Abstract
Credible but conflicting reports address the frequency of prenatal infection by species C adenovirus. This question is important because these viruses persist in lymphoid cells and suppress double-stranded DNA-break repair. Consequently, prenatal adenovirus infections may generate the aberrant clones of lymphocytes that precede development of childhood acute lymphoblastic leukemia (ALL). The present study was designed to overcome technical limitations of prior work by processing cord blood lymphocytes within a day of collection, and by analyzing sufficient numbers of lymphocytes to detect adenovirus-containing cells at the lower limits determined by our previous studies of tonsil lymphocytes. By this approach, adenoviral DNA was identified in 19 of 517 (3.7%) samples, providing definitive evidence for the occurrence of prenatal infection with species C adenoviruses in a significant fraction of neonates predominantly of African American and Hispanic ancestry. Cord blood samples were also tested for the presence of the ETV6-RUNX1 translocation, the most common genetic abnormality in childhood ALL. Using a nested PCR assay, the ETV6-RUNX1 transcript was detected in four of 196 adenovirus-negative samples and one of 14 adenovirus-positive cord blood samples. These findings indicate that this method will be suitable for determining concordance between adenovirus infection and the leukemia-associated translocations in newborns.
Introduction
Leukemia is the most common childhood cancer. Although chromosomal abnormalities associated with childhood acute lymphoblastic leukemia (ALL) often arise before birth [1], the underlying cause of these abnormalities remains unknown [2]. Epidemiological evidence suggests that ALL may be initiated in utero by infection with a common pathogen [3]. Identification of such a pathogen has remained elusive [1,4,5].
Our group published an analysis of Guthrie cards from 49 children who later developed ALL, which identified an increased frequency of adenoviral DNA in leukemic versus normal controls [6]. In that study, the frequency of detection of adenovirus in normal controls, 6%, is in good agreement with the 5.4% detection for adenovirus in amniotic fluid from 1187 sonigraphically normal pregnancies by other investigators [7–10]. However, when we repeated this observation with a larger sample of both leukemic and normal donors, adenoviral DNA was detected in only 2 of a total of 727 samples [11]. Independent testing by other investigators similarly detected adenoviral DNA in Guthrie cards from only 1 of 189 donors [12].
The source of variability of detection of adenoviral DNA in neonatal blood samples is unknown but could arise either from variability in storage conditions of the Guthrie cards or from low numbers of viral DNA-containing cells leading to frequent false negative results. Guthrie cards are a paper substrate to which drops of peripheral blood of newborns are added, dried and stored for decades before sampling for the studies noted above. Guthrie cards may not be handled according to analytical standards required for highly sensitive PCR. As a result, the Guthrie cards could become contaminated by adenoviral DNA and yield a false positive result. To provide more definitive and quantitative evidence for or against a rare, but finite frequency of neonatal infection with human species C adenoviruses, viable human cord blood lymphocytes were collected and analyzed. If storage conditions or low genome copy numbers in past studies limited detection of these viruses, both restrictions should be overcome using relatively large samples of freshly collected material.
In the study described here we use cord blood samples to test for: 1) the presence and amount of adenoviral DNA and, if found, 2) the presence of the most common chromosomal abnormality of childhood ALL, the ETV6-RUNX1 t(12:21) translocation, in the same samples.
Materials and Methods
Clinical samples
Cord blood samples were received from the Grady Memorial Blood Bank under Georgia State University Institutional Review Board exempt approval # H10549. Demographic information for each de-identified sample included the age and apparent ethnicity of the mother and gender of the child.
Lymphocyte purification
The unused portion of venous umbilical cord blood samples (average 3 mL volume) from 517 infants born at the Grady Memorial Hospital in Atlanta, Georgia were collected in heparin-containing tubes and stored at 4°C. Cells were collected by centrifugation for 15 min at 900 x g, washed with cold RPMI medium, concentrated by centrifugation for 5 min at 400 x g, suspended in 6 ml RPMI and layered on top of 4 ml of Lymphocyte Separation Media (Mediatech, Inc., Herndon, VA). Lymphocytes were harvested from the interface after centrifugation for 20 min at 400 x g (with no brake). After centrifugation, the lymphocyte-containing interface was isolated and washed in RPMI. DNA was isolated from 1/3 of the total number of freshly isolated lymphocytes and the remaining cells were stored in liquid nitrogen for subsequent isolation of RNA.
DNA Extraction
A median of 1.2×106 (range: 0.5–30x106) lymphocytes were suspended in lysis buffer (0.45% NP-40, 0.45% Tween 20, 2 mM MgCl2, 50 mM KCl, 10 mM Tris-HCl [pH 8.3], 0.5 mg per ml proteinase K) at a ratio of 20 μL per 106 cells. The cells were incubated at 55°C overnight with intermittent vortexing. Following the 55°C incubation, proteinase K was inactivated at 95°C for 15 min, insoluble matter was removed by centrifugation, and the DNA-containing supernatant was stored at -20°C.
Adenoviral DNA detection by nested PCR
Nested PCR for adenovirus was performed using the following primer sets specific for adenovirus species C hexon gene: outer primers: 5’-ATG GCT ACC CCT TCG ATG ATG C–3’, 5’-GCG TTG TAG GCA GTG CC–3’; inner primers: 5’-GAT GCC GCA GTG GTC TTA–3’, 5’-GTC CAG CAC GCC GCG–3’. These primers, previously identified as P11-P14 [13] for nPCR2, generate a 310 bp product. The first round of PCR used 2.5 μL of DNA template in a 25-μL reaction. Following initial amplification using the outer primers for 30 cycles at an annealing temperature of 56°C, 5 μL of the resulting PCR product was amplified in the second round 50 μL PCR reaction using the inner primers for 35 cycles and an annealing temperature of 60°C.
To avoid sample-to-sample contamination, different rooms and dedicated equipment were used for DNA purification and processing, PCR setup, and gel analysis. The PCR setup hood was treated with UV light for 15 min prior to setting up any PCR amplification. Positive-displacement pipettes were used for PCR setup. In the experiments reported here, no signal was detected in any negative control sample. The PCR products were evaluated after electrophoresis through agarose gels by standard methods. DNA was isolated from groups of 5 to 12 cord blood samples. Negative cord blood samples (also run in triplicate) served as an internal negative control as they were the majority in each gel. Five copies of purified Ad2 DNA (Invitrogen) were spiked into the 4th replicate of every sample to confirm that there was no PCR inhibition in the samples. Positive samples identified from the first nPCR (run in triplicate), and a flanking negative sample, were then re-tested by nPCR resulting in 6 nPCR replicates being evaluated for all positive samples.
Quantitative PCR for adenovirus
Quantitative analysis of species C adenovirus hexon DNA in cord blood lymphocytes was performed using real-time PCR as previously described [13,14]. PCR amplification was carried out in 25 μL reaction mixtures consisting of FastStart Universal Probe Master with Rox (Roche) 0.5 uM of each primer, and 0.3 uM of TaqMan probe. A conserved region of the species C adenovirus hexon gene was detected using the forward primer: 5’- ATG GCT ACC CCT TCG ATG ATG C—3’, reverse primer: 5’- GCG TTG TAG GCA GTG CC—3’ and TaqMan probe: 5’-FAM-CCA CCG AGA CGT ACT TCA GCC T-BHQ-3’. Primers and probes were purchased from Integrated DNA Technologies (Ames, IA). Serial 10-fold dilutions (from 5 x 107 to 5 copies) of purified HAdV-2 DNA (Invitrogen) were used to generate a standard curve for quantitative assessment of cord blood adenoviral DNA content. 2.5 microliters of each DNA sample was used directly in the qPCR assay run on a 7500 Real-Time PCR System (Applied Biosystems). Samples were also tested for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) DNA by real-time PCR using primers as described in [14]. Thermocycling profiles for real-time PCR consisted of 1 cycle of 95°C for 10 min and 50 cycles of 95°C for 15 s, 53°C for 30 s, and 72°C for 35 s in a Bio-Rad iCycler (Bio-Rad). All standards were run in duplicate and test samples were run in triplicate. Negative controls for each quantitative real-time PCR experimental plate included water, the absence of template (in duplicate wells), as well as at least one adenovirus-negative cord blood sample (in triplicate wells).
RNA Extraction
Frozen cord blood lymphocytes were thawed and washed twice in sterile PBS. The PBS wash was removed and the pelleted cells were homogenized using a QIAShredder column (Qiagen). RNA was then purified using the Qiagen RNeasy Mini Kit. RNA concentration was determined using a NanoDrop spectrophotometer and 200 ng of RNA were used as template for a reverse transcription reaction using DyNamo cDNA Synthesis Kit using random hexamers (Thermo Scientific Catalog #F-470L). Reverse transcription was performed in Bio-Rad Mycycler for 25°C for 10 min; 37°C for 45 min and 85°C for 5 min.
Detection of ETV6-RUNX1 translocation
Primers for ETV6-RUNX1 translocation were modified from standards for the detection of minimal residual disease [15]. The primers used here differ by being longer with a higher Tm that is more closely matched for each pair. The pair of nested primers amplify mRNA derived from the prevalent t(12;21) translocation involving a breakpoint within intron 5 of the ETV6 gene and within the large intron of RUNX1. The outer primers were TEL-H: 5’-TGG AGA ATA ATC ACT GCC CAG CGT-3’ (nucleotides 1132–1155 of NCBI Reference Sequence NM_001987) and AML1-G: 5’-GCA TCG TGG ACG TCT CTA GAA GGA TT-3’ (nucleotides 1132–1155 of NCBI Reference Sequence NM_001754). The first product is typically a 334 bp sequence although the exclusion of RUNX1 exon 2 infrequently yields a variant with 39 bp shorter [16]. The inner primers were TEL-I: 5’-TCC GTG GAT TTC AAA CAG TCC AGG CT-5’ (nucleotides 1149–1124 of NM_001987) and AML1-J: 5’-TCG TGG ACG TCT CTA GAA GGA TTC-3’ (nucleotides 288–265 of NM_001754), which amplify a 200 (or 161) bp sequence.
GoTaq Flexi DNA Polymerase (Promega #M8295) was used for both PCRs using a Bio-Rad Mycycler. 5 μL of the 20 μL cDNA product was used as template in the first round 50 μL PCR. Following the first round of amplification using the outer primers for 40 cycles at an annealing temperature of 60°C, 3 μL of PCR product was amplified in the second round 50 μL PCR reaction using the inner primers for 35 cycles and an annealing temperature of 60°C. The UoCB4 cell line was used as a positive control for the ETV6-RUNX1 fusion gene [17]. RNA samples that were analyzed in Winston-Salem were reverse transcribed with Superscript III (Invitrogen) using random hexamers and conditions recommended by the manufacturer. The integrity of the RNA shipped to Winston-Salem was confirmed with an endpoint PCR with primers 5’-TGT CTG GGT TTC ATC CAT CCG ACA-3’ and 5’-GGC ATC TTC AAA CCT CCA TGA TGC T-3’ spanning exons 3 and 4 of the β-2-microglobulin gene that generates a 248 bp product from the cDNA. Amplification of the TEL-RUNX product was performed as described above.
Sequence analysis of the ETV6-RUNX1 amplicon
RT-PCR reaction products were run on a gel and purified with QIAquick Gel Extraction Kit (Qiagen) and submitted to Genewiz (New Jersey) for sequencing with same primers used to generate PCR product.
Statistical analyses
Statistical analyses were performed with the open source language and environment R [18]. Continuous variables that were normally distributed or normally distributed after log-transformation were compared with the Student t test. Categorical variables were compared with the Fisher exact test. Logistic regression analysis was used to evaluate the association between the presence of adenovirus in the cord blood and age of the mother at birth. A 95% confidence interval (CI) for the fraction of dichotomous variables was calculated according to the method of Agresti and Coull [19] as implemented in the Hmisc package in R [20]. A 2-sided P value of less than 0.05 was considered to indicate statistical significance.
Results
From September 2010 to July 2011, umbilical vein cord blood of 517 live births at the Grady Memorial Hospital in Atlanta, Georgia was screened for the presence of adenoviral DNA. A nested PCR assay able to detect 5 copies of the species C adenovirus genome identified virus in 19 of 517 or 3.7% of the samples (95% CI: 2.4–5.7%). Samples were considered adenovirus-positive if a PCR product of the diagnostic size was observed in at least five of six technical replicates evaluated in two separate nested PCR experiments (triplicates tested each time). Representative results showing a positive and several negative samples are seen in Fig. 1. The detection of adenoviral DNA was not related to the number of lymphocytes recovered in the sample (Fig. 2) because the median number of lymphocytes recovered from adenovirus-positive cord blood samples was not significantly different from the number of cells in adenovirus-negative samples (p = 0.7). The presence of adenoviral DNA was not associated with the gender of the child (p = 0.5) or with the apparent race of the mother (p > 0.3). Intriguingly, adenoviral DNA was detected more frequently in children born of younger mothers (7% of mothers 20 years or younger versus 2% of mothers over 30 years at birth). Although not significant (p = 0.07), the results suggest a trend towards adenoviral DNA being more frequently found in children born of younger mothers (Table 1). Adenovirus was detected in children of African American and Hispanic ancestry at comparable frequencies (Table 1). To our knowledge, this is the first report of the prevalence of adenovirus in the lymphocytes of newborn children of African American ancestry. The measured frequency of 3.7% is statistically indistinguishable (p = 0.14) from the 5.4% detection for adenovirus in amniotic fluid from 1187 sonigraphically normal pregnancies obtained in the second trimester [7–10]. These results support the notion that adenovirus is fairly common prenatal infection as indicated by the presence of viral DNA in amniotic fluid.
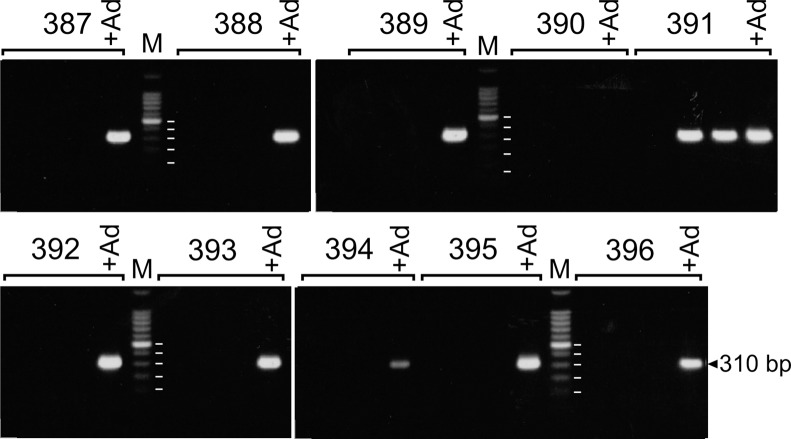
Fig 1. Detection of adenoviral DNA in umbilical vein cord blood.
Lymphocytes enriched by Ficoll density gradient centrifugation were evaluated for adenoviral DNA by a nested PCR assay targeting a conserved region of the hexon gene. Four replicates of each numbered cord blood sample were analyzed. As a positive control, five copies of the type 2 adenovirus genome were added to the fourth replicate (+Ad). Lanes indicated by ‘M’ contained a 100-bp ladder reference. The position of DNA standards corresponding to 500, 400, 300, 200, and 100 bp were determined from a brighter exposure and are indicated. Cord blood samples 390 and 391 represent a negative and positive sample, respectively. These samples were reevaluated because of the failure of the positive control (390) and because the diagnostic 310 bp PCR product was observed in only two of three test samples (391). Samples were considered evaluable if the positive control amplified. Samples were considered to contain adenoviral DNA if the appropriate PCR product was observed in at least five of six technical replicates. All positive samples were evaluated on at least two occasions.
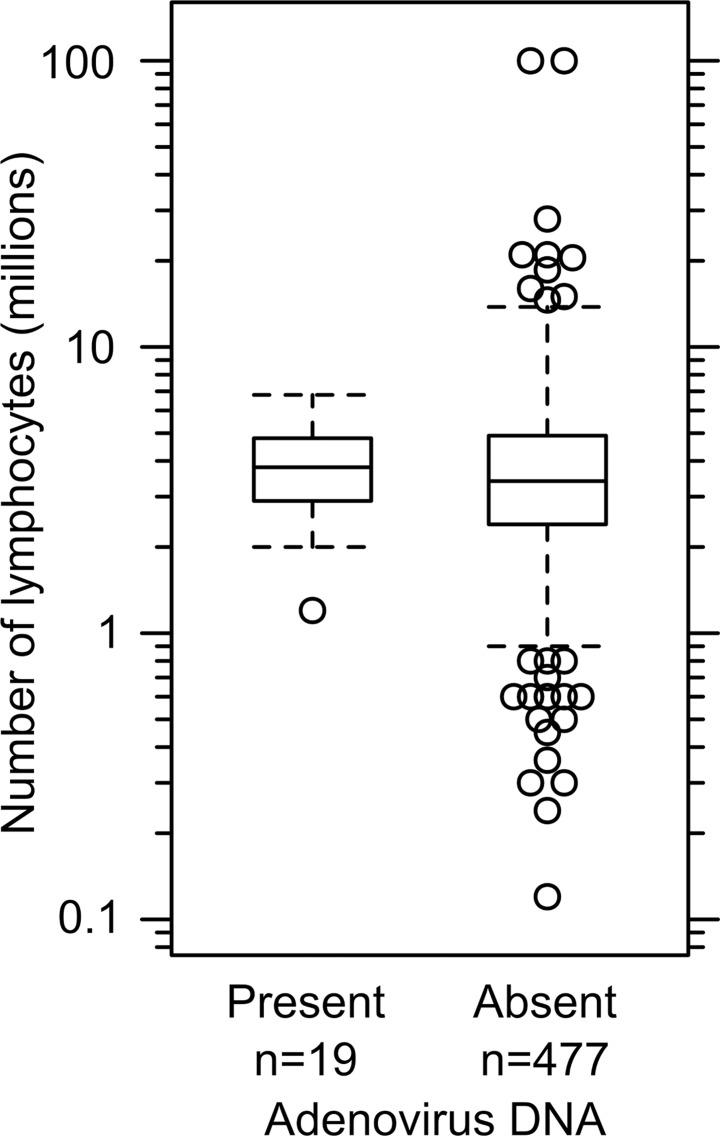
Fig 2. The detection of adenoviral DNA is unrelated to the number of lymphocytes recovered in the cord blood.
The number of lymphocytes in cord blood samples that either contain (Present) or do not contain (Absent) adenoviral DNA show similar distributions. Median values of 3.8×106 and 3.4×106 are indicated by the solid horizontal line. The box spans the interquartile range of log-transformed values. Values beyond 1.5-times the interquartile range are plotted as open circles and whiskers indicate the range of values less than 1.5-times the interquartile range.
Table 1. Adenoviral DNA status as a function of mother’s demographics and infant gender.
| Adenoviral DNA status | ||||
|---|---|---|---|---|
| Mother’s ethnicity | Positive | Negative | % positive | 95% CI 1 |
| African/Black | 15 | 396 | 3.6% | 2.2–5.9% |
| Hispanic | 4 | 77 | 4.9% | 1.9–12% |
| Caucasian | 0 | 13 | 0% | 0–23% |
| Asian | 0 | 7 | 0% | 0–35% |
| Native American | 0 | 1 | 0% | - |
| Unknown | 0 | 4 | 0% | - |
| Mother’s age | ||||
| <16 | 2 | 7 | 22.2% | 6.3–55% |
| 16–20 | 5 | 92 | 5.2% | 2.2–11.5% |
| 21–25 | 5 | 165 | 2.9% | 1.3–6.7% |
| 26–30 | 5 | 136 | 3.5% | 1.5–8.0% |
| 31–35 | 2 | 73 | 2.7% | 0.7–9.2% |
| >35 | 0 | 25 | 0.0% | 0–13.3% |
| Gender | ||||
| Female | 8 | 253 | 3.1% | 1.6–5.9% |
| Male | 11 | 245 | 4.3% | 2.4–7.5% |
| Total | ||||
| 19 | 498 | 3.7% | 2.4–5.7% | |
1The 95% confidence interval for the fraction of adenoviral DNA-positive samples was calculated according to the method of Agresti and Coull [19].
Because adenovirus was definitively detected in some cord blood samples, fourteen adenovirus-positive cord blood samples and 196 adenovirus-negative samples were tested for presence of the most common translocation associated with immature B cell ALL. For this, RNA was extracted from the purified cord blood lymphocytes and reverse transcribed into cDNA. The cDNA was queried with a nested PCR assay designed to detect the ETV6-RUNX1 (TEL-AML1) translocation [15]. Negative controls included RNA from the Burkitt’s lymphoma-derived BJAB cell line, salmon sperm DNA as the template for the nested PCR reaction, and the omission of reverse transcriptase from the cDNA reaction. To serve as a positive control for the ETV6-RUNX1 translocation, RNA from UoC-B4 cells [17] and BJAB cells was mixed at a ratio of 1:100 and used for reverse transcription. RNA samples were shipped to Winston-Salem for an independent assessment. The integrity of all RNA samples shipped to Winston-Salem was confirmed with an end-point PCR assay to detect the β-2 microglobulin transcript as shown by the representative results in Fig. 3A. cDNA derived from the equivalent of 50 ng of RNA was analyzed in triplicate with the nested PCR assay for the ETV6-RUNX1 fusion transcript. A sample was considered to contain the target if a PCR product was detected in at least two of three technical PCR replicates performed independently in both Atlanta and Winston-Salem. Representative results showing detection of the ETV6-RUNX1 fusion transcript in a cord blood sample is seen in Fig. 3B. The identity of each candidate PCR product was determined by sequencing. The sequencing electropherogram (Fig. 3B) confirms the expected fusion between exon 5 of ETV6 and exon 3 of RUNX1. By these criteria, 4 of the 196 adenovirus-negative samples (2.0%, 95%CI: 0.8–5.1%) and one of the 14 of adenovirus-positive cord blood samples (7%, 95% CI: 0.4–32%) contained the ETV6-RUNX1 translocation (Table 2). Although a higher fraction of adenovirus-positive cord blood samples contained the ETV6-RUNX1 translocation, a statistically significant difference (p = 0.3) could not be discerned because of the limited number of samples. Although the non-random selection of samples tested for the ETV6-RUNX1 selection may skew the fraction of cord blood with the fusion transcript, the nested PCR assay used here suggests that over 1% of the children born at Grady Memorial Hospital contained the ETV6-RUNX1 translocation at birth. It should be noted that this value is statistically indistinguishable from the values ranging between 1% and 4% reported by Eguchi-Ishimae et al. [21], Mori et al. [22], Zuna et al. [23] and Škorvaga et al. [24].
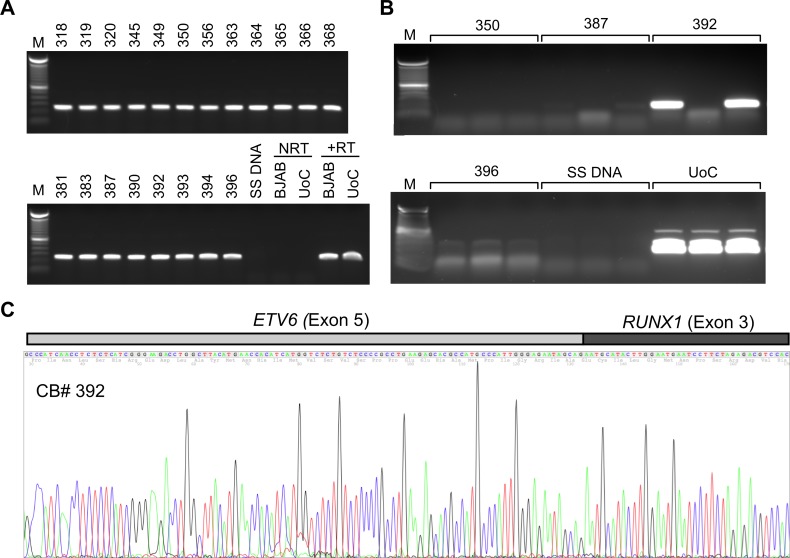
Fig 3. Detection of the ETV6-RUNX1 fusion transcript by reverse transcription followed by PCR.
200 ng of RNA purified from umbilical vein cord blood lymphocytes was reverse transcribed using random hexamers as primers. (A) The integrity of the RNA in the numbered cord blood samples was confirmed by amplification of a 384 bp sequence spanning the junction of exons 3 and 4 of the β-2-microglobulin gene. Positive controls included RNA from the BJAB and UoCB4 cell lines (+RT). Negative controls included salmon sperm DNA (SS DNA) and the exclusion of reverse transcriptase (NRT). Lanes containing the 100 bp DNA ladder reference are indicated by M. (B) A 200 bp amplicon obtained from a nested PCR assay indicates the presence of the ETV6-RUNX1 transcript. Representative results show the products of three technical replicates from four numbered cord blood samples, salmon sperm DNA as a negative control, and a 10-2 dilution of the ETV6-RUNX1-positive UoCB4 cells among BJAB cells as a positive control. Samples in which at least two of three technical replicates were detected from cDNA generated independently in two laboratories and in which no product was observed when reverse transcriptase was omitted were considered to contain the ETV6-RUNX1 fusion transcript. (C) The ETV6-RUNX1 amplicon generated from cord blood sample 392 was subjected to sequencing with the primers used to generate the product. The sequencing electropherogram from one of two reactions demonstrates the structure of the expected fusion transcript.
Table 2. Detection of ETV6-RUNX1 fusion transcript in cord blood RNA.
| ETV6-RUNX1 fusion | ||||
|---|---|---|---|---|
| Present | Absent | % positive | 95% CI 1 | |
| Adenoviral DNA | ||||
| Positive | 1 | 13 | 7.1% | 0.4–31.5% |
| Negative | 4 | 192 | 2.0% | 0.8–5.1% |
| Mother’s ethnicity | ||||
| African/Black | 3 | 145 | 2.0% | 0.7–5.8% |
| Hispanic | 2 | 53 | 3.6% | 1.0–12.3% |
| Other | 0 | 7 | 0.0% | 0–35.4% |
| Gender | ||||
| Female | 2 | 114 | 1.7% | 0.5–6.1% |
| Male | 3 | 91 | 3.2% | 1.1–9% |
| Total | ||||
| 5 | 205 | 2.4% | 1.0–5.5% | |
1The 95% confidence interval for the fraction of ETV6-RUNX1 fusion transcript-positive samples was calculated according to the method of Agresti and Coull [19].
Although the limited number of samples analyzed in this study do not provide sufficient statistical power to establish an association between the detection of adenoviral DNA and the ETV6-RUNX1 translocation, it was possible to measure the amount of viral DNA recovered in some of the samples where enough material remained to do so. Five adenovirus-positive cord blood samples were queried by quantitative PCR. Strikingly, the number of viral genomes in the single sample positive for the ETV6-RUNX1 translocation was 76-fold over the median viral load in the four samples lacking the ETV6-RUNX1 translocation that could be analyzed (Table 3).
Table 3. Adenovirus genome load in selected adenoviral DNA-positive samples.
| Cord blood sample | Viral genomes per 106 cells | ETV6-RUNX1 fusion |
|---|---|---|
| 601 | 136 | - |
| 608 | 168 | - |
| 614 | 7,664 | present |
| 628 | 136 | - |
| 642 | 32 | - |
Discussion
The present study was undertaken to resolve a long-standing conflict in the literature regarding the presence or absence of prenatal species C adenovirus infections in humans. This question is important because these viruses express molecular machinery [25] capable of inducing the genomic translocations that arise prior to birth and lead to leukemia in young children [26–28]. Evidence of prenatal adenovirus infection in samples that also contain the most common translocation of childhood leukemia, ETV6-RUNX1, would be strong support for the involvement of adenovirus in the genesis of this disease. Previous studies looking for adenoviruses in amniocentesis fluid, with some exceptions, find viral DNA in about 5% of samples. By contrast, studies using dried peripheral blood spots from newborn infants (Guthrie cards) generally fail to find adenoviral DNA [11,12].
Based on the results reported here we postulate that the negative results cited above are false negatives due to one of the following three technical limitations. Either the initial sample size was too small to contain a single virus-infected cell, or the samples were treated prior to analysis in ways that cause loss of viral DNA, or the cell types being analyzed were not those that harbor latent or persistent adenovirus. The study presented here was designed to overcome these limitations by using freshly harvested material, by using cord blood which is most likely to contain immature fetal cells, and by using a large initial sample size from which to extract virus using a method known to preserve viral DNA in the samples. This study provides unequivocal evidence for the presence of adenovirus genomes in cord blood at birth in 3.7% of newborns.
Species C adenoviruses establish latent infections in mucosal-associated lymphoid tissues [13]. We and other investigators have identified adenoviral DNA in mononuclear cells from adenoids and tonsils of children as young as two years [13,14,29]. By four years of age virtually every child is carrying one or more adenoviral genotypes in their mucosal tissues [13]. Among eight adenovirus DNA-containing tonsil or adenoid samples that were tested, the frequency of virus-containing mononuclear cells ranged from 3 to 3,400 per 107 cells [13]. In addition, the median number of genomes per infected cell is 280 with 95% confidence limits from 15 to 5,000 [13]. The low and variable frequency of virus-containing cells coupled with the likelihood of encountering multiple copies of the genome per infected cell are key to understanding detection of latent adenovirus in human tissue samples.
We detected adenoviral DNA in 3.7% of 517 cord blood samples (Table 1). This frequency agrees with that reported by several groups that evaluated amniotic fluid for adenovirus DNA. Using a PCR assay, Baschat and associates identified adenoviral DNA in 5.4% of amniotic fluid samples [9]. Three additional studies reported detecting adenoviral DNA in approximately 5% of amniocentesis fluid samples from sonographically normal pregnancies [7–10]. In each of these studies, nucleic acid was purified from 2 to 3 mL of whole amniotic fluid using a modification of the one-step method of Chomczynski and Sacchi [30]. These studies did not report removing the cells by centrifugation before testing for adenovirus DNA. By contrast, two studies that sought adenovirus DNA in cell-free amniotic fluid failed to detect viral DNA [31,32]. At mid-gestation, each mL of amniotic fluid contains as many as 106 intact fetal cells [33] and approximately 3,000 genome-equivalents of cell-free fetal DNA [34]. As noted above, latently infected tonsil lymphocytes were found to contain a median of 280 genomes per infected cell [13]. The nested PCR assay used here has a limit of detection of 5 genomes [14]. Thus, a single latently infected cell among 106 cells in one mL of amniotic fluid should contribute sufficient genomes to be detected by the nested PCR assay. Adenovirus purification takes advantage of the fact that progeny virus remains tightly associated with cells and cellular debris [35]. If the same is true in amniotic fluid, removing the cells by centrifugation would effectively remove adenoviral DNA from the sample.
Dried neonatal blood spots also have been screened for fetal pathogens. In contrast to the study of cord blood lymphocytes described here, most studies evaluating neonatal blood spots have failed to detect a variety of viruses [36] including adenovirus [11,12]. The detection of adenoviral DNA in dried blood spots faces several limitations. First, the recovery of viral DNA from dried blood spots may be less efficient than from fresh cells and that efficiency may decrease with time in storage. Second, this process analyzes a limited number of cells. Four 3.2-mm punches from dried blood spots (used in most studies) corresponds to 6 μl of peripheral blood [37] or 24,000 to 65,000 white blood cells, of which one-fourth are lymphocytes. A reasonable probability (>95%) of identifying a cord blood sample as adenovirus DNA-positive requires only three adenovirus-positive cells with a minimum of 20 viral genomes per cell among the million lymphocytes in a typical sample. Using samples punched from dried archival blood spots, we reported in 2010 the detection of adenovirus DNA in only 0.27% (two of 729) of Guthrie card samples [11]. Indeed, if the effective number of cells recovered from the Guthrie card was 30,000 lymphocytes and the true frequency of adenovirus-positive lymphocytes is approximately three in one million, Poisson considerations dictate that we would have detected adenovirus DNA in less than 0.5% of the samples. Third, the blood deposited on the Guthrie cards was obtained from peripheral blood of infants three to four days after birth. The abundance of adenovirus-positive lymphocytes in the peripheral blood at this time likely differs from that in cord blood at birth. Although adenoviral DNA is found in pediatric adenoid and tonsil lymphocytes [13,14,29,38], it is rarely detected in peripheral blood lymphocytes [39,40]. Interestingly, immature T and B cells are more abundant in pediatric adenoids and tonsils than in other secondary lymphoid tissues [41–43]. Cord blood also contains a greater fraction of immature lymphocytes and lymphocytic progenitors than adult peripheral blood [44,45]. Although cord blood and peripheral blood contain similar frequencies of B cells [45], cord blood contains seven-fold more of the fetal B1 cells (CD5+CD19+) than adult peripheral blood [46]. Finally, immature T cells expressing CD38 are more highly represented in cord blood than in peripheral blood [45,46]. If adenovirus infects or persists in immature lymphocytes, it seems plausible that the lymphocytes of the tonsils and adenoids as well as those of cord blood may serve as a preferred reservoir for the virus.
Compelling evidence indicates that chromosomal aberrations found in pediatric leukemias occur before birth [26–28]. Furthermore, an infectious etiology for ALL has been frequently considered [5,47], including a proposal that an otherwise innocuous viral infection in utero precipitates the development of leukemic mutations [48]. Since the ETV6-RUNX1 translocation is found in nearly 25% of childhood B-cell precursor ALL [49] we analyzed these cord blood samples for an increased frequency of detection of the ETV6-RUNX1 translocation in adenovirus-containing cord blood lymphocytes. We found that one of 14 (7.1%) adenovirus-positive samples contained the translocation compared to four of 196 (2.0%) adenovirus-negative samples (Table 2). The small number of samples evaluated here does not provide sufficient statistical power to indicate if adenovirus DNA is associated with the ETV6-RUNX1 translocation. Nonetheless, it is intriguing to note that this single sample (CB 614) also contained the highest viral DNA load among the samples tested (Table 3). Future studies will determine if there is indeed a relationship between the viral DNA load and the presence of preleukemic translocations. A positive correlation would support the notion that an active prenatal adenovirus infection predisposes a child to leukemia. If this is confirmed, it may be appropriate to routinely screen cord blood with a robust and quantitative measure of adenovirus DNA levels.
Overall, we detected the ETV6-RUNX1 translocation in 2.4% of cord blood samples. This frequency agrees with several reports seeking the ETV6-RUNX1 translocation in cord blood. Applying both a 5’ nuclease real-time qPCR and a nested endpoint PCR assay to cDNA derived from approximately 106 cord blood lymphocytes, Mori and associates identified the ETV6-RUNX1 translocation in six of 567 (≈1%) cord blood samples from England [22]. The cord blood of healthy newborns from Prague was analyzed for the presence of the ETV6-RUNX1, MLL-AF4 and BCR-ABL fusion transcripts. While the MLL-AF4 and BCR-ABL fusion transcripts were not detected in these samples, five of 253 samples (2%) contained the ETV6-RUNX1 fusion transcript [23]. Applying fluorescent in-situ hybridization to a specimen with sufficient numbers of cells, these investigators confirmed the presence of cells bearing the chromosomal ETV6-RUNX1 fusion. A real-time PCR assay identified the ETV6-RUNX1 fusion transcript in approximately 4% of 200 cord blood samples of children born in Bratislava, Slovak Republic [24]. Although not all studies have identified the ETV6-RUNX1 transcript in cord blood [50–52], it was identified at a frequency of 1–4% in those that positively detect this fusion transcript.
To our knowledge, the prenatal frequencies of the ETV6-RUNX1 translocation and adenovirus in the demographic population analyzed here have not been reported. The discovery that three of 148 children of African ancestry (Table 2) contained the pre-leukemic ETV6-RUNX1 translocation is of interest because children of African or partial African ancestry have a lower risk of developing childhood ALL [53]. It has been suggested that the development of childhood B-cell ALL proceeds in a two-stage fashion, with the initial mutation occurring in utero followed by a second event that promotes the development of frank leukemia [3,48,54]. If our finding that the ETV6-RUNX1 pre-leukemic translocation arises at comparable frequency in children of African and European ancestry is confirmed with larger numbers of samples, this outcome may inform the search for factors that contribute to the second event purported to drive the development of leukemia.
Adenovirus is unique among animal viruses because its genome exists in actively infected cells, as well as in cell lines used to model adenovirus persistence, as a linear, double-stranded DNA molecule [55]. Although adenoviral DNA is not found in human lymphomas or leukemias [56], adenovirus profoundly suppresses the ability of the infected cell to respond appropriately to double-stranded DNA-breaks. Notably, the ubiquitous species C adenovirus directs the degradation and inactivation of many cellular proteins that sense and repair double-stranded DNA-breaks, thus disabling both the homologous and non-homologous end-joining DNA-repair pathways [25]. Transient expression of the adenovirus oncoproteins elicits chromosomal abnormalities characteristic of the failure to repair double-stranded DNA-breaks in a timely fashion [57,58]. Thus adenovirus has the potential to elicit chromosomal abnormalities in a “hit-and-run” fashion, which is the induction of an oncogenic cellular change followed by loss of the viral genome [57]. Because it is a common prenatal infectious agent of lymphocytic cells, human adenovirus remains a strong candidate for the virus proposed by Smith as an etiologic agent that contributes the initial step toward the development of childhood ALL.
Acknowledgments
The authors thank Mr. Wen Huang for his excellent technical assistance in the experiments described here. We thank Ms. Denise Palmer, Grady Memorial Hospital, Atlanta, GA for assistance during the collection of cord blood samples from the blood bank following live births.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by the National Cancer Institute, grant number R01 CA127621 to LRG, DAO, CGB. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Eden T (2010) Aetiology of childhood leukaemia. Cancer Treat Rev 36: 286–297. 10.1016/j.ctrv.2010.02.004 [DOI] [PubMed] [Google Scholar]
- 2. Wiemels J (2012) Perspectives on the causes of childhood leukemia. Chem Biol Interact 196: 59–67. 10.1016/j.cbi.2012.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Greaves M (2006) The causation of childhood leukemia: a paradox of progress? Discov Med 6: 24–28. [PubMed] [Google Scholar]
- 4. Zur Hausen H (2009) The search for infectious causes of human cancers: where and why. Virology 392: 1–10. 10.1016/j.virol.2009.06.001 [DOI] [PubMed] [Google Scholar]
- 5. O’Connor SM, Boneva RS (2007) Infectious etiologies of childhood leukemia: plausibility and challenges to proof. Environ Health Perspect 115: 146–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gustafsson B, Huang W, Bogdanovic G, Gauffin F, Nordgren A, et al. (2007) Adenovirus DNA is detected at increased frequency in Guthrie cards from children who develop acute lymphoblastic leukaemia. Br J Cancer 97: 992–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Van den Veyver IB, Ni J, Bowles N, Carpenter RJ Jr, Weiner CP, et al. (1998) Detection of intrauterine viral infection using the polymerase chain reaction. Mol Genet Metab 63: 85–95. [DOI] [PubMed] [Google Scholar]
- 8. Wenstrom KD, Andrews WW, Bowles NE, Towbin JA, Hauth JC, et al. (1998) Intrauterine viral infection at the time of second trimester genetic amniocentesis. Obstet Gynecol 92: 420–424. [DOI] [PubMed] [Google Scholar]
- 9. Baschat AA, Towbin J, Bowles NE, Harman CR, Weiner CP (2003) Prevalence of viral DNA in amniotic fluid of low-risk pregnancies in the second trimester. J Matern Fetal Neonatal Med 13: 381–384. [DOI] [PubMed] [Google Scholar]
- 10. Reddy UM, Baschat AA, Zlatnik MG, Towbin JA, Harman CR, et al. (2005) Detection of viral deoxyribonucleic acid in amniotic fluid: association with fetal malformation and pregnancy abnormalities. Fetal Diagn Ther 20: 203–207. [DOI] [PubMed] [Google Scholar]
- 11. Honkaniemi E, Talekar G, Huang W, Bogdanovic G, Forestier E, et al. (2010) Adenovirus DNA in Guthrie cards from children who develop acute lymphoblastic leukaemia (ALL). Br J Cancer 102: 796–798. 10.1038/sj.bjc.6605581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vasconcelos GM, Kang M, Pombo-de-Oliveira MS, Schiffman JD, Lorey F, et al. (2008) Adenovirus detection in Guthrie cards from paediatric leukaemia cases and controls. Br J Cancer 99: 1668–1672. 10.1038/sj.bjc.6604714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Garnett CT, Talekar G, Mahr JA, Huang W, Zhang Y, et al. (2009) Latent species C adenoviruses in human tonsil tissues. J Virol 83: 2417–2428. 10.1128/JVI.02392-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Garnett CT, Erdman D, Xu W, Gooding LR (2002) Prevalence and quantitation of species C adenovirus DNA in human mucosal lymphocytes. J Virol 76: 10608–10616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van Dongen JJ, Macintyre EA, Gabert JA, Delabesse E, Rossi V, et al. (1999) Standardized RT-PCR analysis of fusion gene transcripts from chromosome aberrations in acute leukemia for detection of minimal residual disease. Report of the BIOMED-1 Concerted Action: investigation of minimal residual disease in acute leukemia. Leukemia 13: 1901–1928. [DOI] [PubMed] [Google Scholar]
- 16. von Goessel H, Jacobs U, Semper S, Krumbholz M, Langer T, et al. (2009) Cluster analysis of genomic ETV6-RUNX1 (TEL-AML1) fusion sites in childhood acute lymphoblastic leukemia. Leuk Res 33: 1082–1088. 10.1016/j.leukres.2008.11.001 [DOI] [PubMed] [Google Scholar]
- 17. Zhang LQ, Downie PA, Goodell WR, McCabe NR, LeBeau MM, et al. (1993) Establishment of cell lines from B-cell precursor acute lymphoblastic leukemia. Leukemia 7: 1865–1874. [PubMed] [Google Scholar]
- 18. R Core Team (2014) R: A language and environment for statistical computing. 3.1.0 ed. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 19. Agresti A, Coull BA (1998) Approximate is better than “exact” for interval estimation of binomial proportions. American Statistician 52: 119–126. [Google Scholar]
- 20. Harrell FE Jr, Dupont C (2012) Hmisc: Harrell Miscellaneous. 3.14–4 ed. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 21. Eguchi-Ishimae M, Eguchi M, Ishii E, Miyazaki S, Ueda K, et al. (2001) Breakage and fusion of the TEL (ETV6) gene in immature B lymphocytes induced by apoptogenic signals. Blood 97: 737–743. [DOI] [PubMed] [Google Scholar]
- 22. Mori H, Colman SM, Xiao Z, Ford AM, Healy LE, et al. (2002) Chromosome translocations and covert leukemic clones are generated during normal fetal development. Proc Natl Acad Sci U S A 99: 8242–8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zuna J, Madzo J, Krejci O, Zemanova Z, Kalinova M, et al. (2011) ETV6/RUNX1 (TEL/AML1) is a frequent prenatal first hit in childhood leukemia. Blood 117: 368–369; author reply 370–361. 10.1182/blood-2010-09-309070 [DOI] [PubMed] [Google Scholar]
- 24. Skorvaga M, Nikitina E, Kubes M, Kosik P, Gajdosechova B, et al. (2014) Incidence of common preleukemic gene fusions in umbilical cord blood in Slovak population. PLoS One 9: e91116 10.1371/journal.pone.0091116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Turnell AS, Grand RJ (2012) DNA viruses and the cellular DNA-damage response. J Gen Virol 93: 2076–2097. 10.1099/vir.0.044412-0 [DOI] [PubMed] [Google Scholar]
- 26. Greaves M (2005) In utero origins of childhood leukaemia. Early Hum Dev 81: 123–129. [DOI] [PubMed] [Google Scholar]
- 27. Taub JW, Ge Y (2004) The prenatal origin of childhood acute lymphoblastic leukemia. Leuk Lymphoma 45: 19–25. [DOI] [PubMed] [Google Scholar]
- 28. Gruhn B, Taub JW, Ge Y, Beck JF, Zell R, et al. (2008) Prenatal origin of childhood acute lymphoblastic leukemia, association with birth weight and hyperdiploidy. Leukemia 22: 1692–1697. 10.1038/leu.2008.152 [DOI] [PubMed] [Google Scholar]
- 29. Alkhalaf MA, Guiver M, Cooper RJ (2013) Prevalence and quantitation of adenovirus DNA from human tonsil and adenoid tissues. J Med Virol 85: 1947–1954. 10.1002/jmv.23678 [DOI] [PubMed] [Google Scholar]
- 30. Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159. [DOI] [PubMed] [Google Scholar]
- 31. Gervasi MT, Romero R, Bracalente G, Chaiworapongsa T, Erez O, et al. (2012) Viral invasion of the amniotic cavity (VIAC) in the midtrimester of pregnancy. J Matern Fetal Neonatal Med 25: 2002–2013. 10.3109/14767058.2012.683899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McLean LK, Chehab FF, Goldberg JD (1995) Detection of viral deoxyribonucleic acid in the amniotic fluid of low-risk pregnancies by polymerase chain reaction. Am J Obstet Gynecol 173: 1282–1286. [DOI] [PubMed] [Google Scholar]
- 33. Delo DM, De Coppi P, Bartsch G Jr, Atala A (2006) Amniotic fluid and placental stem cells. Methods Enzymol 419: 426–438. [DOI] [PubMed] [Google Scholar]
- 34. Bianchi DW, LeShane ES, Cowan JM (2001) Large amounts of cell-free fetal DNA are present in amniotic fluid. Clin Chem 47: 1867–1869. [PubMed] [Google Scholar]
- 35. Tollefson AE, Kuppuswamy M, Shashkova EV, Doronin K, Wold WS (2007) Preparation and titration of CsCl-banded adenovirus stocks. Methods Mol Med 130: 223–235. [DOI] [PubMed] [Google Scholar]
- 36. Gustafsson B, Bogdanovic G (2007) Specific viruses were not detected in Guthrie cards from children who later developed leukemia. Pediatr Hematol Oncol 24: 607–613. [DOI] [PubMed] [Google Scholar]
- 37. Mei JV, Alexander JR, Adam BW, Hannon WH (2001) Use of filter paper for the collection and analysis of human whole blood specimens. J Nutr 131: 1631S–1636S. [DOI] [PubMed] [Google Scholar]
- 38. Neumann R, Genersch E, Eggers HJ (1987) Detection of adenovirus nucleic acid sequences in human tonsils in the absence of infectious virus. Virus Res 7: 93–97. [DOI] [PubMed] [Google Scholar]
- 39. Flomenberg P, Gutierrez E, Piaskowski V, Casper JT (1997) Detection of adenovirus DNA in peripheral blood mononuclear cells by polymerase chain reaction assay. J Med Virol 51: 182–188. [DOI] [PubMed] [Google Scholar]
- 40. Durepaire N, Rogez JP, Verdier M, Rogez S, Weinbreck P, et al. (1997) Detection of adenovirus DNA by polymerase chain reaction in peripheral blood lymphocytes from HIV-infected patients and a control group: preliminary results. J Acquir Immune Defic Syndr Hum Retrovirol 14: 189–190. [DOI] [PubMed] [Google Scholar]
- 41. Strauchen JA, Miller LK (2001) Terminal deoxynucleotidyl transferase-positive cells in human tonsils. Am J Clin Pathol 116: 12–16. [DOI] [PubMed] [Google Scholar]
- 42. Meru N, Jung A, Baumann I, Niedobitek G (2002) Expression of the recombination-activating genes in extrafollicular lymphocytes but no apparent reinduction in germinal center reactions in human tonsils. Blood 99: 531–537. [DOI] [PubMed] [Google Scholar]
- 43. Buscone S, Garavello W, Pagni F, Gaini RM, Cattoretti G (2014) Nasopharyngeal tonsils (adenoids) contain extrathymic corticothymocytes. PLoS One 9: e98222 10.1371/journal.pone.0098222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Harris DT, Schumacher MJ, Locascio J, Besencon FJ, Olson GB, et al. (1992) Phenotypic and functional immaturity of human umbilical cord blood T lymphocytes. Proc Natl Acad Sci U S A 89: 10006–10010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Beck R, Lam-Po-Tang PR (1994) Comparison of cord blood and adult blood lymphocyte normal ranges: a possible explanation for decreased severity of graft versus host disease after cord blood transplantation. Immunol Cell Biol 72: 440–444. [DOI] [PubMed] [Google Scholar]
- 46. Chirumbolo S, Ortolani R, Veneri D, Raffaelli R, Peroni D, et al. (2011) Lymphocyte phenotypic subsets in umbilical cord blood compared to peripheral blood from related mothers. Cytometry B Clin Cytom 80: 248–253. 10.1002/cyto.b.20588 [DOI] [PubMed] [Google Scholar]
- 47. Roman E, Simpson J, Ansell P, Lightfoot T, Smith A (2009) Infectious proxies and childhood leukaemia: findings from the United Kingdom Childhood Cancer Study (UKCCS). Blood Cells Mol Dis 42: 126–128. 10.1016/j.bcmd.2008.10.007 [DOI] [PubMed] [Google Scholar]
- 48. Smith M (1997) Considerations on a possible viral etiology for B-precursor acute lymphoblastic leukemia of childhood. J Immunother 20: 89–100. [DOI] [PubMed] [Google Scholar]
- 49. Romana SP, Poirel H, Leconiat M, Flexor MA, Mauchauffe M, et al. (1995) High frequency of t(12;21) in childhood B-lineage acute lymphoblastic leukemia. Blood 86: 4263–4269. [PubMed] [Google Scholar]
- 50. Olsen M, Madsen HO, Hjalgrim H, Gregers J, Rostgaard K, et al. (2006) Preleukemic TEL-AML1-positive clones at cell level of 10(-3) to 10(-4) do not persist into adulthood. J Pediatr Hematol Oncol 28: 734–740. [DOI] [PubMed] [Google Scholar]
- 51. Lausten-Thomsen U, Madsen HO, Vestergaard TR, Hjalgrim H, Nersting J, et al. (2011) Prevalence of t(12;21)[ETV6-RUNX1]-positive cells in healthy neonates. Blood 117: 186–189. 10.1182/blood-2010-05-282764 [DOI] [PubMed] [Google Scholar]
- 52. Olsen M, Hjalgrim H, Melbye M, Madsen HO, Schmiegelow K (2012) RT-PCR screening for ETV6-RUNX1-positive clones in cord blood from newborns in the Danish National Birth Cohort. J Pediatr Hematol Oncol 34: 301–303. 10.1097/MPH.0b013e3182332268 [DOI] [PubMed] [Google Scholar]
- 53. Chow EJ, Puumala SE, Mueller BA, Carozza SE, Fox EE, et al. (2010) Childhood cancer in relation to parental race and ethnicity: a 5-state pooled analysis. Cancer 116: 3045–3053. 10.1002/cncr.25099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Greaves M (1999) Molecular genetics, natural history and the demise of childhood leukaemia. Eur J Cancer 35: 1941–1953. [DOI] [PubMed] [Google Scholar]
- 55. Zhang Y, Huang W, Ornelles DA, Gooding LR (2010) Modeling adenovirus latency in human lymphocyte cell lines. J Virol 84: 8799–8810. 10.1128/JVI.00562-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fernandez-Soria V, Bornstein R, Forteza J, Parada C, Sanchez-Prieto R, et al. (2002) Inconclusive presence of adenovirus sequences in human leukemias and lymphomas. Oncology Reports 9: 897–902. [PubMed] [Google Scholar]
- 57. Nevels M, Tauber B, Spruss T, Wolf H, Dobner T (2001) "Hit-and-run" transformation by adenovirus oncogenes. J Virol 75: 3089–3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hart LS, Yannone SM, Naczki C, Orlando JS, Waters SB, et al. (2005) The adenovirus E4orf6 protein inhibits DNA double strand break repair and radiosensitizes human tumor cells in an E1B-55K-independent manner. J Biol Chem 280: 1474–1481. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.