Abstract
Importance
Understanding how individuals value health states is central to patient-centered care and to health policy decision making. Generic preference-based measures of health may not effectively capture the impact of ocular diseases. Recently, 6 items from the National Eye Institute Visual Function Questionnaire-25 were used to develop the Visual Function Questionnaire-Utility Index health state classification, which defines visual function health states.
Objective
To describe elicitation of preferences for health states generated from the Visual Function Questionnaire-Utility Index health state classification and development of an algorithm to estimate health preference scores for any health state.
Design
Non-intervention, cross-sectional study.
Setting
General community in four countries (Australia, Canada, United Kingdom, and United States)
Participants
607 adult participants recruited from local newspaper advertisements. In the United Kingdom, an existing database of participants from previous studies was used for recruitment.
Interventions
Eight out of 15,625 possible health states from the Visual Function Questionnaire-Utility Index were valued using time trade-off technique.
Main Outcome Measures
A theta severity score was calculated for Visual Function Questionnaire-Utility Index–defined health states using item response theory analysis. Regression models were then used to develop an algorithm to assign health state preference values for all potential health states defined by the Visual Function Questionnaire-Utility Index.
Results
Health state preference values for the 8 states ranged from 0.343 (standard deviation, 0.395) to 0.956 (0.124). As expected, preference values declined with worsening visual function. Results indicate that the Visual Function Questionnaire-Utility Index describes states that participants view as spanning most of continuum from full health to dead.
Conclusions and Relevance
Visual Function Questionnaire-Utility Index health state classification produces health preference scores that can be estimated in vision-related studies that include National Eye Institute Visual Function Questionnaire-25. These preference scores may be of value for estimating utilities in economic and health policy analyses.
INTRODUCTION
Use of preference-based health-related quality-of-life (HRQL) measures has increased due to the increased utilization of economic evaluation in creating health policy.1,2 The US Public Health Service Panel on Cost-effectiveness in Health and Medicine issued recommendations supporting the use of preference-based measures to calculate quality-adjusted life years (QALYs) for economic evaluations.3 QALYs are used to quantify HRQL outcomes for economic evaluations.4–7 QALYs represent the product of HRQL and survival, allowing effectiveness to be quantified in terms of degree that the intervention changes both. The United Kingdom’s National Institute for Health and Clinical Guidance issued a guidance expressing preference for economic evaluations using generic preference-based utility measures, specifically, the EuroQol (EQ-5D).8 This guidance stated that in the absence of EQ-5D data, empirical mapping to the EQ-5D from other HRQL instruments or the valuation of health states based on other instruments may be used as an alternative.
Because there are many conditions for which utilities are not available, have inconsistent results across studies, or are inadequately represented by available generic preference instruments, interest in expressing preference for different health technologies using disease-targeted measures is growing.6,7,9,10 Disease-targeted measures are viewed as more sensitive to treatment changes and more relevant to the impact on HRQL, especially for chronic medical conditions.6,7,10–16 The EQ-5D, for example, has been shown to have limited sensitivity to visual function in patients with age-related macular degeneration (AMD).17 Recently, innovative methods have been used to estimate utilities from existing instruments such as the Short Form Health Survey (SF-36), the King’s Health Questionnaire, and the Cambridge Pulmonary Hypertension Outcome Review.6,10,18 Using a disease-targeted HRQL measure for preference measurement has the potential advantage of using a more sensitive descriptive system to classify people into health states.
The most widely used ocular disease-targeted HRQL measure is the National Eye Institute Visual Function Questionnaire-25 (NEI VFQ-25).19,20 Six items (ie, 6, 11, 14, 18, 20, and 25) representing 6 of the NEI VFQ-25 subscales were recently converted into a health state classification system called the Visual Function Questionnaire-Utility Index (VFQ-UI).6,21,22 This paper provides an overview of the development of the VFQ-UI a health state classification system, the preference elicitation for selected vision-related health states from the general public, and development of VFQ-UI scoring algorithm.
The objective of this study was to obtain health preferences for VFQ-UI–related health states in over 600 members of the general population in Australia, Canada, United Kingdom (UKI), and United States (US), and to develop an algorithm for estimating utility scores. The utility scores derived using the VFQ-UI algorithm can then be used in estimating QALYs for economic evaluations comparing treatment interventions for ocular diseases and for health technology assessments and policy decisions. Utility scores derived from the disease-targeted NEI VFQ-25 may prove to provide more sensitive preference-based estimates of utilities for patients with varying levels of vision loss compared with generic preference measures such as the EQ-5D or SF-6D.
METHODS
The objective of this study was to generate a preference-based scoring algorithm for estimating utility scores based on the VFQ-UI health state classification. The overall approach for developing the VFQ-UI health state classification system and VFQ-UI scores comprised 3 stages: (1) developing a health state classification system; (2) conducting a valuation study of the health states; and (3) developing the scoring algorithm using the multivariate regression analyses (Figure 1).23
Figure 1.
Developing the VFQ-UI Health State Classification System and Scoring Methods
Abbreviation: VFQ-UI, Visual Function Questionnaire-Utility Index.
Development of the VFQ-UI Health State Classification
A health state classification system is a multidimensional framework that can be used to define health states. Such classifications define a set of health states by selecting one level from each dimension in the system. A secondary analysis was performed using NEI VFQ-25 data collected in several studies of patients with either central (n = 968)24–28 or peripheral vision loss (n = 2451)29,30 (see eAppendix A, Table 1 in the Supplement) to identify the best subset of NEI VFQ-25 items that capture the overall content of the instrument.23 The general health item was removed because it is not specific to vision function. The driving subscale was removed because of high missing data rates and to be more generalizable to countries other than the US, while the ocular pain subscale was excluded due to misfit within both samples.23 Next, we used Rasch analysis31,32 to reduce the NEI VFQ-25 to a simpler descriptive health state classification by identifying the severity level captured by the items.
Rasch analysis was used to transfer categorical item responses to points on a latent scale using a logit model, where the underlying scale is treated as continuous.22 Central and peripheral vision loss datasets that included NEI VFQ-25 data were examined independently, to identify items fitting each vision loss type and any differences in item performance. Rasch models were used to evaluate the unidimensionality of the VFQ-25 domains, item level ordering and fit, and differential item functioning (see Appendix A in the Supplement). After that review, central and peripheral vision loss datasets were pooled to allow for selection of items relevant across ocular indications. Similar analyses were performed on these pooled data. These three sets of analyses were reviewed by clinical and psychometric experts, and decisions were made regarding the final composition of the health state classification.23
Based on these analyses and clinical review, one item from each of six NEI VFQ-25 subscales (near vision activities, distance vision activities, vision-specific social functioning, role difficulties, dependency, and mental health) was selected for the reduced health state classification (ie, VFQ-UI health state classification).
Design and Conduct of the Valuation Study
Development of Health States and Pilot Testing
Based on the VFQ-UI health state classification, eight vision-related health states were developed for the preference valuation study (out of a total possible 15,625).23 These health states were selected to describe best vision function (111111), worst vision function states (555555), and intermediate health states reflecting vision-related functioning and well-being. We pilot tested the health states in interviews with 22 patients with AMD, glaucoma, or macular edema to explore the content validity of the health states. Patient interviews confirmed the relevance and face validity of the health states and that overall they were understandable and accurate (see eAppendix B in the Supplement).
General Population Valuation Sample
The health state valuation survey was conducted with participants from the general public from Australia, Canada, the UK and US. Trained personnel conducted one-on-one interviews. By interviewing the general public as opposed to those with specified eye conditions, we were able to elicit findings more generalizable to the preferences of overall society in the participating countries.
Participants were recruited from local newspaper advertisements. In the UK, an existing database of participants from previous studies was also used for recruitment. Eligible participants must have been aged ≥18 years; current resident of the interview country; able to understand and complete the survey (as judged by the interviewer); and willing and able to give written informed consent. The study protocol and consent form were submitted to a central institutional review board, approved, and met Health Insurance Portability and Accountability Act requirements.
Interview for Preference Elicitation
Participants took part in a health state ranking exercise, a time trade-off (TTO) interview, and were asked to complete the EQ-5D. In the ranking task, participants were asked to take the eight health states along with the anchor states (full health and dead) and put them in order from best to worst. This task allowed participants to familiarize themselves with the health states and to understand their differences.
For the TTO interview, we used a TTO board33 containing timelines for the health state comparisons. The TTO asks participants to imagine they will be in a given vision-related health state for 10 years, and then asks them to compare this vision-related health state to a number of shorter time periods in full health (x) after which time the future is uncertain. The valuation of the targeted state is given as x/10 when the participant reaches a situation where s/he is indifferent or unable to select between the health state and full health alternatives. The TTOs used a 10-year time horizon, given the long-term chronic nature of loss of vision functioning for many ocular diseases (see eAppendix A, in the Supplement).
Target vision-related health states were rated against full health and pits state (worst visual functioning health state defined by the classification) and then pits state against full health and dead.
The pits or worst health state as defined by the VFQ-UI was used as the lower anchor because using dead as the lower anchor during the interviews could be insensitive to the effect of loss of visual acuity. Results need to be presented on a full health/dead scale, so the rating for the pits state on the full health/dead scale was also separately collected during interviews and then used to calibrate previous ratings to the full health/dead scale. The equation for this adjustment is TTOADJ = [TTO + (1 − P)] × P, where TTOADJ is the TTO adjusted score and P is the pits state.21
Participants also completed the EQ-5D,34 a generic preference-based health status measure.
Regression Analyses for Modeling Health State Preferences
Our aim was to develop a scoring algorithm that could assign values for all states defined by the VFQ-UI health state classification. We used an approach summarized by Young et al. for developing preference-based, disease-specific measures when the items in the instrument are not independent (ie, correlated).35 Additional details on the analyses are included in eAppendix A in the supplement.
As described below, we applied item response theory (IRT) analyses to obtain an indicator of severity for each health state defined by the VFQ-UI classification system and then mapped the severity indicator onto the utilities of targeted study health states.22,36 We combined information from two different sets of data: (1) VFQ-UI data used in developing the health state classification from patients with central and peripheral vision loss; and (2) health preference valuation interview study data. Patient data used to develop the health state classification are described in detail elsewhere.23
First, using data set (1) above, we estimated severity (theta) scores from the patient-level responses to the six NEI VFQ-25 items that comprise VFQ-UI using a graded response model.37 Theta represents the location of the health states in terms of vision-related function, where higher scores indicate better functioning.38,39 Regression models were then run to map the relationship between TTO preference scores and selected demographic variables and VFQ-UI thetas. Different regression models were explored to determine whether linear or nonlinear regressions represented a better fit in estimating TTO scores. The regression models included age, gender, and education. All modeling was done using SAS 9.1 (SAS Institute, Inc., Cary, North Carolina). P<0.05 was considered statistically significant.
To evaluate the validity of the VFQ-UI scores, we compared baseline mean VFQ-UI scores from a clinical trial of patients with uveitis by best-corrected visual acuity (BCVA) groups using analysis of covariance, adjusting for gender. BCVA groups were defined as ≥20/40, <20/40 to >20/200, and ≤20/200. The relationship between baseline VFQ-UI and NEI VFQ-25 composite and subscale scores were evaluated using Spearman rank-order correlations.
RESULTS
Preference Survey Respondents
In total, 607 participants in Australia, Canada, UK, and US took part in the valuation survey. The average age within each country ranged from 36 to 52 years, with the Australian sample being the youngest (Table 1). All countries had a larger proportion of women participants. A small proportion had AMD (n=11; 2%).
Table 1.
Time Trade-Off Interview Participant Demographic Characteristics by Country
| Characteristic | Australia (N = 150) | Canada (N = 150) | United Kingdom (N = 152) | United States (N = 155) |
|---|---|---|---|---|
| Age, y | 35.6 (14.5) | 41.2 (15.8) | 41.8 (13.7) | 51.6 (16.3) |
|
| ||||
| Gender, n (%) | ||||
| Men | 57 (38.0) | 65 (43.3) | 55 (36.2) | 69 (44.5%) |
| Women | 93 (62.0) | 85 (56.7) | 97 (63.8) | 86 (55.5%) |
|
| ||||
| Employment status, n (%) | ||||
| Employed, full-time | 55 (36.7) | 74 (49.3) | 69 (45.4) | 59 (38.1) |
| Employed, part-time | 39 (26.0) | 23 (15.3) | 32 (21.1) | 26 (16.8) |
| Homemaker | 2 (1.3) | 6 (4.0) | 2 (1.3) | 3 (1.9) |
| Student | 24 (16.0) | 14 (9.3) | 18 (11.8) | 7 (4.5) |
| Unemployed | 9 (6.0) | 10 (6.7) | 10 (6.6) | 12 (7.7) |
| Retired | 10 (6.7) | 18 (12.0) | 11 (7.2) | 38 (24.5) |
| Disabled or unable to work | 3 (2.0) | 4 (2.7) | 4 (2.6) | 9 (5.8) |
| Other | 2 (1.3) | 0 (0) | 6 (3.9) | 0 (0) |
| Missing | 6 (4.0) | 1 (0.7) | 0 (0) | 1 (0.6) |
|
| ||||
| Highest education, n (%) | ||||
| No formal qualification | 2 (1.3) | N/A | 4 (2.6) | N/A |
| Some high school | N/A | 4 (2.7) | N/A | 4 (2.6) |
| High school | 27 (18.0) | 30 (20.0) | N/A | 26 (16.8) |
| GCSE/O levels or equivalent | N/A | N/A | 21 (13.8) | N/A |
| A levels or equivalent | N/A | N/A | 21 (13.8) | N/A |
| Vocation/work-based qualification | 38 (25.3) | N/A | 14 (9.2) | N/A |
| University degree/College | 74 (49.3) | 84 (56.0) | 75 (49.3) | 71 (45.8) |
| Highest education, n (%) | ||||
| Graduate school | N/A | 24 (16.0) | N/A | 49 (31.6%) |
| Other | 7 (4.7) | 7 (4.7) | 14 (9.2) | 4 (2.6%) |
| Missing | 2 (1.3) | 1 (0.7) | 3(2.0) | 1 (0.6%) |
| Do you have AMD? n (%) | ||||
| No | 145 (96.7) | 148 (98.7) | 148 (97.4) | 150 (96.8) |
| Yes | 3 (2.0) | 1 (0.7) | 3 (2.0) | 4 (2.6) |
| Missing | 2 (1.3) | 1 (0.7) | 1 (0.7) | 1 (0.6) |
| Do you know someone with an eye disease? n (%) | ||||
| No | 131 (87.3) | 114 (76.0) | 130 (85.5) | 95 (61.3) |
| Yes, AMD | 11 (7.3) | 18 (12.0) | 12 (7.9) | 35 (22.6) |
| Yes, glaucoma | 3 (2.0) | 10 (6.7) | 3 (2.0) | 12 (7.7) |
| Yes, other | 4 (2.7) | 7 (4.7) | 6 (3.9) | 12 (7.8) |
| Missing | 1 (0.7) | 1 (0.7) | 1 (0.7) | 1 (0.6) |
Abbreviation: AMD, age-related macular degeneration.
Mean EQ-5D single index scores were similar for all four countries; however, there were differences in ranges, with UK showing the smallest range (0.52–1.0) (Table 2). Mean EQ-5D VAS scores were comparable across the four countries, and as expected were lower than the index scores.40 All rank order scores of the eight health states completed by each participant were in the expected order of no difficulty (111111) to dead (data not shown).
Table 2.
EQ-5D Index and Visual Analog Scale Scores by Country
| Australia (N = 150) | Canada (N = 150) | United Kingdom (N = 152) | United States (N = 155) | |
|---|---|---|---|---|
| EQ-5D-Index | ||||
| Mean (SD) | 0.92 (0.12) | 0.93 (0.11) | 0.93 (0.12) | 0.92 (0.13) |
| Range | 0.41–1.0 | 0.31–1.0 | 0.52–1.0 | 0.45–1.0 |
| IQR | 0.15 | 0.17 | 0.15 | 0.17 |
|
| ||||
| EQ-5D-Visual Analog Scale | ||||
| Mean (SD) | 81.6 (12.8) | 84.5 (11.1) | 84.4 (14.6) | 84.5 (15.3) |
| Range | 25.0–100.0 | 40.0–100.0 | 4.0–100.0 | 15.0–100.0 |
| IQR | 16.0 | 10.0 | 15.0 | 15.0 |
Health State Values
In all, 4,850 health state valuations were elicited (8 health states for each of the 607 participants) with only six valuations either not completed or the indifference point could not be achieved. Descriptive statistics on values by health states after adjustment onto the full health/dead scale are shown in Table 3. Significant country-specific differences were observed for two of the eight health states (Table 3). Differences in mean utilities were seen for health state E (P<0.0001) and health state G (P<0.0001). For both health states, UK participants reported lower scores, Canadian participants provided the highest scores—the maximum differences were 0.05 and 0.15. Mean adjusted health state utilities ranged from 0.343 (SD=0.395) to 0.956 (SD=0.124). The UK had the widest range of mean health state utilities but the lowest valuation for the best health state (111111) (range, 0.264–0.916). Canadian participant valuations resulted in a utility score of 0.377 for the worst health state (555555) but offered the highest score for the best health state 111111 (0.989). Australian utilities ranged from 0.318 to 0.954, and the US utilities ranged from 0.413 to 0.964. These preferences showed a linear decline in utility values as the health states increased in severity. Most participants were willing to trade time for health, and the health state utilities were well distributed with little skewness. Results indicate that the eight evaluated states from VFQ-UI describe states that participants viewed as spanning a large proportion of the continuum from full health to dead.
Table 3.
Summary of VFQ-UI Health State Time Trade-Off Utility Scores
| Health Statea | N | Overall Mean (SD) | Australia Mean (SD) | Canada Mean (SD) | United Kingdom Mean (SD) | United States Mean (SD) |
|---|---|---|---|---|---|---|
| E: 111111b | 606 | 0.956 (0.124) | 0.954 (0.155) | 0.989 (0.028) | 0.916 (0.168) | 0.964 (0.079) |
| G: 211111 | 607 | 0.906 (0.147) | 0.901 (0.148) | 0.955 (0.100) | 0.851 (0.189) | 0.917 (0.117) |
| I: 312211 | 604 | 0.828 (0.173) | 0.829 (0.183) | 0.856 (0.152) | 0.795 (0.185) | 0.832 (0.166) |
| K: 323322 | 607 | 0.750 (0.212) | 0.760 (0.212) | 0.761 (0.194) | 0.717 (0.231) | 0.762 (0.209) |
| M: 333322 | 606 | 0.717 (0.215) | 0.721 (0.198) | 0.721 (0.218) | 0.687 (0.220) | 0.737 (0.220) |
| O: 433354 | 606 | 0.550 (0.283) | 0.590 (0.255) | 0.508 (0.260) | 0.534 (0.302) | 0.567 (0.307) |
| R: 444354 | 607 | 0.402 (0.312) | 0.446 (0.261) | 0.365 (0.290) | 0.378 (0.335) | 0.418 (0.350) |
| T: 555555c | 607 | 0.343 (0.395) | 0.318 (0.364) | 0.377 (0.360) | 0.264 (0.401) | 0.413 (0.436) |
Regression Modeling
Regression models were used to generate algorithms to estimate preference (ie, utility) scores for the range of potential health states defined by the VFQ-UI health state classification. Items 18, 20, and 25 of the NEI VFQ-25 were recoded, so their scoring would be in the same direction as items 6, 11, and 14. The patient data (data set [1] previously described) was analyzed using an IRT graded response model.37 All items fit the graded response model (Table 4), except for item 25 (I worry about doing things that may embarrass myself or others) (data not shown). However, given the large sample size (~3000), it is not surprising that one item showed some evidence of misfit.41
Table 4.
Item Parameters and Fit Statistics for 6-Item VFQ-UI: IRT Analysis of Combined Data
| Slopea | Category Thresholda | Item Fit Statisticsb | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| ID | a | b1 | b2 | b3 | b4 | S-G2 | Prob_G2 | S-X2 | Prob_X2 | df |
| Q6c | 2.51 | −0.36 | 0.67 | 1.54 | 2.31 | 84.15 | 0.002 | 78.59 | 0.006 | 50 |
| Q11d | 2.86 | 0.71 | 1.35 | 2.02 | 2.90 | 62.93 | 0.060 | 63.33 | 0.056 | 47 |
| Q14e | 3.46 | 0.52 | 1.09 | 1.55 | 2.08 | 80.59 | 0.003 | 80.34 | 0.003 | 49 |
| Q18f | 2.31 | 0.29 | 0.94 | 1.75 | 2.37 | 80.81 | 0.013 | 80.36 | 0.015 | 55 |
| Q20g | 3.12 | 0.92 | 1.43 | 1.57 | 2.18 | 60.66 | 0.123 | 58.36 | 0.169 | 49 |
| Q25h | 2.79 | 0.88 | 1.44 | 1.61 | 2.16 | 102.4 | 0.000 | 110.3 | 0.000 | 52 |
We then produced theta scores for each level of each dimension based on this IRT analysis. Level 5, the worst response category (stopped doing because of eyesight, all of the time, or definitely true), was used as the reference category. Thetas are consistent with the ordinality of the VFQ-UI items.
Regression analyses mapping the relationship between the IRT severity score (theta) and TTO preference scores for the eight health states are presented in Table 5. Education and gender were dropped because they were not significant in any regression model. The simplest regression model, model 1, included age, theta as a continuous variable, and theta squared. The theta squared term was not significant, so model 2 was run with a theta cubed term. All of the variables were significant in the model with a cubed theta term. These two models were rerun with the predicted theta term included as a class variable. For both models, all terms were significant. The adjusted R2s were all similar, ranging from 0.40 to 0.42, and other model fit statistics showed that these regression models had a good fit to the observed data.
Table 5.
Regression Models for Estimating Health Preference Scores
| Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|
| Intercept | 0.8336*** | 0.8740*** | 0.8656*** | 0.8841*** |
| Age | 0.0009*** | 0.0009*** | 0.0009*** | 0.0009*** |
| Theta 1 | −0.1667*** | −0.1062*** | −0.0966*** | −0.0867*** |
| Theta 12 | −0.00456* | −0.1122*** | −0.0505*** | −0.0853*** |
| Theta 13 | — | 0.0278*** | — | 0.0114** |
| N | 4842 | 4842 | 4842 | 4842 |
| Adjusted R2 | 0.4065 | 0.4175 | 0.3992 | 0.4002 |
P < 0.05.
P < 0.01.
P < 0.001.
Model 1: Age, predicted theta based on dimensions (continuous variable), theta squared.
Model 2: Age, predicted theta based on dimensions (continuous variable), theta squared, theta cubed.
Model 3: Age, predicted theta based on dimensions (class variable), theta squared.
Model 4: Age, predicted theta based on dimensions (class variable). theta squared, theta cubed.
Validity of VFQ-UI Scores
Assessment of the validity of VFQ-UI outside the development sample was performed using data from a recent interventional study that administered NEI VFQ-25 to patients with uveitis. A detailed description of the clinical trial and key inclusion and exclusion criteria have been reported elsewhere42 but briefly, patients included in the trial were at least 18 years of age with a diagnosis of intermediate or posterior uveitis and decreased visual acuity attributable to uveitis, with vitreous haze score at least +1.5, BCVA between 10 and 75 letters in the study eye measured by the Early Treatment of Diabetic Retinopathy Study method at baseline. Patients were excluded if they had uncontrolled systemic disease, any ocular condition in the study eye that would prevent improvement in VA, or any condition or treatment that would otherwise confound the results of the study. The mean age of the sample was 44.6 years, 65% were female, and majority of the patients were white (61%). The median visual acuity in the study eye was 62.0 letters and 84% received treatment in their worse-seeing eye.
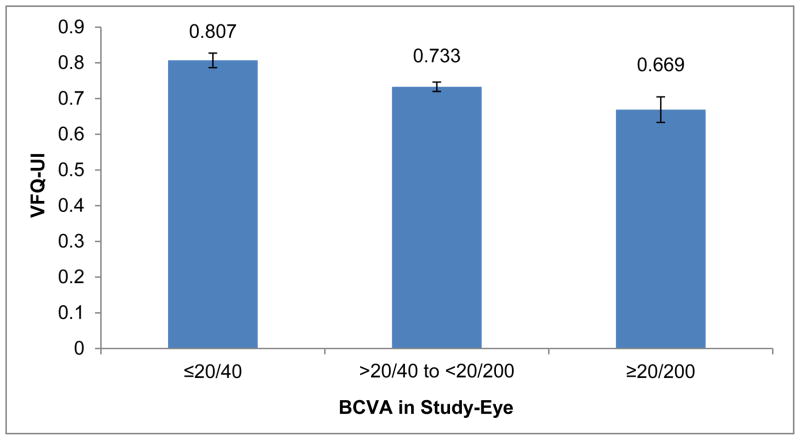
Mean VFQ-UI scores significantly discriminated among study-eye BCVA groups (≥20/40, <20/40 and >20/200, and ≤20/200) at baseline (P < 0.05; Figure 2).43 The VFQ-UI was significantly correlated with NEI VFQ-25 composite (r=0.92) and subscale scores (ranging from a low of r=0.42 for general health to 0.85 for social functioning).
Figure 2.
VFQ-UI Known Groups Validity From Recent Interventional Uveitis Studya
Abbreviations: BCVA, best-corrected visual acuity; VFQ-UI, Visual Function Questionnaire-Utility Index.
aOverall nonstudy eye mean Early Treatment Diabetic Retinopathy Study BCVA (SD) at baseline across all subjects was 76.9 (14.74).
DISCUSSION
We developed a VFQ-UI algorithm based on the VFQ-UI health state classification that will enable estimation of preference scores using responses to six items of the NEI VFQ-25. As a result, preference scores can be estimated when these six items from the NEI-VQ-25 have been completed. The final regression models took into account the patient data used in development of the VFQ-UI health state classification.23 Using patient level data and valuation study data, we mapped the relationship between theta scores and preference scores for VFQ-UI health states. For future research applications using VFQ-UI, we recommend using the model 2 regression equation because it is parsimonious and yielded the largest adjusted R-squared (see eAppendix C Scoring Manual in the Supplement).
This study was conducted to obtain health preferences for VFQ-UI health states in members of the general population in the Australia, Canada, UK, and US. Because the resources allocated for different health technologies come from the public in Australia, Canada, and the UK, it is argued that the preferences should come from the public.3–5,7 In addition, societal preferences are important because generic health preference measures are based on societal data, and it is important to have comparable sources of data for these disease-targeted states. Furthermore, several health technology assessment agencies require preferences based on the general population.8
The analytical method utilized in this study is different from the method used by Brazier and colleagues in developing health state classifications based on the SF-36, Asthma Quality of Life Questionnaire, and other disease-targeted measures.6,10,18,22 Our data could not be analyzed using their approach due to the lack of independence of the selected vision-related dimensions. The approach estimates the theta scores for the target health states using IRT analysis and then models the relationship between these theta scores and the health preference scores. This is comparable to methods used by Young et al. to develop a flushing-specific preference score.9,35
Using data from a separate clinical trial of patients with noninfectious uveitis, we found preliminary support for the validity of the VFQ-UI. VFQ-UI scores were highly correlated with NEI VFQ-25 composite scores and moderately to highly correlated with subscale scores. VFQ-UI scores varied significantly by BCVA groups, with patients with more impaired visual acuity reporting greater impairment in vision-specific preference scores.
One limitation of the valuation survey is that participants were required to attend an interview and therefore were likely healthier than the general population. The survey participants were healthier and more educated than the general population in these countries. SDs for VFQ-UI scores were smaller overall and by country for better visual functioning health states. SDs increased with worsening health states, suggesting that the general population has a broader range of preferences and willingness to trade off years as visual functioning decreases or worsens. The results indicating a high valuation are consistent with public opinion polls in the US that show a high fear of going blind and a high value for vision itself.44 Additionally, although this was a large sample from the general public, collecting utilities from patients who experience vision-related conditions could also be a useful comparison. Further research is needed to compare patient and general public preferences for different vision-related states. Previous research has demonstrated that patients and clinicians have different preferences for vision-related impairment states.45,46
Second, we observed some differences between countries on two of the TTO utilities. However, the rank ordering of the utilities within countries was identical, and the differences may only seem to reflect more optimistic (or pessimistic) valuations across countries. These findings may be attributable to cultural differences among the countries. Individuals were all from western countries, and there may be differences in valuing vision-related health states among Asian, African, North American, and European countries that may affect VFQ-UI scores. Additional research is needed to examine differences in preferences in Asian and other cultures.
Areas for further research include assessing the reliability and validity, including responsiveness to change of utility scores from the VFQ-UI in both central and peripheral vision loss populations as well as for various vision-defined health states used in economic analyses. As the NEI VFQ-25 and the VFQ-UI are completed in terms of vision in both eyes, the VFQ-UI captures utility associated with bilateral vision. Future research can assess utility considering bilateral or monocular conditions and whether a study eye is the better- or worse-seeing eye. In addition, research is needed to assess VFQ-UI estimated health state utility scores and measurement properties compared to generic preference measures such as the EQ-5D, Health Utilities Index Mark 2, Mark 3, SF-6D, and/or the Quality of Well-being.47 A recent study demonstrated that the VFQ-UI was more responsive than generic health preference measures in cataract surgery patients.47
In conclusion, we developed an algorithm for converting VFQ-UI scores into health preferences for use in economic evaluations. These vision-related preference scores are expected to be more responsive to differences among the effects of ophthalmologic interventions than generic health preference measures. The VFQ-UI represents the patient’s perspective on the impact of ocular conditions on functioning and well-being, and VFQ-UI scores allow for comparisons across eye disorders. VFQ-UI health preference scores for different vision-related treatments may be of value to estimate QALYs for economic evaluations and health policy decisions.
Supplementary Material
eAppendix A: Details of methods for preference elicitation and statistical analyses
eAppendix B: Visual Function Questionnaire-Utility Index Valuation Health States
eAppendix C: Visual Function Questionnaire-Utility Index Scoring Manual
Acknowledgments
Financial support
This research was supported by Allergan Inc., Irvine, California. The study sponsor was involved in the design of the study, interpretation of the data, and preparation, review, and approval of the manuscript. Dr Hays was funded in part by the UCLA Resource Center for Minority Aging Research Center for Health Improvement in Minority Elderly (NIH/NIA P30AG021684), UCLA DREW Project EXPORT (NCMHD 2P20MD000182), and the UCLA Older Americans Independence Center (NIH/NIA P30AG028748).
Footnotes
Author contributions
AMR, RY, and DAR made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; have been involved in drafting the manuscript and revising it critically for important intellectual content; and have provided final approval of the version to be published. JWK and JGW made substantial contributions to conception and design, interpretation of data; have been involved in revising the manuscript critically for important intellectual content; and have provided final approval of the version to be published. RDH, JEB, PL, and NB made substantial contributions to interpretation of data; have been involved in revising the manuscript critically for important intellectual content; and have provided final approval of the version to be published.
Ms. Rentz and Ms. Yu had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of interest
Ms. Rentz, Ms. Yu, and Dr. Revicki are employed by United BioSource Corporation (UBC), which provides consulting and other research services to pharmaceutical, device, government and non-government organizations. As UBC employees, they work with a variety of companies and organizations and are expressly prohibited from receiving any payment or honoraria directly from these organizations for services rendered. Dr. Kowalski and Mr. Walt are employees of Allergan, Inc. Dr. Hays received financial support for this study from Allergan, Inc., the UCLA Resource Center for Minority Aging Research Center for Health Improvement in Minority Elderly (NIH/NIA P30AG021684), UCLA DREW Project EXPORT (NCMHD 2P20MD000182), and the UCLA Older Americans Independence Center (NIH/NIA P30AG028748). Dr. Brazier had received payment from Allergan, Inc., as a consultant for this study. Dr. Lee is currently a consultant for Pfizer, Genentech, Quorum Consulting, and Novartis and from March 2010–March 2011 for Allergan; he has in the last 3 years received research grants from Pfizer. He has a proprietary interest in Merck, GlaxoSmithKline, and Vitaspring Health Technologies. Dr. Bressler’s employer, the Johns Hopkins University (JHU), but not Dr. Bressler, received funding from Allergan for this project; the terms of this project are negotiated and administered by JHU’s Office of Research Administration. Under JHU’s policy, support for the costs of research, administered by the institution, does not constitute a financial conflict of interest.
Contributors
-
David S. Boyer, MD, Tammy Gasparyan, Lissette PleitezRetina-Vitreous Associates Medical Group, Beverly Hills, California
-
Jeffrey C. Hong, MD, Marie RodriguezHuntington Eye Medical Group, Pasadena, California
-
Steven Simmons MD, Martin Kaback, MD, and Ralph Sanchez, MDGlaucoma Consultants of the Capital Region, Slingerlands, New York
The authors also thank Beenish Nafees, Sean O’Quinn, Anne Brooks, Scott Doyle, Sarah Hearn, Pamela Murray, Alise Nason, Sherilyn Notte, Paul O’Donohoe, Megan Stafford, and Randell Winnette for their assistance with this study. We also thank Julie Meilak and Aria Gray for their help with proofreading and formatting the manuscript.
References
- 1.Commonwealth Department of Health, Housing, Community Service. Guidelines for the Pharmaceutical Industry on the Submission to the Pharmaceutical Benefits Advisory Committee. Canberra, Australia: Australian Government Publishing Service; 1992. [Google Scholar]
- 2.National Institute of Health and Clinical Excellence. [Accessed January 9, 2009];NICE guide to the methods of technology appraisal. 2004 http://www.nice.org.uk. [PubMed]
- 3.Gold M, Franks P, Erickson P. Assessing the health of the nation. The predictive validity of a preference-based measure and self-rated health. Med Care. 1996;34(2):163–177. doi: 10.1097/00005650-199602000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Lipscomb J, Drummond M, Fryback D, Gold M, Revicki D. Retaining, and enhancing, the QALY. Value Health. 2009;12(suppl 1):S18–26. doi: 10.1111/j.1524-4733.2009.00518.x. [DOI] [PubMed] [Google Scholar]
- 5.Weinstein MC, Torrance G, McGuire A. QALYs: the basics. Value Health. 2009;12(suppl 1):S5–9. doi: 10.1111/j.1524-4733.2009.00515.x. [DOI] [PubMed] [Google Scholar]
- 6.Yang Y, Brazier JE, Tsuchiya A, Young TA. Estimating a preference-based index for a 5-dimensional health state classification for asthma derived from the asthma quality of life questionnaire. Med Decis Making. 2011;31(2):281–291. doi: 10.1177/0272989X10379646. [DOI] [PubMed] [Google Scholar]
- 7.Petrillo J, Cairns J. Converting condition-specific measures into preference-based outcomes for use in economic evaluation. Exp Rev Pharmacoecon Outcomes Res. 2008;8(5):453–461. doi: 10.1586/14737167.8.5.453. [DOI] [PubMed] [Google Scholar]
- 8.National Institute of Health and Clinical Excellence. [Accessed January 28, 2009];Guide to the methods of technology appraisal. 2008 http://www.nice.org.uk.
- 9.Brazier JE, Rowen D, Mavranezouli I, et al. Developing and testing methods for deriving preference-based measures of health from condition-specific measures (and other patient-based measures of outcome) Health Technol Assess. 2012;16(32):1–114. doi: 10.3310/hta16320. [DOI] [PubMed] [Google Scholar]
- 10.Rowen D, Brazier J, Young T, et al. Deriving a preference-based measure for cancer using the EORTC QLQ-C30. Value Health. 2011;14(5):721–731. doi: 10.1016/j.jval.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bansback N, Marra C, Tsuchiya A, et al. Using the health assessment questionnaire to estimate preference-based single indices in patients with rheumatoid arthritis. Arthritis Rheum. 2007;57(6):963–971. doi: 10.1002/art.22885. [DOI] [PubMed] [Google Scholar]
- 12.Buxton MJ, Lacey LA, Feagan BG, Niecko T, Miller DW, Townsend RJ. Mapping from disease-specific measures to utility: an analysis of the relationships between the Inflammatory Bowel Disease Questionnaire and Crohn’s Disease Activity Index in Crohn’s disease and measures of utility. Value Health. 2007;10(3):214–220. doi: 10.1111/j.1524-4733.2007.00171.x. [DOI] [PubMed] [Google Scholar]
- 13.Lenert LA, Sherbourne CD, Sugar C, Wells KB. Estimation of utilities for the effects of depression from the SF-12. Med Care. 2000;38(7):763–770. doi: 10.1097/00005650-200007000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Lo PS, Tong MC, Revicki DA, et al. Rhinitis Symptom Utility Index (RSUI) in Chinese subjects: a multiattribute patient-preference approach. Qual Life Res. 2006;15(5):877–887. doi: 10.1007/s11136-005-4828-x. [DOI] [PubMed] [Google Scholar]
- 15.Revicki DA, Leidy NK, Brennan-Diemer F, Sorensen S, Togias A. Integrating patient preferences into health outcomes assessment: the multiattribute Asthma Symptom Utility Index. Chest. 1998;114(4):998–1007. doi: 10.1378/chest.114.4.998. [DOI] [PubMed] [Google Scholar]
- 16.Revicki DA, Leidy NK, Brennan-Diemer F, Thompson C, Togias A. Development and preliminary validation of the multiattribute Rhinitis Symptom Utility Index. Qual Life Res. 1998;7(8):693–702. doi: 10.1023/a:1008860113818. [DOI] [PubMed] [Google Scholar]
- 17.Espallargues M, Czoski-Murray CJ, Bansback NJ, et al. The impact of age-related macular degeneration on health status utility values. Invest Ophthalmol Vis Sci. 2005;46(11):4016–4023. doi: 10.1167/iovs.05-0072. [DOI] [PubMed] [Google Scholar]
- 18.Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ. 2002;21(2):271–292. doi: 10.1016/s0167-6296(01)00130-8. [DOI] [PubMed] [Google Scholar]
- 19.Mangione CM, Lee PP, Pitts J, Gutierrez P, Berry S, Hays RD. Psychometric properties of the National Eye Institute Visual Function Questionnaire (NEI-VFQ). NEI-VFQ Field Test Investigators. Arch Ophthalmol. 1998;116(11):1496–1504. doi: 10.1001/archopht.116.11.1496. [DOI] [PubMed] [Google Scholar]
- 20.Mangione CM. [Accessed December 5, 2012];NEI VFQ-25 scoring algorithm. www.nei.nih.gov/resources/visionfunction/manual_cm2000.pdf.
- 21.Yang Y, Brazier J, Tsuchiya A, Coyne K. Estimating a preference-based single index from the Overactive Bladder Questionnaire. Value Health. 2009;12(1):159–166. doi: 10.1111/j.1524-4733.2008.00413.x. [DOI] [PubMed] [Google Scholar]
- 22.Young T, Yang Y, Brazier JE, Tsuchiya A, Coyne K. The first stage of developing preference-based measures: constructing a health-state classification using Rasch analysis. Qual Life Res. 2009;18(2):253–265. doi: 10.1007/s11136-008-9428-0. [DOI] [PubMed] [Google Scholar]
- 23.Kowalski J, Rentz AM, Walt J, et al. Development of a classification system for health states from the National Eye Institute Visual Function Questionnaire-25. Qual Life Res. 2012;21:323–334. doi: 10.1007/s11136-011-9938-z. [DOI] [PubMed] [Google Scholar]
- 24.Allergan Inc. A study of the safety and efficacy of a new treatment for macular edema resulting from retinal vein occlusion [data on file] Irvine, CA: [Google Scholar]
- 25.Allergan Inc. A study of safety and efficacy of a new treatment for diabetic macular edema [data on file] Irvine, CA: [Google Scholar]
- 26.Submacular Surgery Trials Research Group. Health-and vision-related quality of life among patients with choroidal neovascularization secondary to age-related macular degeneration at enrollment in randomized trials of submacular surgery: Submacular Surgery Trials Report No. 4. Am J Opthalmol. 2004;138(1):91–108. doi: 10.1016/j.ajo.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 27.Schiffman RM, Walt JG, Jacobsen G, Doyle JJ, Lebovics G, Sumner W. Utility assessment among patients with dry eye disease. Ophthalmology. 2003;110(7):1412–1419. doi: 10.1016/S0161-6420(03)00462-7. [DOI] [PubMed] [Google Scholar]
- 28.Globe DR, Wu J, Azen SP, Varma R. The impact of visual impairment on self-reported visual functioning in Latinos: the Los Angeles Latino Eye Study. Ophthalmology. 2004;111(6):1141–1149. doi: 10.1016/j.ophtha.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 29.Allergan Inc. Memantine in patients with chronic glaucoma [data on file] Irvine, CA: [Google Scholar]
- 30.Muir KW, Santiago-Turla C, Stinnett SS, et al. Health literacy and adherence to glaucoma therapy. Am J Ophthalmol. 2006;142(2):223–226. doi: 10.1016/j.ajo.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 31.Bond TG, Fox CM. Applying the Rasch Model: Fundamental Measurement in the Human Sciences. London, UK: Psychology Press; 2007. [Google Scholar]
- 32.Wright BD, Masters GN. Rating Scale Analysis: Rasch Measurement. Chicago, IL: MESA Press; 1982. [Google Scholar]
- 33.Furlong W, Feeny D, Torrance GW, Barr R, Horsman J. Guide to design and development of health-state utility instrumentation. Hamilton, Ontario: Centre for Health Economics and Policy Analysis, McMaster University; 1990. [Google Scholar]
- 34.Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33(5):337–343. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- 35.Young TA, Rowen D, Norquist J, Brazier JE. Developing preference-based health measures: using Rasch analysis to generate health state values. Qual Life Res. 2010;19(6):907–917. doi: 10.1007/s11136-010-9646-0. [DOI] [PubMed] [Google Scholar]
- 36.Brazier JE, Yang Y, Tsuchiya A, Rowen DL. A review of studies mapping (or cross walking) non-preference based measures of health to generic preference-based measures. Eur J Health Econ. 2010;11(2):215–225. doi: 10.1007/s10198-009-0168-z. [DOI] [PubMed] [Google Scholar]
- 37.Samejima F. Graded response model. In: van der Linden WJ, Hambleton RK, editors. Handbook of Modern Item Response Theory. New York, NY: Springer; 1997. [Google Scholar]
- 38.Thissen D, Chen WH, Bock D. MULTILOG. Lincolnwood, IL: [Google Scholar]
- 39.Bjorner JB, Smith KJ, Orlando M, Stone C, Thissen D, Sun X. IRTFIT: A Macro for Item Fit and Local Dependence Tests under IRT Models. Lincoln, RI: QualityMetric Incorporated; 2006. [Google Scholar]
- 40.Brooks R, Rabin R, de Charro F. The Measurement and Valuation of Health Status Using EQ-5D: A European Perspective. Dordrecht, The Netherlands: Kluwer Academic Publishers; 2003. [Google Scholar]
- 41.Hambleton RK, Swaminathan H, Rogers HJ. Fundamentals of Item Response Theory. Newbury Park, CA: Sage Publications, Inc; 1991. [Google Scholar]
- 42.Lightman S, Foster CS, Robinson MR, et al. Dexamethasone intravitreal implant for noninfectious intermediate or posterior uveitis. Arch Ophthalmol. 2011;129(5):545–553. doi: 10.1001/archophthalmol.2010.339. [DOI] [PubMed] [Google Scholar]
- 43.Naik R, Rentz A, Foster CS, et al. Normative comparison of patient-reported outcomes in noninfectious intermediate or posterior uveitis. JAMA Ophthalmol. 2013;131(2):219–225. doi: 10.1001/2013.jamaophthalmol.102. [DOI] [PubMed] [Google Scholar]
- 44.American Foundation for the Blind. [Accessed December 10, 2010];Attitudes about blindness/severe vision loss. www.afb.org.
- 45.Kymes SM, Lee BS. Preference-based quality of life measures in people with visual impairment. Optom Vis Sci. 2007;84(8):809–816. doi: 10.1097/OPX.0b013e3181337638. [DOI] [PubMed] [Google Scholar]
- 46.Stein JD, Brown MM, Brown GC, Hollands H, Sharma S. Quality of life with macular degeneration: perceptions of patients, clinicians, and community members. Br J Ophthalmol. 2003;87(1):8–12. doi: 10.1136/bjo.87.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feeny D, Spritzer K, Hays RD, et al. Agreement about identifying patients who change over time cautionary results in cataract and heart failure patients. Med Decis Making. 2012;32(2):273–286. doi: 10.1177/0272989X11418671. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix A: Details of methods for preference elicitation and statistical analyses
eAppendix B: Visual Function Questionnaire-Utility Index Valuation Health States
eAppendix C: Visual Function Questionnaire-Utility Index Scoring Manual