Abstract
Background
Treatment strategies for meniscal injury are shifting from meniscectomy to repair, especially cell-based therapy. Delivering selected genes to donor cells can modify differentiation and proliferation. Efficiency of gene transfection and expression may relate to cell type.
Material/Methods
Full-length hIGF-1 cDNA was cloned into eukaryotic expression vector by PCR. Human BMSCs and meniscal fibrochondrocytes were isolated and cultured in vitro and hIGF-1 gene was transfected by FuGene 6. Expression of EGFP and hIGF-1 were determined. Biological activity of the hIGF-1 in medium was assessed by MTT chromatometry. Real-time quantitative PCR and Western blot were used to assess the expression of exogenous genes. Efficacy of gene transfection was detected by immunohistochemistry staining and flow cytometry.
Results
Sequences of hIGF-1 were verified by sequence analysis. Expression of EGFP increased gradually and reached peak intensity 48 h after transfection. Transfection efficiency of BMSCs was higher than meniscal fibrochondrocytes. The population doubling time was decreased in both cell types. Peak concentration of hIGF-1 in the medium of BMSCs and meniscal cells was 32.5±4.8 ng/ml and 24.5±4.6 ng/ml, respectively. Secreted hIGF-1 possessed the ability to enhance proliferation of the cell line. Results of qPCR and Western blot confirmed the expression of hIGF-1. Type II collagen appeared within the cells, and percentage of cells in S stage was increased in both cell types after transfection.
Conclusions
hIGF-1 cDNA can be transfected into BMSCs and meniscal fibrochondrocytes, resulting in gene expression. Expression efficiency in BMSCs was higher than that in fibrochondrocytes.
MeSH Keywords: Genes, vif; Insulin-Like Growth Factor I; Menisci, Tibial; Mesenchymal Stromal Cells
Background
Awareness of the important biomechanical functions of the menisci has led to changing therapeutic strategies for meniscal injury from total meniscectomy to repair or partial meniscectomy [1–3]. Because many reports revealed an early onset of degenerative changes in the knee joint following meniscectomy, orthopaedic surgeons are consequently aiming to preserve as much functional meniscal tissue as possible when treating meniscal injuries [4,5]. Cell-based therapy, including tissue engineering, offers a potentially effective strategy to reconstruct or repair the menisci. To modify the cells, regional gene therapy is an attractive option because the appropriate genes can be delivered to a specific anatomic site. Additionally, the transduced cells are inherently chondro-inductive and may enhance repair processes because both a paracrine and an autocrine response can occur [6,7]. Therefore, the possible responses of the cells to gene transfection may play a key role in this gene-enhanced cell-based strategy.
Bone marrow mesenchymal stem cells (BMSCs) are pluripotent progenitor cells that have been shown to provide a practical source of autologous cells with chondrogenic potential, which can served as a suitable source for meniscal defects repair [8]. Human insulin-like growth factor 1 (hIGF-1) is arguably one of the most critical growth factors in cartilage development and homeostasis [9] and plays a pivotal role in the stimulation of differentiated chondrocyte function and in the local transformation of undifferentiated cells in the subchondral bone pool. Theoretically, cytokine secreted by hIGF-1 gene transfected meniscal fibrochondrocytes or BMSCs can enhance cell metabolism while improving the microenvironment. In addition, it is difficult to affect the other cytokines secretion when using gene-modified cells to repair the damaged meniscus under local action. Responses of the differentiated and undifferentiated cells to the cytokine gene transfection are vital to the strategy for biological reconstruction of cartilage lesions. The effectiveness of the hIGF-1 gene transfected into BMSCs and meniscal fibrochondrocytes may be different. We propose the hypothesis that the efficiency of transfection and expression for exogenous gene maybe related to the differential degree and cell types. Therefore, the BMSCs and meniscal fibrochondrocytes were selected for testing with the hIGF-1 gene.
Material and Methods
hIGF-1 cDNA clone
Primers for the hIGF-1 gene were designed using the full-length cDNA published at the GeneBank (X00173), and contain the restriction enzyme sites for EcoR I and Xho I, as well as the start and termination codons. The upstream primer was 5′ GCCTCGAGGAAGATGCACACCATGTCCTC 3′, and the corresponding sequence was 5′ GCGAATTCCTACATCCTGTAGTTCTTGTTTC 3′. Then polymerase chain reaction (PCR) was performed to amplify the hIGF-1 cDNA and the product was assessed by electrophoresis and DNA sequencing. The fragment was extracted and subcloned into the PMD18-T vector. Both the plasmid PMD18-T -hIGF-1 and pIRES2-EGFP vector were digested with Xho I and EcoR I, then the recombinant vector was amplified in E. coli DH5 alpha selected with kanamycin and verified by electrophoresis and PCR. The recombinant plasmid contains the encephalomyocarditis virus internal ribosomal entry site (IRES), which allows hIGF-1 cDNA and enhanced green fluorescent protein (EGFP) to be translated simultaneously from the same mRNA.
Informed consent
This research was carried out in compliance with the Helsinki Declaration and was approved by the research ethics committee of Qingdao University. Patients were informed before any procedure and material was used for scientific research only after the informed consent was signed.
Culturing the hBMSCs and meniscal fibrochondrocytes
Menisci were retrieved from patients less than 50 years old who received meniscectomy under arthroscopy and divided were into small pieces approximately 2–3 mm3. The meniscal fragments were digested by 0.2% (weight per volume) trypsin for 30 min and then by 0.2% (weight per volume) collagenase for 3 h at 37°C. Cells were then collected and seeded onto tissue-culture dishes and cultured in 4 mL Dulbecco modified Eagle medium (Gibco, Grand Island, New York) containing 10% fetal bovine serum, 1% penicillin and streptomycin (weight per volume) at 37°C in a humidified incubator with 5% CO2.
Bone marrow aspirates were acquired aseptically from the femoral canal of patients under 50 years old who had undergone femur operations. Five to eight ml of bone marrow was mixed with 15 ml of Dulbecco modified Eagle medium containing 10% fetal bovine serum, 104 U/ml heparin and plated onto 35-mm dishes. Adherent cells were cultured in monolayers at 37°C in an incubator with 5% carbon dioxide.
When cells of both types had expanded to cover approximately 90% of the plate, adherent cells were trypsinized, counted, and replated at a density of 2×105cells per 35-mm dishes. To test the cytoactivity, a trypan blue stain rejection test was performed. Growth curves of cells and population doubling time (PDT) were also assessed to observe the propagation of both cell types.
hIGF-1 gene transfer mediated by FuGENE 6
Cells were treated with hyaluronidase at a density of 4U/ml 12 h before and at the start of transfection. The cells were transfected with a plasmid containing the hIGF-1 cDNA by FuGENE6 transfection reagent (Roche Company, Basel, Switzerland) following the manufacturer’s instructions when cells were 80% confluent. The ratio of FuGENE6 and the eukaryotic expression plasmid pIRES2-EGFP-hIGF-1 was 3:1.
Cells were also treated with empty plasmid without hIGF-1 cDNA, FuGENE6 reagent without vector, or DMEM medium alone to determine the influence of the procedure on cells.
Assessment of EGFP expression
At various time points after transfection (4 h, 8 h, 12 h, 24 h, 36 h, 48 h, 72 h, and 24 h after passage), EGFP expression in cells was observed by an inverted fluorescent microscope in a dark room, stimulated by ultraviolet light at a wavelength of 488 nm. To calculate the transfection efficiency, the EGFP-positive cells were counted in consecutive fields of vision and efficiency was determined by EGFP-positive cell number/counted cell number ×100%.
ELISA for the hIGF-1 concentration
Concentration of the hIGF-1 in culture medium was detected using an enzyme-linked immunosorbent assay (ELISA) kit (Diagnostics Laboratory Systems, Webster, TX, USA). Purified hIGF-1 standards and different hIGF-1 samples were loaded onto 96-well plates and incubated overnight at 4°C. After washing with phosphate-buffered saline (PBS), the plates were blocked by 3% (weight/volume) bovine serum albumin (BSA) for 4 h at 4°C. Then the plates were incubated with 100 μl of primary antibody diluted 1:1000 in 1% BSA and incubated with secondary antibody conjugated to alkaline phosphatase. After incubating with substrate at room temperature, the optical density at 405 nm was read on a plate reader.
The biologic activity of expressed cytokine
Biologic activity of the hIGF-1 protein secreted into the medium was assessed by MTT chromatometry. At 48 and 72 h post-transfection, medium was centrifuged and diluted with RPMI-1640 culture medium. Purified hIGF-1 standards were diluted by RPMI-1640 to concentrations of 6.25 ng/ml to 200 ng/ml. Diluted medium samples or purified hIGF-1 standards and culture medium were added into 96-well plates cultured with NIH3T3 cells at a density of 5×104 cells/ml, and incubated overnight. After washing with PBS, 10 μl MTT staining solution and 0.1 ml PBS were added to the plates then incubated for 4 h. Finally, the plates were mixed with 0.1 ml of 10% SDS then optical density at 620 nm was read on a plate reader.
Real time quantitative PCR
Total cellular RNA form 1.3×104 cells transfected with hIGF-1 was extracted by 4 mol/L guanidine thiocyanate and phenol. RNA was digested with RNase free DNase I, extracted twice with phenol/chloroform/isoamyl alcohol, and precipitated in 2.5 volumes of 100% ice-cold ethanol. The cDNA was then synthesized by reverse transcription. The hIGF-1 amplicon was ligated into the pGM-T vector. The plasmids were then extracted and diluted serially 1:10 into 5 standards. Quantitative standards of β2m were set as external references. PCR was performed with cDNA as the template according to the manufacturer’s instructions for the Quant SYBR Green PCR kit. At the end of each reaction, the expression level of hIGF-1 cDNA was analyzed.
Western-blot analysis
Lysates of the transfected cells were centrifuged to remove cells, cellular debris, and protein aggregates. Next, samples were loaded onto a 12% polyacrylamide gel and the proteins were separated by polyacrylamide gel electrophoresis. The separated proteins were then transferred to a nitrocellulose membrane and incubated with blocking buffer (10% nonfat dry milk, 1% 1M Tris [pH 7.4], and 3% 5 M NaCl) for 30 min. Blocking buffer and 1 μg/mL of rabbit anti-hIGF-1 monoclonal antibody (Santa Cruz Biotechnology) were added onto the membrane and incubated overnight at 4°C. The filter was then washed 3 times, incubated with the secondary antibody in the blocking solution, and ECL Western Blotting Detection Reagents (Amersham Biosciences, Buckinghamshire, England) were applied to the nitrocellulose, then exposed to radiographic film.
Immunohistochemistry staining for type II collagen
Immunohistochemistry staining for type II collagen was performed on meniscal chondrocytes and BMSCs grown onto coverslips using a standard indirect 3-step immunoperoxidase technique.
Cold methanol was added onto cells for fixation, and cells were then incubated with 3% hydrogen peroxide for 10 min to block the endogenous peroxidase. Primary antibody (rabbit anti-goat-type II collagen antibody; Santa Cruz Biotechnology) was applied overnight. Then biotinylated secondary antibody was loaded and DAB coloration was performed subsequently.
Non-immune rabbit serum and articular cartilage tissue were also used for negative and positive control.
Flow cytometry
Cells were counted, centrifuged, and washed with PBS, and then cold 70% ethanol was added into the cell pellet. A minimum of 10 000 live cell events were analyzed for cell cycle on a flow cytometer.
Statistical analysis
Differences between the 2 cell types for the results of population doubling time, transfection efficiency, ELISA, and RT-PCR were determined by performing the Student’s t test. Data are expressed as mean ± standard deviation of separate experiments. Differences with a p<0.05 were defined as significant.
Results
Construction of the hIGF-1 gene recombinant vector
Electrophoresis results confirmed the correct size of 410 bp. Sequence analysis was also completely consistent with the published sequence in GeneBank. The PCR detection use of the recombinant vector illustrated the outcome as well.
Characters of the cells
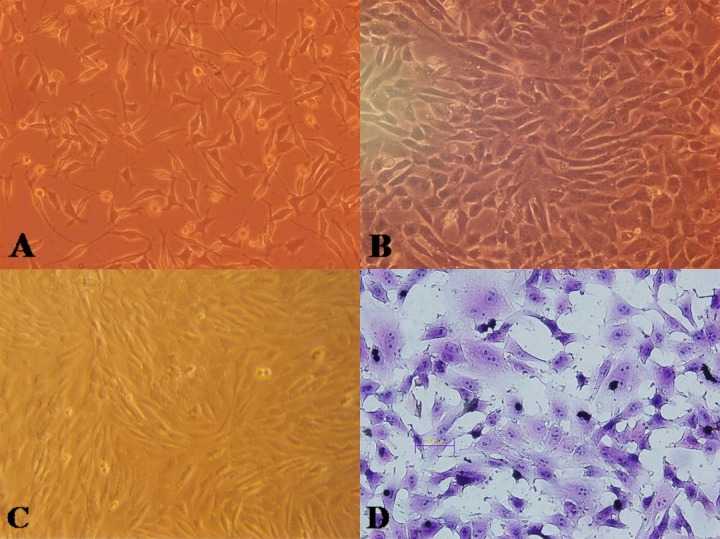
Meniscal fibrochondrocytes adhered onto the dishes 36 h after plating (Figure 1A) and grew to 70–80% confluence within 10 days. Cells in the second generation grew faster with a similar phenotype, such as polygonal, triangular, or elongated fibroblast-like. Trypan blue staining rejection test revealed more than 90% positive cells in the primary and second generations. Population doubling time of the meniscal chondrocytes was calculated as 45.5 h.
Figure 1.
Cell morphology before and after hIGF-1 gene introduction. (A) Before gene intervention, the meniscal cells had consistently distinguished different cell morphologies (original magnification, ×40). (B) After transfection, the proliferation was accelerated as well as enlarged to be slabstone-like (original magnification, ×40). (C) Before transfection, mainly BMSCs were in spindle shape with foci colonies (original magnification, ×40). (D) Cells enlarged with more mitotic phase (HE staining, ×40).
Bone marrow stem cells were found mainly in spindle shape, and could form separate foci colonies (Figure 1C). Cells grew to 70–80% confluence after 7–8 days, and proliferated faster when in their second generation. Trypan blue stain rejection test demonstrated about 92% positive cell rate. Population doubling time of the BMSCs was calculated as 30.5 h.
The EGFP expression and changes in cells after transfection
EGFP expression was seen in both cell types 4 h after transfection, and gradually increased to peak expression at 48 h, at which point intensity began to decrease. The transfection efficiency of meniscal fibrochondrocytes (Figure 2A) was 16±1.5%, compared to 20±2.5% for the BMSCs Figure 2B).
Figure 2.
Expression of the marker gene of EGFP in meniscal fibrochondrocytes (A) and in BMSCs (B).
After transfection, the population doubling time of meniscal fibrochondrocytes decreased from 45.5 h to 33.5 h. Karyokinesis was active in many of the cells with morphological changes. Cells proliferated faster and enlarged after gene introduction with increased percentage of polygonal and triangular morphology (Figure 1B).
The population doubling time of BMSCs decreased from 30.5 h to 26.5 h. A portion of the cells enlarged and changed into triangular, polygonal, or irregular shape (Figure 1D).
Significant (p<0.05) differences existed between the cells in transfection efficiency and discrepancy of PDT after gene introduction.
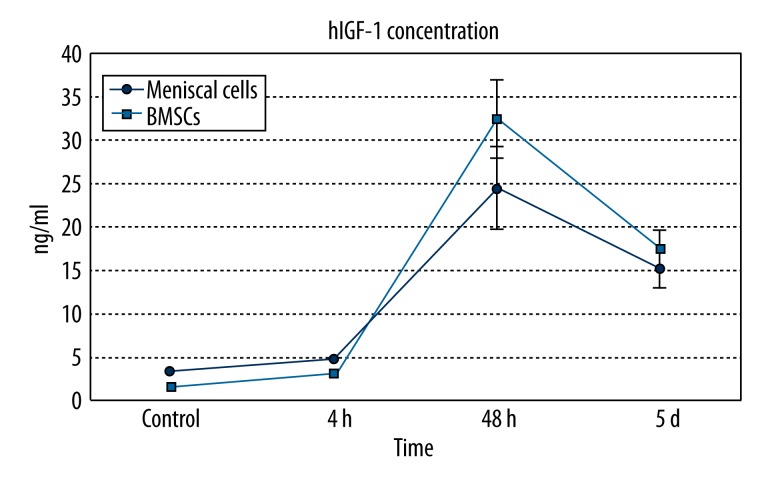
hIGF-1 concentration
The concentration of hIGF-1 in cell culture supernatants was detected to test the efficiency of protein secretion. The hIGF-1 concentration in the medium of untransfected meniscal cells was 3.25±0.14 ng/ml, which increased to 4.85±0.15 ng/ml at 4 h after transfection, peaked at a concentration of 24.5±4.6 ng/ml at 48 h, and then decreased to 15.2±2.2 ng/ml at 5 days after transfection.
In supernatants of untransfected BMSCs, hIGF-1 concentration was 1.50±0.12 ng/ml, rose to 3.05±0.16 ng/ml at 4 h after gene introduction, peaked at 32.5±4.8 ng/ml at 48 h, and then decreased to 17.5±2.3 ng/ml at 5 days.
Compared with meniscal cells, hIGF-1 concentration was higher in BMSCs at each time point (Figure 3).
Figure 3.
Curve graph for the concentration of hIGF-1 protein in the supernatants of cells.
Control groups were also set to detect any possible influence of the empty vector, FuGene 6, and DMEM on cells. The hIGF-1 concentrations of these groups were similar to that of untransfected controls.
Biologic activity of secreted hIGF-1
The biologic activity of the hIGF-1 secreted into medium was tested by its ability to promote the proliferation and division of the NIH 3T3 cell line. Results of the MTT chromatometry revealed the characteristics of the expressed hIGF-1 for enhancing proliferation of NIH 3T3 cells. The changing tendency of the absorbance value was in accordance with that of the concentration of the hIGF-1 detected in the secretions.
Real-time quantitative PCR
Following the transfection of BMSCs and meniscal fibrochondrocytes, the mRNA level of IGF-1 was examined using real-time quantitative PCR. Electrophoresis revealed the amplified fragments at the correct size of 409 bp. Compared with meniscal fibrochondrocytes, the expression level of hIGF-1 cDNA within BMSCs was significantly higher.
Western-blot analysis
The expression of IGF-1 protein was measured through Western blotting. The gray scale of the stained area was measured under identical conditions. Medium of the transfected cells was used to determine the secreted protein by Western blot. Bands appeared at 7.6 kD in both cell types.
Immunohistochemistry staining
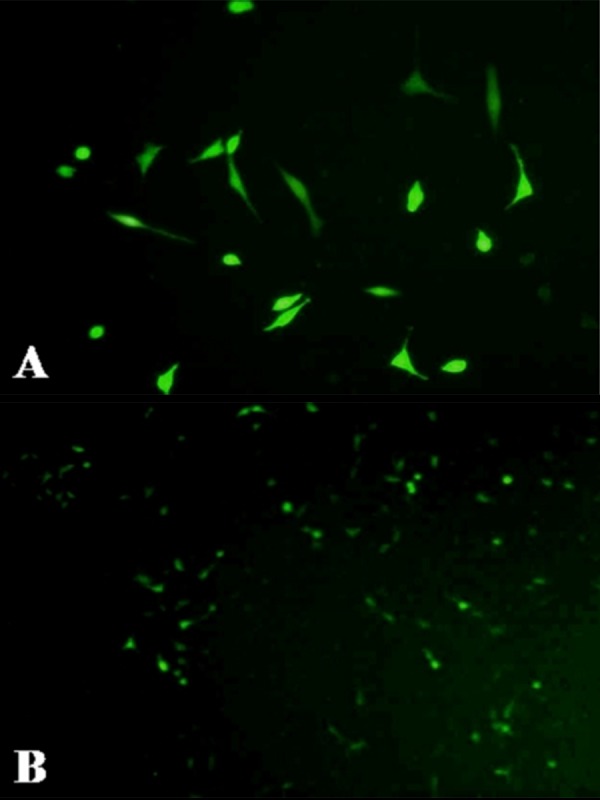
The meniscal fibrochondrocytes (Figure 4A) and BMSCs (Figure 4B) plated on coverslips were stained for type II collagen 72 h after transfection. Both cell types contained brown granules in their cytoplasm, which indicated positive expression of the type II collagen.
Figure 4.
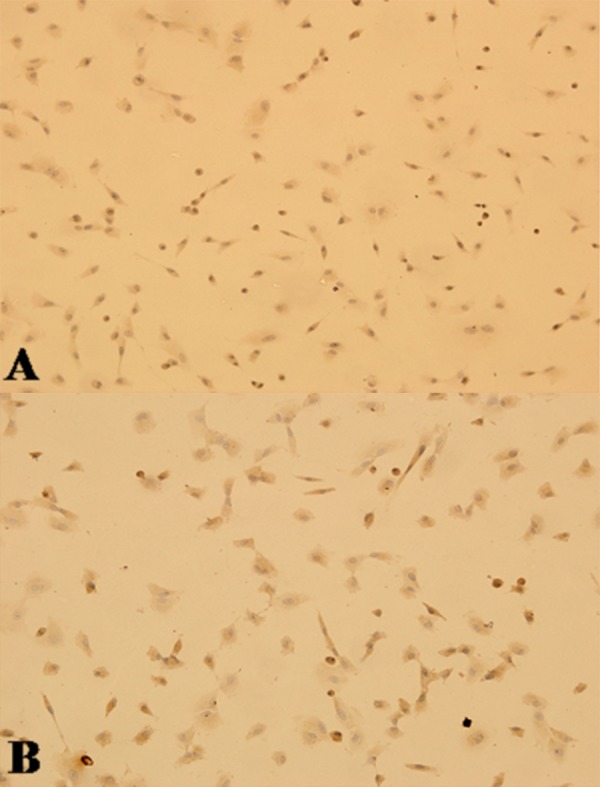
Immunohistochemistry stain for hIGF-1 in the transfected cells revealed multiple brown granules in the cytoplasm of meniscal cells (A) and BMSCs (B).
Flow-cytometry
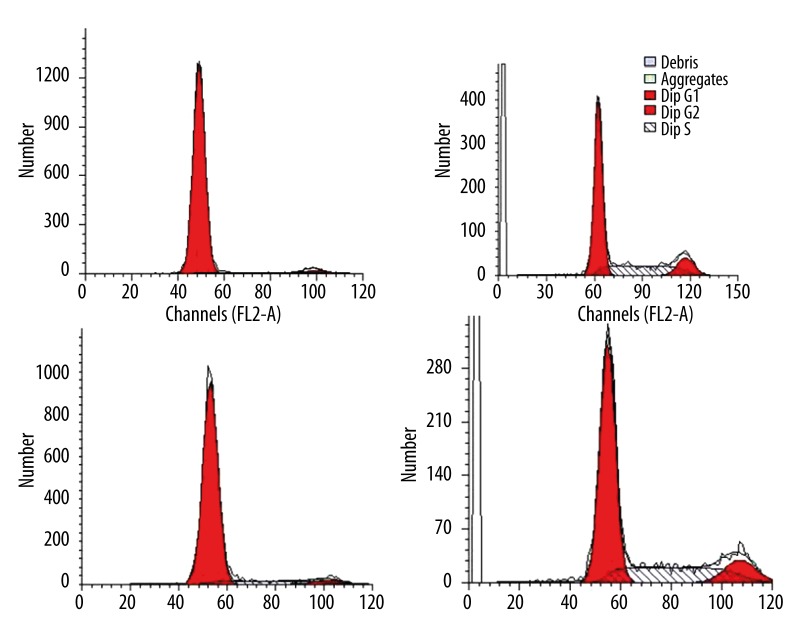
In both cell types, a majority of the cells were in G1 stage before transfection (Figure 5A, 5C), but the percentage of cells in S stage was increased after the introduction of hIGF-1 cDNA-containing vector (Figure 5B, 5D), indicating accelerated propagation.
Figure 5.
Flow cytometry analysis for the cells. Compared with cells before hIGF-1 gene transfection (A for meniscal cells, B for BMSCs), percentage of cells in G2 and S stage was increased (C for meniscal cells, D for BMSCs) after the introduction of hIGF-1 cDNA containing vector.
Discussion
One of the important repair strategies for cartilage injury or defect of hyaline or fibrocartilage is the cell-mediated reconstruction method facilitated by gene transfection [10]. Effective gene expression and the resulting responses of the transfected cells are key factors for therapeutic outcomes of the procedure. Determining which cell type is more appropriate for application is critical, and factors such as differentiation potential, bone marrow mesenchymal stem cells, or differentiated cells like fibrochondrocytes should be considered [11,12].
Our previous research tested the efficacy of gene expression and changes of meniscal fibrochondrocytes in different zones after hIGF-1 gene transfection [13]. Cells from medial zones with a blood supply were mainly polygonal in morphology and cells in the lateral zone without vessels were elongated and fibroblast-like in morphology. In the middle zone, different cell morphologies existed. The speed of growth for cells from the lateral zone was faster than those in the medial zone. The fibrochondrocytes in the lateral zone were more sensitive to hIGF-1 gene transfection in cell proliferation and changing cellular morphology. Diversity of the meniscal cell morphologies may be related to the compression or shear stress and nutrition of the microenvironment. Human IGF-1 has been shown to play a key role in the regulation of chondrocyte proteoglycan metabolism and is an important anabolic modulator of cartilage metabolism. Activity of hIGF-1 is mediated by high-affinity cell surface receptors [14]. Such characteristics make this potent mitogenic cytokine a suitable target gene for gene therapy.
Both of the cell types in this research reacted positively to transfection of hIGF-1 gene. This transfection procedure may play a role in the proliferation and maintenance of cell morphology. BMSCs and fibrochondrocytes were enlarged after hIGF-1 intervention, with more mitotic figures, and more polygonal, ellipse, or short fusiform versions of the cell phenotype occurring in fibrochondrocytes. The PDT represents proliferation rate of cells and was decreased in both cell types after the transfection. Flow-cytometry detection revealed the increased percentage of cells in S and M stage, indicating the vigorous function of synthesizing DNA and protein.
Differences, such as the variation in PDT, transfect efficiency, concentration of secreted cytokines, and transcription level of hIGF-1 cDNA, existed between the BMSCs and fibrochondrocytes. These differences may result from differences in cell membranes between the 2 cell types. The cell membrane of undifferentiated cells may be easier to be transited for liposome complex. In addition, discrepancy of cell division and proliferation affected the expression of transfected gene and secretion of protein. Such differences may play a role in determining the cell type best suited for cell therapy or tissue engineering.
The biological activity of the secreted hIGF-1 in the supernatants was detected by the ability to promote proliferation and division of NIH 3T3 cell line. One of the pivotal factors in an effective gene therapy strategy is whether the gene can be expressed and secreted to affect neighboring cells [15]. Thus, in the procedure of cloning the cDNA for hIGF-1, the oligonucleotide primers must be designed to clone the full-length gene, and the signal peptide (75bp), which plays an essential role for secretion, also needs to be added to ensure that the protein can be guided to transfer from cytoplasm into endoplasmic reticulum for further processes like cutting and folding, and formed the mature protein that can be secreted. Before the secretion, the signal peptide can be cut by enzyme in the inner side of the endoplasmic reticulum membrane. In related studies, gene therapy for meniscus repair were usually under local action and the amount of gene-modified cells was limited, and the secretion levels of IGF in cells does not cause IGF excessive increase in an experimental model. Moreover, in tissue engineering, IGF overexpression could promote cell proliferation ability and accelerate the process of tissue repair. Thus, it is difficult to cause destructive hypertrophic changes in connective tissue.
In the current research, liposomes were used as a tool for transient expression due to their safe characteristics for clinical application. Temporary gene expression may be ideal for many tissue engineering purposes where the process of new tissue formation should be stopped after reconstruction has been completed. Under these conditions, the few weeks of gene expression provided by liposomes may be ideal. Non-viral gene delivery systems can avoid the risk of insertional mutagenesis associated with retrovirus function, the risk of immunogenicity of adenovirus, and the risk of acquiring replication competence [16]. The transgenic efficiency and concentration of cytokines secreted into supernatants were sufficient for cell stimulation and possible differentiation. For optimal interaction with negatively charged cell membranes, the net charge of the lipid-DNA complex has to be positive. For the non-liposomal lipid formulation, FuGENE 6, the effective transfection system of this research, the optimal lipid/DNA ratio was 3:1 for both cell types.
We treated cells with hyaluronidase at a density of 4 U/ml 12 h before and at the start of the transfection procedure to improve transfection efficiency. Hyaluronidase is thought to improve lipid/DNA complex access to the cellular membrane by partially degrading the pericellular matrix and increasing the cellular permeability [17].
Conclusions
Many factors can affect the ultimate results and efficacy of gene therapy, such as gene type, transgenic methods, cytokine size, and cell type. The preliminary results of the current research only cover some facets of complicated details of gene therapy. More in-depth studies should be carried out to determine the best procedure for clinical use.
Footnotes
Source of support: The study was supported by the National Natural Science Foundation of China (No. 81171774, No. 81272056)
References
- 1.Aagaard H, Verdonk R. Function of the normal meniscus and consequences of meniscal resection. Scan J Med Sci Sports. 1999;9(3):134–40. doi: 10.1111/j.1600-0838.1999.tb00443.x. [DOI] [PubMed] [Google Scholar]
- 2.Hunter D. Degeneration of the meniscus and progression of osteoarthritis. HSS J. 2012;8(1):13–14. doi: 10.1007/s11420-011-9243-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roos H, Lauren M, Adalberth T, et al. Knee osteoarthritis after menicectomy: prevalence of radiographic changes after twenty-one years, compared with matched controls. Arthritis Rheum. 1998;41(4):687–93. doi: 10.1002/1529-0131(199804)41:4<687::AID-ART16>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 4.Nepple JJ, Dunn WR, Wright RW. Meniscal repair outcomes at greater than five years: a systematic literature review and meta-analysis. J Bone Joint Surg Am. 2012;94(24):2222–27. doi: 10.2106/JBJS.K.01584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Englund M, Roemer FW, Hayashi D, et al. Meniscus pathology, osteoarthritis and the treatment controversy. Nat Rev Rheumatol. 2012;8(7):412–19. doi: 10.1038/nrrheum.2012.69. [DOI] [PubMed] [Google Scholar]
- 6.Gelse K, von der Mark K, Aigner T, et al. Articular cartilage repair by gene therapy using growth factor-producing mesenchymal cells. Arthritis Rheum. 2003;48(2):430–41. doi: 10.1002/art.10759. [DOI] [PubMed] [Google Scholar]
- 7.Nixon AJ, Haupt JL, Frisbie DD, et al. Gene-mediated restoration of cartilage matrix by combination insulin-like growth factor-I/interleukin-1 receptor antagonist therapy. Gene Ther. 2005;12(2):177–86. doi: 10.1038/sj.gt.3302396. [DOI] [PubMed] [Google Scholar]
- 8.Qi Y, Feng G, Yan W. Mesenchymal stem cell-based treatment for cartilage defects in osteoarthritis. Mol Biol Rep. 2012;39(5):5683–89. doi: 10.1007/s11033-011-1376-z. [DOI] [PubMed] [Google Scholar]
- 9.Fortier LA, Barker JU, Strauss EJ, et al. The role of growth factors in cartilage repair. Clin Orthop Relat Res. 2011;469(10):2706–15. doi: 10.1007/s11999-011-1857-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi S, Mercer S, Eckert GJ, Trippel SB. Regulation of articular chondrocyte aggrecan and collagen gene expression by multiple growth factor gene transfer. J Orthop Res. 2012;30(7):1026–31. doi: 10.1002/jor.22036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang S, Piao J, Jin L, Zhou Y. Does pretreatment of bone marrow mesenchymal stem cells with 5-azacytidine or double intravenous infusion improve their therapeutic potential for dilated cardiomyopathy? Med Sci Monit Basic Res. 2013;19:20–31. doi: 10.12659/MSMBR.883737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li R1, Wei M, Shao J. Effects of verapamil on the immediate-early gene expression of bone marrow mesenchymal stem cells stimulated by mechanical strain in vitro. Med Sci Monit Basic Res. 2013;19:68–75. doi: 10.12659/MSMBR.883790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang HN1, Leng P, Wang YZ, Zhang J. Treating human meniscal fibrochondrocytes with hIGF-1 gene by liposome. Clin Orthop Relat Res. 2009;467(12):3175–82. doi: 10.1007/s11999-009-0870-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi S, Mercer S, Eckert GJ, Trippel SB. Growth factor regulation of growth factors in articular chondrocytes. J Biol Chem. 2009;284(11):6697–704. doi: 10.1074/jbc.M807859200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wan R, Hu J, Zhou Q, et al. Application of co-expressed genes to articular cartilage: new hope for the treatment of osteoarthritis (review) Mol Med Rep. 2012;6(1):16–18. doi: 10.3892/mmr.2012.859. [DOI] [PubMed] [Google Scholar]
- 16.Madry H, Cucchiarini M. Clinical potential and challenges of using genetically modified cells for articular cartilage repair. Croat Med J. 2011;52(3):245–61. doi: 10.3325/cmj.2011.52.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madry H, Trippel SB. Efficient lipid-mediated gene transfer to articular chondrocytes. Gene Ther. 2000;7:286–91. doi: 10.1038/sj.gt.3301086. [DOI] [PubMed] [Google Scholar]