Abstract
Background
Despite the suggestion of a neuropathic component to sickle cell disease (SCD) pain, there are minimal data on the systematic assessment of neuropathic pain in patients with SCD. Neuropathic pain is defined as pain primarily initiated by dysfunction of the peripheral or central nervous system.
Procedure
In a cross-sectional study, we used the painDETECT questionnaire, a one-page validated neuropathic pain screening tool, to determine the presence of neuropathic pain in patients with SCD and to evaluate the relationship between neuropathic pain, age, and gender. We hypothesized that 20% of patients with SCD will experience neuropathic pain and that neuropathic pain will be associated with older age and female gender. The completed painDETECT questionnaire yields a total score between 0–38 (≥19=definite neuropathic pain, 13–18=probable neuropathic pain, ≤12=no neuropathic pain). Scores ≥13 were designated as having evidence of neuropathic pain.
Results
A total of 56 patients participated. Median age was 20.3 years and 77% were female. We found 37% of patients had evidence of neuropathic pain. Age was positively correlated with total score [r=0.43; p=0.001] suggesting older patients experience more neuropathic pain. Females had higher mean total scores [13 vs 8.4; p=0.04]. Significantly more patients with neuropathic pain were taking hydroxyurea [90% vs 59%; p=0.015]. Despite 37% of patients experiencing neuropathic pain, only 5% were taking a neuropathic pain drug.
Conclusions
Neuropathic pain exists in SCD. Valid screening tools can identify patients that would benefit from existing and future neuropathic pain therapies and could determine the impact of these therapies.
Keywords: sickle cell disease, neuropathic pain
Introduction
Pain in sickle cell disease (SCD) is associated with significant morbidity and increased mortality.[1] SCD pain can be acute, increases with age, and in adults, pain often occurs daily and may be consistent with a chronic pain syndrome.[2, 3] Despite many studies documenting the frequency and morbidities associated with pain, the sequence of events leading to the perception and development of pain, the transition from acute to chronic pain, and the variability in phenotypic expression remains poorly understood. Furthermore, this lack of knowledge impedes our progress towards the development of novel therapies or preventative measures for SCD pain.
Verbal pain descriptors (aching, cold, hot, shooting, stabbing)[4, 5] and precipitating factors of SCD pain (cold temperatures, touch, increased wind speed and barometric pressure)[6–8] suggest patients have hypersensitivity to tactile stimuli, a classic neuropathic pain characteristic. Neuropathic pain is defined as pain initiated by dysfunction of the peripheral or central nervous system.[9] This is in contrary to inflammatory or nociceptive pain where tissue damage causes the pain. Hypersensitivity, increased pain from a normally painful stimulus and allodynia, pain from a nonpainful stimulus (i.e. extreme sensitivity to cool stimuli) are both classical components of neuropathic pain.[10, 11] The prevalence of neuropathic pain rises with increasing age[12–15] and neuropathic pain occurs more commonly in females in pain populations other than SCD.[13, 14] Epidemiological studies reveal the prevalence of neuropathic pain is almost 20% in chronic pain populations other than SCD.[14–16]
Although a neuropathic component to SCD pain has been suggested, there are minimal data describing the systematic assessment of neuropathic pain in patients with SCD using valid screening tools. In non-SCD patient populations, valid neuropathic pain screening tools have been used to identify patients with a neuropathic pain phenotype.[17] Importantly, screening can determine prevalence of neuropathic pain in a patient population and direct treatment. Screening tools also allow selection of the correct pain phenotype for inclusion in neuropathic pain treatment trials. Thus, the use of a screening tool for neuropathic pain in patients with SCD has the potential to impact the clinical management of SCD pain. To date, there is only a single published study specifically targeted at the evaluation of neuropathic pain in SCD using a non-specific pain assessment tool. This study utilized PAINReportIt to assess sensory aspects of pain in adults with SCD and focused on location, intensity, quality and pattern.[4] The qualitative aspect of the tool asked patients to choose from 78 verbal pain descriptors that were grouped into neuropathic, nociceptive, or other. This study found over 90% of patients selected at least one neuropathic pain descriptor with a mean of 4.5 neuropathic descriptors chosen.[4] This primarily descriptive study found patients qualitatively describe their pain using words that are neuropathic in nature. However, the tool was not designed to quantitatively differentiate those that are likely or less likely to have neuropathic pain since the PAINReportIt measure is not a specific neuropathic pain screening tool. In addition, this study focused on adults with SCD. Furthermore, it is unknown what patient-level factors are associated with the use of neuropathic pain descriptors.
In summary, limited clinical data suggest a neuropathic component to SCD pain exists. However, it is unknown what proportion of patients with SCD may experience neuropathic pain, whether children experience neuropathic pain, and what patient-level factors are associated with neuropathic pain. Thus, our study objectives were to: 1) determine the presence of clinically evident neuropathic pain in patients with SCD, 2) describe the sensory symptoms that patients with SCD experience when in pain, and 3) evaluate the relationship between neuropathic pain, age, and gender. Based on the literature in non-SCD pain populations, our primary hypothesis was at least 20% of patients with SCD will report experiencing neuropathic pain. Our secondary hypothesis was the presence of neuropathic pain will be associated with older age and be more likely in female patients.
Methods
Study Population
This cross-sectional study was conducted between January 2010 and December 2012. All patients age ≥14 years with SCD (all genotypes) without a history of overt or silent stroke were eligible. This age was chosen since the questionnaire is validated down to 14 years. Currently, there are no published neuropathic pain screening tools for children <14 years. Patients were recruited from the Wisconsin Sickle Cell Center during routine clinic visits while in their baseline state of health to complete the questionnaire only or to complete the questionnaire as part of a larger pain study. Non-English speaking patients were excluded.
The Institutional Review Board of the Children’s Hospital of Wisconsin approved the study and informed consent was obtained from the parent/legal guardian and assent from the child when appropriate. Stipends included $10 Wal-Mart gift card for questionnaire only or $50 Wal-Mart gift card for all study components of larger pain study that were given to each subject.
Measurements
The painDETECT questionnaire was completed. The questionnaire is a cross-sectional, validated neuropathic pain screening tool developed to differentiate neuropathic from non-neuropathic pain.[18] It has been validated by the developer down to 14 years which supports the capability of adolescents to understand the pain descriptors used. The one-page questionnaire is comprised of 12 questions about the severity, course, and quality of pain specifically focused on neuropathic pain symptoms. It has 85% sensitivity, 80% specificity, and 83% positive predictive value to differentiate neuropathic from non-neuropathic pain.[18] This tool is easily completed in a clinic visit and has been used in patients with low back pain, osteoarthritis, diabetic neuropathy, post-herpetic neuralgia, fibromyalgia, carpal tunnel syndrome, and post thoracic surgery.[19–23] Permission for use was granted by the developer.
The questionnaire asks subjects to rate their pain intensity (0–10 scale) in 3 scenarios: currently, strongest pain during past 4 weeks, and average pain during past 4 weeks. Subjects are then asked to choose a picture that best describes the course of their pain (Figure 1). The common pain site(s) are marked on a body diagram and subjects are asked if their pain radiates to other body regions (yes/no). Finally, the remaining 7 questions ask subjects to assess the most commonly experienced sensory symptoms when they have pain (0=never, 1=hardly noticed, 2=slightly, 3=moderately, 4=strongly, 5=very strongly) in the following categories: burning pain (i.e., stinging nettles), spontaneous paresthesias (i.e., tingling, prickling), mechanical allodynia (i.e., light touch is painful), spontaneous pain attacks (i.e., electric shocks), thermal hyperalgesia (i.e., cold or heat sensitivity), and numbness. The pain course picture, presence/absence of radiating pain, and the answers to the sensory symptom questions contribute to a total score between 0–38. The total score is determined by the following method as per the developer’s instructions. The answers to the 7 sensory symptoms questions are weighted based on the response chosen and summed. For example, a response of never receives 0 points, hardly noticed receives 1 point, slightly receives 2 points, etc. From these questions a score is determined out of a potential of 35 total points. This score is then adjusted based on the pain course picture chosen (points added or subtracted) and if patients answered “yes” to radiating pain they received an additional 2 points. The final total score is then determined (range 0–38). Cut points have been determined based on validation studies and scores of 19–38 indicate a definite neuropathic pain component, scores of 13–18 indicate a probable neuropathic pain component, and scores of 0–12 indicate that a neuropathic pain component does not exist. Those that scored ≥13 were defined as having evidence of neuropathic pain. This cutoff was chosen based on previously published literature.[24] The pain intensity scores and the body diagram are descriptive data and are not factored into the total score.
Figure 1. Pictorial Description of Pain Course.
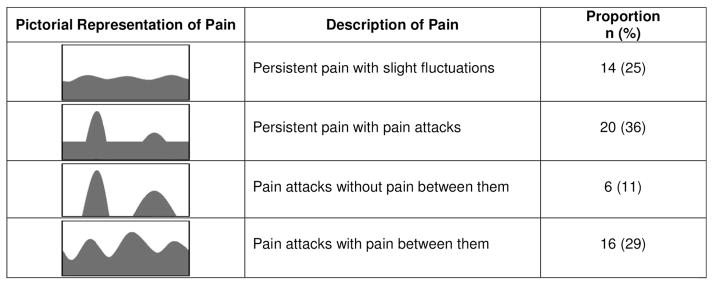
Subjects were asked to choose the picture that best describes their course of pain. The proportion of patients that chose each picture is shown. Adapted from painDETECT questionnaire and Freynhagen et al. CMRO 2006 [18].
Clinical and Laboratory History
Clinical and laboratory data were extracted from the comprehensive SCD center database, from the electronic medical record, and by patient interview. These data included health care utilization for pain in the emergency department or inpatient unit in the 3 months prior to study enrollment, laboratory results, current medications, and history of depression (self-report). The 3 most recent labs obtained closest to data collection were averaged in order to best reflect the patients’ baseline. The majority of these labs were from clinic visits.
Covariates
Covariates of interest were age, gender, and hydroxyurea use at the time of study enrollment. Our SCD center recommends hydroxyurea to all patients with severe sickle cell disease (HbSS/HbSβ0) and others as clinically indicated.
Outcomes
The primary outcome was the painDETECT total score. Secondary outcomes were the association of total score and: 1) age, 2) gender, and 3) use of hydroxyurea.
Data Analysis
Descriptive statistics were performed. The questionnaire was scored based on the developer’s guidelines as outlined above. Total score was found to be normally distributed and age was skewed. As outlined above, the proportion of patients with definite, probable, and no neuropathic pain were calculated and those that scored ≥13 were defined as having evidence of neuropathic pain. Individual item responses about pain sensory symptoms rated 3 or higher (on 5 point Likert scale) were determined to be of clinical significance. Spearman correlation was used to determine the association of age and total score. Independent samples students t-test was used to determine the difference in mean total scores between: males and females and those that were or were not on hydroxyurea. Fisher’s exact test was used to determine the differences in the proportion of patients on hydroxyurea with (total score ≥13) and without (total score ≤12) neuropathic pain.
Results
Study Population
A convenience sample of 56 patients with SCD was approached for participation. All 56 patients approached completed the painDETECT questionnaire. There were no refusals to participate. Median age was 20.3 years (IQR 17–29) and 77% were female. The remainder of the demographics, baseline characteristics, summary of clinical events, and laboratory parameters are displayed in Table I.
Table I.
Demographics and Clinical Characteristics of Study Population
| Variable* | SCD Patients (n=56) | p-value |
|---|---|---|
|
| ||
| Median Age (yrs) | 20.3 (IQR 17–29) | |
|
| ||
| Gender: Female | 43 (77) | |
|
| ||
| Genotype | ||
| HbSS | 36 (64) | |
| HbSC | 14 (25) | |
| HbSβ+thal | 2 (4) | |
| HbSβ0thal | 2 (4) | |
| Other | 2 (4) | |
|
| ||
| Hydroxyurea | 39 (70) | |
|
| ||
| Labs (Mean±SD) | ||
| Hb (g/dL) | 9.8 (1.7) | |
| Reticulocyte count (%) | 7.8 (4.8) | |
| MCV (fL) | ||
| Hydroxyurea | 93 (12.5) | p=0.01 |
| No hydroxyurea | 83 (9.7) | |
|
| ||
| Number of Pain Events in Past 3 Months (requiring hospitalization or ED visit) | ||
| 0 | 29 (52) | |
| 1 | 14 (25) | |
| 2 | 9 (16) | |
| 3 | 1 (2) | |
| 4 | 3 (5) | |
|
| ||
| History of Depression | 1 (2) | |
Data are displayed as number of subjects (%) unless otherwise indicated.
General Outcomes
Mean pain score (0–10 scale) at the time of study completion was 2.9 (±2.6), mean pain score during the past 4 weeks was 5.2 (±2.7), and the strongest pain experienced during the past 4 weeks was 6.5 (±3.2). Thus, all patients reported experiencing pain in the past 4 weeks that was managed at home or in the hospital. Results of the pain course picture chosen are displayed in Figure 1 revealing the majority of patients (89%) chose a picture that supports a component of chronic pain. The most common sites of pain were the lower back/gluteus (48%), chest/torso (45%), bilateral arm (40%), abdomen (39%), upper back (34%), head/neck (30%), and bilateral thigh (25%). We found 59% stated their pain radiated to other body regions from the primary pain site which is a characteristic of neuropathic pain.
Almost 40% of patients have evidence of neuropathic pain
We found 23% of patients had a definite neuropathic pain component with scores ≥19 (range 19–28). An additional 14% of patients had a probable neuropathic pain component with scores ranging between 13–18. Thus, 37% of patients had evidence of neuropathic pain (score ≥13). There was no difference in the proportion of patients with neuropathic pain (score ≥13) between those with HbSS and HbSC disease (33% vs. 50%; p=0.28, Chi-square). Differences between the other genotypes were not analyzed due to small sample size in these groups. The proportions of patients reporting sensory symptoms of clinical significance (rated ≥3 on 5 point Likert scale) are displayed in Figure 2. Only 5% of patients were taking gabapentin, a drug used to treat neuropathic pain.
Figure 2. Proportion of Patients Reporting Sensory Symptoms of Clinical Significance.
Individual item responses about pain sensory symptoms rated 3 or higher (on 5 point Likert scale) were defined to be of clinical significance.
Older age and female gender were associated with neuropathic pain
Age was significantly positively correlated with total score [r=0.43; p=0.001] suggesting older patients experience more neuropathic pain. Females had significantly higher mean total scores than males [13 (±7.1) vs. 8.4 (±6.5); p=0.04].
Patients with neuropathic pain were more likely to be on hydroxyurea
There was a significant relationship between hydroxyurea and neuropathic pain. Significantly more patients with evidence of neuropathic pain (total score ≥13) were taking hydroxyurea compared to the proportion of patients taking hydroxyurea that did not have neuropathic pain (total score ≤ 12) [90% vs. 59%; p=0.015; Fisher’s Exact Test]. Furthermore, those that were taking hydroxyurea had significantly higher mean total scores than those that were not on the drug [13.4 (±7.4) vs. 8.7 (±5.7); p=0.03]. Patients taking hydroxyurea had significantly higher mean MCVs than those that were not on the drug [93 vs. 83; p=0.01, independent samples t-test] documenting adherence to hydroxyurea (Table I).
Discussion
We provide data supporting the existence of neuropathic pain in patients with SCD. Using a one page screening tool specifically targeted at the evaluation of neuropathic pain, we found that almost 40% of our SCD study population had evidence of neuropathic pain. This is almost twice what we originally hypothesized and twice what has been found in epidemiologic studies in chronic pain populations other than SCD.[14–16] Although the underlying pathobiology of SCD pain is not well known, increasing neurobiological evidence in the laboratory and humans[4, 25–28] suggests that neuropathic pain exists. Our study provides clinical data that patients are experiencing neuropathic pain symptoms that are congruent with these laboratory data. We found almost one-third of patients reported evidence of hot or cold sensitivity on the questionnaire (Figure 2). Interestingly, these data parallel the finding of hot and cold pain hypersensitivity (i.e., thermal hyperalgesia) in patients with SCD compared to race-matched controls as measured by quantitative sensory testing.[27, 28] Data in SCD mice also support this finding of thermal hyperalgesia.[25, 26] These parallel and congruent data provide further evidence that abnormal pain processing, either central or peripheral in origin, may be contributing to SCD pain.
As hypothesized, we found that the presence of neuropathic pain increases with age since higher total scores were significantly correlated with older age. These data are consistent with prior published data in painful conditions other than SCD where older age is a risk factor for the development of neuropathic pain.[12–15] Furthermore, in SCD, health care utilization for pain increases with age and older adolescents and adults experience chronic pain.[2, 29] This transition from acute to chronic pain in SCD that occurs with increasing age is an active area of investigation and our data suggest that the development of neuropathic pain may play a role in this transition warranting further investigation. The reason for this association between age and neuropathic pain is hypothesized to be due to abnormalities in pain processing that occur with aging[30, 31]; however, much is unknown and this is an active area of investigation. Our prior work[27] described that older age was also associated with thermal and mechanical pain hypersensitivity in patients with SCD as measured by quantitative sensory testing further affirming our findings.
We also found that neuropathic pain is associated with female gender since female patients had significantly higher mean total scores. This finding is consistent with our hypothesis and also supported by data in painful conditions other than SCD.[13, 14] The reason for this gender difference is unknown and is also an area of active investigation.
Despite pain being the most common complication of SCD, there is a lack of novel treatments for pain. Opioid based compounds have continued to be the mainstay of therapy over the past several decades. Interestingly, despite finding that almost 40% of patients experience neuropathic pain only 5% were taking gabapentin, a drug specifically targeted at treating neuropathic pain. Minimal use of neuropathic pain drugs was also shown by Wilke et al.[4] who found only 14% of their SCD study population reported taking an adjuvant drug that could treat neuropathic pain. The infrequent use of neuropathic pain drugs could be because patients are not systematically screened for the presence of neuropathic pain. Appropriate screening using validated tools, such as what was used in this study, can identify patients with SCD that may benefit from existing neuropathic pain therapies.[32] The three patients in our study that were taking gabapentin had total scores of 2, 7, and 23. Although gabapentin could have attenuated symptoms of neuropathic pain, we included these patients in our analysis since it is not known how the total score changes with neuropathic pain treatment. Including these patients could only have biased the study to finding a lower proportion of patients with neuropathic pain since two of the patients had total scores <12. A measure such as the painDETECT questionnaire could be used to assess the outcome or impact of new treatments or the new application of existing treatments. Thus, measuring the change in total score based on a systematic intervention to treat neuropathic pain would be an important follow-up study.
We found a significantly higher proportion of patients on hydroxyurea in the neuropathic pain group compared to those without evidence of neuropathic pain and those that were on hydroxyurea had significantly higher mean total scores. The reason for this is unclear. However, it may be possible that the use of hydroxyurea is a marker for more severe or refractory pain thus these patients were more likely to be on disease modifying therapy. Supporting this hypothesis, data reveal neuropathic pain is shown to be associated with higher pain intensity and longer duration, is harder to treat, and is more refractory to conventional analgesics.[14, 15] Currently, there are no data supporting the use of hydroxyurea as a treatment for neuropathic pain. Since our sickle cell center recommends hydroxyurea to all patients with severe SCD (HbSS and HbSβ0) and others as clinically indicated, we believe our cohort of patients is representative of our larger population of patients with SCD at our center.
Our study is limited by the cross-sectional design that does not allow for the ability to determine the age in which patients develop neuropathic pain. A prospective study would help answer this question and inform the study of the transition from acute to chronic pain. The painDETECT questionnaire has only been validated in adolescents as young as 14 years, thus we were unable to assess the presence of neuropathic pain in younger children and younger adolescents. Currently there are no published validated neuropathic pain screening tools for younger children and younger adolescents. Our study sample was limited to one site, thus our findings may not be generalizable, however, we have no reason to believe that characteristics of SCD pain differ from site to site. The painDETECT questionnaire was not specifically developed for SCD thus there may be differences in how neuropathic pain manifests in various disease states. However, the questionnaire has been previously used in many other diseases associated with pain[19–23] and it has been validated.[18]. We have no reason to believe that neuropathic pain in SCD is different from neuropathic pain in general. The use of a general tool also allows for the comparison to other diseases that a disease specific tool would not allow. Some patients may have been taking opioids before completing the questionnaire that could cause opioid induced hypersensitivity which can have characteristics of neuropathic pain. This study was not designed to determine the cause of neuropathic pain. It was designed only to determine if it exists in patients with SCD. Future work should be targeted at determining the etiology of neuropathic pain in patients with SCD. A control group was not included since the questionnaire is designed to screen patients with pain thus it would not be valid for healthy controls. A comprehensive neurologic exam was not done in the study. The validation process of the questionnaire included neurologic exams and the questionnaire was purposefully designed as a brief patient-reported outcome screening tool. Furthermore, there is strong evidence that patient-reported outcome measures are extremely important in the evaluation of patients’ symptoms.[33] We did not perform formal depression screening and it is possible that patients with depression experience more neuropathic pain. However, we obtained a self-reported history of depression and only one patient reported a history of depression. We were not able to evaluate the impact of lifetime history of pain events on the existence of neuropathic pain since many of our patients were adults and these clinical data would be limited by recall bias since adults utilize health care at multiple sites limiting the ability to accurately track their care[34] and a significant number of pain events are managed at home.[2, 35]
Conclusions
In summary, we found that almost 40% of our patients with SCD have evidence of neuropathic pain as reported on a validated measure specifically designed to differentiate neuropathic from non-neuropathic pain. We also found that the presence of neuropathic pain was positively associated with older age and female gender consistent with other studies of neuropathic pain in non-SCD painful conditions. Patients with neuropathic pain were more likely to be taking hydroxyurea which may serve as a marker for neuropathic pain being more refractory to treatment and thus these patients were more likely to be receiving disease modifying therapy. Despite the high proportion of patients identified with neuropathic pain in our cohort, only 5% of patients were taking a specific neuropathic pain drug. In conclusion, we provide data that neuropathic pain exists in SCD. Valid screening tools can further phenotype SCD pain and identify patients that could benefit from existing and future novel therapies targeted at the treatment of neuropathic pain. Furthermore, these tools could allow for the ability to determine the impact of these therapies on pain.
Acknowledgments
Funding: This work was supported in part by grants from the National Institutes of Health National Heart, Lung, and Blood Institute 1K23 HL114636-01A1 (AMB) and U54 HL090503 (AMB) and the Midwest Athletes Against Childhood Cancer and Blood Diseases Fund (AMB).
We would like to acknowledge Robbie Kattappuram and Sylvia Torres for their assistance with data collection. We very importantly acknowledge all the patients for their participation in the study.
Footnotes
Conflicts of Interest: The authors declare no competing financial interests.
Authorship Contributions
A.M.B. designed research, performed research, analyzed data, and wrote the manuscript; R.A.F. performed research and critically reviewed the manuscript; J.A.P. designed research and critically reviewed the manuscript.
Disclosure of Conflicts of Interest
The authors declare no competing financial interests.
References
- 1.Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med. 1994;330:1639–1644. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- 2.Smith WR, Penberthy LT, Bovbjerg VE, et al. Daily assessment of pain in adults with sickle cell disease. Ann Intern Med. 2008;148:94–101. doi: 10.7326/0003-4819-148-2-200801150-00004. [DOI] [PubMed] [Google Scholar]
- 3.Walco GA, Dampier CD. Pain in children and adolescents with sickle cell disease: a descriptive study. J Pediatr Psychol. 1990;15:643–658. doi: 10.1093/jpepsy/15.5.643. [DOI] [PubMed] [Google Scholar]
- 4.Wilkie DJ, Molokie R, Boyd-Seal D, et al. Patient-reported outcomes: descriptors of nociceptive and neuropathic pain and barriers to effective pain management in adult outpatients with sickle cell disease. J Natl Med Assoc. 2010;102:18–27. doi: 10.1016/s0027-9684(15)30471-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franck LS, Treadwell M, Jacob E, et al. Assessment of sickle cell pain in children and young adults using the adolescent pediatric pain tool. J Pain Symptom Manage. 2002;23:114–120. doi: 10.1016/s0885-3924(01)00407-9. [DOI] [PubMed] [Google Scholar]
- 6.Nolan VG, Zhang Y, Lash T, et al. Association between wind speed and the occurrence of sickle cell acute painful episodes: results of a case-crossover study. Br J Haematol. 2008;143:433–438. doi: 10.1111/j.1365-2141.2008.07354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith WR, Bauserman RL, Ballas SK, et al. Climatic and geographic temporal patterns of pain in the Multicenter Study of Hydroxyurea. Pain. 2009;146:91–98. doi: 10.1016/j.pain.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Resar LM, Oski FA. Cold water exposure and vaso-occlusive crises in sickle cell anemia. J Pediatr. 1991;118:407–409. doi: 10.1016/s0022-3476(05)82156-0. [DOI] [PubMed] [Google Scholar]
- 9.Freynhagen R, Bennett MI. Diagnosis and management of neuropathic pain. BMJ. 2009;339:b3002. doi: 10.1136/bmj.b3002. [DOI] [PubMed] [Google Scholar]
- 10.Sethna NF, Meier PM, Zurakowski D, et al. Cutaneous sensory abnormalities in children and adolescents with complex regional pain syndromes. Pain. 2007;131:153–161. doi: 10.1016/j.pain.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 11.Treede RD, Meyer RA, Raja SN, et al. Peripheral and central mechanisms of cutaneous hyperalgesia. Prog Neurobiol. 1992;38:397–421. doi: 10.1016/0301-0082(92)90027-c. [DOI] [PubMed] [Google Scholar]
- 12.Boogaard S, De Vet HC, Faber CG, et al. An overview of predictors for persistent neuropathic pain. Expert review of neurotherapeutics. 2013;13:505–513. doi: 10.1586/ern.13.44. [DOI] [PubMed] [Google Scholar]
- 13.Butler S, Jonzon B, Branting-Ekenback C, et al. Predictors of severe pain in a cohort of 5271 individuals with self-reported neuropathic pain. Pain. 2013;154:141–146. doi: 10.1016/j.pain.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Torrance N, Smith BH, Bennett MI, et al. The epidemiology of chronic pain of predominantly neuropathic origin. Results from a general population survey. J Pain. 2006;7:281–289. doi: 10.1016/j.jpain.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Bouhassira D, Lanteri-Minet M, Attal N, et al. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain. 2008;136:380–387. doi: 10.1016/j.pain.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 16.Rayment C, Hjermstad MJ, Aass N, et al. Neuropathic cancer pain: Prevalence, severity, analgesics and impact from the European Palliative Care Research Collaborative-Computerised Symptom Assessment study. Palliat Med. 2012 doi: 10.1177/0269216312464408. [DOI] [PubMed] [Google Scholar]
- 17.Jensen MP. Review of measures of neuropathic pain. Curr Pain Headache Rep. 2006;10:159–166. doi: 10.1007/s11916-006-0041-z. [DOI] [PubMed] [Google Scholar]
- 18.Freynhagen R, Baron R, Gockel U, et al. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin. 2006;22:1911–1920. doi: 10.1185/030079906X132488. [DOI] [PubMed] [Google Scholar]
- 19.Baron R, Tolle TR, Gockel U, et al. A cross-sectional cohort survey in 2100 patients with painful diabetic neuropathy and postherpetic neuralgia: Differences in demographic data and sensory symptoms. Pain. 2009;146:34–40. doi: 10.1016/j.pain.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 20.Steegers MA, Snik DM, Verhagen AF, et al. Only half of the chronic pain after thoracic surgery shows a neuropathic component. J Pain. 2008;9:955–961. doi: 10.1016/j.jpain.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 21.Gwilym SE, Keltner JR, Warnaby CE, et al. Psychophysical and functional imaging evidence supporting the presence of central sensitization in a cohort of osteoarthritis patients. Arthritis Rheum. 2009;61:1226–1234. doi: 10.1002/art.24837. [DOI] [PubMed] [Google Scholar]
- 22.Sonohata M, Tsuruta T, Mine H, et al. The relationship between neuropathic pain, and the function of the upper limbs based on clinical severity according to electrophysiological studies in patients with carpal tunnel syndrome. The open orthopaedics journal. 2013;7:99–102. doi: 10.2174/1874325001307010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koroschetz J, Rehm SE, Gockel U, et al. Fibromyalgia and neuropathic pain--differences and similarities. A comparison of 3057 patients with diabetic painful neuropathy and fibromyalgia. BMC neurology. 2011;11:55. doi: 10.1186/1471-2377-11-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soni A, Batra RN, Gwilym SE, et al. Neuropathic features of joint pain: a community-based study. Arthritis Rheum. 2013;65:1942–1949. doi: 10.1002/art.37962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hillery CA, Kerstein PC, Vilceanu D, et al. Transient receptor potential vanilloid 1 mediates pain in mice with severe sickle cell disease. Blood. 2011;118:3376–3383. doi: 10.1182/blood-2010-12-327429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kohli DR, Li Y, Khasabov SG, et al. Pain-related behaviors and neurochemical alterations in mice expressing sickle hemoglobin: modulation by cannabinoids. Blood. 2010;116:456–465. doi: 10.1182/blood-2010-01-260372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brandow AM, Stucky CL, Hillery CA, et al. Patients with sickle cell disease have increased sensitivity to cold and heat. Am J Hematol. 2013;88:37–43. doi: 10.1002/ajh.23341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Leary JD, Crawford MW, Odame I, et al. Thermal Pain and Sensory Processing in Children With Sickle Cell Disease. Clin J Pain. 2013 doi: 10.1097/AJP.0b013e318292a38e. [DOI] [PubMed] [Google Scholar]
- 29.Panepinto JA, Brousseau DC, Hillery CA, et al. Variation in hospitalizations and hospital length of stay in children with vaso-occlusive crises in sickle cell disease. Pediatr Blood Cancer. 2005;44:182–186. doi: 10.1002/pbc.20180. [DOI] [PubMed] [Google Scholar]
- 30.Edwards RR, Fillingim RB. Age-associated differences in responses to noxious stimuli. J Gerontol A Biol Sci Med Sci. 2001;56:M180–185. doi: 10.1093/gerona/56.3.m180. [DOI] [PubMed] [Google Scholar]
- 31.Edwards RR, Fillingim RB. Effects of age on temporal summation and habituation of thermal pain: clinical relevance in healthy older and younger adults. J Pain. 2001;2:307–317. doi: 10.1054/jpai.2001.25525. [DOI] [PubMed] [Google Scholar]
- 32.Chaparro LE, Wiffen PJ, Moore RA, et al. Combination pharmacotherapy for the treatment of neuropathic pain in adults. Cochrane Database Syst Rev. 2012;7:CD008943. doi: 10.1002/14651858.CD008943.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patrick DL, Burke LB, Powers JH, et al. Patient-reported outcomes to support medical product labeling claims: FDA perspective. Value in health: the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2007;10 (Suppl 2):S125–137. doi: 10.1111/j.1524-4733.2007.00275.x. [DOI] [PubMed] [Google Scholar]
- 34.Panepinto JA, Owens PL, Mosso AL, et al. Concentration of hospital care for acute sickle cell disease-related visits. Pediatr Blood Cancer. 2012;59:685–689. doi: 10.1002/pbc.24028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang WC. Pain at home in sickle cell disease: an underrecognized problem. Journal of Pediatric Hematology/Oncology. 2002;24:610–612. doi: 10.1097/00043426-200211000-00003. [DOI] [PubMed] [Google Scholar]



