Summary
Overexpression of the apoptosis repressor with caspase recruitment domain (ARC, also termed NOL3) protein predicts adverse outcome in patients with acute myeloid leukaemia (AML) and confers drug resistance to AML cells. The second mitochondrial-derived activator of caspases (SMAC, also termed DIABLO) mimetic, birinapant, promotes extrinsic apoptosis in AML cells. SMAC mimetics induce cleavage of cellular inhibitor of apoptosis (cIAP) proteins, leading to stabilization of the nuclear factor-κB (NF-κB)-inducing kinase (MAP3K14, also termed NIK) and activation of non-canonical NF-κB signalling. To enhance the therapeutic potential of SMAC mimetics in AML, we investigated the regulation and role of ARC in birinapant-induced apoptosis. We showed that birinapant increases ARC in AML and bone marrow-derived mesenchymal stromal cells (MSCs). Downregulation of MAP3K14 by siRNA decreased ARC levels and suppressed birinapant-induced ARC increase. Reverse-phase protein array analysis of 511 samples from newly diagnosed AML patients showed that BIRC2 (also termed cIAP1) and ARC were inversely correlated. Knockdown of ARC sensitized, while overexpression attenuated, birinapant-induced apoptosis. Furthermore, ARC knockdown in MSCs sensitized co-cultured AML cells to birinapant-induced apoptosis. Our data demonstrate that ARC is regulated via BIRC2/MAP3K14 signalling and its overexpression in AML or MSCs can function as a resistant factor to birinapant-induced leukaemia cell death, suggesting that strategies to inhibit ARC will improve the therapeutic potential of SMAC mimetics.
Keywords: ARC, MAP3K14, SMAC mimetic, BIRC2, birinapant, AML
Introduction
Inhibitors of apoptosis (IAP) proteins contain one to three common baculovirus repeat domains, and they are important regulators of the apoptosis machinery. The expression of many IAP members is deregulated in various malignant cell types, which contributes to tumourigenesis, drug resistance and poor disease prognosis. Extensive efforts have been made in the last decade to develop strategies to target IAPs, thereby sensitizing malignant cells to spontaneous and/or therapeutic agent-induce cell death.
IAPs are antagonized by the second mitochondrial-derived activator of caspase (SMAC, also termed DIABLO) protein (Du, et al 2000). A large body of evidence has shown that IAPs are over expressed in leukaemia cells and, as such, they are potential targets for leukaemia therapy. We have found that BIRC5 (survivin) and the X-linked inhibitor of apoptosis protein (XIAP), the two most studied IAPs, are highly expressed in acute myeloid leukaemia (AML) cells. Inhibition of BIRC5 and XIAP by antisense oligonucleotides or small-molecule inhibitors promotes death of AML cells and sensitizes them to chemotherapy-induced apoptosis (Carter, et al 2005, Carter, et al 2010, Carter, et al 2003a, Carter, et al 2003b, Gyurkocza, et al 2006). In a clinical setting, using XIAP antisense oligonucleotides, we reported that the inhibition of XIAP induced apoptosis preferentially in CD34+38− AML stem/progenitor cells of AML patients (Carter, et al 2011a). Furthermore, we recently discovered the enhanced expression of cellular inhibitor of apoptosis protein-1 (BIRC2, also known as cIAP1) and the diminished expression of SMAC in AML stem/progenitor cells compared to bulk and CD34+ AML cells. Interestingly, inhibition of IAPs with the SMAC mimetic, birinapant, promoted the death, not only of AML blasts, but also of CD34+38− AML stem/progenitor cells, and sensitized these cells to chemotherapeutic agents, including cytarabine (Carter, et al 2011b, Carter, et al 2014). The bone marrow (BM) microenvironment plays a central role in leukaemogenesis, disease progression, and leukaemia cell drug resistance (Konopleva and Jordan 2011). To mimic this microenvironment, we cultured AML cells with BM-derived mesenchymal stromal cells (MSCs) and found that birinapant promoted the cell death of AML blasts, including CD34+38− AML stem/progenitor cells, even when they were cultured with MSCs under hypoxic conditions (Carter, et al 2014), another mechanism known to protect AML cells from drug-induced cell death (Benito, et al 2011).
SMAC mimetics induce the degradation of cellular inhibitors of apoptosis (cIAPs), which inhibit primarily extrinsic apoptotic cell death and suppress XIAP, which inhibits caspase-9 and caspases-3/7 and blocks activation of both intrinsic and extrinsic apoptosis. Extrinsic apoptosis is also suppressed by FLICE-inhibitory protein (FLIP) (Irmler, et al 1997, Scaffidi, et al 1999) and the apoptosis repressor with caspase recruitment domain (ARC, also termed NOL3) protein (Koseki, et al 1998, Nam, et al 2004). Both proteins inhibit the activation of caspase-8, the initiator caspase for the extrinsic apoptosis pathway. We recently reported that the ARC expression is one of the strongest adverse predictors for overall survival and disease-free survival in AML patients (Carter, et al 2011c) and that ARC confers drug resistance and survival advantage to AML cells in vitro and in vivo (Mak, et al 2014). Therefore, we speculated that targeting ARC would probably sensitize leukaemic cells to SMAC mimetic-induced cell death.
Like other SMAC mimetics (Varfolomeev, et al 2007, Vince, et al 2007), birinapant activates non-canonical nuclear factor-αB (NF-κB) signalling by degrading IAPs and stabilizing NF-κB-inducing kinase (MAP3K14, also termed NIK) (Carter, et al 2014). We observed that ARC levels increased in AML cells treated with birinapant. Given the important role of ARC in AML and the potential of SMAC mimetics in AML therapy, we examined the roles of ARC in birinapant-mediated cell killing by overexpressing or knocking down the protein in AML cells alone or in co-culture with MSCs. We report here that ARC is regulated by BIRC2/MAP3K14 cell signalling and, as such, is a resistance factor to SMAC mimetic birinapant-induced cell death in AML cells. The inhibition of ARC in AML cells sensitizes these cells to birinapant-induced death. Furthermore, the inhibition of ARC in MSCs also rendered AML cells more sensitive to birinapant-induced death, as compared to AML cells co-cultured with control MSCs, suggesting a role of ARC in both leukaemia cells and their microenvironment.
Materials and methods
Cells, cell culture, and treatment of cells
OCI-AML3 cells were kindly provided by Dr. M. Minden (Ontario Cancer Institute, Ontario, Canada); KG-1 cells were purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA); and Molm13 cells were obtained from the German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany). All cells were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum, 2 mmol/l L-glutamine, 100 u/ml penicillin, and 100 µg/ml streptomycin.
Fresh BM or peripheral blood samples from patients with AML and BM samples from normal subjects were acquired after informed consent had been obtained according to the principle of the Declaration of Helsinki. All study protocols were approved by the Institutional Review Board of The University of Texas MD Anderson Cancer Center. Mononuclear cells were purified by Ficoll-Hypaque (Sigma, St. Louis, MO, USA) density-gradient centrifugation and cultured in the same medium as described above. The patient characteristics are summarized in Table I. The MSCs were isolated from human BM as previously described (Studeny, et al 2002).
Table I.
Characteristics of AML Patients Whose Samples Were Treated with Birinapant in vitro
| Patient | Source | Blast % | Clinical treatments and responses |
Cytogenetics | FLT3 |
|---|---|---|---|---|---|
| 1 | PB | 71% | New | Diploid male karyotype 46,XY [20] | |
| 2 | BM | 60% | Relapsed AML | Diploid male karyotype 46,XY[20] | WT |
| 3 | BM | 69% | Refractory AML, with persistent blasts after high dose Ara-C and Clofarabine | 46,XY,t(3;12)(q26.2;p13)[21] | ITD |
| 4 | BM | 51% | Refractory AML arising from MDS, no response to decitabine, Onconova and low dose Ara-C with Hydrea | Diploid male karyotype 46,XY[20] | WT |
| 5 | BM | 69% | Primary refractory AML | Complex cytogenetics | WT |
AML, acute myeloid leukaemia; MDS, myelodysplastic syndrome; PB, peripheral blood; BM, bone marrow; Ara-C, cytarabine; WT, wild-type; ITD, internal tandem duplication.
AML cells from cell lines (0.2 × 106/ml) or mononuclear cells from patient samples (0.5 × 106/ml) were treated with various concentrations of birinapant (TetraLogic Pharmaceuticals, Malvern, PA, USA) alone or in combinations with tumour necrosis factor-α (TNF) (Life Technologies, Grand Island, NY, USA) for up to 72 h. For co-culture experiments, early passage MSCs were pre-plated at 5 × 103/cm2 for 24 h; AML cells were then added and treated. An appropriate amount of dimethyl sulfoxide was used as the treatment control. OCI-AML3 cells were treated with decitabine (DAC) or 5-azacytidine (5-Aza) daily for 48 h.
ARC knockdown MSCs
ARC was knocked down in primary MSCs by lentiviral transduction using a gene-specific shRNAmir-GFP-expressing transfer vector: clone V3LHS_337662, targeting residues 217–235 on RefSeq NM_003946.4 (Open Biosystems, Huntsville, AL, USA). Lentivirus was prepared by cotransfection of HEK293T cells (ATCC) with an equal molar mix of transfer vector and packaging plasmids (psPAX2 and pMD2.G from Addgene, Cambridge, MA, USA) using JetPrime transfection reagent as directed by the manufacturer (Polyplus, Illkirch, France). Fresh lentiviral supernatants were passed through 0.45-µm pore surfactant-free cellulose acetate membranes and then used at once to infect MSCs by incubation overnight at 37°C in 5% CO2. Infected cells were subjected to selection with puromycin (Invitrogen, San Diego, CA, USA) starting at 1 µg/ml. In parallel, MSCs were transduced with lentivirus delivering a non-specific control (Open Biosystems). Knockdown was verified by Western blot analysis and by real-time polymerase chain reaction (PCR).
ARC-knockdown or -overexpressing AML cells
ARC-knockdown OCI-AML3 or Molm13 cells and ARC-overexpressing KG-1 cells were generated by lentiviral transduction using gene-specific shRNAmir-GFP-expressing transfer vectors as previously described (Mak, et al 2014).
Knockdown of MAP3K14 expression by siRNA
OCI-AML3 and Molm13 cells were electroporated with 10, 50, or 100 nmol/l MAP3K14 siRNA or scramble siRNA (both from Thermo Fisher Scientific, Pittsburgh, PA, USA) using an Amaxa apparatus (Lonza, Walkersville, MD, USA) following the manufacturer’s instructions. The on-TARGETplus SMART pool MAP3K14 siRNA consists of a mixture of four target sequences: GGAUUGACCUCACCCAGAA, GAACCGGGCACUACAGCAA, GUCCAAAUACAGUCUCUUA, GCCAGUGGAUUAUGAGUAC.
Reverse transcription polymerase chain reaction (RT-PCR)
MAP3K14 and NOL3 (ARC) RNA levels were measured by reverse-transcription and Taq-Man PCR. RNAs were isolated with TRIzol reagent (Life Technologies) and reverse-transcribed with random hexamers (Roche Applied Science, Indianapolis, IN, USA) by Superscript III reverse transcriptase (Invitrogen) at 50°C for 50 min and then 70°C for 15 min. The PCR amplification mixture (25 µl) contained cDNA, primers and probe for MAP3K14 (Hs00177695-m1) or NOL3 (Hs00358724-g1) and Taq-Man universal PCR master mix (Life Technologies). Thermal cycle conditions included holding the reaction at 50°C for 2 min and at 95°C for 10 min, followed by 50 cycles of 95°C for 15 s and 60°C for 1 min. Results were collected and analysed by an ABI Prism 7900HT fast real-time PCR Sequence Detection System (Life Technologies). The abundance of each transcript relative to that of ABL1 was calculated using the 2−ΔCt method, where ΔCt is the mean Ct of the transcript of interest minus the mean Ct of the transcript for ABL1 (Livak and Schmittgen 2001).
Western blot analysis
Protein levels in treated cells were determined by Western blot analysis, as described previously (Carter, et al 2006a, Carter, et al 2006b, Carter, et al 2014). MAP3K14 antibody was purchased from Cell Signaling Technology (Danvers, MA, USA), BIRC2 antibody from R&D Systems (Minneapolis, MN, USA), and ARC antibody from Imgenex (San Diego, CA, USA). Signals were detected by using the Odyssey Infrared Imaging System and quantitated by Odyssey software version 3.0 (both LI-COR Biosciences, Lincoln, NE, USA). ACTB (β-actin) was used as a loading control.
Protein expression in AML patient samples
Levels of BIRC2 and ARC proteins in samples from 511 patients with newly diagnosed AML were determined by reverse-phase protein array (RPPA) as previously described (Kornblau, et al 2009, Tibes, et al 2006).
Cell viability assay
Viable cell counts were determined by flow cytometry using CountBright absolute counting beads (Life Technologies) on annexin V-negative/7-amino-actinomycin D (7AAD)-negative cell events. Apoptosis was assessed by flow cytometry of phosphatidyl serine externalization (Martin, et al 1995) with annexin V-cyanin 5 (Cy5) (BD Biosciences) using a FACSArray Bioanalyser (BD Biosciences). Cell membrane integrity was simultaneously assessed by 7AAD exclusion in the annexin V-stained cells. For AML cells co-cultured with MSCs, unattached AML cells were obtained by combining cells in suspension and cells collected after washing the wells twice with phosphate-buffered saline. Adherent cells were obtained by trypsinization. The unattached and adherent cells were combined and stained with CD45-APCH7, CD90-phycoerythrin (PE), and annexin V-Cy5 antibodies (all from BD Biosciences). Apoptosis in AML cells was determined by annexin V-Cy5 positivity in CD45+CD90− cells.
Statistical analyses
Triplicate experiments were carried out for cell lines treated with birinapant, TNF, or birinapant plus TNF. The results are expressed as mean ± standard deviation. The combination index (CI), determined by the Chou-Talalay method (Chou and Talalay 1984) and Calcusyn software, was expressed as the mean of the CI values obtained at effective doses ED50, ED75, and ED90. A CI of < 1 was considered synergistic, a CI of 1 additive and a CI of > 1 antagonistic. For patient samples treated with birinapant, the results were expressed as mean ± standard error. Differences between groups were compared by applying Student’s t-test. The statistical significance was set at P ≤ 0.05. For correlation of ARC-BIRC2 expression in RPPA samples from patients, we accounted for multiple testing using a Bonferroni correction and thus accepted Pearson correlation −0.2 ≥ R ≥ 0.2 (P < 0.0001) as significant.
Results
Birinapant treatment increases ARC levels in AML cells
As birinapant primarily induces and ARC inhibits caspase-8-mediated extrinsic apoptosis, we decided to investigate the expression of ARC in AML cells in response to birinapant. While the treatment of AML cells with birinapant promoted a rapid degradation of BIRC2, we observed that this decrease in BIRC2 was accompanied by an increase in ARC protein levels. Treatment with birinapant increased ARC levels in OCI-AML3 and Molm13 AML cells co-cultured with BM derived MSCs as well as in the MSCs themselves (Fig 1).
Fig. 1.
Birinapant treatment decreases BIRC2 and increases ARC protein levels in AML cells and MSCs. OCI-AML3 and Molm13 cells were co-cultured with mesenchymal stem cells (MSCs) and treated with birinapant (bir). Cell lysates from OCI-AML3 and Molm13 cells were collected at 48 and 72 h by combining unattached cells and cells washed off from MSCs; lysates of MSCs were obtained from MSCs co-cultured with Molm13 cells after washing off Molm13 cells. The protein levels were determined by Western blot.
ARC expression is regulated through BIRC2/MAP3K14 signalling
cIAPs promote the inactivation of MAP3K14 via their E3 ubiquitin ligase activity. We have shown that MAP3K14 levels are increased in AML cells treated with birinapant, which is reported to be associated with activation of the non-canonical NF-κB cell-signalling pathway (Carter, et al 2014). To determine whether the increase in ARC in birinapant-treated cells is due to BIRC2 degradation and perhaps an increase in MAP3K14, we first determined whether ARC is regulated by MAP3K14. We transfected OCI-AML3 and Molm13 cells with MAP3K14 siRNA and observed a dose-dependent decrease of MAP3K14 expression at both mRNA and protein levels which was accompanied by decreased NOL3mRNA and ARC protein levels (Fig 2A), suggesting that NOL3/ARC is a downstream target of MAP3K14. Next we electroporated Molm13 cells with 100 nmol/l MAP3K14 siRNA or scrambled control siRNA and after 4 h, treated cells with birinapant for an additional 24 h. We found that birinapant rapidly degraded BIRC2 in both scrambled and MAP3K14 siRNAs-treated cells. MAP3K14 and ARC levels increased in scrambled siRNA-treated Molm13 cells as expected, while the increases in MAP3K14 and ARC were suppressed when MAP3K14 expression was inhibited (Fig 2B), suggesting that ARC is regulated via the BIRC2/MAP3K14 signalling axis.
Fig. 2.
ARC is regulated through the BIRC2/MAP3K14 signalling. (A) Inhibition of MAP3K14 decreases NOL3 mRNA and ARC protein levels. OCI-AML3 and Molm13 cells were transfected with MAP3K14 siRNA or a scramble siRNA control by electroporation. MAP3K14 and NOL3 mRNA levels were determined by Taq-Man RT-PCR and MAP3K14 and ARC protein levels by Western blot 4 h after transfection. (B) Inhibition of MAP3K14 suppresses BIRC2 inhibition-mediated ARC induction. Molm13 cells were electroporated with 100 nmol/l MAP3K14 siRNA for 4 h, then treated with birinapant (bir) for 24 h. Protein levels were determined by Western blot.
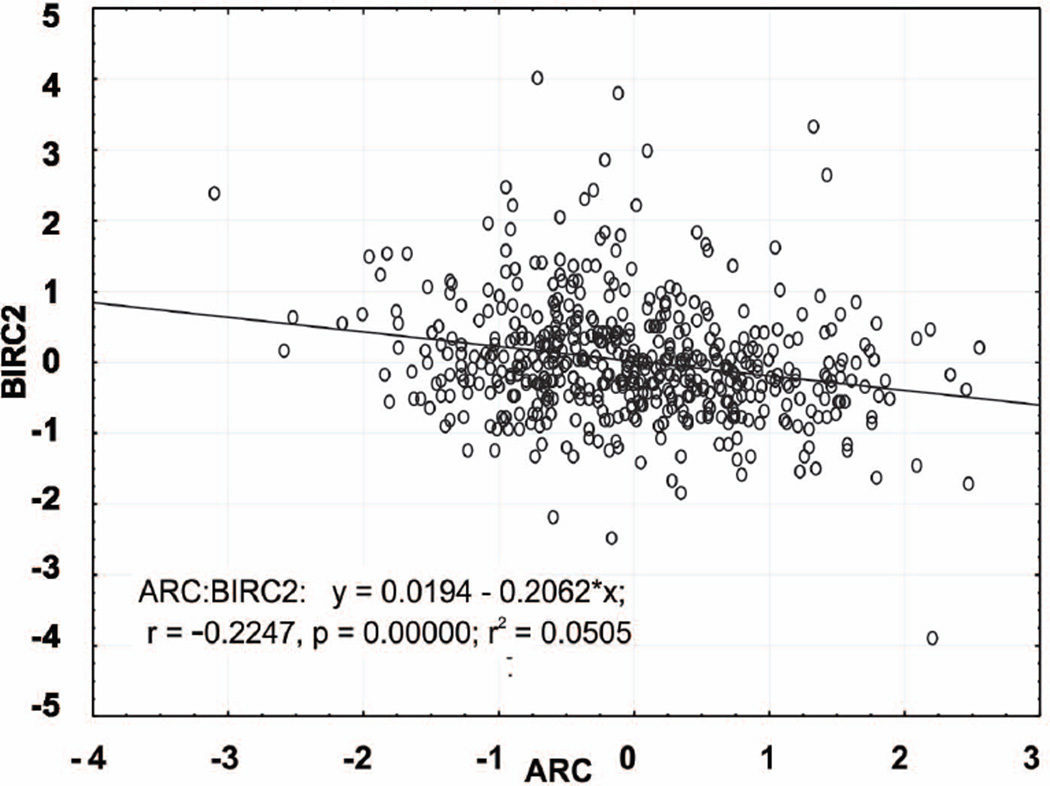
ARC and BIRC2 are inversely correlated in samples obtained from 511 patients with newly diagnosed AML
We previously measured the expression of over 200 proteins by RPPA in samples obtained from 511 patients with newly diagnosed AML, which enabled us to correlate the expression levels of various proteins in the same sample set (Carter, et al 2012). We compared the expression levels of BIRC2 and ARC in these samples and found them to be negatively correlated (R = −0.225, P < 0.0001) (Fig 3). This would support the notion that ARC expression is associated with MAP3K14/BIRC2 signalling (Fig 2).
Fig. 3.
BIRC2 and ARC protein levels negatively correlate in samples from 511 patients with newly diagnosed AML. Protein levels were determined by reverse-phase protein array.
ARC expression modulates birinapant-mediated cell death
Given that the inhibition of caspase-8 activation is one of ARC’s primary mechanisms of action and the level of this protein increased in AML cells treated with birinapant, we hypothesized that ARC could potentially regulate birinapant-mediated apoptosis. To test this hypothesis, we treated ARC-knockdown (K/D) OCI-AML3 and Molm13 and ARC-overexpressing (O/E) KG-1 cells with birinapant, TNF or birinapant plus TNF (Fig 4). We had previously reported that cell death induced by birinapant in AML cell lines requires the presence of death receptor ligands, such as TNF (Carter, et al 2006a, Carter, et al 2006b, Carter, et al 2014). OCI-AML3 and Molm13 cells were insensitive to TNF treatment alone regardless of ARC expression (triangle lines). Knockdown of ARC in the OCI-AML3 and Molm13 cells increased their sensitivity to birinapant (square lines), which was further enhanced by combining birinapant with TNF (circle lines) (Fig 4A). Both the ARC O/E KG-1 and vector control cells were insensitive to TNF (triangle lines) or birinapant (square lines), while the combination of birinapant and TNF markedly induced cell death in KG-1 vector control cells. This cell death induction was suppressed in the ARC O/E KG-1 cells (circle lines) (Fig 4B).
Fig. 4.
ARC is a resistance factor to birinapant-mediated cell death and its level is decreased by demethylating agents in AML cells. ARC knock-down (K/D) Molm13 and OCI-AML3 cells (A), ARC over-expression (O/E) KG-1 cells (B), and their respective control cells were treated with birinapant, TNF, or birinapant plus TNF for 72 h. Cell death was determined by annexin V (AnnV) staining in the presence of 7-amino-actinomycin D (7AAD). *, P ≤ 0.05; bir, birinapant; vec, vector control. (C) Demethylating agents decrease ARC protein level. OCI-AML3 cells were treated with low nmol/l concentrations of decitabine (DAC) or 5-azacytidine (5-Aza) for 48 h. ARC protein levels were determined by Western blot.
We reported previously that demethylating agents sensitize AML cells to birinapant-induced apoptosis partly by inhibiting MAP3K14 levels (Carter, et al 2014). Examination of ARC in OCI-AML3 cells treated with DAC or 5-Aza showed that the demethylating agents also decreased ARC levels in leukaemia cells (Fig 4C).
As birinapant increased the levels of ARC in MSCs co-cultured with AML cells (Fig 1), we next examined whether ARC expression in these cells could contribute to the protection of co-cultured AML cells exposed to birinapant. We co-cultured Molm13, OCI-AML3 and KG-1 cells with ARC K/D or control MSCs and treated them with birinapant, TNF or both. We also co-cultured blast cells obtained from AML patients with ARC K/D or control MSCs and treated those cells with birinapant. Interestingly, the treatments induced higher rates of cell death among AML cells co-cultured with ARC K/D MSCs than in AML cell lines (Fig 5A) or primary AML cells (Fig 5B) co-cultured with control MSCs.
Fig. 5.
Finally, we co-cultured ARC K/D and control OCI-AML3 with ARC K/D or control MSCs and treated them with birinapant plus TNF. As shown in Fig 5C, the ARC K/D OCI-AML3 cells co-cultured with ARC K/D MSCs were significantly more sensitive to the treatment compared to the ARC K/D OCI-AML3 cells co-cultured with control MSCs, and the control OCI-AML3 cells co-cultured with ARC K/D MSCs or with control MSCs.
Discussion
SMAC mimetics are a class of anti-cancer agents that primarily activate the extrinsic apoptosis pathway by antagonizing the IAP family of proteins. These agents have attracted a great deal of attention in recent years and several of them, including birinapant, have entered clinical trials for the treatment of cancers, including leukaemia. Understanding their mechanism of action will help to improve the therapeutic potential of SMAC mimetics. In the present study, we found that the expression of anti-apoptotic ARC is induced by birinapant and regulated through BIRC2/MAP3K14 signalling. Mechanistically, the K/D of ARC expression sensitizes AML cells to birinapant-induced apoptosis, while O/E of the protein decreases their sensitivity to birinapant-induced cell death. Furthermore, we found that K/D of ARC in MSCs decreases their protective effect, on both AML cell lines and primary AML cells, against birinapant-induced cell death. In fact, K/D of ARC in MSCs also decreases their protective effect against other agent-induced cell death (data not shown). We previously reported that ARC in leukaemia cells increases when they are co-cultured with MSCs (Mak, et al 2014). We found that this increase is less when co-cultured with ARC K/D than with control MSCs, suggesting that ARC in MSCs can modulate ARC in leukaemia. Consequently, the inhibition of ARC facilitates birinapant-induced cell death. Furthermore, ARC K/D AML cells were more sensitive to birinapant plus TNF when they were co-cultured with ARC K/D MSCs than with control MSCs, suggesting a therapeutic benefit from concurrent inhibition of ARC in both AML cells and MSCs. As degradation of BIRC2 leading to the activation of MAP3K14 is a common mechanism of action for SMAC mimetics, the inhibition of ARC should sensitize cells to other SMAC mimetics. Our results suggest that the combination of IAP inhibition and ARC inhibition may be more effective in inducing apoptosis in leukaemia cells than inhibiting IAP alone.
Interestingly, a large set of AML patient samples (n = 511) showed a reverse correlation between BIRC2 and ARC protein levels. Birinapant decreases primarily BIRC2 and, to a lesser degree, cIAP2 and XIAP in AML cells (Carter, et al 2014). We reported previously that ARC expression was inversely correlated with XIAP in the same sample set (Carter, et al 2011c) and found that the inhibition of XIAP by antisense oligonucleotide increased ARC levels (results not shown). ARC was also found to be degraded by MDM2 E3 ligase activity (Foo, et al 2007). XIAP, BIRC2, and BIRC3 all have E3 ligase activities and regulate multiple proteins by promoting their proteasomal degradation. Therefore, another possible mechanism of birinapant-mediated ARC induction could be that the decreased expression of IAP proteins diminishes their proteasomal activity, leading to accumulation of ARC proteins. Indeed, treating AML cells with proteasome inhibitors increased ARC protein levels (results not shown).
We also found that ARC expression is regulated by MAP3K14 signalling, which has not been reported. This finding further supports the notion that inhibition of ARC will not only lower the threshold of activation of extrinsic apoptosis signalling, but also remove a resistance factor to this pathway. A recent study showed that SMAC mimetics stabilize MAP3K14 and activate MAP3K14-NF-κB2, which leads to the induction of CFLAR (Cheung, et al 2011), another inhibitor of caspase-8 and the extrinsic apoptosis pathway. We recently reported that combinations of birinapant and demethylating agents synergistically promoted the death of AML cells, including AML stem/progenitor cells in vitro and in vivo, and that demethylating agents decrease the levels of MAP3K14 and its downstream target CFLAR (Carter, et al 2011b, Carter, et al 2014). In the present study, we found that demethylating agents also decrease the level of ARC. Birinapant is under clinical development for the treatment of certain cancers, and demethylating agents are already in clinical use for AML in elderly patients. Based on our pre-clinical findings, a clinical trial has recently been initiated that combines birinapant and the demethylating agent 5-Aza in myelodysplastic syndrome and AML (NCT01828346). Our findings suggest that a combination of birinapant and an agent that specifically targets ARC is warranted, and could prove to have a clinical benefit. Although currently no ARC inhibitors exist, combinations of birinapant with agents indirectly inhibiting ARC expression will hopefully improve the effects of birinapant.
Acknowledgements
This work was supported, in part, by grants from the National Institutes of Health (P01 CA055164 and MD Anderson’s Cancer Center Support Grant CA016672) and by the Paul and Mary Haas Chair in Genetics (to MA); and by the MD Anderson Cancer Center Leukemia SPORE grant (CA100632) (to BZC). We thank Numsen Hail, Deanna A. Alexander, and Kathryn L. Hale for assisting with the manuscript preparation.
Footnotes
Author contributions PYM performed experiments and analysed data. DHM, VR, RJ performed experiments. SMK analysed data. MA edited and approved the final version and supported the study. BZC, designed the study, analysed data and wrote the paper.
Disclosures The authors have no disclosures.
References
- Benito J, Shi Y, Szymanska B, Carol H, Boehm I, Lu H, Konoplev S, Fang W, Zweidler-McKay PA, Campana D, Borthakur G, Bueso-Ramos C, Shpall E, Thomas DA, Jordan CT, Kantarjian H, Wilson WR, Lock R, Andreeff M, Konopleva M. Pronounced hypoxia in models of murine and human leukemia: high efficacy of hypoxia-activated prodrug PR-104. Plos One. 2011;6:e23108. doi: 10.1371/journal.pone.0023108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BZ, Milella M, Tsao T, McQueen T, Schober WD, Hu W, Dean NM, Steelman L, McCubrey JA, Andreeff M. Regulation and targeting of antiapoptotic XIAP in acute myeloid leukemia. Leukemia. 2003a;17:2081–2089. doi: 10.1038/sj.leu.2403113. [DOI] [PubMed] [Google Scholar]
- Carter BZ, Wang RY, Schober WD, Milella M, Chism D, Andreeff M. Targeting survivin expression induces cell proliferation defect and subsequent cell death involving the mitochondrial pathway in myeloid leukemic cell. Cell Cycle. 2003b;2:488–493. [PubMed] [Google Scholar]
- Carter BZ, Gronda M, Wang Z, Welsh K, Pinilla C, Andreeff M, Schober WD, Nefzi A, Houghten RA, Brandwein J, Minden MD, Schuh A, Wells RA, Chun K, Reed JC, Schimmer AD. Small-molecule XIAP inhibitors derepress downstream effector caspases and induce apoptosis of acute myeloid leukemia cells. Blood. 2005;105:4043–4050. doi: 10.1182/blood-2004-08-3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BZ, Mak D, Schober WD, Cabreira-Hansen M, Beran M, McQueen T, Chen W, Andreeff M. Regulation of survivin expression through bcr-abl/MAPK cascade: targeting survivin overcomes Imatinib resistance and increases Imatinib sensitivity in Imatinib responsive CML cells. Blood. 2006a;107:1555–1563. doi: 10.1182/blood-2004-12-4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BZ, Mak DH, Shi Y, Schober WD, Wang RY, Konopleva M, Koller E, Dean NM, Andreeff M. Regulation and targeting of Eg5, a mitotic motor protein in blast crisis CML: overcoming imatinib resistance. Cell Cycle. 2006b;5:2223–2229. doi: 10.4161/cc.5.19.3255. [DOI] [PubMed] [Google Scholar]
- Carter BZ, Mak DH, Schober WD, Koller E, Pinilla C, Vassilev LT, Reed JC, Andreeff M. Simultaneous activation of p53 and inhibition of XIAP enhance the activation of apoptosis signaling pathways in AML. Blood. 2010;115:306–314. doi: 10.1182/blood-2009-03-212563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BZ, Mak DH, Morris SJ, Borthakur G, Estey E, Byrd AL, Konopleva M, Kantarjian H, Andreeff M. XIAP antisense oligonucleotide (AEG35156) achieves target knockdown and induces apoptosis preferentially in CD34+38− cells in a phase 1/2 study of patients with relapsed/refractory AML. Apoptosis. 2011a;16:67–74. doi: 10.1007/s10495-010-0545-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BZ, Mak DH, Qiu Y, Kornblau S, Mak PY, Weng D, McKinlay MA, Andreeff M. Antagonizing IAPs by SMAC Mimetic TL32711 Induces Apoptosis in AML Cells Including AML Stem/Progenitor Cells Alone and in Combination with Chemotherapy. Blood. 2011b;118:32a. [Google Scholar]
- Carter BZ, Qiu YH, Zhang N, Coombes KR, Mak DH, Thomas DA, Ravandi F, Kantarjian HM, Koller E, Andreeff M, Kornblau SM. Expression of ARC (apoptosis repressor with caspase recruitment domain), an antiapoptotic protein, is strongly prognostic in AML. Blood. 2011c;117:780–787. doi: 10.1182/blood-2010-04-280503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BZ, Qiu Y, Huang X, Diao L, Zhang N, Coombes KR, Mak DH, Konopleva M, Cortes J, Kantarjian HM, Mills GB, Andreeff M, Kornblau SM. Survivin is highly expressed in CD34(+)38(−) leukemic stem/progenitor cells and predicts poor clinical outcomes in AML. Blood. 2012;120:173–180. doi: 10.1182/blood-2012-02-409888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BZ, Mak PY, Mak DH, Shi Y, Qiu Y, Bogenberger JM, Mu H, Tibes R, Yao H, Coombes KR, Jacamo RO, McQueen T, Kornblau SM, Andreeff M. Synergistic targeting of AML stem/progenitor cells with IAP antagonist birinapant and demethylating agents. J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/djt440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung HH, St Jean M, Beug ST, Lejmi-Mrad R, LaCasse E, Baird SD, Stojdl DF, Screaton RA, Korneluk RG. SMG1 and NIK regulate apoptosis induced by Smac mimetic compounds. Cell Death Dis. 2011;2:e146. doi: 10.1038/cddis.2011.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Du C, Fang M, Li Y, Li L, Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000;102:33–42. doi: 10.1016/s0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- Foo RS, Chan LK, Kitsis RN, Bennett MR. Ubiquitination and degradation of the anti-apoptotic protein ARC by MDM2. J Biol Chem. 2007;282:5529–5535. doi: 10.1074/jbc.M609046200. [DOI] [PubMed] [Google Scholar]
- Gyurkocza B, Plescia J, Raskett CM, Garlick DS, Lowry PA, Carter BZ, Andreeff M, Meli M, Colombo G, Altieri DC. Antileukemic activity of shepherdin and molecular diversity of hsp90 inhibitors. J.Natl.Cancer Inst. 2006;98:1068–1077. doi: 10.1093/jnci/djj300. [DOI] [PubMed] [Google Scholar]
- Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, Bodmer JL, Schroter M, Burns K, Mattmann C, Rimoldi D, French LE, Tschopp J. Inhibition of death receptor signals by cellular FLIP. Nature. 1997;388:190–195. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- Konopleva MY, Jordan CT. Leukemia stem cells and microenvironment: biology and therapeutic targeting. J.Clin.Oncol. 2011;29:591–599. doi: 10.1200/JCO.2010.31.0904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornblau SM, Tibes R, Qiu YH, Chen WJ, Kantarjian HM, Andreeff M, Coombes KR, Mills GB. Functional proteomic profiling of AML predicts response and survival. Blood. 2009;113:154–164. doi: 10.1182/blood-2007-10-119438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koseki T, Inohara N, Chen S, Nunez G. ARC, an inhibitor of apoptosis expressed in skeletal muscle and heart that interacts selectively with caspases. Proc.Natl.Acad.Sci.U.S.A. 1998;95:5156–5160. doi: 10.1073/pnas.95.9.5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(−Delta Delta C) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mak PY, Mak DH, Mu H, Shi Y, Ruvolo P, Ruvolo V, Jacamo R, Burks JK, Wei W, Huang X, Kornblau SM, Andreeff M, Carter BZ. Apoptosis repressor with caspase recruitment domain is regulated by MAPK/PI3K and confers drug resistance and survival advantage to AML. Apoptosis. 2014;19:698–707. doi: 10.1007/s10495-013-0954-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S, Reutelingsperger C, McGahon A, Rader J, van Schie R, LaFace D, Green D. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and ABL. J Exp Med. 1995;182:1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam YJ, Mani K, Ashton AW, Peng CF, Krishnamurthy B, Hayakawa Y, Lee P, Korsmeyer SJ, Kitsis RN. Inhibition of both the extrinsic and intrinsic death pathways through nonhomotypic death-fold interactions. Mol Cell. 2004;15:901–912. doi: 10.1016/j.molcel.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Scaffidi C, Schmitz I, Zha J, Korsmeyer SJ, Krammer PH, Peter ME. Differential modulation of apoptosis sensitivity in CD95 type I and type II cells. J Biol Chem. 1999;274:22532–22538. doi: 10.1074/jbc.274.32.22532. [DOI] [PubMed] [Google Scholar]
- Studeny M, Marini FC, Champlin RE, Zompetta C, Fidler IJ, Andreeff M. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res. 2002;62:3603–3608. [PubMed] [Google Scholar]
- Tibes R, Qiu Y, Lu Y, Hennessy B, Andreeff M, Mills GB, Kornblau SM. Reverse phase protein array: validation of a novel proteomic technology and utility for analysis of primary leukemia specimens and hematopoietic stem cells. Mol Cancer Ther. 2006;5:2512–2521. doi: 10.1158/1535-7163.MCT-06-0334. [DOI] [PubMed] [Google Scholar]
- Varfolomeev E, Blankenship JW, Wayson SM, Fedorova AV, Kayagaki N, Garg P, Zobel K, Dynek JN, Elliott LO, Wallweber HJ, Flygare JA, Fairbrother WJ, Deshayes K, Dixit VM, Vucic D. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell. 2007;131:669–681. doi: 10.1016/j.cell.2007.10.030. [DOI] [PubMed] [Google Scholar]
- Vince JE, Wong WW, Khan N, Feltham R, Chau D, Ahmed AU, Benetatos CA, Chunduru SK, Condon SM, McKinlay M, Brink R, Leverkus M, Tergaonkar V, Schneider P, Callus BA, Koentgen F, Vaux DL, Silke J. IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell. 2007;131:682–693. doi: 10.1016/j.cell.2007.10.037. [DOI] [PubMed] [Google Scholar]