026 |
| Age | 118 | 1.02 (1.00–1.05) | 0.038 | — | >0.05 A |
| Sex | 118 | 1.67 (0.79–3.54) | 0.181 | — | — B |
| Last meal | 118 | 0.99 (0.94–1.07) | 0.99 | — | >0.05 A |
CI = confidence intervals
BH4 = tetrahydrobiopterin
BH2 = dihydrobiopterin
PfHRP-2 = P. falciparum histidine-rich protein-2
Note: Phenylalanine, Urine BH4, Urine BH2 and PfHRP-2 were log-transformed to enable regression analysis. Number of observations included in multivariate model was 96.
A = included in multivariate logistic regression analysis, but not retained in the final model due to p-value > 0.05 in multivariate analysis.
B = not included in multivariate logistic regression analysis because of non-significance in bivariate logistic regression analysis.
The urine BH4:BH2 ratio is not included in the logistic regression model due to co-linearity with BH4. When the multivariate model included the BH4:BH2 ratio in lieu of urine BH4, the odds ratio of CM was comparable to the odds ratio of CM seen with urine BH4: a unit decrease in Log10-urine BH4:BH2 ratio was associated with a 3.63-fold (95% CI 1.95–6.72) increase in the odds of CM (p<0.001).
Discussion
Systemic BH4 concentrations are significantly lower in children with CM compared to HC, UM, and NMC. BH4 concentrations were likewise significantly lower in fatal CM when compared across the spectrum of UM and CM survivors, and a decrease in urine BH4 was independently associated with a 3.85-fold increase in odds of CM. While two prior studies reported conflicting results on BH4 levels in cerebrospinal fluid [17,49], our study is the first published analysis of systemic biopterin levels in severe human malaria. Our findings have important implications regarding the underlying reasons for the decreased NO bioavailability and microvascular dysfunction observed in severe malaria [16,19,21]. These data also represent a novel link between the extensive vascular medicine research regarding the effects of BH4 and BH2 on the endothelium [27] and the vasculopathic mechanisms of severe malaria [16].
Our prior studies have demonstrated that NO is important in protecting against severe malaria, including CM, and have demonstrated several mechanisms by which NO bioavailability is decreased in malaria. These mechanisms include: low overall NO production [21]; low NOS2 protein levels in peripheral blood mononuclear cells [21]; low NOS activity in PBMC [22]; NOS2 single nucleotide polymorphisms that modulate NO production [23]; increased cell-free hemoglobin, an NO quencher [50]; decreased NOS substrate arginine [6,24]; and increased asymmetric dimethyl arginine (ADMA) [25,26], an endogenous inhibitor of NOS activity [51]. Our present study documents another potential mechanism for low NO production in severe malaria—diminished levels of NOS cofactor, BH4. Our prior studies of NO in malaria have focused on NOS2 (inducible NOS), which is expressed in multiple tissues, including brain and endothelium. Our current findings apply not only to NOS2, but to all three NOS isoforms (endothelial, inducible and neuronal), as BH4 is an obligate co-factor for all three NOS isoforms and deficiency of BH4 can result in diminished NOS activity and cause uncoupling in all NOS isoforms. We observed that total urine biopterins (which reflect total body biopterins) were increased in mild and severe malaria. Expression of PBMC mRNA for CGH1, the rate-limiting enzyme in biopterin synthesis, was at least as high in malaria as in HC. While pterin synthesis in human leukocytes may not reflect biopterin bioavailability in other tissues (e.g., the endothelium), this finding is consistent with our observation of increased total urine biopterins in malaria. Beyond the absolute totals, we think the more significant observation is the markedly skewed distribution of biopterin species in CM cases—we found that nearly 2/3 of biopterins were oxidized to inactive forms, a finding not previously reported. Tanzanian children with CM had significantly lower urine BH4 concentrations and a lower BH4:BH2 ratio compared to HC, UM and NMC.
Quantification of the different redox states of biopterins in malaria—fully reduced, BH4, and its oxidized metabolites, BH2 and B0—may be a novel method for measuring oxidative stress in severe malaria and possibly other critical illnesses. The same rationale applies to the NH2:N0 ratio. Our finding of increased levels of neopterin in UM and CM is consistent with prior reports of neopterin concentrations in malaria [52,53]. While the pathophysiologic role of neopterin remains unclear, the ratio of reduced to oxidized neopterin (NH2:N0) might represent a novel parameter to quantify mononuclear phagocyte redox state. The decrease in both BH4:BH2 and NH2:N0 ratios indicates increased systemic oxidative stress in CM.
As a NOS co-factor, BH4 performs both structural and biochemical functions. It stabilizes the NOS homodimer, thereby enabling NOS catalytic function, and it donates an electron to form a transient BH4∙+ radical which is required for oxidation of L-arginine to L-citrulline [27]. However, in the absence of BH4, NOS catalyzes a reaction in which NADPH-derived electrons reduce heme iron thereby binding and activating oxygen [54]. Without BH4-dependent L-arginine oxidation, activated oxygen is released from the heme catalytic center of NOS as superoxide. This generation of superoxide is termed NOS “uncoupling,” as L-arginine oxidation to L-citrulline is no longer coupled to oxygen activation for NO synthesis and release [27]. In an environment where both superoxide (uncoupled NOS) and NO (coupled NOS) are generated, both products may react to yield peroxynitrite [55,56]. Peroxynitrite and superoxide induce further uncoupling by oxidizing available BH4 to BH2 [57], thereby decreasing the availability of BH4 for NO synthesis. This uncoupling is compounded by BH2 accumulation, which competes with BH4 at its NOS binding site, further inhibiting NOS-catalyzed NO synthesis [37]. Thus a feed-forward mechanism, initiated by low intracellular BH4 levels, ensues and leads to oxidative injury caused by superoxide and peroxynitrite [58]. Experimental models of eNOS activity indicate that the BH4:BH2 ratio, which we found to be significantly decreased in CM compared to all other groups, is the measurement that most strongly correlates with NOS uncoupling [37].The hyperphenylalaninemia that we previously described in UM and CM [18] was one of the initial observations that led us to test the hypothesis of impaired BH4 in CM, since BH4 is an essential cofactor for the enzymatic hydroxylation of phenylalanine to tyrosine. We confirmed our prior finding of elevated plasma phenylalanine in UM and CM. Our present findings suggest that this elevation in malaria is secondary to decreased systemic concentrations of BH4 and increased concentrations of BH2.
Decreased BH4 and increased BH2 systemic levels in children with CM may be relevant to the central pathogenic mechanisms in severe malaria—endothelial activation and dysfunction and the sequestration of pRBCs, unparasitized RBCs, mononuclear cells, and platelets resulting in microcirculatory congestion of post-capillary venules [14,16,19,59]. Endothelial cells low in intracellular BH4 and high in BH2 favor decreased NO production by NOS, and low NO synthesis is associated with endothelial dysfunction [6]. The effect of decreased NO production due to NOS uncoupling may exacerbate microvascular sequestration in several ways. Low NO states are associated with decreased RBC deformability [60]. In vitro endothelial cell models have shown that increased bioavailability of NO is associated with decreased cytoadherence of P. falciparum-infected RBCs via down-regulation of endothelial receptors for P. falciparum erythrocyte membrane protein [61,62]. NO deficiency causes increased vascular tone with decreased vessel diameter and possible flow impedance. Additionally, NOS uncoupling due to low BH4 and high BH2 would also favor generation of peroxynitrite, a highly reactive species thought to exacerbate endothelial cell dysfunction [63]. Given that low BH4 levels may be contributing to endothelial dysfunction and sequestration in severe malaria, adjunctive therapy to reduce BH2 and regenerate BH4 warrants further study [64,65]. The need for such studies is also supported by an experimental animal model of cerebral malaria demonstrating that the blunted cerebral arteriolar response to NOS agonists is partially recovered by supplementation with BH4 [66].
While our study has many strengths, including rigorous case definitions for the four clinical groups and rigorous methods for sample collection, processing and analysis, we acknowledge several limitations. The specificity of the WHO case definition for CM may be as low as 77% [13]. The causes and severity of illness in the NMC group were heterogeneous, making comparisons with this group more tenuous. Since we were able to measure plasma creatinine in only 16 UM and 39 CM patients, we did not include plasma creatinine in the final multivariate logistic regression model assessing for an independent association between urine BH4 concentrations and malaria severity. We therefore cannot exclude that the significant association between BH4 and malaria severity is dependent upon renal function. We performed a multivariate logistic regression sub-model for the 48 malaria patients who had urine biopterin and plasma creatinine results. In this sub-model we found that only respiratory rate (OR 1.11 [95% CI 1.01–1.21]; p = 0.04) and plasma creatinine (OR 201.42 {95% CI 8.15–4978.96]; p = 0.001) were independently associated with odds of cerebral malaria (BH4 OR 0.34 [95% CI 0.09–1.28;p = 0.11). This indicates that plasma creatinine has a statistically significant association with malaria severity, but the wide confidence intervals show a lack of power for more precisely assessing this relationship and its impact on the association between urine BH4 and malaria severity. While the lack of plasma creatinine results is a key limitation of our final multivariate logistic regression model, we note the following in regards to the urine BH4 measurements and renal function: 1) only two children (both with CM) had clinically significant renal impairment; 2) A linear regression model of urine BH4 showed no significant variance by plasma creatinine among the 85 children in whom both a urine BH4 measurement and a plasma creatinine measurement. 3) Total urine biopterins were increased in CM patients, which argues against the higher mean plasma creatinine values in CM as accounting for the decreased levels of BH4 in the urine of CM patients. Inclusion of plasma creatinine in the model would have been optimal, but the reasons detailed above, we think exclusion of creatinine from the multivariate logistic regression model is justified and preferred.
Additional limitations include the fact that we did not measure NO bioavailability directly and that our findings do not demonstrate a causal link between endothelial cell dysfunction and low systemic BH4. Unlike our companion study demonstrating decreased systemic BH4 in Indonesian adults with severe falciparum malaria, we do not have measures of endothelial function in this study to compare with the decreased levels of systemic BH4 we have observed in Tanzanian children with CM. Among a subset of the cohort we were able to measure Ang-2, a mediator of endothelial cell activation that is associated with reduced NO-bioavailability in both severe malaria and sepsis [10,67]. The markedly elevated plasma Ang-2 levels and the abnormal urine biopterin metabolite levels observed among children with malaria were not significantly correlated. A small sample size may have limited our ability to show a significant correlation between Ang-2 and BH2. While we speculate that the highly perturbed BH4: BH2 ratio is due to oxidative stress, we do not have additional purported measures of oxidative stress with which to compare biopterin redox status. An alternative explanation for this the low BH4: BH2 ratio is impaired recycling via DHFR conversion of BH2 to BH4.
In conclusion, we found that BH4, an essential cofactor for NO production, is low in children with CM, and that low BH4 concentrations are independently associated with disease severity in children with malaria. Low BH4 may contribute to the pathogenesis of severe malaria as it represents an important mechanism of low NO bioavailability. Furthermore, low BH4 together with elevated BH2 likely leads to generation of reactive oxygen species. These potential sequelae of low systemic BH4 likely contribute to endothelial dysfunction and pRBC sequestration—hallmark pathophysiologic features of severe malaria. Interventions that replenish BH4 or redistribute the BH4:BH2 ratio toward reduced BH4 merit investigation as adjunctive therapies to improve outcomes in pediatric severe falciparum malaria.
Materials and Methods
Study design and site
We conducted a prospective observational study in Dar es Salaam, Tanzania. Children were enrolled from the clinics and the inpatient wards of Amana and Mwanayamala District Hospitals into the following four groups: UM, CM, NMC (see criteria below), and HC. These two district hospitals are separated by 6.5 kilometers, and both are located in semi-urban areas of Dar es Salaam. Once enrolled, children with CM or with NMC were transferred to the clinical research unit at the Hubert Kairuki Memorial University Hospital for further evaluation and care. Approval for this study was obtained from the institutional review boards of Hubert Kairuki Memorial University, United Republic of Tanzania National Medical Research Institute, University of Utah, and Duke University. Informed consent was obtained from parents or guardians of all children, and the U.S. Department of Health and Human Services guidelines for human subjects research were followed.
Enrollment criteria
Children were 6 months to 6 years in age. The World Health Organization (WHO) case definition for CM was used as the inclusion criteria for the CM cohort: any level of P. falciparum parasitemia on peripheral blood smear; unarousable coma (Blantyre Coma Score ≤ 2) not attributable to hypoglycemia (blood glucose level < 40 mg/dL) and persisting more than 60 minutes after any convulsion; no other identifiable cause of coma [68]. Inclusion criteria for UM were as follows: a clinical syndrome consistent with malaria and a documented fever (temperature ≥ 38° C) or history of fever within 48 hours from time of enrollment; P. falciparum parasitemia > 10,000 parasites/μL on Giemsa-stained blood film plus a positive P. falciparum RDT (Paracheck-Pf; Omega Diagnostics); no other cause of fever identified; no WHO warning signs suggestive of severe disease [68]. These warning signs were the following: inability to suckle, eat and/or drink; excessive vomiting; abnormal respiratory rate or respiratory distress as evidenced by accessory muscle use, suprasternal retractions, or intercostal retractions; recent history of convulsions; altered mental status; inability to sit unaided.
Exclusion criteria for both groups with malaria were any of the following: microscopic evidence of mixed infection with any other Plasmodium species; bacterial co-infection as evidenced by septicemia or urinary tract infection; oral or intravenous quinine or oral artemesinin combination therapy initiated > 18 hours prior to enrollment; hemoglobin < 5 mg/dL, as erythrocyte transfusions were not readily available at our study sites. Children with CM were excluded if they had evidence of bacterial meningitis as indicated by isolation of a pathogen from CSF culture or by CSF analysis.
Similar aged healthy children and children presenting with NMC were prospectively enrolled as control groups. The healthy children were enrolled from outpatient well-baby clinics at the two district hospitals. Eligible children had no signs or symptoms of active illness, no febrile illness within the previous two weeks, no history or evidence of an active inflammatory condition, and negative P. falciparum RDT (Paracheck-Pf; Omega Diagnostics). Children with NMC were eligible if they had a CNS condition for which a lumbar puncture for CSF analysis was clinically indicated as part of diagnostic evaluation and management (e.g. suspected meningitis, encephalitis, hemorrhage, trauma, metabolic, toxic, recurrent seizures as cause for altered mental status). All children enrolled in this group had a Giemsa-stained blood film negative for Plasmodium and a negative P. falciparum RDT (Paracheck-Pf; Omega Diagnostics). A subset of children in this group were classified as likely bacterial or fungal meningitis based on detection of a microbiologic pathogen in CSF and one of the following findings on CSF analysis: white blood cell count > 6/μL, neutrophils present on cytospin Wright stain prep, or glucose < 2/3 plasma glucose level.
Clinical evaluation and management
At presentation, demographic information, clinical history, and examination were documented using standardized case report forms. Capillary blood samples were obtained for Giemsa-stained thick and thin blood films as well as on-site malaria rapid diagnostic testing. Venous samples for routine laboratory analysis included complete blood count (Beckman-Coulter Act 10), sodium, potassium, chloride, bicarbonate, blood urea nitrogen, creatinine, and venous blood gas (i-STAT 1; Abbott Laboratories). Urine collected for pterin quantification (see below) was also subjected to urine dipstick analysis (Multistix 10 SG; Siemens Healthcare Diagnostics) and urine culture. These blood and urine laboratory results were immediately available to the clinician. Blood cultures were obtained in all children with UM and CM to rule out concomitant bacteremia. Lumbar puncture with opening pressure measurement was done in all patients with coma to evaluate for meningitis. CSF analysis included determination of glucose and protein levels, cell count with differential by trained microscopists, and bacterial and fungal cultures.
Children with UM and CM received anti-malarial therapy and other supportive care as per standard Tanzanian national protocols at the time of the study (artemesinin combined oral therapy and intravenous quinine, respectively). Treatment was initiated as soon as the diagnosis of malaria was suspected. Children with CM and NMC were re-examined daily until death or hospital discharge.
Urine collection and storage
Urine was collected from subjects at the time of enrollment. Strict adherence to the collection procedure was followed in every case. All collection and handling of urine samples was done in the dark or under dim lighting (collection bags covered with a black, light-impermeable cloth) to prevent photo-oxidation. For quantification of urine biopterins and neopterins, 5–20 ml urine was collected voluntarily into a sterile urine collection cup or with use of a pediatric bag (U-bag urine collector, Hollister Pediatric) affixed to the perineum with adhesive. In both cases, issuing urine was immediately collected into solid dithioerythritol (DTE, approximately 50 mg/ml urine) and diethylene triamine penta-acetic acid (DETAPAC, approximately 5 mg/ml urine) and mixed well to dissolve the powders as described previously [35]. Immediately after collection, urine samples were placed into an insulated transport cooler charged with large cooling packs preconditioned at -80° C. This resulted in freezing of urine samples within minutes. The samples were then stored in a -80° C freezer and then transported to the USA in a liquid nitrogen dry shipper, and subsequently stored at -80° C until pterin analysis. This procedure exceeded in stringency the conditions required to prevent oxidation of reduced pterins previously described [35,69]. Reagent BH4 exposed to this procedure was stable at concentrations in the range measured on clinical urine samples; no detectable oxidation of BH4 measured by our analytic methods occurred. Conversely, exposure of oxidized reagent pterins to this procedure did not result in any measureable reduction. Thus the analysis of our samples revealed quantities of urine pterins as they existed in vivo.
Quantification of urine pterins
Thawed urine samples were mixed and directly subjected to analysis for BH4, BH2 and B0 and for NH2 and N0 by high-performance liquid chromatography (HPLC) using sequential electrochemical and fluorescence detection. The procedure relies on reversed phase HPLC separation of pterins, BH4, BH4, NH2, B0 and N0 in samples of urine. BH4 is oxidized to quinonoid dihydrobiopterin (q-BH2) and then reduced back to BH4. The current generated on the reduction is monitored and used to determine BH4 concentration using an ESA Coularray electrochemical integration system. BH2 and NH2 are oxidized to B0 and N0 respectively by electrochemistry and then measured by fluorescence using EZ Chrom integration system. B0 and N0 are not affected by electrochemical electrodes and are measured by their natural fluorescence using the EZ Chrom system. Further details of the analytical methods are as reported previously [70] (see S2 Fig. and see Fig. 2 & 3 in citation 41 for example chromatograms).
Plasma PfHRP-2 measurement
PfHRP-2, a measure of total parasite biomass, was quantified by ELISA as previously described [6,10] using primary and secondary monoclonal antibodies to P. falciparum HRP-2 (MPFM-55A and MPFG-55P; Immunology Consultant Laboratories). Concentrations were derived from standard curves utilizing purified PfHRP-2 kindly provided by D. Sullivan (Johns Hopkins University School of Public Health). Samples with ODs outside the linear part of the curve were repeated at an appropriate dilution until an accurate concentration was determined. The lower limit of detection was 1.5 ng/mL.
Plasma Angiopoietin-2 measurement
Ang-2 was measured by ELISA (R&D Systems) as noted before [10].
Measurement of mRNA for GTPCH-1 by qRT-PCR
PBMC were isolated using standard methods. RNA was extracted from PBMC and subjected to real-time reverse transcriptase-polymerase chain reaction (RT-PCR) techniques. We used the equivalent of 100 ng RNA per reaction. Stratagene VILO kit was used for the RT reaction, and the Roche faststart universal probe master kit for PCR (Roche Applied Science). Primer/probe sets were designed by the Roche universal probe library application. Primers were then made by IDT while probes were from the Roche universal probe library (http://universalprobelibrary.com) (Roche Applied Science). The quantitative PCR were performed] on an ABI7300 machine. Samples were run in triplicate, or in duplicate if sample amount was limiting. The GAPDH and HPRT1 genes were selected as endogenous control genes. The average Ct of the endogenous controls for each sample was used in the dCt calculation. For individual ddCt values, we used each sample dCt and the averaged healthy control dCt. For group ddCt values, we used the average dCt for each group to calculate ddCt for the group. The data is expressed as 2-ddCt, which is the fold change as compared to the average of the healthy controls. Table 4 displays the relevant sequences for the primers and probes.
Table 4. Primers and probes for GTP-cyclohydrolase I mRNA reverse transcriptase polymerase chain reaction.
| Gene | Forward Primer | Reverse Primer | Probe number | Probe cat# | Probe sequence |
|---|---|---|---|---|---|
| GTPCH1 | gctgtagcaatcacggaagc | cacctcgcattaccatacaca | 67 | 4688660001 | tgctggag |
| GAPDH | agccacatcgctcagacac | gcccaatacgaccaaatcc | 60 | 4688589001 | tggggaag |
| HPRT1 | tgaccttgatttattttgcatacc | cgagcaagacgttcagtcct | 73 | 4688961001 | gctgagga |
Plasma amino acid analysis
Blood samples were collected into heparin tubes, mixed and immediately centrifuged to sediment blood cells. Supernatant plasma was removed to freezing tubes and placed into the transport vessel described above, after which they were stored at -80° C until thawing for analysis. Amino acids were quantified by ion-exchange chromatography with detection using spectrophotometry after reaction with ninhydrin. All amino acid analysis was performed at the Biochemical Genetics Section, ARUP Laboratories, University of Utah in collaboration with Dr. Marzia Pasquale.
Statistical methods
Statistical analysis was performed with STATA software (version 12.0; StataCorp). Results are presented as mean with 95% confidence intervals for normally distributed continuous variables or median with interquartile range for variables with non-parametric distribution. For continuous variables with normal distribution, differences across groups were compared by ANOVA with Bonferroni adjustment for multiple comparisons and differences between groups were compared using Student’s t-test. For continuous variables with a non-parametric distribution, differences across groups were compared using Kruskal-Wallis test and differences between groups were compared using the Wilcoxon rank-sum test. Correlation between variables with non-parametric distributions was assessed using Spearman correlation coefficients, and we used the partial correlation test to assess for correlations while controlling for malaria severity. Differences in proportions between groups were assessed with Chi-square test. Multivariate linear regression was used to control for confounding variables that could affect urine BH4 and plasma phenylalanine concentrations. Bivariate logistic regression was used to analyze continuous variables for co-variation by a binomial variable (e.g., co-variation of urine BH4 concentration by the presence or absence of pyuria). For children with UM and CM, backward stepwise multivariate logistic regression was performed to determine whether phenylalanine or urine biopterin species were independently associated with disease severity. Bivariate logistic regression was performed for available biologically plausible variables known to be associated with severe malaria: hemoglobin, PfHRP-2, and respiratory rate and bicarbonate, as indicators of metabolic acidosis [3,71,72]. Variables were included in the multivariate logistic regression model if p < 0.20 on bivariate analysis, and were retained in the multivariate model if the p-value generated in the multivariate analysis was <0.05. The multivariate model also controlled for age, sex, weight and duration of fasting. Plasma creatinine was not included in the multivariate model because creatinine results were only available for 14 UM and 34 CM patients with urine biopterin results. In a multivariate logistic regression sub-model of these 48 malaria patients we did include creatinine in order to assess the relationship between estimated renal function and odds of cerebral malaria. Continuous variables with non-parametric distributions were log-transformed to meet normality assumptions for use in the logistic regression models. Goodness-of-fit was assessed by Hosmer-Lemeshow test. A two-sided p-value of < 0.05 was employed as the cut-off for statistical significance throughout the manuscript unless otherwise indicated (e.g., Bonferoni adjustments when making comparisons across multiple groups).
Supporting Information
Plasma Angiopoeitin-2 (Ang-2) plotted against urine dihydrobiopterin (BH2). Squares represent healthy controls (HC, n = 9), triangles represent uncomplicated malaria (UM, n = 5), circles represent cerebral malaria (CM, n = 13), and stars represent non-malaria central nervous system conditions (NMC, n = 9).
(TIF)
A: Electrochemical detection of of tetrahydrobiopterin (BH4) and dihydroneopterin (NH2) in urine. B: Electrochemical detection of BH4 and NH2 in standards. C: Fluorescence detection of neopterin (N0), dihydroneopterin (NH2), biopterin (B0), and dihydrobiopterin (BH2) in urine. D: Fluorescence detection of N0, NH2, B0, and BH2 in standards.
(TIF)
Acknowledgments
We thank all patients and their families for participation in our research, and the staff at the Hubert Kairuki Memorial University Hospital in Dar es Salaam. We are indebted to the "Hyperphenylalaninemia in cerebral malaria" study staff for their technical and administrative support: Rehema Sombi, Kokushubila Kairuki, Keto Mshigeni, Stephen Biginagwa, Loyce Kahungya, Kaizerege Karoma, Irene Maokola, Sophia Mbangukura, Stella Shalua and George Kihwili. We especially thank the study coordinators: Bernard John, Rebecca Johnson and Ruth Stanton for their excellent assistance. We thank Read Taintor for supplying urine culture kits, and Ms. Lesa Hall for excellent graphics assistance. We thank Dr. Marzia Pasquale for providing plasma amino acid analysis, Dr. Molly Leecaster for guidance on statistical analysis, and Dr. Kathryn Swoboda for scientific guidance throughout the project. Dr. John Hibbs kindly provided a critical review of the manuscript.
Data Availability
All relevant data are within the paper and Supporting Information.
Funding Statement
The work was supported by the following agencies and grants: National Institutes of Health (U01AI057565 and R01AI041764 [DLG and JBW]); Fogarty International Center Global Health Fellowship (R25TW009343 [MPR]); the Veterans Affairs Research Service (JBW and DLG); and Australian NHMRC Fellowships (NMA and TWY). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. WHO (2013) World Health Organization World Malaria Report 2013. Geneva: WHO; http://www.who.int/malaria/publications/world_malaria_report_2013/report/en/. Accessed 4 March 2014. [Google Scholar]
- 2. Dondorp AM, Fanello CI, Hendriksen IC, Gomes E, Seni A, et al. (2010) Artesunate versus quinine in the treatment of severe falciparum malaria in African children (AQUAMAT): an open-label, randomised trial. Lancet 376: 1647–1657. 10.1016/S0140-6736(10)61924-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marsh K, Forster D, Waruiru C, Mwangi I, Winstanley M, et al. (1995) Indicators of life-threatening malaria in African children. N Engl J Med 332: 1399–1404. [DOI] [PubMed] [Google Scholar]
- 4. WHO (2010) World Health Organization Guidelines for the treatment of malaria, 2nd ed. Geneva: WHO; http://www.who.int/malaria/publications/atoz/9789241547925/en/index.html. Accessed 28 July 2013. 10.1186/1475-2875-9-212 [DOI] [Google Scholar]
- 5. Idro R, Marsh K, John CC, Newton CR (2010) Cerebral malaria: mechanisms of brain injury and strategies for improved neurocognitive outcome. Pediatr Res 68: 267–274. 10.1203/00006450-201011001-00524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yeo TW, Lampah DA, Gitawati R, Tjitra E, Kenangalem E, et al. (2007) Impaired nitric oxide bioavailability and L-arginine reversible endothelial dysfunction in adults with falciparum malaria. J Exp Med 204: 2693–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hollestelle MJ, Donkor C, Mantey EA, Chakravorty SJ, Craig A, et al. (2006) von Willebrand factor propeptide in malaria: evidence of acute endothelial cell activation. Br J Haematol 133: 562–569. [DOI] [PubMed] [Google Scholar]
- 8. Moxon CA, Chisala NV, Wassmer SC, Taylor TE, Seydel KB, et al. (2014) Persistent endothelial activation and inflammation after Plasmodium falciparum Infection in Malawian children. J Infect Dis 209: 610–615. 10.1093/infdis/jit419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Turner GD, Morrison H, Jones M, Davis TM, Looareesuwan S, et al. (1994) An immunohistochemical study of the pathology of fatal malaria. Evidence for widespread endothelial activation and a potential role for intercellular adhesion molecule-1 in cerebral sequestration. Am J Pathol 145: 1057–1069. [PMC free article] [PubMed] [Google Scholar]
- 10. Yeo TW, Lampah DA, Gitawati R, Tjitra E, Kenangalem E, et al. (2008) Angiopoietin-2 is associated with decreased endothelial nitric oxide and poor clinical outcome in severe falciparum malaria. Proc Natl Acad Sci U S A 105: 17097–17102. 10.1073/pnas.0805782105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pongponratn E, Turner GD, Day NP, Phu NH, Simpson JA, et al. (2003) An ultrastructural study of the brain in fatal Plasmodium falciparum malaria. Am J Trop Med Hyg 69: 345–359. [PubMed] [Google Scholar]
- 12. Silamut K, Phu NH, Whitty C, Turner GD, Louwrier K, et al. (1999) A quantitative analysis of the microvascular sequestration of malaria parasites in the human brain. Am J Pathol 155: 395–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Taylor TE, Fu WJ, Carr RA, Whitten RO, Mueller JS, et al. (2004) Differentiating the pathologies of cerebral malaria by postmortem parasite counts. Nat Med 10: 143–145. [DOI] [PubMed] [Google Scholar]
- 14. Dondorp AM, Ince C, Charunwatthana P, Hanson J, van Kuijen A, et al. (2008) Direct in vivo assessment of microcirculatory dysfunction in severe falciparum malaria. J Infect Dis 197: 79–84. 10.1086/523762 [DOI] [PubMed] [Google Scholar]
- 15. Hanson J, Lam SW, Mahanta KC, Pattnaik R, Alam S, et al. (2012) Relative contributions of macrovascular and microvascular dysfunction to disease severity in falciparum malaria. J Infect Dis 206: 571–579. 10.1093/infdis/jis400 [DOI] [PubMed] [Google Scholar]
- 16. White NJ, Pukrittayakamee S, Hien TT, Faiz MA, Mokuolu OA, et al. (2014) Malaria. The Lancet 383: 723–735. 10.1016/S0140-6736(13)60024-0 [DOI] [PubMed] [Google Scholar]
- 17. Dobbie M, Crawley J, Waruiru C, Marsh K, Surtees R (2000) Cerebrospinal fluid studies in children with cerebral malaria: an excitotoxic mechanism? Am J Trop Med Hyg 62: 284–290. [DOI] [PubMed] [Google Scholar]
- 18. Lopansri BK, Anstey NM, Stoddard GJ, Mwaikambo ED, Boutlis CS, et al. (2006) Elevated plasma phenylalanine in severe malaria and implications for pathophysiology of neurological complications. Infect Immun 74: 3355–3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yeo TW, Lampah DA, Kenangalem E, Tjitra E, Price RN, et al. (2013) Impaired skeletal muscle microvascular function and increased skeletal muscle oxygen consumption in severe falciparum malaria. J Infect Dis 207: 528–536. 10.1093/infdis/jis692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weinberg JB, Lopansri BK, Mwaikambo E, Granger DL (2008) Arginine, nitric oxide, carbon monoxide, and endothelial function in severe malaria. Curr Opin Infect Dis 21: 468–475. 10.1097/QCO.0b013e32830ef5cf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Anstey NM, Weinberg JB, Hassanali MY, Mwaikambo ED, Manyenga D, et al. (1996) Nitric oxide in Tanzanian children with malaria: inverse relationship between malaria severity and nitric oxide production/nitric oxide synthase type 2 expression. J Exp Med 184: 557–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Boutlis CS, Tjitra E, Maniboey H, Misukonis MA, Saunders JR, et al. (2003) Nitric oxide production and mononuclear cell nitric oxide synthase activity in malaria-tolerant Papuan adults. Infect Immun 71: 3682–3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hobbs MR, Udhayakumar V, Levesque MC, Booth J, Roberts JM, et al. (2002) A new NOS2 promoter polymorphism associated with increased nitric oxide production and protection from severe malaria in Tanzanian and Kenyan children. Lancet 360: 1468–1475. [DOI] [PubMed] [Google Scholar]
- 24. Lopansri BK, Anstey NM, Weinberg JB, Stoddard GJ, Hobbs MR, et al. (2003) Low plasma arginine concentrations in children with cerebral malaria and decreased nitric oxide production. Lancet 361: 676–678. [DOI] [PubMed] [Google Scholar]
- 25. Yeo TW, Lampah DA, Tjitra E, Gitawati R, Darcy CJ, et al. (2010) Increased asymmetric dimethylarginine in severe falciparum malaria: association with impaired nitric oxide bioavailability and fatal outcome. PLoS Pathog 6: e1000868 10.1371/journal.ppat.1000868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Weinberg JB, Yeo TW, Mukemba JP, Florence SM, Volkheimer AD, et al. (2014) Dimethylarginines: endogenous inhibitors of nitric oxide synthesis in children with falciparum malaria. J Infect Dis 210:913–922. 10.1093/infdis/jiu156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Crabtree MJ, Channon KM (2011) Synthesis and recycling of tetrahydrobiopterin in endothelial function and vascular disease. Nitric Oxide 25: 81–88. 10.1016/j.niox.2011.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Blau NB TB, Cotton RH, Hyland K. Disorders of Tetrahydrobiopterin and Related Biogenic Amines In: Scriver CR, Beaudet AL, Sly WS and Valle D, eds The Metabolic and Molecular Basis of Inherited Disease 8th Edition. New York, New York, USA: McGraw-Hill; 2001:1725–1737. [Google Scholar]
- 29. Longo N (2009) Disorders of biopterin metabolism. J Inherit Metab Dis 32: 333–342. 10.1007/s10545-009-1067-2 [DOI] [PubMed] [Google Scholar]
- 30. Kaufman S (1999) A model of human phenylalanine metabolism in normal subjects and in phenylketonuric patients. Proc Natl Acad Sci U S A 96: 3160–3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zurfluh MR, Giovannini M, Fiori L, Fiege B, Gokdemir Y, et al. (2005) Screening for tetrahydrobiopterin deficiencies using dried blood spots on filter paper. Mol Genet Metab 86 Suppl 1: S96–103. [DOI] [PubMed] [Google Scholar]
- 32. Opladen T, Abu Seda B, Rassi A, Thony B, Hoffmann GF, et al. (2011) Diagnosis of tetrahydrobiopterin deficiency using filter paper blood spots: further development of the method and 5 years experience. J Inherit Metab Dis 34: 819–826. 10.1007/s10545-011-9300-1 [DOI] [PubMed] [Google Scholar]
- 33. Ohashi A, Suetake Y, Saeki Y, Harada T, Aizawa S, et al. (2012) Rapid clearance of supplemented tetrahydrobiopterin is driven by high-capacity transporters in the kidney. Mol Genet Metab 105: 575–581. 10.1016/j.ymgme.2012.01.009 [DOI] [PubMed] [Google Scholar]
- 34. Blair JA, Pearson AJ (1974) Some observations on effects of light and solvent polarity on kinetics of tetrahydrobiopterin autoxidation. J Chem Soc Perkin Trans 2: 1786–1787. [Google Scholar]
- 35. Howells DW, Hyland K (1987) Direct analysis of tetrahydrobiopterin in cerebrospinal fluid by high-performance liquid chromatography with redox electrochemistry: prevention of autoxidation during storage and analysis. Clin Chim Acta 167: 23–30. [DOI] [PubMed] [Google Scholar]
- 36. Klatt P, Schmid M, Leopold E, Schmidt K, Werner ER, et al. (1994) The pteridine binding site of brain nitric oxide synthase. Tetrahydrobiopterin binding kinetics, specificity, and allosteric interaction with the substrate domain. J Biol Chem 269: 13861–13866. [PubMed] [Google Scholar]
- 37. Crabtree MJ, Smith CL, Lam G, Goligorsky MS, Gross SS (2008) Ratio of 5,6,7,8-tetrahydrobiopterin to 7,8-dihydrobiopterin in endothelial cells determines glucose-elicited changes in NO vs. superoxide production by eNOS. Am J Physiol Heart Circ Physiol 294: H1530–1540. 10.1152/ajpheart.00823.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Huber C, Batchelor JR, Fuchs D, Hausen A, Lang A, et al. (1984) Immune response-associated production of neopterin. Release from macrophages primarily under control of interferon-gamma. J Exp Med 160: 310–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Werner ER, Werner-Felmayer G, Fuchs D, Hausen A, Reibnegger G, et al. (1990) Tetrahydrobiopterin biosynthetic activities in human macrophages, fibroblasts, THP-1, and T 24 cells. GTP-cyclohydrolase I is stimulated by interferon-gamma, and 6-pyruvoyl tetrahydropterin synthase and sepiapterin reductase are constitutively present. J Biol Chem 265: 3189–3192. [PubMed] [Google Scholar]
- 40. Ziegler I, Schott K., Lubbert M., Herrmann F., Schwulera U., and Bacher A. (1990) Control of tetrahydrobiopterin synthesis in T lymphocytes by synergistic action of interferon-gamma and interleukin-2. J Biol Chem 265: 17026–17030. [PubMed] [Google Scholar]
- 41. Weinberg JB, Misukonis MA, Shami PJ, Mason SN, Sauls DL, et al. (1995) Human mononuclear phagocyte inducible nitric oxide synthase (iNOS): analysis of iNOS mRNA, iNOS protein, biopterin, and nitric oxide production by blood monocytes and peritoneal macrophages. Blood 86: 1184–1195. [PubMed] [Google Scholar]
- 42. Fuchs D, Weiss G, Reibnegger G, Wachter H (1992) The role of neopterin as a monitor of cellular immune activation in transplantation, inflammatory, infectious, and malignant diseases. Crit Rev Clin Lab Sci 29: 307–341. [DOI] [PubMed] [Google Scholar]
- 43. Hyland K (1985) Estimation of tetrahydro, dihydro and fully oxidised pterins by high-performance liquid chromatography using sequential electrochemical and fluorometric detection. J Chromatogr 343: 35–41. [DOI] [PubMed] [Google Scholar]
- 44. Fiedler U, Scharpfenecker M, Koidl S, Hegen A, Grunow V, et al. (2004) The Tie-2 ligand angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel-Palade bodies. Blood 103: 4150–4156. [DOI] [PubMed] [Google Scholar]
- 45. Fiedler U, Reiss Y, Scharpfenecker M, Grunow V, Koidl S, et al. (2006) Angiopoietin-2 sensitizes endothelial cells to TNF-alpha and has a crucial role in the induction of inflammation. Nat Med 12: 235–239. [DOI] [PubMed] [Google Scholar]
- 46. Giuliano JS Jr., Lahni PM, Harmon K, Wong HR, Doughty LA, et al. (2007) Admission angiopoietin levels in children with septic shock. Shock 28: 650–654. [PMC free article] [PubMed] [Google Scholar]
- 47. Conroy AL, Glover SJ, Hawkes M, Erdman LK, Seydel KB, et al. (2012) Angiopoietin-2 levels are associated with retinopathy and predict mortality in Malawian children with cerebral malaria: a retrospective case-control study*. Crit Care Med 40: 952–959. 10.1097/CCM.0b013e3182373157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lowenstein CJ, Morrell CN, Yamakuchi M (2005) Regulation of Weibel-Palade body exocytosis. Trends Cardiovasc Med 15: 302–308. [DOI] [PubMed] [Google Scholar]
- 49. Weiss G, Thuma PE, Biemba G, Mabeza G, Werner ER, et al. (1998) Cerebrospinal fluid levels of biopterin, nitric oxide metabolites, and immune activation markers and the clinical course of human cerebral malaria. J Infect Dis 177: 1064–1068. [DOI] [PubMed] [Google Scholar]
- 50. Yeo TW, Lampah DA, Tjitra E, Gitawati R, Kenangalem E, et al. (2009) Relationship of cell-free hemoglobin to impaired endothelial nitric oxide bioavailability and perfusion in severe falciparum malaria. J Infect Dis 200: 1522–1529. 10.1086/644641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Vallance P, Leone A, Calver A, Collier J, Moncada S (1992) Endogenous dimethylarginine as an inhibitor of nitric oxide synthesis. J Cardiovasc Pharmacol 20 Suppl 12: S60–62. [DOI] [PubMed] [Google Scholar]
- 52. Reibnegger G, Boonpucknavig V, Fuchs D, Hausen A, Schmutzhard E, et al. (1984) Urinary neopterin is elevated in patients with malaria. Trans R Soc Trop Med Hyg 78: 545–546. [DOI] [PubMed] [Google Scholar]
- 53. te Witt R, van Wolfswinkel ME, Petit PL, van Hellemond JJ, Koelewijn R, et al. (2010) Neopterin and procalcitonin are suitable biomarkers for exclusion of severe Plasmodium falciparum disease at the initial clinical assessment of travellers with imported malaria. Malar J 9: 255 10.1186/1475-2875-9-255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Adak S, Wang Q, Stuehr DJ (2000) Arginine conversion to nitroxide by tetrahydrobiopterin-free neuronal nitric-oxide synthase. Implications for mechanism. J Biol Chem 275: 33554–33561. [DOI] [PubMed] [Google Scholar]
- 55. Pryor WA, Squadrito GL (1995) The chemistry of peroxynitrite: a product from the reaction of nitric oxide with superoxide. Am J Physiol 268: L699–722. [DOI] [PubMed] [Google Scholar]
- 56. Xia Y, Dawson VL, Dawson TM, Snyder SH, Zweier JL (1996) Nitric oxide synthase generates superoxide and nitric oxide in arginine-depleted cells leading to peroxynitrite-mediated cellular injury. Proc Natl Acad Sci U S A 93: 6770–6774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bendall JK, Alp NJ, Warrick N, Cai S, Adlam D, et al. (2005) Stoichiometric relationships between endothelial tetrahydrobiopterin, endothelial NO synthase (eNOS) activity, and eNOS coupling in vivo: insights from transgenic mice with endothelial-targeted GTP cyclohydrolase 1 and eNOS overexpression. Circ Res 97: 864–871. [DOI] [PubMed] [Google Scholar]
- 58. Alkaitis MS, Crabtree MJ (2012) Recoupling the cardiac nitric oxide synthases: tetrahydrobiopterin synthesis and recycling. Curr Heart Fail Rep 9: 200–210. 10.1007/s11897-012-0097-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dondorp AM, Pongponratn E, White NJ (2004) Reduced microcirculatory flow in severe falciparum malaria: pathophysiology and electron-microscopic pathology. Acta Trop 89: 309–317. [DOI] [PubMed] [Google Scholar]
- 60. Bor-Kucukatay M, Wenby RB, Meiselman HJ, Baskurt OK (2003) Effects of nitric oxide on red blood cell deformability. Am J Physiol Heart Circ Physiol 284: H1577–1584. [DOI] [PubMed] [Google Scholar]
- 61. Pino P, Vouldoukis I, Dugas N, Conti M, Nitcheu J, et al. (2004) Induction of the CD23/nitric oxide pathway in endothelial cells downregulates ICAM-1 expression and decreases cytoadherence of Plasmodium falciparum-infected erythrocytes. Cell Microbiol 6: 839–848. [DOI] [PubMed] [Google Scholar]
- 62. Serirom S, Raharjo WH, Chotivanich K, Loareesuwan S, Kubes P, et al. (2003) Anti-adhesive effect of nitric oxide on Plasmodium falciparum cytoadherence under flow. Am J Pathol 162: 1651–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Alp NJ, Channon KM (2004) Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease. Arterioscler Thromb Vasc Biol 24: 413–420. [DOI] [PubMed] [Google Scholar]
- 64. Antoniades C, Shirodaria C, Warrick N, Cai S, de Bono J, et al. (2006) 5-methyltetrahydrofolate rapidly improves endothelial function and decreases superoxide production in human vessels: effects on vascular tetrahydrobiopterin availability and endothelial nitric oxide synthase coupling. Circulation 114: 1193–1201. [DOI] [PubMed] [Google Scholar]
- 65. Huang A, Vita JA, Venema RC, Keaney JF Jr. (2000) Ascorbic acid enhances endothelial nitric-oxide synthase activity by increasing intracellular tetrahydrobiopterin. J Biol Chem 275: 17399–17406. [DOI] [PubMed] [Google Scholar]
- 66. Ong PK, Melchior B, Martins YC, Hofer A, Orjuela-Sanchez P, et al. (2013) Nitric oxide synthase dysfunction contributes to impaired cerebroarteriolar reactivity in experimental cerebral malaria. PLoS Pathog 9: e1003444 10.1371/journal.ppat.1003444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Davis JS, Yeo TW, Piera KA, Woodberry T, Celermajer DS, et al. (2010) Angiopoietin-2 is increased in sepsis and inversely associated with nitric oxide-dependent microvascular reactivity. Crit Care 14: R89 10.1186/cc9020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. WHO (2000) Severe falciparum malaria. Trans R Soc Trop Med Hyg 94: Supplement 1. [Google Scholar]
- 69. Hyland K, Howells DW (1988) Analysis and clinical significance of pterins. J Chromatogr 429: 95–121. [DOI] [PubMed] [Google Scholar]
- 70. Howells DW, Smith I, Hyland K (1986) Estimation of tetrahydrobiopterin and other pterins in cerebrospinal fluid using reversed-phase high-performance liquid chromatography with electrochemical and fluorescence detection. J Chromatogr 381: 285–294. [DOI] [PubMed] [Google Scholar]
- 71. Hendriksen IC, White LJ, Veenemans J, Mtove G, Woodrow C, et al. (2013) Defining falciparum-malaria-attributable severe febrile illness in moderate-to-high transmission settings on the basis of plasma PfHRP2 concentration. J Infect Dis 207: 351–361. 10.1093/infdis/jis675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Rubach MP, Mukemba J, Florence S, John B, Crookston B, et al. (2012) Plasma Plasmodium falciparum histidine-rich protein-2 concentrations are associated with malaria severity and mortality in Tanzanian children. PLoS One 7: e35985 10.1371/journal.pone.0035985 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Plasma Angiopoeitin-2 (Ang-2) plotted against urine dihydrobiopterin (BH2). Squares represent healthy controls (HC, n = 9), triangles represent uncomplicated malaria (UM, n = 5), circles represent cerebral malaria (CM, n = 13), and stars represent non-malaria central nervous system conditions (NMC, n = 9).
(TIF)
A: Electrochemical detection of of tetrahydrobiopterin (BH4) and dihydroneopterin (NH2) in urine. B: Electrochemical detection of BH4 and NH2 in standards. C: Fluorescence detection of neopterin (N0), dihydroneopterin (NH2), biopterin (B0), and dihydrobiopterin (BH2) in urine. D: Fluorescence detection of N0, NH2, B0, and BH2 in standards.
(TIF)
Data Availability Statement
All relevant data are within the paper and Supporting Information.