Key Points
Eosinophils have been traditionally perceived as terminally differentiated cytotoxic effector cells. Recent studies have provided a more sophisticated understanding of eosinophil effector functions and a more nuanced view of their contributions to the pathogenesis of various diseases, including asthma and respiratory allergies, eosinophilic gastrointestinal diseases, hypereosinophilic syndromes and parasitic infection.
Eosinophils are granulocytes that develop in the bone marrow from pluripotent progenitors in response to cytokines, such as interleukin-5 (IL-5), IL-3 and granulocyte–macrophage colony-stimulating factor (GM-CSF). Mature eosinophils are released into the peripheral blood and enter tissues in response to cooperative signalling between IL-5 and eotaxin family chemokines.
Eosinophils in peripheral blood and tissues are uniquely identified by their bilobed nuclei, their large specific granules that store cytokines, cationic proteins and enzymes, and their expression of the IL-5 receptor and CC-chemokine receptor 3 (CCR3). In addition, the receptors sialic acid-binding immunoglobulin-like lectin 8 (SIGLEC-8) and SIGLEC-F are expressed by human and mouse eosinophils, respectively.
IL-5 has a central and profound role in all aspects of eosinophil development, activation and survival. IL-5 is produced by T helper 2 (TH2) cells, and more recently the contributions of the epithelium-derived innate cytokines thymic stromal lymphopoietin (TSLP), IL-25 and IL-33 in promoting eosinophilia via the induction of IL-5 have also been recognized.
Although eosinophil responses are influenced by cytokines produced by T cells, eosinophils in turn modulate the functions of B and T cells. Eosinophils also communicate with a range of innate immune cells (such as mast cells, dendritic cells, macrophages and neutrophils). Eosinophils serve to bridge innate and adaptive immunity by regulating the production of chemoattractants and cytokines (including CC-chemokine ligand 17 (CCL17), CCL22, a proliferation-inducing ligand (APRIL) and IL-6) and via antigen presentation.
Both successful and unsuccessful attempts to target eosinophils have yielded remarkable insights into their contribution to disease pathogenesis. Many eosinophil-associated inflammatory conditions have been shown to be heterogeneous in nature. As such, successful therapeutic strategies will depend on the correlation of disease activity with dysregulated eosinophil function as well as the identification of the crucial molecules that regulate eosinophil accumulation in the affected tissues.
Supplementary information
The online version of this article (doi:10.1038/nri3341) contains supplementary material, which is available to authorized users.
Subject terms: Granulocytes, Innate immunity, Infection, Allergy
This Review describes the unique biology of the eosinophil. The authors explain how eosinophils interact with other leukocyte populations to promote protective immunity following infection. They also discuss the pathological roles of eosinophils in allergic-type diseases, such as asthma and the hypereosinophilic syndromes.
Supplementary information
The online version of this article (doi:10.1038/nri3341) contains supplementary material, which is available to authorized users.
Abstract
Eosinophils have been traditionally perceived as terminally differentiated cytotoxic effector cells. Recent studies have profoundly altered this simplistic view of eosinophils and their function. New insights into the molecular pathways that control the development, trafficking and degranulation of eosinophils have improved our understanding of the immunomodulatory functions of these cells and their roles in promoting homeostasis. Likewise, recent developments have generated a more sophisticated view of how eosinophils contribute to the pathogenesis of different diseases, including asthma and primary hypereosinophilic syndromes, and have also provided us with a more complete appreciation of the activities of these cells during parasitic infection.
Supplementary information
The online version of this article (doi:10.1038/nri3341) contains supplementary material, which is available to authorized users.
Main
Eosinophils were first described in 1879 by Paul Ehrlich, who noted their unusual capacity to be stained by acidophilic dyes. Interestingly, our appreciation of this unique property of eosinophils is clear and steadfast, but a comprehensive understanding of the function of these cells in health and disease remains elusive. Some basic characteristics of eosinophils are established and accepted. It is clear that eosinophils are granulocytes that develop in the bone marrow from pluripotent progenitors. They are released into the peripheral blood in a phenotypically mature state, and they are capable of being activated and recruited into tissues in response to appropriate stimuli, most notably the cytokine interleukin-5 (IL-5) and the eotaxin chemokines. Eosinophils spend only a brief time in the peripheral blood (they have a half-life of ∼18 hrs)1 before they migrate to the thymus or gastrointestinal tract, where they reside under homeostatic conditions2. In response to inflammatory stimuli, eosinophils develop from committed bone marrow progenitors, after which they exit the bone marrow, migrate into the blood and subsequently accumulate in peripheral tissues, where their survival is prolonged (reviewed in REFS 3, 4, 5).
However, much remains unclear. For example, the long-held belief that eosinophils promote immunity to helminths has been called into question by results from animal studies, some of which suggest that eosinophils may be serving to promote the needs and longevity of specific parasites6,7. Likewise, eosinophils are clearly recruited to and activated in lung tissue as part of the pathophysiology of asthma, and most current evidence suggests that eosinophils contribute to airway dysfunction and tissue remodelling in this disorder8,9. Evolution tells us that the ability to induce pathology cannot be a 'raison d'être' for any existing cell lineage, and recent findings on the antimicrobial and antiviral activities of eosinophils suggest that the pathology that arises from dysregulated eosinophilia in the airways may be collateral damage related to host defence. Similarly, although there are now two unique eosinophil-deficient mouse strains10,11, there are no known unique eosinophil-deficiency states in humans to help us to decipher the importance of these cells in vivo.
This Review examines the most recent advances in our understanding of the contributions of eosinophils to the maintenance of health, and how dysregulated eosinophil function promotes various disease states. These advances were made possible by reagents, systems and methods that target eosinophil function and by the first clinical trials using humanized monoclonal antibodies specific for IL-5 (Table 1). These tools have been invaluable for shaping our current views on eosinophil function and for generating new hypotheses for future examination.
Table 1.
Tools for the eosinophil biologist
| System | Specific reagent or model | Characteristics | Refs |
|---|---|---|---|
| Cytological stains | Modified Giemsa | Stains the nucleus blue and granules bright red | 148 |
| Sirius red | Stains the nucleus blue and granules red | 149 | |
| Fast green and neutral red | Stains the nucleus red and granules bright green | 150 | |
| Laboratory antibodies for the detection of eosinophils by flow cytometry | Antibody specific for mouse IL-5Rα | Binds to the receptor for IL-5 | 151 |
| Antibody specific for mouse SIGLEC-F | Binds to a sialic acid-binding immunoglobulin-like lectin expressed by eosinophils | 148 | |
| Antibody specific for mouse CCR3 | Binds to the receptor for the eotaxins | 152 | |
| Laboratory antibodies for the detection of eosinophils by immunohistochemistry | Antibody specific for human EPX | Monoclonal; does not cross-react with myeloperoxidase | 153 |
| Antibody specific for mouse major basic protein | Binds to an eosinophil granule protein | 154 | |
| ELISA assays | ELISA for human EDN | Targets an eosinophil granule protein | 155 |
| ELISA for mouse EPX | Targets an eosinophil granule protein | 156 | |
| In vivo eosinophil depletion | Treatment with TRFK5 antibody | Rat monoclonal antibody that targets mouse IL-5 | 157 |
| Treatment with antibodies specific for mouse CCR3 | Rat antibodies that target mouse CCR3 | 158 | |
| Treatment with antibodies specific for mouse SIGLEC-F | Depletes eosinophils by targeting a sialic acid-binding immunoglobulin-like lectin | 159 | |
| Methods for generating eosinophils ex vivo | Culture of human CD34+ bone marrow cells with IL-5, IL-3 and GM-CSF | A cytokine-based method for generating eosinophils from human CD34+ cells in vitro | 133 |
| Culture of mouse bone marrow cells with SCF, FLT3L and IL-5 | A cytokine-based method for generating eosinophils from unselected mouse bone marrow progenitors in vitro | 160 | |
| Mouse strains for manipulating eosinophils | ΔdblGATA mice | Deletion of a palindromic GATA-binding site in the promoter of Gata1 results in the unique loss of cells of the eosinophil lineage | 10 |
| TgPHIL mice | The expression of diphtheria toxin A under the control of the Epx promoter results in the loss of eosinophil promyelocytes in the bone marrow | 11 | |
| Il5−/− mice | Il5 gene deletion; no eosinophilia in response to TH2 cell-inducing stimuli, although baseline eosinophil counts remain normal | 161 | |
| Il5ra−/− mice | Il5ra gene deletion; no eosinophilia in response to IL-5 | 162 | |
| Cd2-IL-5-transgenic mice | IL-5 overexpression is driven by the lymphocyte Cd2 promoter, resulting in systemic eosinophilia | 163 | |
| Cd3δ-IL-5-transgenic (NJ.1638) mice | IL-5 overexpression is driven by the T cell Cd3δ promoter and enhancer region, resulting in systemic eosinophilia | 164 | |
| Ccl11−/− mice | Ccl11 gene deletion, resulting in diminished recruitment of eosinophils to the lungs and gastrointestinal tract | 165 | |
| Ccl24−/− mice | Ccl24 gene deletion; CCL24 is the dominant chemokine for allergen-associated eosinophil recruitment to the lungs | 166 | |
| Ccl11−/−Ccl24−/− mice | Dual deletion results in profoundly diminished eosinophil recruitment in response to allergen sensitization and challenge | 167 | |
| IL-5/CCL24 double-transgenic mice | Overexpression of IL-5 (as in NJ.1638 mice) and CCL24 (under the control of the lung-specific Cc10 promoter) elicits profound pulmonary eosinophilia and eosinophil degranulation in situ | 168 | |
| Ccr3−/− mice | Gene deletion of the receptor for eotaxins, resulting in diminished recruitment of eosinophils to tissues | 169 | |
| Siglecf−/− mice | Exaggerated eosinophil responses and delayed resolution of lung eosinophilia in response to allergen challenge | 170 | |
| Humanized monoclonal antibodies for clinical applications | IgG1-isotype antibodies specific for human IL-5 (mepolizumab and reslizumab (humanized TRFK5)) | Indirectly target eosinophils by depleting IL-5 | 96,137,171 |
| IgG1-isotype antibody specific for human IL-5Rα (benralizumab) | Mediates the antibody-dependent cytotoxic destruction of eosinophils by targeting IL-5Rα | 96 | |
| CCL, CC-chemokine ligand; CCR3, CC-chemokine receptor 3; EDN, eosinophil-derived neurotoxin; ELISA, enzyme-linked immunosorbent assay; EPX, eosinophil peroxidase; FLT3L, FMS-like tyrosine kinase 3 ligand; GATA1, GATA-binding protein 1; GM-CSF, granulocyte–macrophage colony-stimulating factor; IL, interleukin; IL-5Rα, IL-5 receptor subunit-α; SCF, stem cell factor; SIGLEC-F, sialic acid-binding immunoglobulin-like lectin F; TH2, T helper 2. | |||
The unique biology of the eosinophil
Relatively few mature eosinophils are found in the peripheral blood of healthy humans (less than 400 per mm3), but these cells can be readily distinguished from the more prevalent neutrophils by virtue of their bilobed nuclei and large specific granules (Fig. 1). Human eosinophil granules contain four major proteins: eosinophil peroxidase, major basic protein (MBP) and the ribonucleases eosinophil cationic protein (ECP) and eosinophil-derived neurotoxin (EDN). The granules also store numerous cytokines, enzymes and growth factors. Other prominent features of eosinophils include primary granules that contain Charcot–Leyden crystal protein (also known as galectin 10 and eosinophil lysophospholipase) and lipid bodies, which are the sites of synthesis of cysteinyl leukotrienes, thromboxane and prostaglandins.
Figure 1. The eosinophil.
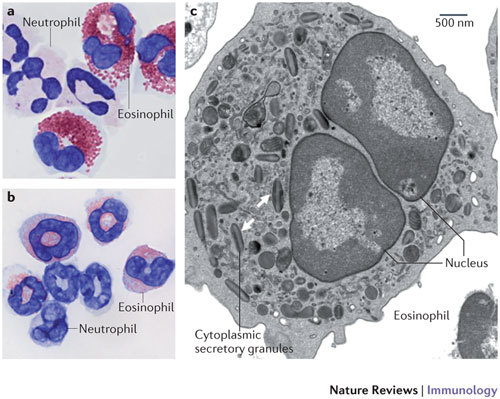
a | Human eosinophils from peripheral blood stained with modified Giemsa exhibit characteristic bilobed nuclei and large red-stained cytoplasmic secretory granules. The cells with multilobed nuclei and without large granules are neutrophils. Original magnification, ×100. b | The image shows eosinophils and neutrophils isolated from the spleen of a Cd2-interleukin-5-transgenic mouse and stained with modified Giemsa. c | The image shows a transmission electron micrograph of a mouse eosinophil. Cytoplasmic secretory granules are indicated by the arrows; the central core of these granules contains cationic major basic protein, and their periphery contains the remaining major cationic proteins, cytokines, chemokines, growth factors and enzymes. Original magnification, ×6,000.
Eosinophils have been identified and characterized in all vertebrate species, but their morphology, repertoire of cell-surface receptors and intracellular contents vary significantly between species. Of particular note, there are several crucial differences between mouse and human eosinophils that must be taken into account when interpreting mouse model studies of human disease12 (Fig. 1).
Eosinophils express surface receptors for ligands that support growth, adhesion, chemotaxis, degranulation and cell-to-cell interactions (Fig. 2). Many of the signalling pathways involved in these responses have been detailed in recent reviews3,4,13. Among the main receptors that define the unique biology of the eosinophil are interleukin-5 receptor subunit-α (IL-5Rα) and CC-chemokine receptor 3 (CCR3), as well as sialic acid-binding immunoglobulin-like lectin 8 (SIGLEC-8) in humans and SIGLEC-F (also known as SIGLEC-5) in mice. Pattern-recognition receptors (PRRs) are also likely to be important for eosinophil function, a subject that remains to be fully explored (Box 1).
Figure 2. Cellular features of eosinophils.
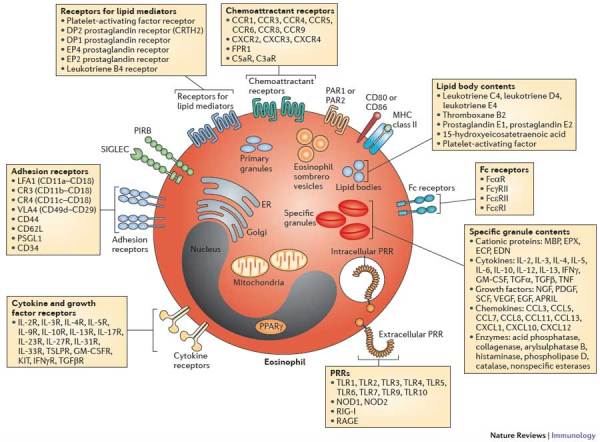
Eosinophils are equipped with features that promote interactions with the environment. In one such interaction, eosinophils release the contents of their specific granules in response to external stimuli. Some of these granule contents are released via membrane-bound vesicles known as eosinophil sombrero vesicles. Eosinophils also synthesize lipid mediators for release in cytoplasmic lipid bodies and store Charcot–Leyden crystal protein (CLC) in primary granules. Although not highly biosynthetic, mature eosinophils have minimal numbers of mitochondria and a limited endoplasmic reticulum (ER) and Golgi, as well as a nucleus. Eosinophils express a wide variety of receptors that modulate adhesion, growth, survival, activation, migration and pattern recognition. Mouse eosinophils do not express CLC or Fcε receptor 1 (FcεR1) and have divergent homologues of sialic acid-binding immunoglobulin-like lectin 8 (SIGLEC-8) and the granule ribonucleases eosinophil-derived neurotoxin (EDN) and eosinophil cationic protein (ECP)12. APRIL, a proliferation-inducing ligand; CCL, CC-chemokine ligand; CCR, CC-chemokine receptor; CXCL, CXC-chemokine ligand; CXCR, CXC-chemokine receptor; EGF, epidermal growth factor; EPX, eosinophil peroxidase; FPR1, formyl peptide receptor 1; GM-CSF, granulocyte–macrophage colony-stimulating factor; IFN, interferon; IL, interleukin; MBP, major basic protein; NGF, nerve growth factor; NOD, nucleotide-binding oligomerization domain protein; PAR, proteinase-activated receptor; PDGF, platelet-derived growth factor; PIRB, paired immunoglobulin-like receptor B; PPARγ, peroxisome proliferator-activated receptor-γ; PRR, pattern-recognition receptor; PSGL1, P-selectin glycoprotein ligand 1; RAGE, receptor for advanced glycation end-products; RIG-I, retinoic acid-inducible gene I; TGF, transforming growth factor; TLR, Toll-like receptor; TNF, tumour necrosis factor; SCF, stem cell factor; VEGF, vascular endothelial growth factor.
Factors that promote eosinophilia
IL-5 has a central and profound role in all aspects of eosinophil development, activation and survival (Box 2). Likewise, CC-chemokine ligand 11 (CCL11; also known as eotaxin), which is a ligand for CCR3, promotes eosinophilia both cooperatively with IL-5 and via IL-5-independent mechanisms14,15. Recently, several new factors that promote eosinophilic inflammation in vivo have been identified.
The epithelial cell-derived cytokines thymic stromal lymphopoietin (TSLP), IL-25 (also known as IL-17E) and IL-33 promote eosinophilia by inducing IL-5 production. TSLP is an IL-2 family cytokine that signals through a heterodimeric receptor that comprises the IL-7 receptor α-chain and a specific TSLP receptor β-chain. The TSLP receptor is expressed widely, by myeloid dendritic cells (DCs), CD4+ and CD8+ T cells, B cells, mast cells and airway epithelial cells. The TSLP receptor is also expressed by human eosinophils and modulates their survival and activation16.
IL-25 is produced primarily by activated T helper 2 (TH2) cells and mast cells and induces the production of TH2-type cytokines (including IL-5) from TH2 cells, as well as from the newly described populations of mouse innate lymphoid cells, which include nuocytes and natural helper cells17,18,19. In this manner, IL-25 can amplify the development, recruitment and survival of eosinophils in allergic states. Abundant expression of both IL-25 and the IL-25 receptor was also detected in a recent study of bronchial and skin biopsies from allergic human subjects20, and eosinophils themselves were identified as the primary source of IL-25 in patients with severe systemic vasculitis (Churg–Strauss syndrome)21.
IL-33 — which is a member of the IL-1 cytokine family — is expressed by epithelial cells, endothelial cells, fibroblasts and adipocytes, and is an endogenous danger signal known as an alarmin. IL-33 specifically modulates TH2-type pro-inflammatory signals following its release from necrotic cells. The IL-33 receptor ST2 (also known as IL-1RL1) is found primarily on TH2 cells, but IL-33-dependent responses from mouse nuocytes, natural helper cells and innate type 2 helper cells, and in human eosinophils themselves, have been described22,23,24. Furthermore, an IL-33- and IL-25-responsive innate lymphoid cell population has recently been defined in humans25. Although the biology of this cytokine has not been fully elucidated, IL-33 typically contributes to the synthesis and release of IL-5 from one or more of the aforementioned target cells, and thereby promotes systemic eosinophilia.
IL-23 is a member of the IL-12 family of cytokines that promotes the function of TH17 cells and also regulates allergic airway inflammation. Silencing the expression of IL-23 in mice that were sensitized and challenged with ovalbumin resulted in decreased recruitment of eosinophils to the lung tissue in association with diminished levels of IL-17 and IL-4 (Ref. 26). Accordingly, the overexpression of IL-23 was shown to augment antigen-stimulated eosinophil recruitment27. However, another study found that IL-23 suppressed eosinophilia in a mouse model of fungal infection, a response that was IL-17 independent28.
High-mobility group protein B1 (HMGB1) is another example of an alarmin that promotes eosinophilia. However, in contrast to IL-33, there is no evidence that eosinophil activation in response to HMGB1 involves IL-5. First identified as a nuclear protein and transcription factor, HMGB1 is expressed ubiquitously and mediates inflammatory responses via its receptors. The HMGB1 receptors that have been identified so far are receptor for advanced glycation end-products (RAGE), Toll-like receptor 2 (TLR2) and TLR4. Importantly, eosinophil mobilization and activation were observed in response to HMGB1 in tumour cell lysates29. Further work is needed in this area, as a better appreciation of the way in which eosinophils are activated in response to HMGB1 and other, related damage-associated molecular patterns (DAMPs) may explain the eosinophil recruitment that is observed in the setting of tissue destruction associated with myalgias and myopathies.
Eosinophil degranulation
Degranulation — that is, the release of granule contents into the extracellular space — is a prominent eosinophil function. Previously, the release of secretory mediators was assumed to take place primarily through cytolytic degranulation, a mechanism through which a pathogenic assault (real or perceived) results in the complete emptying of the eosinophil's arsenal of preformed cationic proteins. Interestingly, a careful analysis of electron micrographs of eosinophils degranulating in tissues suggested a more controlled process, which was given the name 'piecemeal degranulation'30 to reflect the fact that the eosinophil was able to release bits or pieces of its granule contents in response to a given stimulus, while remaining otherwise intact and apparently viable. Piecemeal degranulation is now accepted as the most commonly observed physiological form of eosinophil degranulation. Eosinophils undergoing piecemeal degranulation in response to cytokines, such as interferon-γ (IFNγ) and CCL11, develop cytoplasmic secretory vesicles, known as eosinophil sombrero vesicles31 (Fig. 2), and remain viable and fully responsive to subsequent stimuli.
A recent study has provided substantial insights into the molecular mechanism of piecemeal degranulation32. Specifically, IL-4 released from CCL11-activated eosinophils first forms a complex with IL-4 receptor subunit-α (IL-4Rα) within the granule membrane, and IL-4Rα thereby chaperones IL-4 to the membrane vesicles before its release from the cell. Although receptor-mediated trafficking pathways have not yet been defined for other eosinophil mediators33, this study provides an insight into the potential for exquisite molecular modulation of piecemeal degranulation32.
Eosinophils also release intact granules, which are capable of storing and releasing mediators in response to physiological secretagogues in the cell-free state34. Cell-free granules have been identified in tissues in association with eosinophil-associated disorders35, although their functional significance and their ability to respond to activating stimuli in situ await further evaluation.
Interactions of eosinophils with other leukocytes
During their transit from the bloodstream to the tissue, eosinophils use selectins and integrins to interact with endothelial cells, and they interact with epithelial cells at mucosal surfaces in a similar manner; these subjects have been reviewed extensively36. Eosinophils also interact with and modulate the functions of other leukocytes (Fig. 3).
Figure 3. Eosinophils modulate the function of other leukocytes.
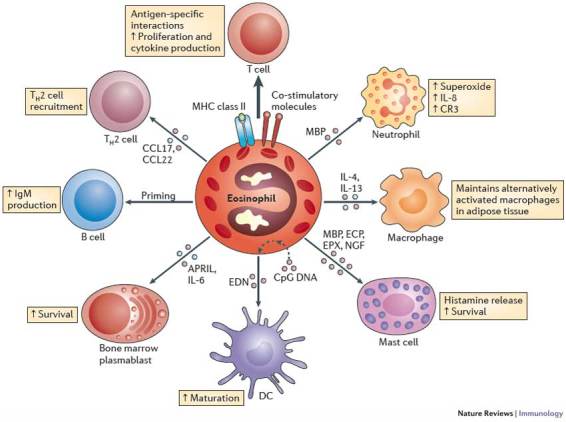
Eosinophils not only respond to signals, but also have a definitive impact on the actions of other leukocytes. Eosinophils can express MHC class II and co-stimulatory molecules, process antigens and stimulate T cells to proliferate and produce cytokines in an antigen-specific manner39. Furthermore, acting together with dendritic cells (DCs), eosinophils regulate the recruitment of T helper 2 (TH2) cells in response to allergen sensitization and challenge by producing CC-chemokine ligand 17 (CCL17) and CCL22 (Refs 40, 41). Eosinophils also prime B cells for antigen-specific IgM production39 and sustain long-lived plasma cells in mouse bone marrow via the production of a proliferation-inducing ligand (APRIL) and interleukin-6 (IL-6)44,45. Eosinophils that are stimulated by CpG DNA induce DC maturation51. Indeed, the eosinophil granule protein eosinophil-derived neurotoxin (EDN) promotes the maturation and activation of DCs52,53. Major basic protein (MBP) released from eosinophils activates neutrophils, causing them to release superoxide and IL-8 and increase their expression of the cell-surface integrin complement receptor 3 (CR3)146. Eosinophils also maintain alternatively activated macrophages in adipose tissue by producing IL-4 and IL-13 (Ref. 50). The eosinophil granule proteins MBP, eosinophil cationic protein (ECP) and eosinophil peroxidase (EPX) activate mast cells, resulting in the release of histamine. Likewise, eosinophil-derived nerve growth factor (NGF) prolongs mast cell survival147.
Interaction with lymphocytes. Eosinophils clearly respond to signals (such as IL-5) that are provided by T cells. Two recent studies indicate that T cells also respond to signals provided by eosinophils37,38. Although not 'professional' antigen-presenting cells, eosinophils can express cell-surface components that are required for antigen presentation (such as MHC class II molecules and the co-stimulatory molecules CD80 and CD86). Indeed, eosinophils can process antigens and stimulate T cells in an antigen-specific manner, resulting in T cell proliferation and cytokine release39. Furthermore, in experiments performed in both wild-type mice and transgenic mice that lack eosinophils (TgPHIL mice), eosinophils can augment allergic inflammation by regulating the production of TH2-type chemoattractants (including CCL17 and CCL22), which promote the recruitment of TH2 cells, and also through their interactions with DCs40,41. In addition, eosinophils release preformed cytokines (such as IL-4, IL-13 and IFNγ) that promote either TH2 or TH1 cell responses42.
Eosinophils also promote humoral immune responses. Indeed, they are capable of priming B cells for the production of antigen-specific IgM43. Most recently, the production of a proliferation-inducing ligand (APRIL) and IL-6 by eosinophils was shown to be crucial for the support of long-lived plasma cells in mouse bone marrow44. Interestingly, activated eosinophils from the bone marrow of adjuvant-immunized mice were found to be even more effective at supporting plasma cell survival than those from adjuvant-naive mice45.
Interactions with innate immune cells.Alternatively activated macrophages have a pivotal role in recruiting eosinophils to the tissues46,47 through the release of YM1 (also known as CHI3L3), a chitinase-like selective eosinophil chemoattractant48,49. Eosinophils likewise recruit alternatively activated macrophages to, and maintain their viability in, adipose tissue50, promote the maturation of monocyte-derived DCs in vitro51, and are required for the accumulation of myeloid DCs and the systemic production of TH2-type cytokines in mice with allergic airway disease. The eosinophil secretory mediator EDN promotes the activation and migration of DCs52,53.
Eosinophils communicate extensively with tissue-resident mast cells. Eosinophils and mast cells are found in close proximity to one another under homeostatic conditions in the gut, and they also colocalize in the allergic lung and in the inflamed gut in patients with Crohn's disease54. The bidirectional signalling that occurs between eosinophils and mast cells involves several immunomodulatory mediators. These include stem cell factor (also known as KIT ligand), granule proteins, cytokines (such as granulocyte–macrophage colony-stimulating factor (GM-CSF), IL-3, IL-5 and tumour necrosis factor (TNF)), nerve growth factor and mast cell proteases. Actual physical coupling of eosinophils and mast cells has been observed both in vitro and in vivo, and this interaction prolongs eosinophil survival54.
Eosinophil responses to pathogens and parasites
Eosinophils and helminths: who wins? The historic view that eosinophils promote host defence against helminths arose largely from histological images of eosinophils and parasites in tissue specimens and from in vitro studies that documented the antiparasitic activities of the eosinophil granule proteins MBP and ECP. With the development of reagents that block eosinophilia in mice (such as IL-5-specific antibodies) and of IL-5- or eosinophil-deficient mice, the picture has become more complex. For instance, the helminth Schistosoma mansoni, although not a natural mouse pathogen, can infect wild-type mice and can elicit a profound TH2-type cytokine-mediated pathology and cause the accumulation of eosinophils in tissues55. Although the eosinophil granule proteins ECP and MBP are toxic to both schistosomules and the larvae of S. mansoni, the manipulation of eosinophils in mouse models had no significant impact on disease development during S. mansoni infection56,57. However, in Strongyloides stercoralis and Angiostrongylus cantonensis infection models, eosinophil depletion resulted in prolonged survival of tissue-based larval forms of the parasites58,59. Thus, the role of eosinophils in mouse models of helminth infection remains unclear and controversial.
The interaction of eosinophils and helminths during infection in human subjects has been examined using a genomics approach60. The 434G>C polymorphism in the gene encoding ECP results in substitution of the cationic amino acid arginine for the neutral amino acid threonine at position 97. The genotype 434CC — which encodes the more neutral and somewhat less cytotoxic form of ECP — is found commonly among Ugandans, who live in a region endemic for S. mansoni infection. By contrast, the 434CC genotype is quite rare in Sudan, where S. mansoni is not endemic. Although this result suggests that there is no selective advantage for those individuals whose eosinophils might provide stronger antischistosomal host defence, the authors of this study determined that individuals with the 434CC genotype developed substantially less liver fibrosis secondary to S. mansoni infection. As such, the selective advantage may be for those individuals whose eosinophils promote less collateral tissue damage when faced with a similar pathogen burden. Similarly, cerebral malaria, a severe outcome of infection with Plasmodium falciparum, is also associated with eosinophilia and elevated serum levels of ECP. The haplotype strongly associated with susceptibility to severe disease encodes arginine at position 97 and thus the more cationic form of ECP61. The explanation of this finding awaits further clarification of the role of eosinophils in cerebral malaria.
The most recent developments in this field have exploited current concepts of eosinophils as immunomodulatory cells. In wild-type mice, infection with Trichinella spiralis induces eosinophil recruitment to the infected tissues and the formation of nurse cells in skeletal muscle. In eosinophil-deficient ΔdblGATA and TgPHIL mice, T. spiralis larvae do not survive, largely owing to the diminished recruitment of TH2 cells and a concomitant increase in the activity of inducible nitric oxide synthase (iNOS) and the synthesis of nitric oxide in local macrophages6,7. One interpretation of these results is that the parasites recruit eosinophils to support their own persistence and survival; another possibility is that eosinophils are recruited to maintain homeostatic balance by limiting the development of TH1-type immune responses that lead to oxidative damage and tissue destruction. How the parasite elicits this response and whether this finding is unique to Trichinella species are important subjects for future consideration. In addition, it will be interesting to address whether the mechanisms by which T. spiralis recruits eosinophils to muscle tissue, the activation state of the eosinophils at this site and the mediators released in situ are similar to those involved in eosinophilic inflammatory myopathies.
Eosinophils and bacteria: pathogens, probiotics and the microbiome. Early experiments carried out in vitro documented the bactericidal properties of the cationic eosinophil granule proteins MBP and ECP62,63. Subsequent studies exploring the mechanisms involved showed that ECP has a specific affinity for bacterial lipopolysaccharide and peptidoglycan and can agglutinate Gram-negative bacterial pathogens64. More recently, in vivo studies of the interaction of eosinophils with bacteria documented the catapult-like release of structures resembling neutrophil extracellular traps (NETs) from eosinophils, and this was associated with protection from the lethal sequelae of caecal ligation65. In contrast to NETs, which are composed primarily of nuclear DNA and neutrophil-specific proteins, eosinophil NET-like structures are composed of mitochondrial DNA, MBP and ECP66. Whether eosinophils and their secretory mediators have physiological bactericidal functions in vivo requires further study. Although eosinophil-enriched IL-5-transgenic mice were protected from the lethal sequelae of Pseudomonas aeruginosa infection67, recent findings suggest that IL-5-mediated protection during bacterial sepsis might be mediated by cells other than eosinophils68.
Recently, tremendous interest has developed regarding the immunomodulatory impact of probiotic or health-promoting bacteria. Although the mechanisms remain uncertain, oral administration of live probiotic Lactobacillus or Bifidobacterium species suppressed eosinophil recruitment in mouse models of allergic airway disease69,70. However, the therapeutic impact of probiotics in human studies of allergic disease has been less impressive. Indeed, in a recent prospective study in which allergic children were provided with oral supplementation with Lactobacillus rhamnosus GG or a placebo control, no significant differences were recorded in the number of asthma exacerbations per year, the number of days on medication, the peripheral blood eosinophil count or the serum ECP levels71.
In parallel, the interactions between commensal bacteria and tissue-resident eosinophils in the intestine have been the subject of recent investigations. Mice raised under germ-free conditions exhibited exaggerated eosinophilia in a model of allergic airway inflammation; this phenotype was reversed when the gastrointestinal tract was colonized with normal microflora72. Likewise, a large prospective study involving over 400 healthy infants73 concluded that individuals with greater bacterial diversity in the gastrointestinal tract had a lower risk of developing allergic sensitization later in life.
Eosinophils and viruses. Human respiratory viruses — such as influenza virus, parainfluenza virus, respiratory syncytial virus (RSV), coronaviruses and, most prominently, rhinoviruses — are among the most common causes of asthma exacerbation. Although asthma typically involves dysregulated eosinophil recruitment, and eosinophils are generally perceived as promoting disease pathology in this setting, the outcome of eosinophil–virus interactions has not been fully explored. A recent concept to emerge is that eosinophils and their secretory mediators may have a role in promoting antiviral host defence. An initial study showed that eosinophil secretory mediators decrease the ability of RSV to infect target host epithelial cells74. This was followed by a later report75 that found that eosinophils that were induced by allergen sensitization decreased viral loads during parainfluenza virus infection in a guinea pig asthma model. Accelerated clearance of RSV has been demonstrated in the lungs of eosinophil-enriched Cd2-IL-5-transgenic mice (which overexpress IL-5 under the control of the Cd2 promoter)76, and activated eosinophils protect mice from the lethal sequelae of acute pneumovirus infection (C. Percopo, K.D.D., S. Ochkur, J. Lee, J. Domachowske and H.F.R., unpublished observations). Moreover, both human and mouse eosinophils release immunomodulatory mediators, notably IL-6, in response to infection with respiratory virus pathogens77,78.
Hypereosinophilia is a frequent finding in late-stage HIV infection, typically in association with allergic and/or immune dysfunction and low CD4+ T cell counts79. Furthermore, one study documented large numbers of CD8+CD30+ T cell clones expressing TH2-type cytokines (including IL-5) in HIV-positive donors80, although another did not confirm this finding81. Interestingly, the granule protein EDN has been shown to have HIV-inhibitory activity82. However, the precise mechanisms by which eosinophils and their secretory mediators interact with viral pathogens remain to be elucidated.
Eosinophils and disease
There is extensive literature on eosinophil dysregulation associated with diseases such as asthma and eosinophilic oesophagitis. Although we know a substantial amount regarding how eosinophils develop and how they are recruited into various organs and tissues, there is a lack of understanding regarding the roles of eosinophils in eosinophil-associated diseases — even the relatively common ones. Targeting eosinophils therapeutically has revealed the complex and heterogeneous nature of eosinophil-associated diseases. We have selected the examples that follow to illustrate these principles; a more extensive list of diseases associated with eosinophilia is included in Supplementary information S1 (table) (see also Ref. 83).
Eosinophils and asthma. Asthma is a chronic inflammatory disease that is characterized by reversible airway obstruction and airway hyperreactivity in response to nonspecific spasmogenic stimuli. Eosinophils are a common feature of the inflammatory response that occurs in asthma, as they are recruited to the lungs and airways by cytokines that are released from activated TH2 cells and by a range of chemokines, most notably those of the eotaxin family.
A role for eosinophils in promoting the pathogenesis of some forms of asthma is supported by a large body of literature, primarily from studies of acute and chronic allergen-challenged mouse models of allergic airway disease84,85. Antigen sensitization and challenge, typically with ovalbumin or Aspergillus species, induces an allergic airway disease that replicates many of the hallmark features of allergic asthma, including increased numbers of cytokine-secreting TH2 cells and eosinophils in the airways, mucus hypersecretion and airway hyperreactivity. Chronic exposure to these antigens results in features of airway remodelling, including fibrosis and thickening of the basement membrane. Collectively, these studies suggest that targeting eosinophils themselves, eosinophil migration and/or eosinophilopoiesis should provide therapeutic benefit for the treatment of asthma.
These findings ultimately led to the development of two humanized IL-5-specific monoclonal antibodies, mepolizumab and reslizumab, which block the binding of IL-5 to IL-5Rα. In two of the earliest studies86,87, mepolizumab was administered to patients with mild atopic asthma and to healthy volunteers. In response, eosinophil numbers in the bronchial mucosa decreased by 50%, an observation that correlated with reduced levels of the prominent pro-fibrotic eosinophil secretory cytokine, transforming growth factor-β1 (TGFβ1), and with diminished deposition of extracellular matrix proteins. Similarly, another study showed that mepolizumab suppressed eosinophil maturation in the bone marrow and resulted in fewer CD34+IL-5Rα+ eosinophil progenitors in the lungs87.
In initial clinical trials, small cohorts of patients with mild or moderate asthma were treated with mepolizumab or reslizumab, respectively88,89. Both monoclonal antibodies were well tolerated by patients, and both reduced eosinophil numbers in the blood and airways. However, no objective measures of clinical improvement emerged (Box 3).
In part owing to the results from these clinical trials, the complex nature of the inflammatory response in asthma has been revisited90,91. Four distinct phenotypes based on the inflammatory cell profile in induced sputum have been introduced (Box 3) and, likewise, categories of asthma endogenous phenotypes (referred to as endotypes) based on molecular mechanisms and environmental influences have been defined. In subsequent studies, the therapeutic potential of IL-5-specific monoclonal antibodies was explored in a subset of asthmatics who were steroid dependent and had persistent sputum eosinophilia91,92,93,94,95. In these trials, the numbers of eosinophils in sputum fell to almost zero, a finding that correlated with decreased frequencies of exacerbations, a steroid-sparing effect, improved lung function and long-term improvements in asthma control.
These studies highlight the heterogeneous nature of asthma90,91 and, most importantly, define a clinical phenotype — known as steroid-resistant eosinophilic asthma — in which eosinophils make a clear and direct contribution to current disease and its management. New approaches that target eosinophils directly, such as the cytotoxic IL-5Rα-specific monoclonal antibody benralizumab, or indirectly, such as the IL-13-specific monoclonal antibody lebrikizumab, may further enhance therapeutic outcomes96,97.
Eosinophilic oesophagitis. Eosinophils are normally found in the gastrointestinal tract, notably in the caecum, but not in the oesophagus. First described by Landres and colleagues in 1978, eosinophilic oesophagitis is the most common of the eosinophil-associated gastrointestinal diseases. In 2007, an international consortium — the First International Gastrointestinal Eosinophil Research Symposium (FIGERS) — published consensus guidelines for diagnosis, which were revised in 2011. These criteria include: clinical evidence of oesophageal dysfunction (including dysphagia, abdominal pain and/or food bolus impaction); 2–4 biopsy samples from the proximal and distal oesophagus with ≥15 eosinophils per field at ×400 magnification; and no response to 6–8 weeks of high-dose proton-pump inhibitor therapy, ruling out gastro-oesophageal reflux disease. As we focus here on eosinophil-mediated mechanisms, we refer readers to a recent review on the complete natural history of eosinophilic oesophagitis98.
All evidence points to dysregulated eosinophilia as being central to the pathophysiology of eosinophilic oesophagitis. The aetiology appears to be dependent on the TH2-type cytokines IL-5 and IL-13. Patients often report concurrent allergic responses to food and airborne allergens, along with a family history of allergy, and there is an unexplained male predominance. Although absolute eosinophil numbers in biopsy samples at any given time may or may not correlate directly with disease severity, evidence of eosinophil activation — including the presence of extracellular granules and degranulated cationic proteins (such as MBP) — is prominent in tissue biopsy samples99. The eosinophil chemoattractant CCL26 (also known as eotaxin 3) is a prominent biomarker of eosinophilic oesophagitis. Indeed, CCL26 is highly upregulated in diseased tissues and also in peripheral blood cells in patients with this disorder. A single-nucleotide polymorphism (2,496T>G) in the 3′ untranslated region of the gene encoding CCL26 has been associated with increased susceptibility to eosinophilic oesophagitis, although the mechanisms involved are not yet known100. Susceptibility to eosinophilic oesophagitis has also been correlated with polymorphisms in the gene encoding TSLP101.
There are several mouse models of eosinophilic oesophagitis. Some of these models use oral or intranasal delivery of allergens to elicit tissue pathology, and others promote eosinophil recruitment to the oesophagus via the overexpression of IL-5 or IL-13. Among these models, one uses repeated intranasal delivery of fungal or insect aeroallergens102,103, which induces the expression of TH2-type cytokines and the eotaxin family member CCL11 (mice do not express CCL26), resulting in eosinophil recruitment to the oesophagus. Another mouse model involves systemic sensitization with ovalbumin in aluminium hydroxide adjuvant followed by repeated intra-oesophageal challenge104, which induces eosinophil recruitment associated with angiogenesis, basal zone hyperplasia and tissue fibrosis. Interestingly, although the administration of eosinophil-depleting SIGLEC-F-specific antibodies to these mice inhibits eosinophil recruitment and the associated tissue remodelling104, in another investigation, in which oesophageal remodelling was driven by lung-specific expression IL-13, no role for eosinophils was observed105. Similarly, ablation of CD4+ T cells — which presumably leads to a reduction in the levels of TH2-type cytokines — has only a limited impact on the recruitment of eosinophils to the oesophagus after chronic administration of Aspergillus species antigens102. Among the issues to be addressed in future studies is the role of eosinophil degranulation into the oesophageal tissue in these mouse models. Furthermore, mouse models that incorporate relevant clinical symptoms, such as failure to thrive, would certainly be of significant value.
Current therapies for patients with eosinophilic oesophagitis include the introduction of an elemental diet and treatment with steroids, which target the global inflammatory response and have an impact on eosinophil-derived cytokines106. Therapies that specifically target eosinophils are also being tested. For example, a randomized placebo-controlled double-blind trial in which adults with eosinophilic oesophagitis were treated with a humanized IL-5-specific monoclonal antibody (mepolizumab) resulted in a reduction in oesophageal inflammation and the reversal of tissue remodelling, but only minimal relief of symptoms107. Similar results were obtained in a prospective study in children, with clinical improvement observed in both experimental and placebo groups108. Interestingly, mepolizumab did not deplete eosinophils found in the duodenal mucosa of these patients109. However, the aforementioned studies suggest that this disorder may be primarily regulated by CCL26. As with asthma, the stratification of patients into subgroups that respond to specific therapies may ultimately improve clinical outcomes.
Eosinophilic myopathies. These conditions are among the most rare and poorly characterized of the eosinophil-related disorders, and include eosinophilic fasciitis (also known as Shulman's syndrome), toxic oil syndrome and eosinophilia–myalgia syndrome110 (Box 4). Although eosinophils are associated with these conditions, it is not clear how they are recruited to the affected tissue or what their contributions are to the pathology observed.
Eosinophilic myositis is a relatively rare condition in which the infiltration of muscle tissue by eosinophils is observed, sometimes in association with peripheral blood and bone marrow eosinophilia. The disease can result from helminth infection, or it can be toxin induced or idiopathic in nature. Recently, specific mutations in the gene encoding calpain 3 were identified in association with idiopathic eosinophilic myositis111. Calpain 3 is a muscle-specific neutral cysteine protease that interacts with intracellular myofibrillar proteins and has a role in sarcomere adaptation. However, there is no direct or obvious relationship between the actions of this enzyme and eosinophils or eosinophilia. It is not clear why mutations in calpain 3 result in signals that elicit eosinophil accumulation, what these signals might be, whether eosinophils are a primary or indirect target, and whether eosinophils are promoting tissue damage or altering the local immune status. One possibility is that inflamed, damaged muscle tissue releases endogenous alarmins (such as IL-33 and/or HMGB1) that activate innate immune signalling pathways that lead to peripheral blood and tissue eosinophilia. Of note, limb-girdle muscular dystrophy type 2A, which is a common autosomal recessive form of muscular dystrophy, has also been directly linked to mutations in the gene encoding calpain 3. Although eosinophils do not have a prominent role in this disorder, transient eosinophilia has been reported in the early stages of the disease. Similarly, no infiltration of eosinophils into muscle tissue was reported in calpain 3-deficient mice112, although this observation should be reassessed in other mouse strains.
Hypereosinophilic syndromes. Hypereosinophilic syndromes are disorders of eosinophil haematopoiesis that result in hypereosinophilia (defined as >1,500 eosinophils per mm3) in peripheral blood in the absence of any known aetiology. Although these disorders were recognized early on as clinically heterogeneous, recent studies have revealed the molecular basis for a few of the distinct phenotypes. The identification of myeloproliferative hypereosinophilic syndrome (MHES) emerged from the dramatic therapeutic responses observed in a subset of patients with hypereosinophilic syndrome following empirical treatment with imatinib, a tyrosine kinase inhibitor first developed for the treatment of chronic myeloid leukaemia113. This clinical observation led to the detection of a deletion in chromosome 4 that results in the fusion of the genes encoding pre-mRNA 3′-end-processing factor FIP1 (FIP1L1) and platelet-derived growth factor receptor-α (PDGFRA)114. This leads to the production of a FIP1L1–PDGFRA fusion protein that constitutively activates proliferation and survival pathways, resulting in the clonal proliferation of eosinophils, elevated serum levels of tryptase and vitamin B12 (also known as cobalamin), severe peripheral eosinophilia and end-organ damage, the most severe form of which is endomyocardial fibrosis. Other fusion kinases have also been identified in individuals with MHES; other individuals display clinical symptoms consistent with MHES but without a clear molecular diagnosis. Thus far, all of the PDGFRA- or PDGFRB-derived mutant fusion proteins that have been identified in humans have been associated with eosinophilia, for reasons that remain obscure.
The constitutive cellular activation and proliferation promoted by the FIP1L1–PDGFRA fusion protein has been explored in cell-culture models. For example, Ba/F3 immortalized mouse pro-B cells require the cytokine IL-3 for survival and proliferation in culture, but stable expression of the FIP1L1–PDGFRA fusion gene activates intracellular signalling pathways and eliminates the requirement for this cytokine113. Likewise, imatinib inhibits the growth of the human eosinophil leukaemia EoL-1 cell line, which expresses the FIP1L1–PDGFRA fusion protein115. Most intriguingly, the uncontrolled activity of the fusion protein lies within the PDGFRA component, as the fusion eliminates an inhibitory juxtamembrane region encoded by exon 12 of the PDGFRA gene, resulting in constitutive signalling by PDGFRA in the absence of its ligand116.
In contrast to the myeloproliferative variants, eosinophilia in lymphocytic-variant hypereosinophilic syndrome (LHES) results from aberrantly activated T cell clones that constitutively produce eosinophilopoietic cytokines, including IL-5. The resulting eosinophilia is thus reactive. The aberrant T cell clones (which typically have a CD3−CD4+ phenotype) are also associated with elevated serum levels of IgE and CCL17, and elicit predominantly skin manifestations, including pruritus, eczema, erythroderma, urticaria and angio-oedema. Individuals with this diagnosis respond to treatment with steroids, with cytotoxic agents (such as hydroxyurea) and with mepolizumab117, which reduced the requirement for corticosteroids in clinical studies.
These two defined variants of hypereosinophilic syndrome currently represent a minority of cases. Indeed, a recent study showed that FIP1L1–PDGFRA fusions were associated with only 11% of cases of hypereosinophilic syndrome, and LHES accounted for only 17% of cases118. The classification of hypereosinophilic syndrome is currently a work in progress, and attempts are being made to balance the clinical diagnosis with the predicted response to therapy119.
In an initial mouse model, bone marrow transplantation using haematopoietic progenitors that had been retrovirally transduced with FIP1L1–PDGFRA resulted in myeloproliferative disease120. Another group created a model that combines features of both myeloproliferative and lymphocytic-variant disease121 by transducing haematopoietic progenitors from Cd2-IL-5-transgenic mice with FIP1L1–PDGFRA, which resulted in profound peripheral eosinophilia in association with tissue infiltration. Most recently, mice lacking the serine/threonine kinase NIK (also known as MAP3K14) were found to develop a CD4+ T cell-dependent blood and tissue eosinophilia122. However, future studies will be necessary to determine whether these mouse models will be useful in identifying the disease mechanisms underlying distinct hypereosinophilic syndromes.
Eosinophils: changing perspectives
The field of eosinophil research is one of changing perspectives and emerging new directions. Eosinophils are clearly capable of more sophisticated immune functions than previously thought, as shown by their nuanced degranulation responses to distinct stimuli and their complex interactions with other leukocytes and pathogens. Both successful and unsuccessful attempts to target eosinophils have yielded remarkable insights into disease pathogenesis. Asthma and hypereosinophilic syndromes are now understood to be complex heterogeneous disorders that require tailored therapeutic strategies. Assessing the role of endogenous and exogenous PRR ligands in eosinophil responses and clarifying the relationship between eosinophil degranulation and tissue remodelling will be important goals for future research. A better understanding of these and other aspects of eosinophil biology will aid the development of new therapeutic strategies for diseases characterized by eosinophil dysregulation.
Box 1 | Receptors important for eosinophil function.
Interleukin-5 receptor subunit-α
The T helper 2 (TH2) cell-associated cytokine interleukin-5 (IL-5) has a unique and profound impact on nearly all aspects of eosinophil biology. Originally known as T cell replacing factor and murine B cell growth factor II, and later as eosinophil differentiation factor, IL-5 is produced by activated TH2 cells and, in smaller amounts, by mast cells, natural killer (NK) cells, natural killer T (NKT) cells and eosinophils themselves. In addition, several new sources of IL-5 have been identified in mouse models. These sources include KIT+ innate natural helper cells17, nuocytes17 and IL-25- or IL-33-responsive innate IL-5-producing cells18. IL-5 functions synergistically with the TH2-type cytokines IL-4 and IL-13, and with the eosinophil chemoattractants CC-chemokine ligand 11 (CCL11), CCL24 and CCL26 (also known as eotaxin, eotaxin 2 and eotaxin 3, repectively) to promote eosinophil activation and recruitment into tissues in acute inflammatory responses5,123.
As such, IL-5 receptor subunit-α (IL-5Rα) is the most prominent cytokine receptor associated with eosinophils112,124. In humans and mice, IL-5Rα is expressed by eosinophils and basophils. Mouse B1 cells also express IL-5Rα, and it functions to promote the proliferation and survival of these cells. The IL-5 receptor is heterodimeric; the α-subunit couples with a signalling β-subunit that is shared with the receptors for IL-3 and granulocyte–macrophage colony- stimulating factor (GM-CSF). IL-5 receptor signalling promotes the development of eosinophils from committed progenitors, induces eosinophil activation and sustains eosinophil survival in peripheral blood and tissues.
Humanized IL-5-specific monoclonal antibodies (namely, mepolizumab and reslizumab) and a humanized IL-5Rα-specific monoclonal antibody (namely, benralizumab) are under exploration for the therapeutic management of dysregulated eosinophilia92,93,94,95,96,125.
Chemokine receptors
CC-chemokine receptor 3 (CCR3) mediates eosinophil chemotaxis in response to the eotaxins, CCL11, CCL24 and CCL26 (Ref. 126). CCR3 can also be activated by CCL5 (also known as RANTES), CCL7 (also known as MCP3), CCL8 (also known as MCP2) and CCL12 (also known as MCP5). Eosinophils also express CCR1 — which is the primary receptor for CCL3 (also known as MIP1α) and CCL5 — and the platelet-activating factor receptor.
SIGLEC-8 and SIGLEC-F
Sialic acid-binding immunoglobulin-like lectin 8 (SIGLEC-8) is a cell-surface immunoglobulin-like lectin that is expressed predominantly by human eosinophils. Mouse eosinophils express a functional paralogue, SIGLEC-F127. SIGLEC-8 and SIGLEC-F are members of a larger family of structurally related carbohydrate-binding proteins. Although the function of these proteins from the perspective of the eosinophil remains uncertain, antibodies specific for SIGLEC-8 or its recently identified carbohydrate ligand (6-sulpho sialyl Lewis X) promote selective eosinophil apoptosis. In particular, SIGLEC-8- specific antibodies exert this effect in physiologically relevant in vivo models128. Thus, these SIGLEC proteins represent important targets for potential therapeutic ablation129.
Pattern-recognition receptors
Several families of pattern-recognition receptors (PRRs) are expressed by eosinophils130. Toll-like receptors (TLRs) are expressed by both human and mouse eosinophils, although at lower levels than by neutrophils and macrophages. TLR7 — which is localized in endosomes and detects single-stranded RNA — is by far the most prominent TLR expressed by eosinophils. It is not yet clear what exact role TLR7 has in promoting eosinophil function in vivo. However, priming eosinophils with IL-5 promotes responsiveness to the TLR7 ligand R837 and enhances the release of the pro-inflammatory cytokine IL-8 via unknown mechanisms. Activation of TLR7 regulates the adhesion, migration and chemotaxis responses of eosinophils and prolongs eosinophil survival131.
Box 2 | Eosinophil development.
The signals that promote the differentiation of eosinophils from bone marrow progenitors and commitment to the eosinophil lineage are not completely understood. Current models point to a unique role for interleukin-5 (IL-5) — with contributions from IL-3 and granulocyte–macrophage colony-stimulating factor (GM-CSF) — in promoting the expansion of the eosinophil lineage from committed progenitors in the bone marrow (reviewed in Ref. 132).
Numerous studies have focused on transcription factor networks and the hierarchical expression of transcription factors that promote eosinophil development. Notable interactions are those that involve members of the GATA-binding protein family (including GATA1 and GATA2), as well as CCAAT/enhancer-binding proteins (such as C/EBPα and C/EBPε) and PU.1. Expression or overexpression of GATA1 or GATA2 promotes eosinophil lineage commitment and the development of myeloid progenitor cells, and deletion of a GATA-binding enhancer site in the mouse Gata1 gene results in a unique loss of the eosinophil lineage. Functional interactions between GATA1, PU.1 and C/EBPε have been reported in eosinophil promyelocyte cell lines and, more recently, researchers identified both activator and repressor isoforms of C/EBPε that modulate the differentiation of human CD34+ progenitor cells into eosinophils in vitro133. However, these transcription factors also have roles in supporting the development of other haematopoietic lineages. There are no known transcription factors that are uniquely dedicated to promoting eosinophil lineage commitment and differentiation.
Eosinophil lineage-committed progenitor cells have recently been identified in the bone marrow of healthy humans134,135. These lineage-committed progenitors are defined as CD34+CD38+IL-3Rα+CD45RA−IL-5Rα+ and generate only eosinophils under ex vivo culture conditions that are enriched in stem cell factor, IL-3, IL-5, GM-CSF, erythropoietin and thrombopoietin. Most surprisingly, human IL-5Rα+ eosinophil lineage-committed progenitor cells are direct descendants of IL-5Rα−common myeloid progenitors and constitute a lineage that is distinct from granulocyte–macrophage progenitors (GMPs), which give rise to neutrophils and basophils in culture. By contrast, mouse eosinophil haematopoiesis proceeds somewhat differently, as eosinophil lineage-committed progenitor cells are a subpopulation of mouse GMPs.
Eosinophils can also develop from CD34+ progenitor cells that are found outside the bone marrow, notably in lung tissue. The mobilization of CD34+ progenitors from the bone marrow to the lungs has been observed in mouse models of allergic airway inflammation. Progenitors in the lungs can then give rise to mature eosinophils via a process that also depends directly on IL-5 (Ref. 136).
Box 3 | Eosinophils and asthma: complexity, controversy and consensus.
Eosinophil accumulation in the airway wall and lumen is a prominent feature of asthma. However, the part played by eosinophils in promoting the cardinal features of this disorder has been the subject of recent controversy. Most available evidence from mouse models suggests that the activation of eosinophils contributes directly to the mucous production, bronchoconstriction and airway dysfunction and remodelling that are characteristic of allergic asthma. As such, eosinophils and molecules that regulate eosinophil development and recruitment are perceived as appropriate targets for therapeutic ablation137,138.
One conflicting perspective emerged from studies of allergic airway disease in the two eosinophil-deficient mouse models (see Table 1). TgPHIL mice that were sensitized and challenged with an allergen responded as anticipated, with diminished mucous production and lower levels of airway hyperreactivity compared with wild-type mice11. By contrast, initial results from ΔdblGATA mice suggested that eosinophils had no role in promoting acute airway responses139. These differences, once highly controversial, have since been attributed to variations in the mouse background strain140.
At the same time, results from the first safety and efficacy trials of humanized monoclonal antibodies specific for interleukin-5 (IL-5) were published87,88. The target populations for these trials were broadly defined, and included individuals with mild to moderate asthma. In these cohorts, the IL-5-specific antibodies were quite effective at removing eosinophils from the blood and the airways; however, no objective clinical benefits emerged. Although it was possible to conclude that eosinophils are unimportant in functional asthma pathogenesis, it was also evident that a large portion (up to 50%) of the eosinophils present in lung tissue were not removed and remained in the tissue both during and following the completion of the IL-5-specific antibody therapy.
The recognition of heterogeneity within the group of diseases currently classified as asthma has led to the introduction of the concept of disease endotypes91, as well as of specific inflammatory phenotypes (namely, neutrophilic asthma, eosinophilic asthma, mixed granulocytic asthma and paucigranulocytic asthma)90. One of the most recent findings is that patients with poorly controlled, steroid-resistant eosinophilic asthma respond to IL-5-specific monoclonal antibody therapy with eosinophil clearance and marked improvements in important objective measures of disease92,93,94,95.
Box 4 | Eosinophilia–myalgia syndrome.
Eosinophilia–myalgia syndrome (EMS) is a multisystem disorder that was first formally documented in a 1989 report of three cases in which eosinophilia and myalgias were connected to the ingestion of L-tryptophan dietary supplements141. Symptoms included severe muscle pain accompanied by profound peripheral eosinophilia. By 1990, more than 1,000 cases had been identified. The US Centers for Disease Control and Prevention defined EMS by three criteria: peripheral eosinophilia of ≥109 eosinophils per litre of blood; generalized myalgias of sufficient severity to interfere with daily activities; and the absence of an infectious or neoplastic aetiology. Although a specific impurity (1,1′-ethylidenebis[tryptophan]; also known as peak E) in L-tryptophan from one dietary supplement supplier was identified142, there has never been closure on a number of issues, including the disease-eliciting potential of this impurity (or of L-tryptophan itself) in a robust animal model of disease. Similarly, there is no definitive information on the molecular signals that promote eosinophilia and eosinophil tissue infiltration in EMS, nor is it clear whether the eosinophils were in fact causing the acute and/or chronic symptoms. Other theories have emerged, including those featuring tryptophan metabolites such as indoleamine as inhibitors of histamine degradation, leading to eosinophilia and myalgias143. Likewise, age (>45 years) and the HLA alleles DRB1*03, DRB1*04 and DQA1*0601 have been identified as risk factors for the development of EMS144.
The US Food and Drug Administration called a halt to L-tryptophan sales in the United States in 1990; sales of this dietary supplement resumed in 2005. No epidemic has ensued since that time, although one recent case report has appeared145 in which L-tryptophan was associated with eosinophilia, the recruitment and degranulation of eosinophils in muscle tissue, and muscle fibrosis, consistent with the diagnosis of EMS.
Supplementary information
Disorders associated with eosinophilia and/or eosinophil accumulation in organs and tissues (PDF 100 kb)
Acknowledgements
The authors thank R. Dreyfuss (Medical Arts Branch, Office of the Director, US National Institutes of Health (NIH)) for photographic images and E. R. Fischer (Research Technologies Section, Rocky Mountain Laboratories, US National Institute of Allergy and Infectious Diseases (NIAID), NIH) for preparing the transmission electron micrograph of the mouse eosinophil. H.F.R. receives funding from the NIAID Division of Intramural Research (grants AI000941 and AI000943); P.S.F. receives funding from the National Health and Medical Research Council of Australia and fellowship support from the Harvard Club of Australia.
Glossary
- Innate lymphoid cells
Cells that produce cytokines typically attributed to T helper cell subsets (for example, IL-5) but that have no rearranged antigen-specific receptors.
- Alarmin
A term used to describe endogenous molecules that interact with pattern- recognition receptors and thereby signal danger to the host. These molecules are typically released from necrotic cells and complement the function of the more familiar pathogen-associated molecular patterns. Examples discussed in this Review include HMGB1 and IL-33. Another name for an alarmin is a damage- or danger-associated molecular pattern.
- Cytolytic degranulation
A mechanism through which eosinophils lyse, thereby releasing either free granule proteins or fully intact granules. This renders the cells non-viable. Intact granules released in this manner can respond to physiological secretagogues.
- Piecemeal degranulation
A mechanism through which eosinophils (as well as basophils and mast cells) release specific mediators from cytoplasmic granules by transporting them to the cell surface in membrane-bound cytoplasmic vesicles. The eosinophils remain viable and fully responsive to subsequent stimuli.
- Secretagogues
Substances that induce the secretion of another substance from a cell or storage granule.
- Promyelocyte
A cell in the bone marrow that has differentiated from a haematopoietic stem cell and that will ultimately generate mature granulocytes, including neutrophils, basophils and eosinophils. A promyelocyte can be identified in bone marrow smears as a relatively large cell with a full, non-condensed nucleus and lineage-specific cytoplasmic granules.
- Common myeloid progenitors
(CMPs). In current models of haematopoiesis, the most primitive cells are multipotent, self-renewing haematopoietic stem cells. By definition, CMPs are the subset of progenitor cells that are capable of generating all myeloid cells (that is, monocytes, macrophages, dendritic cells, erythrocytes, megakaryocytes, platelets, basophils, eosinophils and neutrophils) under appropriate cytokine stimulation, but that are no longer capable of generating cells of the lymphoid lineages (such as B cells, T cells and NK cells).
- Granulocyte–macrophage progenitors
(GMPs). By definition, GMPs are the subset of progenitor cells that are capable of generating monocytes, macrophages and all granulocyte lineages (that is, basophils, eosinophils and neutrophils), but not the other lineages. However, as noted in the text, human eosinophils are not derived from the cells currently identified as GMPs.
- Alternatively activated macrophages
One of the major differences between these cells and classically activated macrophages is that these macrophages are not primed with IFN. Instead, alternatively activated macrophages are stimulated by TH2-type cytokines (such as IL-4 or IL-13) and present soluble antigens to T cells. Alternatively activated macrophages release CCL17, CCL18, CCL22, IL-10, TGF, YM1, YM2 and RELM, and they characteristically function to promote the resolution of inflammation.
- Nurse cells
As used in this Review, this term refers to skeletal muscle cells that have been infected with the larval forms of Trichinella species parasites. A capillary network forms around the nurse cells, which provides crucial support for the parasites as they develop.
- Neutrophil extracellular traps
(NETs). Fibrous networks that are released into the extracellular environment by neutrophils. They are composed mainly of DNA, but also contain proteins from neutrophil granules. NETs act as a mesh that traps microorganisms and exposes them to neutrophil-derived effector molecules.
Biographies
Helene F. Rosenberg received a combined M.D. and Ph.D. degree from Cornell University Medical College, New York, USA, and the Rockefeller University, New York, USA. She is currently a senior investigator and the Section Chief for the Inflammation Immunobiology Section of the Laboratory of Allergic Diseases, US National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH), Bethesda, Maryland, USA. Her research programme includes a focus on eosinophil biology as part of a larger programme on the inflammatory responses to and novel immunomodulatory therapies for severe respiratory virus infection.
Kimberly D. Dyer received her Ph.D. from Georgetown University, Washington DC, USA. She is currently a staff scientist in the Laboratory of Allergic Diseases at the NIAID, NIH, Bethesda, Maryland, USA. Her research focuses on the differentiation and biology of eosinophils with a special emphasis on in vitro culture systems.
Paul S. Foster received his Ph.D. and Doctorate of Science from the Australian National University, Canberra, Australia. He is currently the Director of the Priority Research Centre for Asthma and Respiratory Disease, University of Newcastle, Australia, and holds the Chair of Immunology in the School of Biomedical Sciences and Pharmacy, Faculty of Health, University of Newcastle, Australia. His research focuses on understanding the molecular and cellular basis of asthma, allergy and infectious disease of the lung. He is particularly interested in translational approaches directed towards the development of novel anti-inflammatory therapies.
Related links
FURTHER INFORMATION
Competing interests
The authors declare no competing financial interests.
References
- 1.Steinbach KH, et al. Estimation of kinetic parameters of neutrophilic, eosinophilic, and basophilic granulocytes in human blood. Blut. 1979;39:27–38. doi: 10.1007/BF01008072. [DOI] [PubMed] [Google Scholar]
- 2.Lamousé-Smith ES, Furuta GT. Eosinophils in the gastrointestinal tract. Curr. Gastroenterol. Rep. 2006;8:390–395. doi: 10.1007/s11894-006-0024-6. [DOI] [PubMed] [Google Scholar]
- 3.Hogan SP, et al. Eosinophils: biological properties and role in health and disease. Clin. Exp. Allergy. 2008;38:709–750. doi: 10.1111/j.1365-2222.2008.02958.x. [DOI] [PubMed] [Google Scholar]
- 4.Blanchard C, Rothenberg ME. Biology of the eosinophil. Adv. Immunol. 2009;101:81–121. doi: 10.1016/S0065-2776(08)01003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foster PS, et al. Elemental signals regulating eosinophil accumulation in the lung. Immunol. Rev. 2001;179:173–181. doi: 10.1034/j.1600-065X.2001.790117.x. [DOI] [PubMed] [Google Scholar]
- 6.Fabre V, et al. Eosinophil deficiency compromises parasite survival in chronic nematode infection. J. Immunol. 2009;182:1577–1583. doi: 10.4049/jimmunol.182.3.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gebreselassie NG, et al. Eosinophils preserve parasitic nematode larvae by regulating local immunity. J. Immunol. 2012;188:417–425. doi: 10.4049/jimmunol.1101980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wegmann M. Targeting eosinophil biology in asthma therapy. Am. J. Respir. Cell. Mol. Biol. 2011;45:667–674. doi: 10.1165/rcmb.2011-0013TR. [DOI] [PubMed] [Google Scholar]
- 9.Jacobsen EA, Ochkur SI, Lee NA, Lee JJ. Eosinophils and asthma. Curr. Allergy Asthma Rep. 2007;7:18–26. doi: 10.1007/s11882-007-0026-y. [DOI] [PubMed] [Google Scholar]
- 10.Yu C, et al. Targeted deletion of a high-affinity GATA-binding site in the GATA-1 promoter leads to selective loss of the eosinophil lineage in vivo. J. Exp. Med. 2002;195:1387–1395. doi: 10.1084/jem.20020656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee JJ, et al. Defining a link with asthma in mice congenitally deficient in eosinophils. Science. 2004;305:1773–1776. doi: 10.1126/science.1099472. [DOI] [PubMed] [Google Scholar]
- 12.Lee JJ, et al. Human versus mouse eosinophils: “that which we call an eosinophil, by any other name would stain as red”. J. Allergy Clin. Immunol. 2012;130:572–584. doi: 10.1016/j.jaci.2012.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shamri R, Xenakis JJ, Spencer LA. Eosinophils in innate immunity: an evolving story. Cell Tissue Res. 2011;343:57–83. doi: 10.1007/s00441-010-1049-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mould A, Matthaei K, Young I, Foster P. Relationship between interleukin-5 and eotaxin in regulating blood and tissue eosinophilia in mice. J. Clin. Invest. 1997;99:1064–1071. doi: 10.1172/JCI119234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collins PD, Marleau S, Griffiths-Johnson DA, Jose PJ, Williams TJ. Cooperation between interleukin-5 and the chemokine eotaxin to induce eosinophil accumulation in vivo. J. Exp. Med. 1995;182:1169–1174. doi: 10.1084/jem.182.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong CK, Hu S, Cheung PF, Lam CW. Thymic stromal lymphopoietin induces chemotactic and prosurvival effects in eosinophils: implications in allergic inflammation. Am. J. Respir. Cell. Mol. Biol. 2010;43:305–315. doi: 10.1165/rcmb.2009-0168OC. [DOI] [PubMed] [Google Scholar]
- 17.Moro K, et al. Innate production of TH2 cytokines by adipose tissue-associated c- Kit+Sca-1+ lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 18.Neill DR, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ikutani M, et al. Identification of innate IL-5-producing cells and their role in lung eosinophil regulation and antitumor immunity. J. Immunol. 2012;188:703–713. doi: 10.4049/jimmunol.1101270. [DOI] [PubMed] [Google Scholar]
- 20.Corrigan CJ, et al. Allergen-induced expression of IL-25 and IL-25 receptor in atopic asthmatic airways and late-phase cutaneous responses. J. Allergy Clin. Immunol. 2011;128:116–124. doi: 10.1016/j.jaci.2011.03.043. [DOI] [PubMed] [Google Scholar]
- 21.Terrier B, et al. Interleukin-25: a cytokine linking eosinophils and adaptive immunity in Churg-Strauss syndrome. Blood. 2010;116:4523–4531. doi: 10.1182/blood-2010-02-267542. [DOI] [PubMed] [Google Scholar]
- 22.Mirchandani AS, Salmond RJ, Liew FY. Interleukin-33 and the function of innate lymphoid cells. Trends Immunol. 2012;33:389–396. doi: 10.1016/j.it.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 23.Cherry WB, Yoon J, Bartemes KR, Iijima K, Kita H. A novel IL-4 family cytokine, IL-33, potently activates human eosinophils. J. Allergy Clin. Immunol. 2008;121:1484–1490. doi: 10.1016/j.jaci.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsuba-Kitamura S, et al. Contribution of IL-33 to induction and augmentation of experimental allergic conjunctivitis. Int. Immunol. 2010;22:479–489. doi: 10.1093/intimm/dxq035. [DOI] [PubMed] [Google Scholar]
- 25.Mjösberg JM, et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nature Immunol. 2011;12:1055–1062. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- 26.Li Y, et al. Silencing IL-23 expression by small hairpin RNA protects against asthma in mice. Exp. Mol. Med. 2011;43:197–204. doi: 10.3858/emm.2011.43.4.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peng J, Yang XO, Chang SH, Yang J, Dong C. IL-23 signaling enhances Th2 polarization and regulates allergic airway inflammation. Cell Res. 2010;20:62–71. doi: 10.1038/cr.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szymczak WA, Sellers RS, Pirofski LA. IL-23 dampens theallergic response to Cryptococcus neoformans through IL-17-independent and -dependent mechanisms. Am. J. Pathol. 2012;180:1547–1559. doi: 10.1016/j.ajpath.2011.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lotfi R, Lee JJ, Lotze MT. Eosinophilic granulocytes and damage-associated molecular pattern molecules (DAMPs): role in the inflammatory response within tumors. J. Immunother. 2007;30:16–28. doi: 10.1097/01.cji.0000211324.53396.f6. [DOI] [PubMed] [Google Scholar]
- 30.Dvorak AM, Estrella P, Ishizaka T. Vesicular transport of peroxidase in human eosinophilic myelocytes. Clin. Exp. Allergy. 1994;24:10–18. doi: 10.1111/j.1365-2222.1994.tb00910.x. [DOI] [PubMed] [Google Scholar]
- 31.Melo RC, et al. Human eosinophils secrete preformed, granule-stored interleukin-4 through distinct vesicular compartments. Traffic. 2005;6:1047–1057. doi: 10.1111/j.1600-0854.2005.00344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spencer LA, et al. Cytokine receptor-mediated trafficking of preformed IL-4 in eosinophils identifies an innate immune mechanism of cytokine secretion. Proc. Natl Acad. Sci. USA. 2006;103:3333–3338. doi: 10.1073/pnas.0508946103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lacy P, Stow JL. Cytokine release from innate immune cells: association with diverse membrane trafficking pathways. Blood. 2011;118:9–18. doi: 10.1182/blood-2010-08-265892. [DOI] [PubMed] [Google Scholar]
- 34.Neves JS, et al. Eosinophil granules function extracellularly as receptor-mediated secretory organelles. Proc. Natl Acad. Sci. USA. 2008;105:18478–18483. doi: 10.1073/pnas.0804547105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neves JS, Weller PF. Functional extracellular eosinophil granules: novel implications in eosinophil immunobiology. Curr. Opin. Immunol. 2009;21:694–699. doi: 10.1016/j.coi.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walsh GM. Antagonism of eosinophil accumulation in asthma. Recent Pat. Inflamm. Allergy Drug Discov. 2010;4:210–213. doi: 10.2174/187221310793564263. [DOI] [PubMed] [Google Scholar]
- 37.Mackenzie J, Mattes J, Dent L, Foster P. Eosinophils promote allergic disease of the lung by regulating CD4+ Th2 lymphocyte function. J. Immunol. 2001;167:3146–3155. doi: 10.4049/jimmunol.167.6.3146. [DOI] [PubMed] [Google Scholar]
- 38.Mattes J, et al. Intrinsic defect in T cell production of interleukin (IL)-13 in the absence of both IL-5 and eotaxin precludes the development of eosinophilia and airways hyperreactivity in experimental asthma. J. Exp. Med. 2002;195:1433–1444. doi: 10.1084/jem.20020009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang HB, Ghiran I, Matthaei K, Weller PF. Airway eosinophils: allergic inflammation recruited professional antigen-presenting cells. J. Immunol. 2007;179:7585–7592. doi: 10.4049/jimmunol.179.11.7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jacobsen EA, et al. Allergic pulmonary inflammation in mice is dependent on eosinophil-induced recruitment of effector T cells. J. Exp. Med. 2008;205:699–710. doi: 10.1084/jem.20071840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jacobsen EA, Zellner KR, Colbert D, Lee NA, Lee JJ. Eosinophils regulate dendritic cells and Th2 pulmonary immune responses following allergen provocation. J. Immunol. 2011;87:6059–6068. doi: 10.4049/jimmunol.1102299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spencer LA, et al. Human eosinophils constitutively express multiple Th1,Th2 and immunoregulatory cytokines that are secreted rapidly and differentially. J. Leukoc. Biol. 2009;85:117–123. doi: 10.1189/jlb.0108058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang HB, Weller PF. Pivotal advance: eosinophils mediate early alum adjuvant-elicited B cell priming and IgM production. J. Leukoc. Biol. 2008;83:817–821. doi: 10.1189/jlb.0607392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chu VT, et al. Eosinophils are required for the maintenance of plasma cells in the bone marrow. Nature Immunol. 2011;12:151–159. doi: 10.1038/ni.1981. [DOI] [PubMed] [Google Scholar]
- 45.Chu VT, Berek C. Immunization induces activation of bone marrow eosinophil required for plasma cell survival. Eur. J. Immunol. 2012;42:130–137. doi: 10.1002/eji.201141953. [DOI] [PubMed] [Google Scholar]
- 46.Voehringer D, van Rooijen N, Locksley RM. Eosinophils develop in distinct stages and are recruited to peripheral sites by alternatively activated macrophages. J. Leukoc. Biol. 2007;81:1434–1444. doi: 10.1189/jlb.1106686. [DOI] [PubMed] [Google Scholar]
- 47.Dasgupta P, Keegan AD. Contribution of alternatively activated macrophages to allergic lung inflammation: a tale of mice and men. J. Innate Immun. 2012;4:478–488. doi: 10.1159/000336025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Falcone FH, et al. Brugia malayi homolog of macrophage migration inhibitory factor reveals an important link between macrophages and eosinophil recruitment during nematode infection. J. Immunol. 2001;167:5348–5354. doi: 10.4049/jimmunol.167.9.5348. [DOI] [PubMed] [Google Scholar]
- 49.Webb D, et al. Expression of the Ym2 lectin-binding protein is dependent on interleukin (IL)-4 and IL-13 signal transduction: identification of a novel allergy-associated protein. J. Biol. Chem. 2001;276:41969–41976. doi: 10.1074/jbc.M106223200. [DOI] [PubMed] [Google Scholar]
- 50.Wu D, et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–247. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lotfi R, Lotze M. Eosinophils induce DC maturation, regulating immunity. J. Leukoc. Biol. 2008;83:456–460. doi: 10.1189/jlb.0607366. [DOI] [PubMed] [Google Scholar]
- 52.Yang D, et al. Eosinophil-derived neurotoxin (EDN), an antimicrobial protein with chemotactic activities for dendritic cells. Blood. 2003;102:3396–3403. doi: 10.1182/blood-2003-01-0151. [DOI] [PubMed] [Google Scholar]
- 53.Yang D, et al. Eosinophil-derived neurotoxin acts as an alarmin to activate the TLR2–MyD88 signal pathway in dendritic cells and enhances Th2 immune responses. J. Exp. Med. 2008;205:79–90. doi: 10.1084/jem.20062027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Elishmereni M, et al. Physical interactions between mast cells and eosinophils: a novel mechanism enhancing eosinophil survival in vitro. Allergy. 2011;66:376–385. doi: 10.1111/j.1398-9995.2010.02494.x. [DOI] [PubMed] [Google Scholar]
- 55.Pearce EJ, MacDonald AS. The immunobiology of schistosomiasis. Nature Rev. Immunol. 2002;2:499–511. doi: 10.1038/nri843. [DOI] [PubMed] [Google Scholar]
- 56.Sher A, Coffman RL, Hieny S, Cheever AW. Ablation of eosinophil and IgE responses with anti-IL-5 or anti-IL-4 antibodies fails to affect immunity against Schistosoma mansoni in the mouse. J. Immunol. 1990;145:3911–3916. [PubMed] [Google Scholar]
- 57.Swartz JM, et al. Schistosoma mansoni infection in eosinophil lineage-ablated mice. Blood. 2006;108:2420–2427. doi: 10.1182/blood-2006-04-015933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sasaki O, Sugaya H, Ishida K, Yoshimura K. Ablation of eosinophils with anti-IL-5 antibody enhances the survival of intracranial worms of Angiostrongylus cantonensis in the mouse. Parasite Immunol. 1993;15:349–454. doi: 10.1111/j.1365-3024.1993.tb00619.x. [DOI] [PubMed] [Google Scholar]
- 59.Rotman HL, et al. Strongyloides stercoralis: eosinophil-dependent immune-mediated killing of third stage larvae in BALB/cByJ mice. Exp. Parasitol. 1996;82:267–278. doi: 10.1006/expr.1996.0034. [DOI] [PubMed] [Google Scholar]
- 60.Eriksson J, et al. The 434(G>C) polymorphism within the coding sequence of eosinophil cationic protein (ECP) correlates with the natural course of Schistosoma mansoni infection. Int. J. Parasitol. 2007;37:1359–1366. doi: 10.1016/j.ijpara.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 61.Adu B, et al. Polymorphisms in the RNASE3 gene are associated with susceptibility to cerebral malaria in Ghanaian children. PLoS ONE. 2011;6:e29465. doi: 10.1371/journal.pone.0029465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lehrer RI, et al. Antibacterial properties of eosinophil major basic protein and eosinophil cationic protein. J. Immunol. 1989;142:4428–4434. [PubMed] [Google Scholar]
- 63.Rosenberg HF. Recombinant human eosinophil cationic protein: ribonuclease activity is not essential for cytotoxicity. J. Biol. Chem. 1995;270:7876–7881. doi: 10.1074/jbc.270.14.7876. [DOI] [PubMed] [Google Scholar]
- 64.Torrent M, Navarro S, Moussaoui M, Boix E. Eosinophil cationic protein high-affinity binding to bacteria-wall lipopolysaccharides and peptidoglycans. Biochemistry. 2008;47:3544–3555. doi: 10.1021/bi702065b. [DOI] [PubMed] [Google Scholar]
- 65.Yousefi S, et al. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nature Med. 2008;14:949–953. doi: 10.1038/nm.1855. [DOI] [PubMed] [Google Scholar]
- 66.Nizet V. Innate immunity turned inside-out: antimicrobial defense by phagocyte extracellular traps. J. Mol. Med. 2009;87:775–783. doi: 10.1007/s00109-009-0481-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Linch SN, et al. Mouse eosinophils possess potent antibacterial properties in vivo. Infect. Immun. 2009;77:4976–4982. doi: 10.1128/IAI.00306-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Linch SN, et al. IL-5 is protective during sepsis in an eosinophil-independent manner. Am. J. Respir. Crit. Care Med. 2012;186:246–254. doi: 10.1164/rccm.201201-0134OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huang J, et al. The effects of probiotics supplementation timing on an ovalbumin-sensitized rat model. FEMS Immunol. Med. Microbiol. 2010;60:132–141. doi: 10.1111/j.1574-695X.2010.00727.x. [DOI] [PubMed] [Google Scholar]
- 70.Yu J, et al. The effects of Lactobacillus rhamnosus on the prevention of asthma in a murine model. Allergy Asthma Immunol. Res. 2010;2:199–205. doi: 10.4168/aair.2010.2.3.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rose MA, Schubert R, Schulze J, Zielen S. Follow-up of probiotic Lactobacillus GG effects on allergic sensitization and asthma in infants at risk. Clin. Exp. Allergy. 2011;41:1819–1821. doi: 10.1111/j.1365-2222.2011.03876.x. [DOI] [PubMed] [Google Scholar]
- 72.Herbst T, et al. Dysregulation of allergic airway inflammation in the absence of microbial colonization. Am. J. Respir. Crit. Care Med. 2011;184:198–205. doi: 10.1164/rccm.201010-1574OC. [DOI] [PubMed] [Google Scholar]
- 73.Bisgaard H, et al. Reduced diversity of the intestinal microbiota during infancy is associated with increased risk of allergic disease at school age. J. Allergy Clin. Immunol. 2011;128:646–652. doi: 10.1016/j.jaci.2011.04.060. [DOI] [PubMed] [Google Scholar]
- 74.Domachowske JB, Dyer KD, Bonville CA, Rosenberg HF. Recombinant human eosinophil-derived neurotoxin/RNase 2 functions as an effective antiviral agent against respiratory syncytial virus. J. Infect. Dis. 1998;177:1458–1464. doi: 10.1086/515322. [DOI] [PubMed] [Google Scholar]
- 75.Adamko DJ, Yost BL, Gleich GJ, Fryer AD, Jacoby DB. Ovalbumin sensitization changes the inflammatory response to subsequent parainfluenza infection. Eosinophils mediate airway hyperresponsiveness, M2 muscarinic receptor dysfunction, and antiviral effects. J. Exp. Med. 1999;190:1465–1478. doi: 10.1084/jem.190.10.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Phipps S, et al. Eosinophils contribute to innate antiviral immunity and promote clearance of respiratory syncytial virus. Blood. 2007;110:1578–1586. doi: 10.1182/blood-2007-01-071340. [DOI] [PubMed] [Google Scholar]
- 77.Davoine F, et al. Virus-induced eosinophil mediator release requires antigen-presenting and CD4+ T cells. J. Allergy Clin. Immunol. 2008;122:69–77. doi: 10.1016/j.jaci.2008.03.028. [DOI] [PubMed] [Google Scholar]
- 78.Dyer KD, Percopo CM, Fischer ER, Gabryszewski SJ, Rosenberg HF. Pneumoviruses infect eosinophils and elicit MyD88-dependent release of chemoattractant cytokines and interleukin-6. Blood. 2009;114:2649–2656. doi: 10.1182/blood-2009-01-199497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Skiest DJ, Keiser P. Clinical significance of eosinophilia in HIV-infected individuals. Am. J. Med. 1997;102:449–453. doi: 10.1016/S0002-9343(97)00048-X. [DOI] [PubMed] [Google Scholar]
- 80.Manetti R, et al. CD30 expression by CD8+ T cells producting type 2 helper cytokines. Evidence for large numbers of CD8+CD30+ T cell clones in human immunodeficiency virus infection. J. Exp. Med. 1994;180:2407–2411. doi: 10.1084/jem.180.6.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Empson M, Bishop GA, Nightingale B, Garsia R. Atopy, anergic status, and cytokine expression in HIV-infected subjects. J. Allergy Clin. Immunol. 1999;103:833–842. doi: 10.1016/S0091-6749(99)70427-6. [DOI] [PubMed] [Google Scholar]
- 82.Rugeles MT, et al. Ribonuclease is partly responsible for the HIV-1 inhibitory effect activated by HLA alloantigen recognition. AIDS. 2003;17:481–486. doi: 10.1097/00002030-200303070-00002. [DOI] [PubMed] [Google Scholar]
- 83.Bochner BS, et al. Workshop report from the National Institutes of Health Taskforce on the Research Needs of Eosinophil-Associated Diseases (TREAD) J. Allergy Clin. Immunol. 2012;130:587–596. doi: 10.1016/j.jaci.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bochner BS, Gleich GJ. What targeting eosinophils has taught us about their role in diseases. J. Allergy Clin. Immunol. 2010;126:16–25. doi: 10.1016/j.jaci.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Foster PS, Rosenberg HF, Asquith KL, Kumar RK. Targeting eosinophils in asthma. Curr. Mol. Med. 2008;8:585–590. doi: 10.2174/156652408785748013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Flood-Page P, et al. Anti-IL-5 treatment reduces deposition of ECM proteins in the bronchial subepithelial basement membrane of mild atopic asthmatics. J. Clin. Invest. 2003;112:1029–1036. doi: 10.1172/JCI17974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Menzies-Gow A, et al. Anti-IL-5 (mepolizumab) therapy induces bone marrow eosinophil maturational arrest and decreases eosinophil progenitors in the bronchial mucosa of atopic asthmatics. J. Allergy Clin. Immunol. 2003;111:714–719. doi: 10.1067/mai.2003.1382. [DOI] [PubMed] [Google Scholar]
- 88.Leckie MJ, et al. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet. 2000;356:2144–2148. doi: 10.1016/S0140-6736(00)03496-6. [DOI] [PubMed] [Google Scholar]
- 89.Flood-Page P, et al. A study to evaluate safety and efficacy of mepolizumab in patients with moderate persistent asthma. Am. J. Respir. Crit. Care Med. 2007;176:1062–1071. doi: 10.1164/rccm.200701-085OC. [DOI] [PubMed] [Google Scholar]
- 90.Gibson PG. Inflammatory phenotypes in adult asthma: clinical applications. Clin. Respir. J. 2009;3:198–206. doi: 10.1111/j.1752-699X.2009.00162.x. [DOI] [PubMed] [Google Scholar]
- 91.Anderson GP. Endotyping asthma: new insights into key pathogenic mechanisms in a complex, heterogeneous disease. Lancet. 2008;372:1107–1109. doi: 10.1016/S0140-6736(08)61452-X. [DOI] [PubMed] [Google Scholar]
- 92.Nair P, et al. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N. Engl. J. Med. 2009;360:985–993. doi: 10.1056/NEJMoa0805435. [DOI] [PubMed] [Google Scholar]
- 93.Haldar P, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N. Engl. J. Med. 2009;360:973–984. doi: 10.1056/NEJMoa0808991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Castro M, et al. Reslizumab for poorly controlled, eosinophilic asthma: a randomized, placebo-controlled study. Am. J. Respir. Crit. Care Med. 2011;184:1125–1132. doi: 10.1164/rccm.201103-0396OC. [DOI] [PubMed] [Google Scholar]
- 95.Pavord ID, et al. Mepoliuzmab for severe eosinophilic asthma (DREAM): a multicenter, double-blind, placebo-controlled trial. Lancet. 2012;380:651–659. doi: 10.1016/S0140-6736(12)60988-X. [DOI] [PubMed] [Google Scholar]
- 96.Molfino NA, Gossage D, Kolbeck R, Parker JM, Geba GP. Molecular and clinical rationale for therapeutic targeting of interleukin-5 and its receptor. Clin. Exp. Allergy. 2012;42:712–737. doi: 10.1111/j.1365-2222.2011.03854.x. [DOI] [PubMed] [Google Scholar]
- 97.Corren J, et al. Lebrikizumab treatment in adults with asthma. N. Engl. J. Med. 2011;365:1088–1098. doi: 10.1056/NEJMoa1106469. [DOI] [PubMed] [Google Scholar]
- 98.Furuta GT. Eosinophilic esophagitis: update on clinicopathological manifestations and pathophysiology. Curr. Opin. Gastroenterol. 2011;27:383–388. doi: 10.1097/MOG.0b013e328347bb10. [DOI] [PubMed] [Google Scholar]
- 99.Mueller S, Aigner T, Neureiter D, Stolte M. Eosinophil infiltration and degranulation in oesophageal mucosa from adult patients with eosinophilic oesophagitis: a retrospective and comparative study on pathological biopsy. J. Clin. Pathol. 2006;59:1175–1180. doi: 10.1136/jcp.2005.031922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Blanchard C, et al. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J. Clin. Invest. 2006;116:536–547. doi: 10.1172/JCI26679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sherrill JD, et al. Variants of thymic stromal lymphopoietin and its receptor associate with eosinophilic esophagitis. J. Allergy Clin. Immunol. 2010;126:160–165. doi: 10.1016/j.jaci.2010.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mishra A, Schlotman J, Wang M, Rothenberg ME. Critical role for adaptive T cell immunity in experimental eosinophilic esophagitis in mice. J. Leukoc. Biol. 2007;81:916–924. doi: 10.1189/jlb.1106653. [DOI] [PubMed] [Google Scholar]
- 103.Rayapudi M, et al. Indoor insect allergens are potent inducers of experimental eosinophilic esophagitis in mice. J. Leukoc. Biol. 2010;88:337–346. doi: 10.1189/jlb.0110025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rubinstein E, et al. Siglec-F inhibition reduces esophageal eosinophilia and angiogenesis in a mouse model of eosinophilic esophagitis. J. Pediatr. Gastroenterol. Nutr. 2011;53:409–416. doi: 10.1097/MPG.0b013e3182182ff8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zuo L, et al. IL-13 induces esophageal remodeling and gene expression by an eosinophil-independent, IL-13Rα2-inhibited pathway. J. Immunol. 2010;185:660–669. doi: 10.4049/jimmunol.1000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lucendo AJ, De Rezende L, Comas C, Caballero T, Bellón T. Treatment with topical steroids downregulates IL-5, eotaxin-1/CCL11, and eotaxin-3/CCL26 gene expression in eosinophilic esophagitis. Am. J. Gastroenterol. 2008;103:2184–2193. doi: 10.1111/j.1572-0241.2008.01937.x. [DOI] [PubMed] [Google Scholar]
- 107.Straumann A, et al. Anti-interleukin-5 antibody treatment (mepolizumab) in active eosinophilic oesophagitis: a randomised, placebo-controlled, double-blind trial. Gut. 2010;59:21–30. doi: 10.1136/gut.2009.178558. [DOI] [PubMed] [Google Scholar]
- 108.Assa'ad AH, et al. An antibody against IL-5 reduces numbers of esophageal intraepithelial eosinophils in children with eosinophilic esophagitis. Gastroenterology. 2011;141:1593–1604. doi: 10.1053/j.gastro.2011.07.044. [DOI] [PubMed] [Google Scholar]
- 109.Conus S, Straumann A, Bettler E, Simon HU. Mepolizumab does not alter levels of eosinophils, T cells, and mast cells in the duodenal mucosa in eosinophilic esophagitis. J. Allergy Clin. Immunol. 2010;126:175–177. doi: 10.1016/j.jaci.2010.04.029. [DOI] [PubMed] [Google Scholar]
- 110.Varga J, Kahari VM. Eosinophilia–myalgia syndrome, eosinophilic fasciitis, and related fibrosing disorders. Curr. Opin. Rheumatol. 1997;9:562–570. doi: 10.1097/00002281-199711000-00013. [DOI] [PubMed] [Google Scholar]
- 111.Krahn M, et al. CAPN3 mutations in patients with idiopathic eosinophilic myositis. Ann. Neurol. 2006;59:905–911. doi: 10.1002/ana.20833. [DOI] [PubMed] [Google Scholar]
- 112.Kramerova I, Kudryashova E, Tidball JG, Spencer MJ. Null mutation of calpain 3 (p94) in mice causes abnormal sarcomere formation in vivo and in vitro. Hum. Mol. Genet. 2004;13:1373–1388. doi: 10.1093/hmg/ddh153. [DOI] [PubMed] [Google Scholar]
- 113.Cools J, et al. A tyrosine kinase created by fusion of the PDGFRA and FIP1L1 genes as a therapeutic target of imatinib in idiopathic hypereosinophilic syndrome. N. Engl. J. Med. 2003;348:1201–1214. doi: 10.1056/NEJMoa025217. [DOI] [PubMed] [Google Scholar]
- 114.Valent P, et al. Pathogenesis and classification of eosinophil disorders: a review of recent developments in the field. Expert Rev. Hematol. 2012;5:157–176. doi: 10.1586/ehm.11.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cools J, et al. The EOL-1 cell line as an in vitro model for the study of FIP1L1-PDGFRA-positive chronic eosinophilic leukemia. Blood. 2004;103:2802–2805. doi: 10.1182/blood-2003-07-2479. [DOI] [PubMed] [Google Scholar]
- 116.Stover EH, et al. Activation of FIP1L1–PDGFRα requires disruption of the juxtamembrane domain of PDGFRα and is FIP1L1-independent. Proc. Natl Acad. Sci. USA. 2006;103:8078–8083. doi: 10.1073/pnas.0601192103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Roufosse F, et al. Mepolizumab as a corticosteroid-sparing agent in lymphocytic variant hypereosinophilic syndrome. J. Allergy Clin. Immunol. 2010;126:828–835. doi: 10.1016/j.jaci.2010.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ogbogu PU, et al. Hypereosinophilic syndrome: a multicenter, retrospective analysis of clinical characteristics and response to therapy. J. Allergy Clin. Immunol. 2009;124:1319–1325. doi: 10.1016/j.jaci.2009.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Valent P, et al. Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. J. Allergy Clin. Immunol. 2012;130:607–612. doi: 10.1016/j.jaci.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cools J, et al. PKC412 overcomes resistance to imatinib in a murine model of FIP1L1–PDGFRα-induced myeloproliferative disease. Cancer Cell. 2003;3:459–469. doi: 10.1016/S1535-6108(03)00108-9. [DOI] [PubMed] [Google Scholar]
- 121.Yamada Y, Cancelas JA, Rothenberg ME. Murine model of hypereosinophilic syndromes/chronic eosinophilic leukemia. Int. Arch. Allergy Immunol. 2009;149(Suppl. 1):102–107. doi: 10.1159/000211381. [DOI] [PubMed] [Google Scholar]
- 122.Häcker H, Chi L, Rehg JE, Redecke V. NIK prevents the development of hypereosinophilic syndrome-like disease in mice independent of IKKα activation. J. Immunol. 2012;188:4602–4610. doi: 10.4049/jimmunol.1200021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pease JE, Williams TJ. Eotaxin and asthma. Curr. Opin. Pharmacol. 2001;1:248–253. doi: 10.1016/S1471-4892(01)00044-3. [DOI] [PubMed] [Google Scholar]
- 124.Takatsu K, Kouro T, Nagai Y. Interleukin-5 in the link between innate and acquired immune response. Adv. Immunol. 2009;101:191–236. doi: 10.1016/S0065-2776(08)01006-7. [DOI] [PubMed] [Google Scholar]
- 125.Wechsler ME, et al. Novel targeted therapies for eosinophilic disorders. J. Allergy Clin. Immunol. 2012;130:563–571. doi: 10.1016/j.jaci.2012.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lloyd CM, Rankin SM. Chemokines in allergic airway disease. Curr. Opin. Pharmacol. 2003;3:443–448. doi: 10.1016/S1471-4892(03)00069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bochner BS. Siglec-8 on human eosinophils and mast cells, and Siglec-F on murine eosinophils, are functionally related inhibitory receptors. Clin. Exp. Allergy. 2009;39:317–324. doi: 10.1111/j.1365-2222.2008.03173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kiwamoto T, Kawasaki N, Paulson JC, Bochner BS. Siglec-8 as a drugable target to treat eosinophil and mast cell-associated conditions. Pharmacol. Ther. 2012;135:327–336. doi: 10.1016/j.pharmthera.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hudson SA, Bovin NV, Schnaar RL, Crocker PR, Bochner BS. Eosinophil-selective binding and proapoptotic effect in vitro of a synthetic Siglec-8 ligand, polymeric 6′- sulfated sialyl Lewis X. J. Pharmacol. Exp. Ther. 2009;330:608–612. doi: 10.1124/jpet.109.152439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kvarnhammar AM, Cardell LO. Pattern recognition receptors in human eosinophils. Immunology. 2012;136:11–20. doi: 10.1111/j.1365-2567.2012.03556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cardell LO. Role of atopic status in Toll-like receptor (TLR)7- and TLR9-mediated activation of human eosinophils. J. Leukoc. Biol. 2009;85:719–727. doi: 10.1189/jlb.0808494. [DOI] [PubMed] [Google Scholar]
- 132.Ackerman SJ, Bochner BS. Mechanisms of eosinophilia in the pathogenesis of hypereosinophilic disorders. Immunol. Allergy Clin. North Am. 2007;27:357–375. doi: 10.1016/j.iac.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bedi R, Du J, Sharma AK, Gomes I, Ackerman SJ. Human C/EBP-ε activator and repressor isoforms differentially reprogram myeloid lineage commitment and differentiation. Blood. 2009;113:317–327. doi: 10.1182/blood-2008-02-139741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mori Y, et al. Identification of the human eosinophil lineage-committed progenitor: revision of phenotypic definition of the human common myeloid progenitor. J. Exp. Med. 2009;206:183–193. doi: 10.1084/jem.20081756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Iwasaki H, et al. Identification of eosinophil lineage-committed progenitors in the murine bone marrow. J. Exp. Med. 2005;201:1891–1897. doi: 10.1084/jem.20050548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Southam DS, et al. Increased eosinophil-lineage committed progenitors in the lung of allergen-challenged mice. J. Allergy Clin. Immunol. 2005;115:95–102. doi: 10.1016/j.jaci.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 137.Busse WW, Ring J, Huss-Marp J, Kahn JE. A review of treatment with mepolizumab, an anti-IL-5 mAb, in hypereosinophilic syndromes and asthma. J. Allergy Clin. Immunol. 2010;125:803–813. doi: 10.1016/j.jaci.2009.11.048. [DOI] [PubMed] [Google Scholar]
- 138.Foster P, Hogan S, Ramsay A, Matthaei K, Young I. Interleukin-5 deficiency abolishes eosinophilia, airways hyperreactivity, and lung damage in a mouse asthma model. J. Exp. Med. 1996;183:195–201. doi: 10.1084/jem.183.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Humbles AA, et al. A critical role for eosinophils in allergic airways remodeling. Science. 2004;305:1776–1779. doi: 10.1126/science.1100283. [DOI] [PubMed] [Google Scholar]
- 140.Walsh ER, et al. Strain-specific requirement for eosinophils in the recruitment of T cells to the lung during the development of allergic asthma. J. Exp. Med. 2008;205:1285–1292. doi: 10.1084/jem.20071836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hertzman PA, et al. Association of the eosinophilia–myalgia syndrome with the ingestion of tryptophan. N. Engl. J. Med. 1990;322:869–873. doi: 10.1056/NEJM199003293221301. [DOI] [PubMed] [Google Scholar]
- 142.Mayeno AN, et al. Characterization of “peak E”, a novel amino acid associated with eosinophilia–myalgia syndrome. Science. 1990;250:1707–1708. doi: 10.1126/science.2270484. [DOI] [PubMed] [Google Scholar]
- 143.Smith MJ, Garrett RH. A heretofore undisclosed crux of eosinophilia–myalgia syndrome: compromised histamine degradation. Inflamm. Res. 2005;54:435–450. doi: 10.1007/s00011-005-1380-7. [DOI] [PubMed] [Google Scholar]
- 144.Okada S, et al. Immunogenetic risk and protective factors for development of L-tryptophan-associated eosinophilia–myalgia syndrome and associated symptoms. Arthritis Rheum. 2009;61:1305–1311. doi: 10.1002/art.24460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Allen JA. Post-epidemic eosinophilia–myalgia syndrome associated with L-tryptophan. Arthritis Rheum. 2011;63:3633–3639. doi: 10.1002/art.30514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Haskell MD, Moy JN, Gleich GJ, Thomas LL. Analysis of signaling events associated with activation of neutrophil superoxide anion production by eosinophil granule major basic protein. Blood. 1995;86:4627–4637. [PubMed] [Google Scholar]
- 147.Munitz A, Levi-Schaffer F. Eosinophils: 'new' roles for 'old' cells. Allergy. 2004;59:268–275. doi: 10.1111/j.1398-9995.2003.00442.x. [DOI] [PubMed] [Google Scholar]
- 148.Dyer KD, Garcia-Crespo KE, Killoran KE, Rosenberg HF. Antigen profiles for the quantitative assessment of eosinophils in mouse tissues by flow cytometry. J. Immunol. Methods. 2011;369:91–97. doi: 10.1016/j.jim.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Meyerholz DK, Griffin MA, Castilow EM, Varga SM. Comparison of histochemical methods for murine eosinophil detection in an RSV vaccine-enhanced inflammation model. Toxicol. Pathol. 2009;37:249–255. doi: 10.1177/0192623308329342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yamaguchi Y, et al. Models of lineage switching in hematopoietic development: a new myeloid-committed eosinophil cell line (YJ) demonstrates trilineage potential. Leukemia. 1998;12:1430–1439. doi: 10.1038/sj.leu.2401115. [DOI] [PubMed] [Google Scholar]
- 151.Histoshi Y, et al. Distribution of IL-5 receptor-positive B cells. Expression of IL-5 receptor on Ly-1(CD5)+ B cells. J. Immunol. 1990;144:4218–4225. [PubMed] [Google Scholar]
- 152.Wise EL, Bonner KT, William TJ, Pease JE. A single nucleotide polymorphism in the CCR3 gene ablates receptor export to the plasma membrane. J. Allergy Clin. Immunol. 2010;126:150–157. doi: 10.1016/j.jaci.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 153.Willetts L, et al. Immunodetection of occult eosinophils in lung tissue biopsies may help predict survival in acute lung injury. Respir. Res. 2011;12:116. doi: 10.1186/1465-9921-12-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Macias MP, et al. Identification of a new murine eosinophil major basic protein (mMBP) gene: cloning and characterization of mMBP-2. J. Leukoc. Biol. 2000;67:567–576. doi: 10.1002/jlb.67.4.567. [DOI] [PubMed] [Google Scholar]
- 155.Ito W, et al. Hepatocyte growth factor suppresses production of reactive oxygen species and release of eosinophil-derived neurotoxin from human eosinophils. Int. Arch. Allergy Immunol. 2008;147:331–337. doi: 10.1159/000144041. [DOI] [PubMed] [Google Scholar]
- 156.Ochkur SI, et al. The development of a sensitive and specific ELISA for mouse eosinophil peroxidase: assessment of eosinophil degranulation ex vivo and in models of human disease. J. Immunol. Methods. 2012;375:138–147. doi: 10.1016/j.jim.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Blyth DI, Wharton TF, Pedrick MS, Savage TJ, Sanjar S. Airway subepithelial fibrosis in a murine model of atopic asthma: suppression by dexamethasone or anti-interleukin-5 antibody. Am. J. Respir. Cell. Mol. Biol. 2000;23:241–246. doi: 10.1165/ajrcmb.23.2.3999. [DOI] [PubMed] [Google Scholar]
- 158.Grimaldi JC, et al. Depletion of eosinophils in mice through the use of antibodies specific for C-C chemokine receptor 3 (CCR3) J. Leukoc. Biol. 1999;65:846–853. doi: 10.1002/jlb.65.6.846. [DOI] [PubMed] [Google Scholar]
- 159.Song DJ, et al. Anti-Siglec-F antibody reduces allergen-induced eosinophilic inflammation and airway remodeling. J. Immunol. 2009;183:5333–5341. doi: 10.4049/jimmunol.0801421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Dyer KD, et al. Functionally competent eosinophils differentiated ex vivo in high purity from normal mouse bone marrow. J. Immunol. 2008;181:4004–4009. doi: 10.4049/jimmunol.181.6.4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kopf M, et al. IL-5-deficient mice have a developmental defect in CD5+ B-1 cells and lack eosinophilia but have normal antibody and cytotoxic T cell responses. Immunity. 1996;4:15–24. doi: 10.1016/S1074-7613(00)80294-0. [DOI] [PubMed] [Google Scholar]
- 162.Yoshida T, et al. Defective B-1 cell development and impaired immunity against Angiostrongylus cantonensis in IL-5Rα-deficient mice. Immunity. 1996;4:483–494. doi: 10.1016/S1074-7613(00)80414-8. [DOI] [PubMed] [Google Scholar]
- 163.Dent LA, Strath M, Mellor AL, Sanderson CJ. Eosinophilia in transgenic mice expressing interleukin 5. J. Exp. Med. 1990;172:1425–1431. doi: 10.1084/jem.172.5.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lee NA, et al. Expression of IL-5 in thymocytes/ T cells leads to the development of a massive eosinophilia, extramedullary eosinophilopoiesis, and unique histopathologies. J. Immunol. 1997;158:1332–1344. [PubMed] [Google Scholar]
- 165.Rothenberg ME, MacLean JA, Pearlman E, Luster AD, Leder P. Targeted disruption of the chemokine eotaxin partially reduces antigen-induced tissue eosinophilia. J. Exp. Med. 1995;185:785–790. doi: 10.1084/jem.185.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Pope SM, et al. Identification of a cooperative mechanism involving interleukin-13 and eotaxin-2 in experimental allergic lung inflammation. J. Biol. Chem. 2005;280:13952–13961. doi: 10.1074/jbc.M406037200. [DOI] [PubMed] [Google Scholar]
- 167.Pope SM, Zimmermann N, Stringer KF, Karow ML, Rothenberg ME. The eotaxin chemokines and CCR3 are fundamental regulators of allergen-induced pulmonary eosinophilia. J. Immunol. 2005;175:5341–5350. doi: 10.4049/jimmunol.175.8.5341. [DOI] [PubMed] [Google Scholar]
- 168.Ochkur SI, et al. Coexpression of IL-5 and eotaxin-2 in mice creates an eosinophil-dependent model of respiratory inflammation with characteristics of severe asthma. J. Immunol. 2007;178:7879–7889. doi: 10.4049/jimmunol.178.12.7879. [DOI] [PubMed] [Google Scholar]
- 169.Humbles AA, et al. The murine CCR3 receptor regulates both the role of eosinophils and mast cells in allergen-induced airway inflammation and hyperresponsiveness. Proc. Natl Acad. Sci. USA. 2002;99:1479–1484. doi: 10.1073/pnas.261462598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zhang M, et al. Defining the in vivo function of Siglec-F, a CD33-related Siglec expressed on mouse eoisnophils. Blood. 2007;109:4280–4287. doi: 10.1182/blood-2006-08-039255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Leckie MJ. Anti-interleukin-5 monoclonal antibodies: preclinical and clinical evidence in asthma models. Am. J. Resp. Med. 2003;2:245–259. doi: 10.1007/BF03256653. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disorders associated with eosinophilia and/or eosinophil accumulation in organs and tissues (PDF 100 kb)


