Abstract
Understanding the implications of different management strategies is necessary to identify best conservation trajectories for ecosystems exposed to anthropogenic stressors. For example, science-based risk assessments at large scales are needed to understand efficacy of different vector management approaches aimed at preventing biological invasions associated with commercial shipping. We conducted a landscape-scale analysis to examine the relative invasion risk of ballast water discharges among different shipping pathways (e.g., Transoceanic, Coastal or Domestic), ecosystems (e.g., freshwater, brackish and marine), and timescales (annual and per discharge event) under current and future management regimes. The arrival and survival potential of nonindigenous species (NIS) was estimated based on directional shipping networks and their associated propagule pressure, environmental similarity between donor-recipient ecosystems (based on salinity and temperature), and effects of current and future management strategies (i.e., ballast water exchange and treatment to meet proposed international biological discharge standards). Our findings show that current requirements for ballast water exchange effectively reduce invasion risk to freshwater ecosystems but are less protective of marine ecosystems because of greater environmental mismatch between source (oceanic) and recipient (freshwater) ecoregions. Future requirements for ballast water treatment are expected to reduce risk of zooplankton NIS introductions across ecosystem types but are expected to be less effective in reducing risk of phytoplankton NIS. This large-scale risk assessment across heterogeneous ecosystems represents a major step towards understanding the likelihood of invasion in relation to shipping networks, the relative efficacy of different invasion management regimes and seizing opportunities to reduce the ecological and economic implications of biological invasions.
Introduction
Aquatic invasive species are nonindigenous organisms that establish and spread in a new environment, frequently resulting in negative impacts on biodiversity [1], ecological structure and function [2], and imperilment of fauna [3]. As the severity of impacts is influenced by multiple interacting drivers that are difficult to predict, prevention through vector management has often been cited as the best conservation strategy [4, 5]. Despite these recommendations, little attention has been directed at understanding efficacy of different vector management approaches, particularly at landscape scales, impeding the progress of science-based regulation and precluding assessments evaluating current and future ecological risk.
The unintentional release of nonindigenous species (NIS) in ballast water moved by transoceanic ships has long been recognized as a primary vector of aquatic invasions [6–9]. As a result, many countries have enacted ballast water management at landscape scales (i.e., at the scale of global or national shipping networks; [10]) to reduce the risk of species invasion and concomitant ecological change. Currently, the main requirement entails ballast water exchange (BWE), where vessels replace ballast water loaded at a foreign port with offshore oceanic water in an effort to reduce the abundance and diversity of coastal plankton transported between ports, thereby decreasing the likelihood of invasion because the number of individuals discharged in ballast water (propagule pressure) is an important determinant of invasion success [11].
The efficacy of BWE as a management strategy, however, is highly variable. Empirical studies indicate that BWE purges 70–100% of planktonic organisms entrained at the source port depending on ship type, method and location of exchange, and type of organism [12–16]. Ballast water exchange is considered most protective for freshwater recipient ports due to osmotic shock induced by the alternation of marine and fresh waters [17], and less effective for vessels conducting coastal exchange (50–200 nautical miles offshore) in comparison to mid-ocean exchange (> 200 nautical miles offshore) since plankton densities can be high in nearshore waters [15, 18, 19]. Furthermore, BWE is not a viable option for intra-regional vessels that remain within 50 nautical miles of shore; however, few studies have examined the ecological implications of BWE across landscape scales and global shipping networks involving many permutations of taxa, propagule pressure, and ecological conditions of source and recipient locations.
Due to concerns about limited ecological protection afforded by BWE, pending ratification of an international convention, vessels will soon be required to manage ballast water to meet numeric discharge standards limiting the density of organisms released to recipient ecosystems (the D-2 standards; [20]). It is expected that most vessels will install on-board multi-stage treatment systems such as filtration + electro-chlorination [21, 22], which are expected to physically remove large particles and chemically inactivate small particles in ballast water to meet the D-2 standards: less than 10 viable organisms ≥ 50 μm in minimum dimension per m3 (nominally ‘zooplankton’) and less than 10 viable organisms ≥ 10 μm and < 50 μm in minimum dimension per mL (nominally ‘phytoplankton’) [20]. Since plankton densities can be highly variable after BWE, in theory, treatment should offer risk reduction by consistently reducing plankton density to low levels. In addition, treatment is considered a safer and more broadly applicable management tool for transoceanic, coastal and domestic vessels. Concerns have been raised, however, that the D-2 standards are not stringent enough, as a proportion of ships can meet the standards without any ballast water management [10]. Further, concerns about the loss of the salinity barrier imposed by BWE have prompted Canadian and American regulators to propose enhanced requirements (BWE plus treatment) for vessels arriving to fresh water.
In general, the probability that a particular species will become established decreases as propagule pressure decreases [23–25], however, invasions are a stochastic process and the shape of the propagule pressure-establishment relationship is highly context-dependent [11, 26, 27]. The relationship is further complicated by the wide range of possible combinations of the number of individuals released per event, the frequency of release events, and the specific physical-chemical requirements and ecological interactions between arriving propagules and the recipient community [28–30]. As a result, it has not been possible to allocate a numeric invasion probability with confidence for specific inoculum densities. Even though the relative importance of different combinations has not been quantified, it has been postulated that invasion success is greater for multiple release events over time and space than for single, high-density releases, since repeated introductions may allow founding populations to overcome stochastic demographic and environmental limitations [11, 24, 31].
Using a mechanistic approach, the objective of this study is to evaluate the relative invasion risk to diverse ecosystems at landscape scales (i.e., across different directional shipping networks arriving to freshwater, brackish, and marine ports in Canada and the Laurentian Great Lakes) in relation to current and proposed vector management regimes. We estimate potential for arrival and survival of NIS and the change in these metrics anticipated under future requirements for ballast water treatment, recognizing that relative invasion risk is dependent on both the density of NIS carried by individual ships and the cumulative arrival of ships across time.
Methods
Estimating NIS arrival potential
A comprehensive database of the number and volume of ballast water discharges by merchant vessels (≥50m length with ballast capacity ≥8 m3) at ports in Canada and the Great Lakes was compiled using data extracted and cross-referenced from multiple Canadian and American government sources [27]. Because data were not available for the same year in all regions, comparative analyses were conducted using a 12-month time frame of recent data (2006, 2007 or 2008), representing an annual estimate of the arrival potential of NIS. Merchant vessels were grouped into geographic pathways based on their region(s) of operation (Table 1; Fig. 1). Biological data (density, diversity, biogeographic status of species in ballast water) were obtained from coordinated surveys conducted by the Canadian Aquatic Invasive Species Network (CAISN) and Fisheries and Oceans Canada over three years (2007 to 2009). Standardized protocols were used across the country to assess multiple taxonomic groups (diatoms, dinoflagellates and invertebrates) in ballast water sampled from different shipping pathways [17, 32–37], including unpublished data from Munawar, Fisheries and Oceans Canada. On average, 40 samples were collected for each region for zooplankton and 20 samples for phytoplankton (Table 1). For the East Coast, ballast water samples were collected in the ports of Sept-Îles, Port Cartier, Baie-Comeau (Quebec), St. John (New Brunswick), Canso, Halifax, Hantsport and Liverpool (Nova Scotia). For the West Coast, ships were sampled at the Port of Vancouver. In the Great Lakes, ships were sampled in Nanticoke, Hamilton (Ontario, Canada) and Toledo (Ohio, United States) [33]. Ships were selected on an opportunistic basis.
Table 1. Definitions of shipping pathways in Canada and the Great Lakes, with corresponding ballast water management requirements (modified from Casas-Monroy et al. [27]).
| Pathway | Operation Region | Management Requirement | N |
|---|---|---|---|
| Arctic Coastal Domestic | GLSLR, Atlantic and Arctic regions | No exchange/flush required | 13/NA |
| Eastern Coastal Domestic | GLSLR and Atlantic regions | No exchange/flush required | 37/7 |
| Lakers | GLSLR region and St. Lawrence Estuary (from Duluth to Sept Iles) | No exchange/flush required | 87/6 |
| Atlantic International Exempt | Atlantic region and coastal U.S. north of Cape Cod | No exchange/flush required | 11/14 |
| Pacific International Exempt | Pacific region, with last port-of-call in the coastal U.S. north of Cape Blanco | No exchange/flush required | 17/23 |
| Arctic International Transoceanic | GLSLR, Atlantic and Arctic regions and global ports outside Canada | Exchange/flush >200 nm offshore and >2000m depth prior to entering Canadian EEZ | 23/22 |
| GLSLR International Transoceanic | GLSLR region and global ports outside Canada | 16/17 | |
| Atlantic International Coastal U.S. | Atlantic region and coastal U.S. (south of Cape Cod) | Exchange/flush >50 nm offshore and >500m depth prior to entering Canadian EEZ | 23/23 |
| Atlantic International Transoceanic | Atlantic region and global ports outside Canada | Exchange/flush >200 nm offshore and >2000m depth prior to entering Canadian EEZ | 22/22 |
| Pacific International Coastal U.S. | Pacific region and coastal U.S. (south of Cape Blanco) | Exchange/flush >50 nm offshore and >500m depth prior to entering Canadian EEZ | 17/23 |
| Pacific International Transoceanic | Pacific region and global ports outside Canada | Exchange/flush >200 nm offshore and >2000m depth prior to entering Canadian EEZ | 23/24 |
GLSLR = Great Lakes-St. Lawrence River, EEZ = Exclusive Economic Zone. N = Number of samples for biological assessment of Zooplankton/Phytoplankton. NA = not assessed.
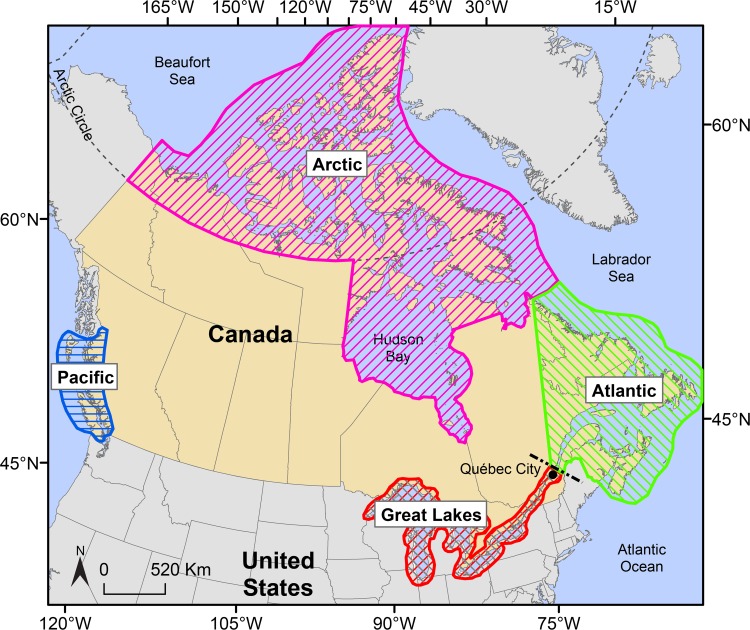
Fig 1. Geographic regions used to define shipping pathways and to quantify relative invasion risk among freshwater, brackish, and marine ecosystems (modified from Casas-Monroy et al. [27]).
Arrival potential was assessed separately for zooplankton and phytoplankton at two timescales: the number of NIS discharged per event and the cumulative number of NIS discharged per year. Since plankton densities sampled from ballast water displayed high variation across ships within a given pathway, we conducted a simple resampling process to generate a mean arrival potential statistic for each timescale. First, we constructed a probability distribution of the observed density of NIS from tank samples within a given shipping pathway. Next, we randomly selected a density value from the probability distribution, which was multiplied by a randomly selected value of ship discharge volume. The result of this multiplication was one trial of the total number of NIS released to the recipient ecosystem associated with a single shipping event. This process was repeated across all of n discharges in a given year, and then across 1000 iterations. The mean value at each timescale represents the average number of NIS discharged (i.e., mean abundance of NIS released per event; mean released cumulatively within a given year). Five categorical bins ranging from lowest to highest arrival potential were created by determining the 20th, 40th, 60th, 80th, and 100th percentiles from the entire distribution of values from the resampling process (e.g., for annual arrivals, percentiles were calculated from the 11,000 annual data points calculated for all pathways) [27]. We utilized the mean arrival values (which are influenced by right-tail skew in the distribution) derived during the Monte Carlo process to assign pathways into the percentile bins because discharges with very high NIS density, although rare, can be very important for invasion success [38].
Estimating survival potential
Survival potential was estimated by comparing environmental similarity between paired source and recipient ports for each directional ballast water movement in the shipping database. We focused our analysis on salinity and climate (i.e., water temperature) because these are fundamental variables for the survival and reproduction of aquatic organisms (e.g., [39–41], and because including additional factors that are related to invasion risk for only a subset of all potential NIS can affect the sensitivity of the environmental similarity measure [42–44]. Global annual mean salinity was determined for each coastal source and recipient port using the online World Ocean Atlas database [45], while salinity of inland ports (e.g., Great Lakes ports) was obtained from Keller et al. [43]. Global ports were classified into salinity categories as follows: 0.0–5.0 parts per thousand (‰) as “fresh water”; 5.1–18.0 ‰ as “brackish”; 18.1 ‰ and higher as “marine”. To reflect the environmental effect of BWE, based on Canadian regulations requiring vessels to exchange ballast water resulting in a minimum final salinity of at least 30 ‰ [46], the source salinity was changed to 30 ‰ for all vessel transits that conducted BWE to reflect that the source of the ballast water was no longer from a coastal port, but from the ocean. A matrix approach was used to determine similarity of salinity between all source-recipient pairs [47]. The score has three metrics and ranged from “lowest” similarity of salinity for a port-pair with highly divergent salinities (e.g., freshwater—marine) to “highest” similarity if both ports had the same salinity classification (e.g., freshwater—freshwater). Following Spalding et al. [48] and Keller et al. [43], climate categories were assigned according to latitudinal port position as follows: Tropical (0°–20°N/S), Warm-Temperate (20°–40°N/S), Cold-Temperate (40°–60°N/S), and Polar (>60°N/S). Again a matrix approach was used to determine climate similarity between each source-recipient port-pair. The score has three metrics and ranged from “lowest” climate similarity for a port-pair with highly divergent climates (e.g., Polar-Tropical) to “highest” similarity if both ports were located in the same or adjacent climate category. The overall survival potential for each vessel transit was estimated based on the lowest score of either salinity or climate matching to reflect the influence of the most limiting environmental variable. The final survival potential for each shipping pathway was then assigned the level (highest, intermediate or lowest) having the greatest number of observations across voyages [27].
Estimating relative invasion risk
The potentials for arrival and survival were combined into a final relative invasion risk for each pathway using a risk matrix (Table 2), modified from Chan et al. [44]. The risk was calculated using a 5 (arrivals) x 3 (survivals) matrix that reduces the final ratings to three levels. For example, given a lowest arrival potential and a highest survival potential, the overall introduction potential would be intermediate, because highest survival probabilities are offset by the small number of arriving individuals. Invasion risk was calculated separately for each pathway in each region, considering both annual and per-event arrivals and differential propagule pressures of zooplankton and phytoplankton.
Table 2. Matrix used to combine arrival potential and survival potential into final relative risk rankings.
| Arrival Potential | ||||||
|---|---|---|---|---|---|---|
| Lowest | Lower | Intermediate | Higher | Highest | ||
| Survival potential | Highest | Intermediate | Intermediate | Highest | Highest | Highest |
| Intermediate | Lowest | Intermediate | Intermediate | Intermediate | Highest | |
| Lowest | Lowest | Lowest | Lowest | Intermediate | Intermediate | |
For example, given a lowest arrival potential and a highest survival potential, the overall introduction potential would be intermediate. At the opposite, given a highest rank of arrival potential and intermediate survival, the final relative risk invasion would be highest (modified from Casas-Monroy et al. [27]).
Future invasion risk
Since Canada (and all other countries bound by the international convention, once fully ratified) will soon transition to a new ballast water management regime that is expected to enhance protection against ballast-mediated NIS, we repeated the process of estimating invasion risk using estimates of zooplankton/phytoplankton NIS densities expected after ballast water treatment. The anticipated NIS densities were estimated assuming the proportion of taxa (i.e., NIS vs. other taxa) will remain the same when the total abundance of all organisms is reduced to meet the D-2 standards. In addition, a salinity correction (30 ‰) was applied only for international transits (transoceanic or coastal) arriving to freshwater ports in line with requirements for “BWE plus treatment” on the Great Lakes [49–50].
Results
Baseline arrival potential
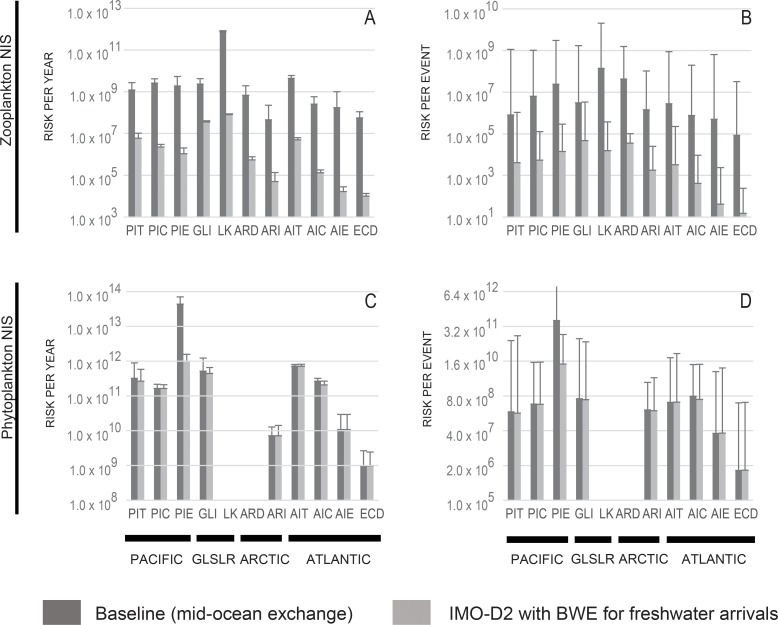
Merchant vessels conducted roughly 11,000 ballast water discharge events at 309 Canadian and Great Lakes’ ports, discharging 116,159,585 m3 of ballast water during the twelve-month period assessed. Our results revealed a broad variance across pathways in terms of number of discharge events and annual volume of ballast water discharged (Table 3). Lakers and international transoceanic vessels are the most active pathways, in contrast with Arctic Coastal Domestic vessels. Hence, when factoring in biological data, the cumulative arrival potential for zooplankton NIS varied broadly, with the highest ranked pathways—Lakers and Atlantic International Transoceanic—transporting more than 8.33 x 1011 NIS per year, and the lowest ranked pathways—Arctic International Transoceanic—transporting less than 9.69 x 107 NIS per year (Fig. 2A). Less variation was observed for the per event timescale, with the highest ranked pathways—Arctic Coastal Domestic and Lakers—transporting more than 8.24 x 109 NIS per ship and the lowest ranked pathways—Eastern Coastal Domestic, Atlantic International Coastal U.S., and Pacific International Transoceanic—having less than 1.26 x 106 NIS per ship (ranked as intermediate arrival potential) (Fig. 2B).
Table 3. Number of discharge events and annual volume of ballast water discharged by shipping pathways in Canada and the Great Lakes (modified from Casas-Monroy et al. [27]).
| Pathway | Total Volume of Ballast Water Discharged | Number of discharge events |
|---|---|---|
| Arctic Coastal Domestic | 78,125 | 16 |
| Arctic International Transoceanic | 197,589 | 30 |
| Eastern Coastal Domestic | 5,952,615 | 667 |
| GLSLR International Transoceanic | 2,914,206 | 759 |
| Lakers | 52,418,330 | 5227 |
| Atlantic International Coastal U.S. | 7,665,502 | 343 |
| Atlantic International Exempt | 5,652,994 | 357 |
| Atlantic International Transoceanic | 23,253,391 | 1530 |
| Pacific International Coastal U.S. | 2,324,543 | 415 |
| Pacific International Exempt | 592,089 | 79 |
| Pacific International Transoceanic | 15,110,203 | 1488 |
Fig 2. Estimated abundances of nonindigenous zooplankton (upper panels) and phytoplankton (lower panels) species transported in ballast water under current (dark gray) and future (light gray) management scenarios.
Abundances were calculated on annual (left panels) and per event (right panels) timescales. PIT = Pacific International Transoceanic, PIC = Pacific International Coastal, PIE = Pacific International Exempt; GLSR = Great Lakes-St. Lawrence River International Transoceanic, LK = Lakers, ARD = Arctic Coastal Domestic, ARI = Arctic International Transoceanic, AIT = Atlantic International Transoceanic, AIC = Atlantic International Coastal, AIE = Atlantic International Exempt, ECD = Eastern Coastal Domestic.
Cumulative annual arrival potential for phytoplankton NIS was also highly variable, with the highest ranked pathways—Atlantic International Transoceanic and Pacific International Exempt—introducing more than 7.02 x 1013 NIS per year, while the lowest ranked pathway—Lakers—introduces minimal NIS annually (Fig. 2C). No phytoplankton NIS were detected for Lakers in this study although the sample size was very small. The per-event arrival potential for phytoplankton NIS had an even larger range than annual levels, with highest ranked pathways—Arctic International Transoceanic, GLSLR International Transoceanic, Atlantic International Coastal U.S., Atlantic International Transoceanic, Pacific International Coastal U.S., Pacific International Exempt, and Pacific International Transoceanic—transporting more than 1.08 x 1013 NIS per ship, and the lowest ranked pathway—Lakers—again transporting negligible NIS (Fig. 2D).
Baseline survival potential
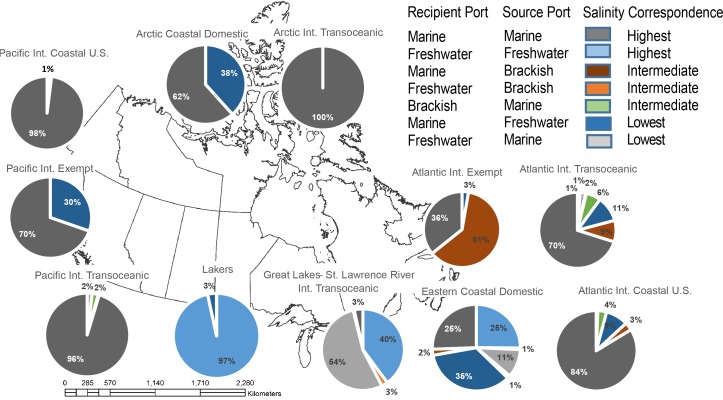
A total of 20,140 comparisons were conducted to evaluate environmental similarity between ballast water source-recipient port-pairs. Results indicate that 88% of vessel transits occurred between locations within the same salinity category. In total, seven pathways were ranked with highest salinity similarity, having more than 60% of vessel transits moving between locations of the same salinity category (Fig. 3). Only one pathway (Atlantic International Exempt) was ranked as intermediate with 61% of transits moving between locations where salinity was offset by one category. Finally, one pathway (GLSLR International Transoceanic) was ranked as lowest similarity with 54% of transits having dissimilar source-recipient salinities.
Fig 3. Estimated similarity of salinity between source and recipient locations of discharged ballast water under current requirements for ballast water exchange, summarized by pathway.
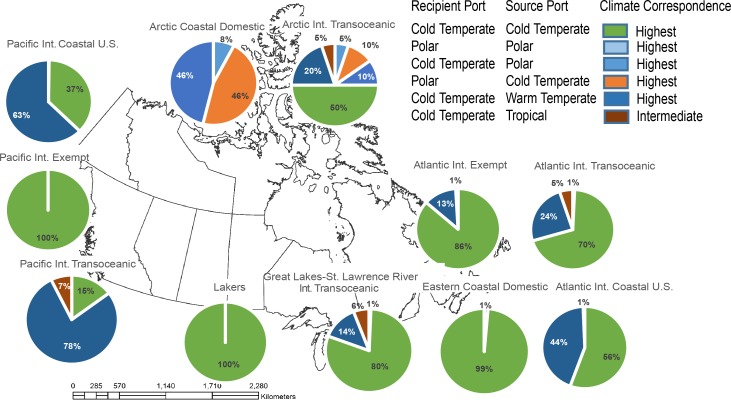
Climate similarity results indicate that 87% of port-pair comparisons had highest similarity for climate. In total, all ten pathways were classified with highest climate similarity, having more than 60% of vessel transits moving between locations of the same climate category (Fig. 4). Our analysis showed that less than 10% of vessel transits occurred between locations having dissimilar climate.
Fig 4. Estimated similarity of climate between source and recipient locations of discharged ballast water, summarized by pathway.
Note that no combinations of Tropical-Polar transits were observed, so the lowest realized ranking is “Intermediate”. The same climate scenario was used for both current and future management scenarios.
The overall survival potential, based on the combination of salinity and climate similarity, was driven primarily by salinity rankings; the outcome for overall survival potential is therefore visually very similar to the results displayed in Fig. 3. Six pathways were ranked with highest survival potential, with 65% of transits moving between environmentally similar locations. Only one pathway (Atlantic International Exempt) was ranked with intermediate survival potential based on 60% of transits. Finally, two pathways (Arctic Coastal Domestic and GLSLR International Transoceanic) were ranked with lowest survival potential.
Final relative invasion risk
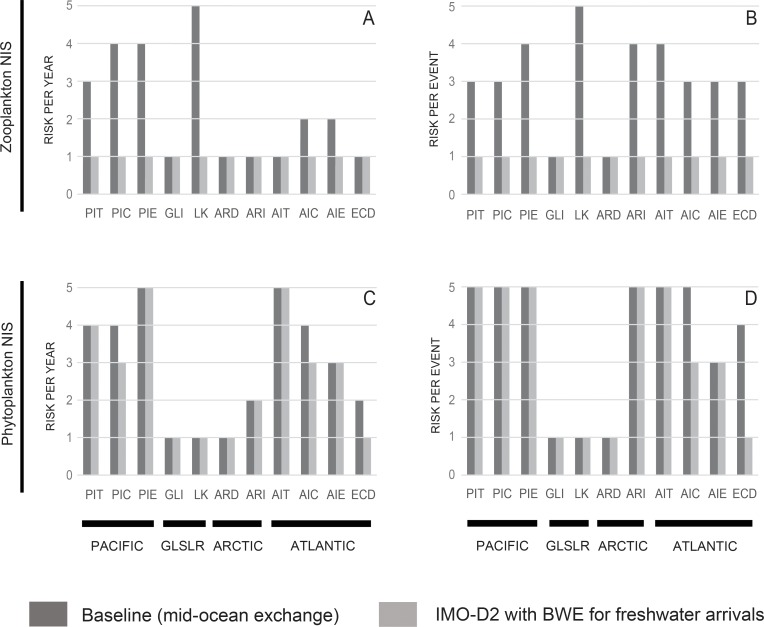
Our relative invasion risk assessment discriminated pathways into the following five groups: pathways presenting highest invasion risk for zooplankton NIS but lowest invasion risk for phytoplankton NIS on both temporal scales (Lakers), pathways posing higher invasion risk for zooplankton NIS at both temporal scales but highest for phytoplankton NIS (Pacific International Exempt) on an annual basis, pathways posing highest invasion risk for phytoplankton NIS at per event-scale (Pacific International Transoceanic, Pacific International Coastal U.S., Pacific International Exempt, Arctic International Transoceanic, Atlantic International Transoceanic, Atlantic International Coastal U.S.), pathways posing intermediate risk for zooplankton NIS at per event scale (Pacific International Transoceanic, Pacific International Coastal U.S., Atlantic International Coastal U.S., Atlantic International Exempt and Eastern Coastal Domestic), and finally, pathways posing lowest invasion risk for both taxonomic groups at both temporal scales (Great Lakes International Transoceanic and Arctic Coastal Domestic). The results of the final relative invasion risk assessment are summarized in Fig. 5 (A-D).
Fig 5. Final relative invasion risk estimated for nonindigenous zooplankton (upper panels) and phytoplankton (lower panels) species estimated under current (dark gray) and future (light gray) management scenarios.
Invasion risk was calculated on annual (left panels) and per event (right panels) timescales. PIT = Pacific International Transoceanic, PIC = Pacific International Coastal, PIE = Pacific International Exempt; GLSR = Great Lakes Saint Lawrence International Transoceanic, LK = Lakers, ARD = Arctic Coastal Domestic, ARI = Arctic International Transoceanic, AIT = Atlantic International Transoceanic, AIC = Atlantic International Coastal, AIE = Atlantic International Exempt, ECD = Eastern Coastal Domestic. Risk scale bar denotes risk from lowest = 1 to highest = 5.
Future risk with the IMO D-2 standards
Application of the IMO D-2 standards is expected to decrease abundances of zooplankton NIS for all pathways up to 99% in magnitude (Fig. 2A, B). In comparison to the current scenario, all pathways were ranked as lowest future arrival potential for zooplankton NIS on both annual and per event timescales. Although the ten pathways assessed already have mean densities of phytoplankton NIS below the future discharge standard, abundances of phytoplankton NIS may be expected to further decrease from 0.4% to 97% after application of IMO D-2 standards, since the variance in abundance will be reduced (Fig. 2C, 2D). In comparison to the current scenario, there are few changes in the ranking of marine international pathways for annual arrival potential (i.e., Atlantic International Coastal U.S. decreases its risk to intermediate), and no rankings change for per-event scenarios.
With the transition to treatment, rather than BWE, future survival potential based on similarity of salinity and climate is expected to decrease to intermediate for domestic pathways (i.e., Eastern Coastal Domestic) and one international pathway transiting between Canada and U.S. (i.e., Atlantic International Coastal U.S.) (data not shown). Again, the survival potential is driven by salinity of source-recipient locations. In the future scenario, where BWE is no longer required for vessels arriving to marine ports, environmental similarity decreases since many of the source ports are freshwater (see supplementary information, S1 Dataset, Raw data used for the relative invasion risk assessment).
Finally, Fig. 5A-5D) summarizes the final relative invasion risk assessment for the future scenario. Our results reveal that all pathways are expected to pose lowest invasion risk for zooplankton NIS, while future invasion risk for phytoplankton NIS ranges from lowest to highest. In comparison to the current scenario, the final relative invasion risk for zooplankton NIS decreased for seven pathways, but for phytoplankton NIS, decreased for only one pathway (Eastern Coastal Domestic).
Discussion
The present relative risk framework incorporates NIS arrival and survival metrics to generate a landscape-scale portrait of the relative invasion risk across freshwater and marine ecosystems in Canada and the Great Lakes under current and future management regimes. Our findings show that current requirements for BWE reduce invasion risk to freshwater ecosystems, but are less effective in reducing risk to marine ecosystems because of greater environmental mismatch between source (oceanic) and recipient (freshwater) ecoregions. Future requirements for ballast water treatment are expected to reduce risk of zooplankton NIS introductions across ecosystem types but may be less effective in reducing risk of phytoplankton NIS. The proposed requirement for BWE plus treatment for vessels arriving to freshwater ports in the Great Lakes is expected to maintain very low survival potential of introduced organisms while systematically reducing propagule pressure; these expected benefits are supported by recent empirical tests [51].
This risk assessment indicates that international transoceanic ships show lowest invasion risk in the Great Lakes. Given that arrival potential by this pathway was similar or greater than that of all other pathways, our model confirms that the salinity mismatch induced by BWE is highly protective of this freshwater region [17]. In contrast, our model illustrates that domestic shipping (Lakers) can pose very high risk by facilitating secondary transfers of NIS, which can introduce novel genotypes in established populations, leading to greater impacts of NIS [52, 53] and patterns of dispersal that extend beyond a natural baseline.
The present model indicates the importance of short-sea coastal pathways on the Atlantic and Pacific coasts (currently exempt from BWE due to geographic proximity of ports), which appear to transport large numbers of NIS into Canadian marine waters at both timescales. Although, this pathway operates within a limited geographic extent, several source ports are considered primary introduction areas or “hotspots” (i.e., Great Bay, New Hampshire or Puget Sound, Washington) [54], with a high number of NIS that could be secondarily transported to Canadian ports. In both North America and Europe, recent studies have also pointed to coastal traffic as a critical vector introducing NIS [14, 15, 18, 55].
The model in the present study also reveals a high environmental similarity between source and recipient ports located along ocean coasts which are linked by pathways with global ports. This environmental similarity analysis is restricted to fundamental variables such as salinity and temperature in contrast with previous species-specific risk assessments. Using these two variables (salinity and climate as temperature proxy), we found that more than 80% of comparisons have highest environmental match. Over the coming century, sea surface warming at high latitudes is estimated to increase the level of environmental match between ports [56]. Consequently, an increase in shipping traffic connecting regions with high environmental match will expose regions of Canada to a larger number of NIS with high probability to establish populations, especially as a result of climate change.
This risk assessment suggests that shipping pathways managed by BWE do not necessarily exhibit lower risk than pathways without BWE; in part due to the number of transits—pathways with lower mean abundances of NIS can still pose a higher risk if there are many individual vessel transits. As noted in the introduction, the effect of BWE can be highly variable across individual ship transits [12, 15, 16, 18, 57]. In fact, in some studies BWE appears to have increased the risk of introduction potential of NIS, particularly for phytoplankton on the northwest Atlantic coast (e.g., 33, 58, 59]. Roy et al. [37] also reported that diversity of phytoplankton NIS was higher on ships that had undertaken BWE, with some species being of oceanic origin. Similarly, on the Pacific coast, Cordell et al. [12] reported that BWE had no significant influence on coastal zooplankton species, but increased the abundance of oceanic zooplankton species. Causes for BWE inefficiency may include structural limitations inside ballast tanks that restrict exchange of water, or offshore transport of coastal taxa [27]. Additionally, enforcement efforts (e.g. ballast water logs inspection, salinity measurements, salt flushing) are considerably lower for Canada’s marine coasts compared with the Great Lakes region [27].
Our future risk projections indicate that ballast water treatment at the level of the D-2 standards will dramatically reduce arrival potential for zooplankton NIS for all pathways in all regions but will have a lesser effect on arrival potential for phytoplankton NIS (reducing expected abundances of NIS for only five pathways). Hence, these results could be an indication that phytoplankton standards may not be as robust as zooplankton standards. According to the best available science at the time of drafting the D-2 standards, the limit for phytoplankton was set three orders of magnitude lower than the mean density observed in unmanaged ballast water while for zooplankton the limit was set two orders of magnitude lower [60]. The apparent dichotomy in our efficacy projections may be because our risk assessment evaluates the probability of NIS being introduced, whereas the D-2 standard does not discriminate between nonindigenous and more cosmopolitan taxa. In addition, recent advances in methods for the sampling and analysis of ballast water samples may have resulted in a shift of median plankton densities observed, compared to studies completed more than a decade ago. Evaluating the appropriateness of any particular ballast water discharge standard is a management exercise that involves risk tolerance, and is beyond the scope of this scientific risk assessment. The take home message of our analysis therefore should be that transition from BWE to treatment will result in improved protection against aquatic NIS across ecosystems and shipping pathways, with greater risk reduction expected for zooplankton as compared to phytoplankton.
The results presented in this research are based on recent shipping patterns and environmental metrics; any changes to one or both factors will lead to changes in relative invasion risk. Efforts to increase trade and shipping traffic would result in higher propagule arrival and could establish new connections with global source ports sharing high environmental similarity with recipient ports. Further, climate change scenarios predict both thermal and physical changes that could impact analyses of environmental similarity between source-recipient port-pairs. A reanalysis of environmental similarity between donor and recipient port-pairs, using environmental variables projected under climate change scenarios, may be useful to further refine predictions of future invasion risk [57].
Although biological invasions are highly stochastic, quantifying the arrival and survival of nonindigenous propagules across heterogeneous ecosystems represents a major step towards understanding the likelihood of invasion in relation to shipping networks, the relative efficacy of invasion management regimes, and seizing opportunities to reduce the ecological and economic implications of species invasion. Large-scale models assessing the invasion process can provide a general framework in order to better predict potential success of invading species [61] by including stochastic events [62]. [29]
Supporting Information
The file is divided in three sections: 1) ballast water discharge volumes for all the ships arriving to the Canadian regions using a 12-month time frame of recent data (2006, 2007 and 2008) representing an annual estimate of the arrival potential NIS. 2) Biological data obtained from coordinated surveys conducted by the Canadian Aquatic Invasive Species Network (CAISN) and Fisheries and Oceans Canada (DFO) over three years (2007 to 2009). 3) List of source-recipient port-pair and results of the overall survival potential, based on the combination of salinity and climate similarity.
(XLSX)
Acknowledgments
We thank M. Koops, H. MacIsaac, N. Mandrak, A. Quigg and anonymous reviewers for insightful discussions and comments that improved both the study and the manuscript.
Data Availability
All data is available as a dataset accessible through Figshare.com. The link to our supplementary file is: figshare.com/s/48b4252889ff11e499a406ec4bbcf141. The DOI is: 10.6084/m9.figshare.1276404).
Funding Statement
Funding for this study was provided by Transport Canada and Fisheries and Oceans Canada, both directly and through the Natural Sciences and Engineering Research Council of Canada Visiting Fellowships in Canadian Government Laboratories program, and a Natural Sciences and Engineering Research Council of Canada Discovery Grant (SAB), #371454-2010 RGPIN. (https://www.tc.gc.ca, http://www.dfo-mpo.gc.ca, http://www.nserc-crsng.gc.ca). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Sala OE, Chapin FS III, Armesto JJ, Berlow R, Bloomfield J, Dirzo R, et al. Global biodiversity scenarios for the year 2100. Science. 2000;287: 1770–1774. [DOI] [PubMed] [Google Scholar]
- 2. Johnson TB, Bunnell DB, Knight CT. A potential new energy pathway in central Lake Erie: the Round Goby connection. J Great Lakes Res. 2005;31: 238–251. [Google Scholar]
- 3. Dextrase A, Mandrak NE. Impacts of invasive alien species on freshwater fauna at risk in Canada. Biol Invasions. 2006;8: 13–24. [Google Scholar]
- 4. Hulme PE. Trade, transport and trouble: managing invasive species pathways in an era of globalization. J Appl Ecol. 2006;46: 10–18. [Google Scholar]
- 5. Leung B, Lodge DM, Finnoff D, Shogren JF, Lewis MA, Lamberti G. An ounce of prevention or a pound of cure: bioeconomic risk analysis of invasive species. Proceedings: Biological Sciences. 2002;269: 2407–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Katsanevakis S, Zenetos A, Belchior C, Cardoso AC. Invading European Seas: assessing pathways of introduction of marine aliens. Ocean Coast Manage. 2013;76: 64–74. [Google Scholar]
- 7. Steichen JL, Windham R, Brinkmeyer R, Quigg A. Ecosystem under pressure: Ballast water discharge into Galveston Bay, Texas (USA) from 2005 to 2010. Mar Poll Bull. 2012;64: 779–789. [DOI] [PubMed] [Google Scholar]
- 8. Mead A, Carlton JT, Griffiths CL, Rius M. Revealing the scale of marine bioinvasions in developing regions: a South African re-assessment. Biol Invasions. 2011;13: 1991–2008. [Google Scholar]
- 9. Ruiz GM, Fofonoff PW, Carlton JT, Wonham MJ, Hines AH. Invasion of coastal marine communities in North America: Apparent patterns, processes, and biases. Annu Rev Ecol Syst. 2000;31: 481–531. [Google Scholar]
- 10. Albert RJ, Lishman JM, Saxena JR. Ballast water regulations and the move toward concentration-based numeric discharge limits Ecol Appl. 2013;23: 289–300. [DOI] [PubMed] [Google Scholar]
- 11. [NRC] National Research Council. Assessing the relationship between propagule pressure and invasion risk in ballast water The National Academies Press; Washington, D.C.; 2011. [Google Scholar]
- 12. Cordell JR, Lawrence DJ, Ferm NC, Tear LM, Smith SS, Herwig RP. Factors influencing densities of non-indigenous species in the ballast water of ships arriving at ports in Puget Sound, Washington, United States. Aquat Conserv: Mar Freshwat Ecosyst. 2009;19: 322–343. [Google Scholar]
- 13. Dickman M, Zhang F. Mid-ocean exchange of container vessel ballast water. 2: Effects of vessel type in the transport of diatoms and dinoflagellates from Manzanillo, Mexico, to Hong Kong, China. Mar Ecol Prog Ser. 1999;176: 253–262. [Google Scholar]
- 14. Lavoie DM, Smith LD, Ruiz GM. The potential for intracoastal transfer of nonindigenous species in the ballast water of ships. Estuar. Coast Shelf Sci. 1999;48: 551–564. [Google Scholar]
- 15. McCollin T, Shanks AM, Dunn J. Changes in zooplankton abundance and diversity after ballast water exchange in regional seas. Mar Pollut Bull. 2008;56: 834–844. 10.1016/j.marpolbul.2008.02.004 [DOI] [PubMed] [Google Scholar]
- 16. Simard N, Plourde S, Gilbert M, Gollasch S. Net efficacy of open ocean ballast water exchange on plankton communities. J Plankton Res. 2011;33: 1378–1395. [Google Scholar]
- 17. Bailey SA, Deneau MG, Jean L, Wiley CJ, Leung B, MacIsaac HJ. Evaluating efficacy of an environmental policy to prevent biological invasions. Environ Sci Technol. 2011;45: 2554–2561. 10.1021/es102655j [DOI] [PubMed] [Google Scholar]
- 18. Lawrence DJ, Cordell JR. Relative contributions of domestic and foreign sourced ballast water to propagule pressure in Puget Sound, Washington, USA. Biol Conserv. 2010;143: 700–709. [Google Scholar]
- 19. Levings CD, Foreman MGG. Ecological and oceanographic criteria for alternate ballast water exchange zones in the Pacific Region. DFO Can Sci Advis Sec Res Doc. 2004;118. [Google Scholar]
- 20.[IMO] International Maritime Organization International convention for the control and management of ships’ ballast water and sediments; 2004. Available: http://ww.imo.org/About/Conventions/ListOfConventions/Pages/Default.aspx. Accessed 26 September 2014.
- 21. Gregg M, Rigby G, Hallegraeff G. Review of two decades of progress in the development of management options for reducing or eradicating phytoplankton, zooplankton and bacteria in ship’s ballast water. Aquat Invasions. 2009;4: 521–565. [Google Scholar]
- 22. Tsolaki E, Diamadopoulos E. Technologies for ballast water treatment: a review. J Chem Technol Biot. 2010;85: 19–32. [Google Scholar]
- 23. Drake JM, Lodge DM. Allee effects, propagule pressure and the probability of establishment: risk analysis for biological invasions. Biol Invasions. 2006;8: 365–375. [Google Scholar]
- 24. Simberloff D. The role of propagule pressure in biological invasions. Annu Rev Ecol Syst. 2009;40: 81–102. [Google Scholar]
- 25. Gertzen EL, Leung B, Yan N D. Propagule pressure, Allee effects and the probability of establishment of an invasive species (Bythotrephes longimanus) Ecosphere. 2011;2: art30. 10.1890/ES10-00170.1 [DOI] [Google Scholar]
- 26. Wonham MJ, Byers JE, Grosholz ED, Leung B. Modeling the relationship between propagule pressure and invasion risk to inform policy and management. Ecol Appl. 2013;23: 1691–1706. [DOI] [PubMed] [Google Scholar]
- 27. Casas-Monroy O, Linley RD, Adams JK, Chan FT, Drake DAR, Bailey SA. National Risk Assessment for Introduction of Aquatic Nonindigenous Species to Canada by Ballast Water. DFO Can Sci Advis Sec Res Doc. 2014;128 Available: http://www.dfo-mpo.gc.ca/Library/352598.pdf. Accessed 21 November 2014. [Google Scholar]
- 28. Brockerhoff E, Kimberley M, Liebhold AM, Haack RA, Cavey JF. Predicting how altering propagule pressure changes establishment rates of biological invaders across species pools. Ecology. 2014;95: 594–601. [DOI] [PubMed] [Google Scholar]
- 29. Bradie J, Leung B. Pathway-level models to predict non-indigenous species establishment using propagule pressure, environmental tolerance and trait data. J Appl Ecol. 2014. 10.1111/1365-2664.12376 25641980 [DOI] [Google Scholar]
- 30.Duncan, RP, Blackburn TM, Rossinelli S, Bacher S. Quantifying invasion risk: the relationship between establishment probability and founding population size. Methods Ecol Evol. 2014. 10.1111/2041-210X.12288. [DOI]
- 31. Bailey SA, Vélez-Espino LA, Johannsson OE, Koops MA, Wiley CJ. Estimating establishment probabilities of Cladocera introduced at low density: An evaluation of the proposed ballast water discharge standards. Can J Fish Aquat Sci. 2009;66: 261–276. [Google Scholar]
- 32. Adebayo AA, Zhan A, Bailey SA, MacIsaac HJ. Domestic ships as a potential pathway of nondigenous species introduction from the Saint Lawrence River to the Great Lakes. Biol Invasions. 2014;16: 793–801. 10.1007/s11307-014-0752-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Casas-Monroy O. Introduction des dinoflagellés non-indigènes dans les écosystèmes aquatiques canadiens via les réservoirs de ballast de navires. Ph.D. thesis. Univ. of Quebec at Rimouski. 2012. Available: http://www.semaphore.uqar.ca/870/. Accessed 24 November 2014.
- 34. DiBacco C, Humphrey DB, Nasmith LE, Levings CD. Ballast water transport of non-indigenous zooplankton to Canadian ports. ICES J Mar Sci. 2011;69: 483–491. [Google Scholar]
- 35.Humphrey DB. Characterizing ballast water as a vector for nonindigenous zooplankton transport. M.Sc. thesis, Faculty of Graduate Studies (Oceanography), The University of British Columbia, Vancouver, B.C. 2008. Available: https://circle.ubc.ca/handle/2429/2391 Accessed 25 September 2014.
- 36. Klein G, Kaczmarska I, Ehrman JM. The Diatom chatoceros in ships' ballast waters-survivorship of stowaways. Acta Botanica Croatia. 2009;68: 325–338. [Google Scholar]
- 37. Roy S, Parenteau M, Casas-Monroy O, Rochon A. Coastal ship traffic: a significant introduction vector for potentially harmful dinoflagellates in eastern Canada. Can J Fish Aquat Sci. 2012;69: 627–644. [Google Scholar]
- 38. Lewis M. Variability, patchiness, and jump dispersal in the spread of an invading population In: Spatial Ecology: the role of space in population dynamics and interspecific interactions. Princeton: University Press; 1997. [Google Scholar]
- 39. Anger K. Effects of temperature and salinity on the larval development of the Chinese mitten crab Eriocheir sinensis (Decapoda: Grapsidae). Mar Ecol Prog Ser. 1991;72: 103–110. [Google Scholar]
- 40. Browne RA, Wanigasekera G. Combined effects of salinity and temperature on survival and reproduction of five species of Artemia. J Exp Mar Biol Ecol. 2000;244: 29–44. [Google Scholar]
- 41. Verween A, Vincs M, Degraer S. The effect of temperature and salinity on the survival of Mytilopsis leucophaeata larvae (Mollusca, Bivalvia): The search for environmental limits. J Exp Mar Biol Ecol. 2007;348: 111–120. [Google Scholar]
- 42. Barry SC, Hayes KR, Hewitt CL, Behrens HL, Dragsund E, Bakke SM. Ballast water risk assessment: principles, processes, and methods. ICES J Mar Sci. 2008;65: 121–131. [Google Scholar]
- 43. Keller RP, Drake JM, Drew MB, Lodge DM. Linking environmental conditions and ship movements to estimate invasive species transport across the global shipping network. Divers Distrib. 2011;17: 93–102. [Google Scholar]
- 44. Chan FT, Bailey SA, Wiley CJ, MacIsaac HJ. Relative risk assessment for ballast-mediated invasions at Canadian Arctic port. Biol Invasions. 2013;15: 295–308. [Google Scholar]
- 45.Antonov JI, Locarnini RA, Boyer TP, Mishonov AV, Garcia HE. In: World Ocean Atlas 2005. Volume 2: Salinity. S. Levitus, editor. NOAA Atlas NESDIS 62, U.S.; 2006. pp. 182.
- 46.Transport Canada. A guide to Canada’s ballast water control and management regulations TP 13617E. 2007. Available: https://www.tc.gc.ca/eng/marinesafety/tp-tp13617-menu-2138.htm/. Accessed 16 July 2014.
- 47. Gollasch S, Leppäkoski E. Risk assessment and management scenarios for ballast water mediated species introductions into Baltic Sea. Aquat Invasions. 2007;2: 313–340. [Google Scholar]
- 48. Spalding MD, Fox HE, Allen GR, Davidson N, Ferdeña ZA, Finlayson M, et al. Marine Ecoregions of the World: a Bioregionalization of Coastal and Shelf Area. BioScience. 2007;57: 573–583. [Google Scholar]
- 49.Transport Canada Discussion paper: Canadian implementation of the ballast water convention. 2012. Available: http://www.tc.gc.ca/media/documents/marinesafety/Canada_Ballast_Water_Implementation_Discussion_Paper_Revised.pdf. Accessed 16 July 2013.
- 50. [US EPA] United States Environmental Protection Agency. Vessel general permit for discharges incidental to the normal operation of vessels (VGP) US EPA; 2013. [Google Scholar]
- 51. Briski E, Allinger LE, Balcer M, Cangelosi A, Fanberg L, Markee TP, et al. A multi-dimensional approach to invasive species prevention. Environ Sci Technol. 2013;47: 1216–1221. [DOI] [PubMed] [Google Scholar]
- 52. Lockwood JL, Casey P, Blackburn T. The role of propagule pressure in explaining species invasions. Trends Ecol Evol. 2005;20: 223–228. [DOI] [PubMed] [Google Scholar]
- 53. Briski E, Wiley CJ, Bailey SA. Role of domestic shipping in the introduction or secondary spread of nonindigenous species: biological invasions within the Laurentian Great Lakes J Appl Ecol. 2012;49: 1124–1130. [Google Scholar]
- 54. Ruiz GM, Fofonoff PW, Ashton G, Minton MS, Miller AW. Geographic variation in marine invasions among large estuaries: effects of ships and time. Ecol Appl. 2013;23: 311–320 [DOI] [PubMed] [Google Scholar]
- 55. Simkanin C, Davidson I, Falkner M, Sytsma M, Ruiz G. Intra-coastal ballast water flux and the potential for secondary spread of non-native species on the US West Coast. Mar Pollut Bull. 2009; 58: 366–374. 10.1016/j.marpolbul.2008.10.013 [DOI] [PubMed] [Google Scholar]
- 56. Ware C, Berge J, Sundet JH, Kirkpatrick JB, Coutts ADM, Jelmert A, et al. Climate change, non-indigenous species and shipping: assessing the risk of species introduction to a high-Arctic archipelago. Diversity and Distirb. 2014;20: 10–19. [Google Scholar]
- 57. Taylor MD, MacKenzie LM, Dodgshun TJ, Hopkins GA, de Zwart EJ, Hunt CD. Trans-Pacific shipboard trials on planktonic communities as indicators of open ocean ballast water exchange. Mar Ecol Prog Ser. 2007;350: 41–54. [Google Scholar]
- 58. Carver CE, Mallet AL. An assessment of the risk of ballast water-mediated introduction of non-indigenous phytoplankton and zooplankton into Atlantic Canadian waters Mallet Research Services, Dartmouth, Nova Scotia: 2002. Available: http://www.ceaa.gc.ca/B4777C6B-docs/WP-1785-057.pdf. Accessed 25 February 2011. [Google Scholar]
- 59.Chan FT, Bradie J, Briski E, Bailey SA, Simard N, MacIsaac HJ. Assessing introduction risk using species’ rank abundance distribution. Proc R Soc B. 2005. 10.1098/rspb.2014.1517. [DOI] [PMC free article] [PubMed]
- 60.[ICES] International Council for the Exploration of the Sea. Comments on draft Regulation E-2 Concentrations of organisms delivered in ships’ ballast water in the absence of any treatment: Establishing a baseline for consideration of treatment efficacy. Submitted to the Marine Environment Protection Committee, International Maritime Organization. London U.K.; 2003.
- 61. Jerde CL, Lewis MA. Waiting for invasions: a framework for the arrival of nonindigenous species. Am. Nat. 2007;170: 1–9. [DOI] [PubMed] [Google Scholar]
- 62. Carlton JT. Patterns, process and prediction in marine invasion ecology. Biol Conserv. 1996;78: 97–106. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The file is divided in three sections: 1) ballast water discharge volumes for all the ships arriving to the Canadian regions using a 12-month time frame of recent data (2006, 2007 and 2008) representing an annual estimate of the arrival potential NIS. 2) Biological data obtained from coordinated surveys conducted by the Canadian Aquatic Invasive Species Network (CAISN) and Fisheries and Oceans Canada (DFO) over three years (2007 to 2009). 3) List of source-recipient port-pair and results of the overall survival potential, based on the combination of salinity and climate similarity.
(XLSX)
Data Availability Statement
All data is available as a dataset accessible through Figshare.com. The link to our supplementary file is: figshare.com/s/48b4252889ff11e499a406ec4bbcf141. The DOI is: 10.6084/m9.figshare.1276404).