Abstract
New evidence for the regulation of vitamin C homeostasis has emerged from several studies of human genetic variation. Polymorphisms in the genes encoding sodium-dependent vitamin C transport proteins are strongly associated with plasma ascorbate levels and likely impact tissue cellular vitamin C status. Furthermore, genetic variants of proteins that suppress oxidative stress or detoxify oxidatively damaged biomolecules, i.e., haptoglobin, glutathione-S-transferases, and possibly manganese superoxide dismutase, affect ascorbate levels in the human body. There also is limited evidence for a role of glucose transport proteins. In this review, we examine the extent of the variation in these genes, their impact on vitamin C status, and their potential role in altering chronic disease risk. We conclude that future epidemiological studies should take into account genetic variation in order to successfully determine the role of vitamin C nutriture or supplementation in human vitamin C status and chronic disease risk.
Keywords: ascorbate, SVCT, haptoglobin, GST, polymorphism, SNP
INTRODUCTION
Maintaining adequate vitamin C (ascorbic acid) levels in the body is essential for collagen, catecholamine, and carnitine biosynthesis; absorption of nonheme iron; and antioxidant protection and hence plays a critical role in normal functioning of the body and optimum health (62). Numerous factors, both endogenous and exogenous, contribute to vitamin C body status, including vitamin C transporters that regulate the vitamin’s bioavailability and plasma and tissue concentrations; enzymatic reactions in which vitamin C is used up by reducing redox-active metal cofactors; and a host of environmental factors and endogenous stresses, such as oxidative stress, infection, and inflammation.
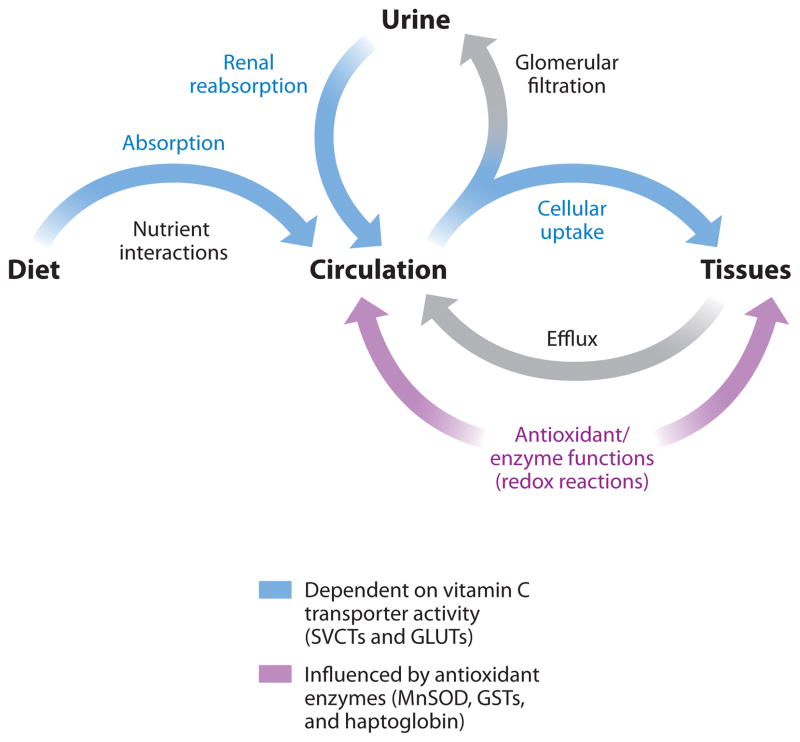
Thus, over the past decades, several proteins have been identified as critical regulators of vitamin C homeostasis. These proteins affect ascorbate levels by different mechanisms, such as vitamin C transport, transition metal regulation, enzymatic redox activity, and antioxidant protection (Figure 1). However, much of this work has been performed in animal models and cell culture, the results of which often are difficult to translate to humans or are confounded by artifacts in study design. Controlled studies of vitamin C status in humans are difficult to perform, challenged by design, and limited in interpretation (56).
Figure 1.
Vitamin C homeostasis. Vitamin C levels in the circulation and tissues are influenced by several regulatory mechanisms in the body. Proteins involved in vitamin C homeostasis fall under the general-categories transporters (blue) that transport vitamin C across cell membranes and those that modulate oxidative stress (red) and would interact with ascorbate due to its antioxidant properties. GLUT, glucose transporter protein; GST, glutathione S-transferase; MnSOD, manganese superoxide dismutase; SVCT, sodium-dependent vitamin C transporter.
Despite these difficulties, new evidence for the regulation of vitamin C status has emerged from studies of human genetic variations. Although a relatively new and burgeoning field, there is strong evidence that several genetic alterations, in the form of single-nucleotide polymorphisms (SNPs), gene duplications, or gene deletions, affect ascorbate levels in the human body. The phenotypes of these genomic changes are supporting previous evidence of the physiological functions of vitamin C and its role in human health.
Although the list will undoubtedly grow with future research, this review focuses on variations within the genes for which there is direct evidence of alterations in vitamin C plasma levels. These genes can be subdivided into two categories based on their interactions with ascorbic acid. First, genes involved in the direct transport and regulation of vitamin C concentrations in tissues and extracellular fluids are discussed. This discussion focuses on the known polymorphisms in the sodium-dependent vitamin C transporters (SVCTs) of the SLC23 family (SLC23A1 and SLC23A2). The second category of genes is related to the antioxidant and redox activities of vitamin C and involves genetic variants affecting iron homeostasis and oxidative stress. Hence, we examine iron dysregulation through haptoglobin (HP) gene polymorphisms and genetic variants impacting the activity of glutathione S-transferases (GSTs). In addition, as candidates for future studies, we present some limited data on the influence of genetic changes in manganese superoxide dismutase (SOD2) and glucose transport proteins (SLC2 family) on ascorbate levels in humans.
SODIUM-DEPENDENT VITAMIN C TRANSPORTERS
The two sodium-dependent vitamin C transport proteins, SVCT1 and SVCT2, are specific for the cotransport of sodium ions and ascorbic acid across cell membranes. Although sodium-dependent vitamin C transport in cells had been characterized decades before (35), the genes encoding these members of the solute carrier family 23 (SLC23A1 and SLC23A2) were not identified until 1999 (86). Initially characterized in rodents, human analogs were isolated and mapped shortly afterward (26, 73, 91).
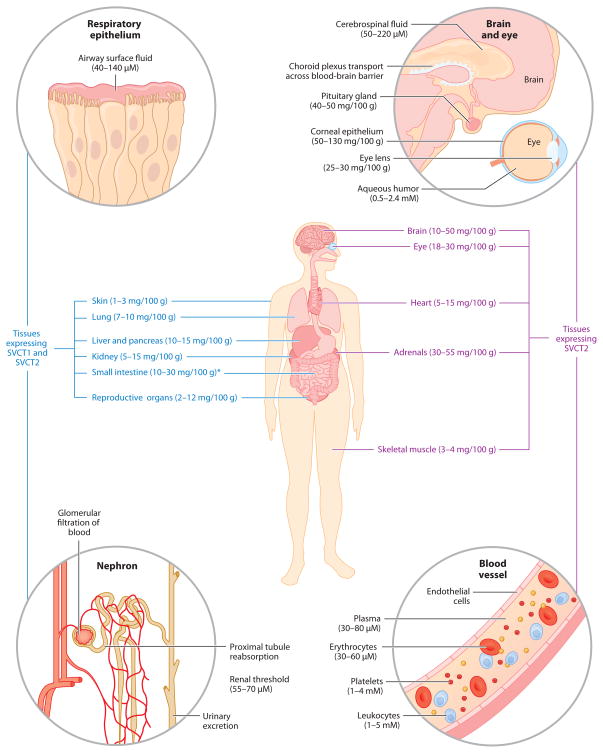
Transcriptional regulation of the SLC23 genes controls the tissue distribution of SVCTs and is responsible for the maintenance of vitamin C levels in nearly all cells, tissues, and extracellular fluids (Figure 2). The only cells without functional SVCT proteins are erythrocytes, which selectively lose SVCT2 expression during erythropoiesis (59). Consequently, ascorbate levels in erythrocytes are low, generally in equilibrium with the surrounding blood plasma.
Figure 2.
Tissue distribution of vitamin C and its transport proteins in the human body. Ascorbate concentrations are from limited human data and are represented as mg/100 g wet weight for tissues and molarity (mM or μM) for intracellular and extracellular fluids. Asterisk indicates that the tissue concentrations of ascorbate in the small intestine are heavily dependent on recent dietary intake. Sodium-dependent vitamin C transporter (SVCT) distribution is based on mRNA expression data in animals and human tissues. Republished with permission from Saunders/Elsevier, from Michels, A and Frei B, Vitamin C, in Biochemical, Physiological, and Molecular Aspects of Human Nutrition, third edition, ed. MH Stipanuk, MA Caudill, pp. 626–54, copyright 2012 (62).
Due to the direct interaction of SVCTs with ascorbic acid and their roles in absorption and tissue accumulation of vitamin C, genetic alterations in SLC23A1 and A2 appear to have the greatest effect on human vitamin C status of all the genetic factors investigated to date. Each SLC23A1 and SLC23A2 is thought to have emerged via gene duplication before vertebrate evolution and expansion (26). The divergent evolution of the two genes has led to distinct differences in the expression, regulation, and function of SVCT1 and SVCT2, and therefore the two genes encoding these proteins are considered separately. It should be noted that genetic variations in both of these genes is the latest area of research in this field, with these and other polymorphisms having the greatest potential for phenotypic effects that will impact vitamin C homeostasis and human health.
SVCT1 (SLC23A1)
SVCT1, encoded by the gene SLC23A1, is predominantly responsible for high-capacity vitamin C transport across membranes. Found on the apical side of polarized epithelial cells (8, 58), SVCT1 is highly expressed in the liver, kidney, lung, small intestine, and pancreas (86, 91). Of these tissues, it is the expression of SVCT1 in the kidney proximal tubule that is considered most important for the regulation of whole-body vitamin C status, as it is involved in the renal reabsorption of vitamin C following glomerular filtration of blood plasma (Figures 2 and 3a). Mice carrying a genetic knockout of SLC23A1 excrete large amounts of ascorbic acid in the urine and have low plasma and tissue ascorbate levels (17). Because we cannot synthesize ascorbic acid in our body, loss of SVCT1 expression may cause a far more severe vitamin C deficiency in humans than in ascorbic acid–synthesizing animals. However, there are currently no reports of spontaneous loss of SVCT1 in humans.
Figure 3.
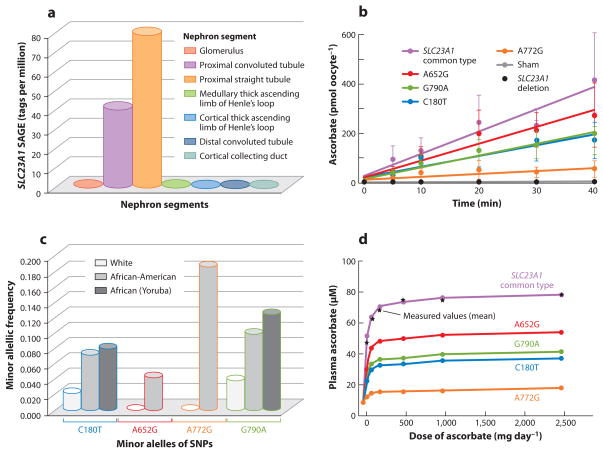
SLC23A1 activity and human genetic variation. (a) Distribution of SLC23A1 in kidney nephron segments by Serial Analysis of Gene Expression (SAGE) libraries containing expression data from microdissected glomeruli and six different nephron segments; values are expressed as tags per million. (b) Effect of SLC23A1 SNPs on ascorbate transport. Xenopus laevis oocytes were microinjected with the following SLC23A1 cRNAs: common type; sham injected; human deletion construct; and SNPs A652G (rs34521685), G790A (rs33972313), A772G (rs35817838), and C180T (rs6886922). (c) Population prevalences of SLC23A1 polymorphisms. Shown are averaged minor allelic frequencies of SLC23A1 genotypes in African (n = 48), American African (n = 438), and white (n = 1,874) individuals, using pooled genotype data. (d) Modeled effects of SLC23A1 polymorphisms on plasma ascorbate concentrations in humans. Values in healthy young women for common type SLC23A1 are measured and calculated fasting steady-state plasma ascorbate concentrations. For women with SNPs, values are calculated from wild-type data comparisons to transport data in panel b. Reprinted with permission of the American Society for Clinical Investigations, from Corpe C.P., Tu H., Eck P., Wang J., Faulhaber-Walter R., Schnermann J., Margolis S., Padayatty S., Sun H., Wang Y., Nussbaum R.L., Espey, M.G., and Levine, M. Vitamin C transporter Slc23a1 links renal reabsorption, vitamin C tissue accumulation, and perinatal survival in mice. April 1:120(4). Copyright 2010 (17).
Outside of an absence in renal reabsorption, it is unclear what other impact the loss of SVCT1 expression may have in humans. SLC23A1 knockout mice experience increased perinatal mortality, but with no apparent physiological or cell-specific phenotype (17). Unlike SVCT2, SVCT1 appears to be expressed in specific locations within tissues. For instance, SVCT1 expression in the kidney is limited to the proximal tubule of the nephron (Figure 3a). It is possible that the loss of SVCT1 would only impact ascorbate levels in specific cellular environments or specific cell types within tissues. Although SVCT1 expression has been noted in the skin, bile duct, lung, pancreas, and reproductive organs (29, 45, 57, 91), it is not clear what consequences a loss of SVCT1 would have in these tissues. Another possibility is that the apparent lack of a phenotype in SLC23A1-null animals may be obscured, as all cell types that express SVCT1 also express SVCT2 (Figure 2). SVCT2 or glucose transporters (see below) may compensate for the lack of SVCT1, albeit with a limited capacity. For example, although SVCT1 is expressed in the intestine, SLC23A1 genetic knockout mice are still capable of absorbing ascorbic acid from the diet (17).
Genomic Characterization of SLC23A1
SLC23A1 consists of 16 kb found on human chromosome 5 (26). The gene has 15 exons that encode for a 598 amino acid protein. A nonfunctional splice variant with four extra amino acids has been described in intestinal cells but has not been well characterized (91). The last intron and exon of SLC23A1 overlap with the promoter and first exon and intron of the gene for polyadenylate-binding protein-interacting protein 2 (PAIP2), which encodes a transcriptional repressor protein (26). No genetic interaction between PAIP2 and SLC23A1 has been reported.
The promoter region of SLC23A1 is small, with critical regulatory elements defined only within a few hundred base pairs of the transcription start site. Two independent studies have found functional binding sites for hepatocyte nuclear factor 1 (HNF1) in this region, and regulation of SVCT1 by HNF1α has been demonstrated in HepG2 cells (63, 75). This would support regulation of the protein by HNF4α as well. Although binding of other transcription factors has been postulated, none have been identified. In some cells, SVCT1 expression declines with increasing exposure to ascorbate, but the regulatory factors mediating this response are unknown.
Over 150 SNPs have been identified in SLC23A1, although many of these have not been verified in large populations to determine global allele frequencies. Prevalence of 13 SNPs has been noted in Caucasian, African American, and, to a lesser degree, Asian populations (9, 26). Overall, African American populations show the greatest diversity of SNPs present in SLC23A1—many of which are not detectable in Caucasians (9). However, as studies into the genetic variations in SLC23A1 continue, a different portrait of SVCT1 genetic variability may emerge.
At least four of the SNPs present in SLC23A1 are found within the coding region (exons 3, 7, and 8), which results in one synonymous and three nonsynonymous changes to the protein (Table 1). All nonsynonymous polymorphisms produce a functional SVCT1 protein, but each of these proteins displays functional declines in ascorbate transport when expressed in Xenopus laevis oocytes (17) (Figure 3b). For example, the amino acid substitution encoded by the rs35817838 SNP results in a methionine-to-valine missense mutation at position 258. Oocytes expressing this particular variant demonstrate a 75% reduction in the rate of intracellular ascorbate accumulation (Figure 3b; A772G). This SNP is found in very low frequency in the general population, primarily in African Americans (Figure 3c; A772G). It is currently unclear if there are individuals homozygous for the expression of this SLC23A1 variant, and its impact on vitamin C homeostasis in humans is unknown.
Table 1.
Phenotypic effects of single-nucleotide polymorphisms (SNPs) found in the human SLC23A1 gene
| Reference SNP ID | Location | Genetic varianta | References | Alterations in phenotypeb |
|---|---|---|---|---|
| rs10063949 | 5′ UTR | c.-97–487 A>G | (27, 28, 85, 94, 97) | Increased plasma and serum ascorbate levels in the BWHHS cohort but not in the EPIC cohort |
| rs6886922 | Exon 3 | c.180 C>T | (17) | Modeled in Xenopus oocytes: 40%–50% reduction in ascorbate transport |
| rs34521685 | Exon 7 | c.652 A>G | (17) | Isoleucine-to-valine substitution at position 218 of SVCT1. Modeled in Xenopus oocytes: 40%–50% reduction in ascorbate transport |
| rs35817838 | Exon 8 | c.772 A>G | (17, 27) | Methionine-to-valine substitution at position 258 of SVCT1. Modeled in Xenopus oocytes: 75% reduction in ascorbate transport |
| rs33972313 | Exon 8 | c.790 G>A | (17, 27, 85) | Valine-to-methionine substitution at position 264 of SVCT1. Modeled in Xenopus oocytes: 40%–50% reduction in ascorbate transport. Strongly associated with decreased plasma and serum ascorbate levels in multiple cohorts |
| rs11950646 | Intron 9 | c.1074–101 A>G | (82, 94) | Increased risk of follicular lymphoma observed in a U.S. cohort |
| rs4257763 | Intron 10 | c.1179+109 T>C | (9, 85) | Decreased serum ascorbate levels in the BWHHS cohort |
| rs34063983 | Intron 11 | c.1310–41 A>G | (27) | None |
| rs6876106 | Intron 13 | c.1550–2088 C>T | (94) | None |
| rs6596473 | Intron 13 | c.1549+2515 G>C | (9, 27, 82, 85, 94) | Trend toward decreased plasma ascorbate levels in two studies. Increased risk of follicular lymphoma in a U.S. cohort |
| rs6596471 | Intron 14 | c.*19+2088 T>C | (85) | None |
Variations listed relative to the coding region in the reference sequence NM_005847.4.
Effects of genetic polymorphism as described in the references provided.
Abbreviations: BWHHS, British Women’s Heart and Health Study; EPIC, European Prospective Investigation into Cancer and Nutrition; SVCT, sodium-dependent vitamin C transport protein
Genetic Variation in SLC23A1 and Vitamin C Status
SVCT1 is considered to play a central role in regulating whole-body vitamin C status and is involved in bulk vitamin C transport throughout the body (Figure 2). Although results from SLC23A1 knockout mice suggest that absorption of ascorbic acid may not be impaired by the loss of SVCT1, the lack of renal reabsorption results in reduced plasma levels of ascorbate. Therefore, genetic variations in SLC23A1 may lower the renal threshold for vitamin C and increase its urinary excretion, limiting steady-state plasma and body vitamin C levels. The reduced rate of ascorbate transport has been demonstrated in Xenopus oocytes as described above (Figure 3b). It is expected that individuals carrying these SNPs would have lower plasma ascorbate levels even with high dietary vitamin C intake (Figure 3d).
In 2010, two studies were published demonstrating the effects of SNPs in SLC23A1 on circulating concentrations of vitamin C (Table 1). Lower serum ascorbate levels were found in Caucasian and East Asian subjects displaying the rs4257763 SNP variant (9). Similarly, this SNP was associated with lower ascorbate levels in subjects participating in the British Women’s Heart and Health Study (BWHHS) (91). Strong trends toward a decline in circulating ascorbate levels were also observed in both aforementioned studies (9, 91) when a different intronic SNP (rs6596473) was examined (Table 1). Interestingly, this same SNP was also associated with a decreased risk of follicular lymphoma (82), as further discussed below. Another SNP (rs10063949) was also found in the BWHHS cohort to be associated with a small but significant increase in circulating ascorbate levels, but this per allele effect was not observed in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk cohort (85).
The most robust finding for the influence of SLC23A1 genetic variation on plasma or serum ascorbate levels was found by Timpson et al. (85) in a multiple cohort evaluation of more than 15,000 individuals in the United Kingdom. One of the SNPs examined (rs33972313) was associated with consistent, reproducible declines in circulating ascorbate levels across all study cohorts. As discussed below, this SNP encodes an amino acid change in the SVCT1 protein that results in decreased vitamin C transport activity. Overall, in these human populations, this genetic feature was associated with an approximately 6 μM lower plasma or serum ascorbate concentration per allele present. These declines were noted despite differences in vitamin C intake, smoking status, alcohol consumption, geographical location, and socioeconomic status.
The rs33972313 variant is a nonsynonymous SNP in SLC23A1 that results in a valine-to-methionine substitution at position 264 of SVCT1 (17). The rs33972313 SNP is found in Caucasians, but it is present at a higher frequency in African American and Yoruba African populations (Figure 3c; G790A). This variation results in an approximate 50% decline in the rate of ascorbate accumulation in cells (Figure 3b). It is postulated that the overall effects of this mutation in humans would increase excretion of ascorbic acid from the body and alter the dose-response relationship between plasma ascorbate levels and dietary vitamin C intake (Figure 3d). However, no individuals carrying this variant or homozygous for this allele have been examined to determine the strength of this relationship with vitamin C homeostasis.
To date, the association of SLC23A1 genetic variation and plasma levels of vitamin C is the strongest evidence for the influence of SVCT1 on human vitamin C status. Since the regulation of plasma vitamin C homeostasis ultimately influences the availability of ascorbic acid to all tissues and other fluids in the body, it is possible that further associations between these SNPs in SLC23A1 and physiological function or chronic disease risk will be discovered. However, caution must be taken in such analyses. Associations should not be based merely on genetic variation, but also on measured plasma or serum levels of ascorbic acid, and possibly on urinary excretion rates. Dietary records of vitamin C intake do not adequately reflect vitamin C plasma levels or body status (30, 43) and should not be used to evaluate the possible role of vitamin C in chronic disease risk. Hence, any future studies into phenotypic changes associated with SLC23A1 genetic variation will require measuring the effects on circulating vitamin C levels. Without this data, it will be difficult to draw any conclusions in relation to vitamin C status.
Genetic Variation in SLC23A1 and Chronic Disease Risk
Early studies of genetic variation in SLC23A1 had focused on associations with disease risk. Because higher dietary intake of vitamin C has been associated with lower risk of cancers of the digestive tract (13), and SVCT1 plays an important role in ascorbic acid uptake from the diet, SNPs in SLC23A1 may play a role in gastrointestinal cancer development. However, neither incidence of gastric cancer (94) nor advanced colorectal adenoma (28) was related to SLC23A1 genotype. These results are not surprising given the functional redundancy of vitamin C absorption, as both SVCT1 and SVCT2 are expressed in the digestive tract.
Since many of the genetic variations in SLC23A1 are strongly associated with decreased plasma vitamin C levels, they might affect cancer development in tissues other than the gastrointestinal tract by altering the supply of ascorbic acid in the circulation. Only one study supports this idea: Skibola et al. (82) observed an increased risk of follicular lymphoma associated with two SNPs (rs6596473 and rs11950646), one of which (rs6596473) is associated with decreased plasma ascorbate levels (9, 85) (Table 1). Interestingly, the increased cancer risk was observed in a U.S. but not a German cohort (82). The reason for this discrepancy is not known but may be due to the small population size, lower lymphoma rate in the European cohort, or interactions with diet and environmental exposures that were not assessed in the study.
Higher vitamin C intakes or plasma levels are consistently associated with decreased incidence and risk of death from cardiovascular diseases (30, 32, 47, 65, 90, 95). Furthermore, several studies have shown that vitamin C supplementation and higher plasma ascorbate levels are associated with decreased blood pressure, improved endothelial function, lowered circulating C-reactive protein levels, and increased serum high-density lipoprotein cholesterol levels (5, 7, 31, 44). Given these strong associations with vitamin C status, it is surprising that no studies have investigated the effects of SNPs in SLC23A1 on cardiovascular function. There is a significant need to determine the impact of genetic variation in the SLC23 family on the risk of cardiovascular diseases, including coronary artery disease, ischemic stroke, and hypertension.
Taken together, the current data suggest that there is only a weak association, at best, between SLC23A1 polymorphisms and disease risk. However, it should be noted that in all of the aforementioned studies, plasma levels of ascorbate were not measured, and dietary vitamin C intake was evaluated in only two of the studies (28, 94). It is often assumed that dietary vitamin C intake accurately reflects vitamin C body status, but the presence of SNPs in SLC23A1 may alter SVCT1-based absorption of vitamin C from the diet. Because steady-state levels of plasma ascorbate are affected by polymorphisms in SLC23A1 (see above), the supply of ascorbate to tissues may also be affected. This suggests that in studies of chronic disease risk, the influence of SLC23A1 SNPs must be evaluated with regard to changes in measured, circulating levels of ascorbate.
Furthermore, it must be noted that the studies on SLC23A1 variants and cancer risk were limited to Caucasian populations. The only nonsynonymous SNP in SLC23A1 found at high frequency in Caucasian populations is the rs33972313 variant, which—as mentioned above—results in a valine-to-methionine substitution at position 264 of SVCT1 and decreased vitamin C transport activity (17). This SNP was not evaluated for cancer risk. Since other changes in the SVCT1 protein are found at greater frequency in African and African American populations (Figure 3C), there may be associations between risk for certain diseases and genetic variations in SLC23A1 that have yet to be discovered.
SVCT2 (SLC23A2)
Encoded by the gene SLC23A2, SVCT2 is a vitamin C transport protein found in nearly every tissue and cell of the body (Figure 2). SVCT2 is a high-affinity ascorbate transport protein responsible for the accumulation of vitamin C into tissues that have the highest concentrations of ascorbate: the brain, eyes, and adrenals (86). SVCT2 is also the only vitamin C transport protein expressed in leukocytes (70) and platelets (79); however, it is notably absent from erythrocytes (59).
Mouse models in which SLC23A2 has been ablated survive until birth but die soon afterward from cerebral hemorrhaging and respiratory failure due to lack of lung expansion (83). Thus, it is believed that the expression of SVCT2 and adequate ascorbate levels in the brain are critical for normal fetal development. Studies in animal models and human cell lines support a role of redox signaling in the transcriptional regulation of SVCT2 (69, 70, 80). Intracellular ascorbic acid levels regulate the expression of SVCT2 in many tissues, although the brain appears to be an exception (61).
Genomic Characterization of SLC23A2
SLC23A2 is approximately 10 times larger than SLC23A1, exceeding 150 kbp on chromosome 20 in humans (26, 73). Corresponding to this increased gene size, larger 5′ and 3′ untranslated regions (UTRs) and wider intronic sequences have been noted in SLC23A2. Two distinct promoter regions exist in this gene, allowing for alternative sites for the initiation of transcription and different lengths of mRNA produced (76). SLC23A2 has 17 exons, with the alternative starting exons labeled exon 1a and exon 1b. Transcription through both exons has been detected in human cells, but exon 1b predominates in mature mRNA transcripts. Transcriptional regulation of both promoter regions has been described through KLF, Sp1, YY1, and factors of the EGR family (71, 72), though the functional aspects of this regulation are unclear. Additional regulation may come in the form of microRNA binding to the 3′ UTR of the full SLC23A2 transcript. A recently published study links the expression of miR-30b and miR-30d with lowered SLC23A2 expression (33). These microRNAs have been implicated in regulating p53 and catalase expression in response to hydrogen peroxide exposure (38, 50) and may mediate redox regulation of SVCT2 expression.
Due to the relatively large size of the gene, variants within the genetic structure are relatively frequent and widespread. Over 2,200 SNPs have been identified, and at least 250 of those are present in greater than 5% of all populations tested. Published reports have mentioned 30 different SNPs that span the entire length of the gene (26). Although some specific variants have not been detected in different groups, many SNPs are present in individuals of different ethnic backgrounds.
Overall, most of the SNPs analyzed to date fall in intronic or untranslated regions of SLC23A2 (Table 2) and do not directly affect the coding of the SVCT2 protein. SNPs that do lie within the protein coding region of the gene have all been synonymous polymorphisms, suggesting that amino acid changes in SVCT2 are generally unfavorable mutations. However, SNPs not involved in protein coding are not precluded from impacting cellular SVCT2 levels. For example, one relatively rare SNP (rs16990301) found in a Yoruba African population appears to influence regulation by the miR-30d microRNA, leading to decreased SVCT2 mRNA levels (33).
Table 2.
Phenotypic effects of single-nucleotide polymorphisms (SNPs) found in the human SLC23A2 gene
| Reference SNP ID | Location | Genetic varianta | References | Alterations in phenotypeb |
|---|---|---|---|---|
| rs1279683 | Intron 1 | c.-375–947 C>T | (97) | Associated with decreased plasma ascorbate levels and increased risk of developing glaucoma |
| rs12479919 | Intron 2 | c.-282+1312 G>A | (2, 27, 28, 94) | Trend toward decreased risk of gastric cancer; considered part of a high-risk bladder cancer phenotype |
| rs2681118 | Intron 2 | c.-282+14050 C>A | (27, 28, 94) | None |
| rs6139591 | Intron 3 | c.-155+80 C>T | (9, 27, 28, 94) | Trend toward increased risk of preterm delivery; no association with serum ascorbate levels |
| rs2681116 | Intron 3 | c.-155+108 A>G | (9, 27, 28, 94) | None, including no association with serum ascorbate levels |
| rs13037458 | Intron 3 | c.-155+224 T>G | (28, 94) | None |
| rs4813725 | Intron 3 | c.-154–4623 G>A | (27, 28, 94) | None |
| rs1776948 | Intron 3 | c.-154–17751 C>T | (82) | None |
| rs1715365 | Intron 4 | c.109–5464 G>A | (27, 28) | None |
| rs6133175 | Intron 5 | c.207+1767 T>C | (82) | None |
| rs1776964 | Exon 7 | c.375 C>T | (27, 28, 94) | None |
| rs4987219 | Intron 9 | c.642+453 G>C | (15, 27, 28) | Decreased risk of head and neck cancer with concurrent HPV infection |
| rs1110277 | Exon 12 | c.1002 C>T | (27, 28, 94) | None |
| rs16990301 | Exon 17 | c.*2203 G>A | (33) | Limited interactions with regulating miRNA; associated with a decreased expression of SVCT2 in cells |
| rs35560557 | Exon 17 | c.*2724 C>T | (27, 28) | None |
Variations listed relative to the coding region in the reference sequence NM_005116.6.
Effects of genetic polymorphism as described in the references provided.
Abbreviations: HPV, human papillomavirus; miRNA, microRNA; SVCT, sodium-dependent vitamin C transport protein.
Genetic Variation in SLC23A2 and Vitamin C Status
Unlike variation in SLC23A1, genetic changes in SLC23A2 are not expected to have a large impact on ascorbate homeostasis in the circulation, as SVCT2 regulates the tissue accumulation of the vitamin from the plasma. To date, only two studies have addressed this question. The first found no correlation between serum ascorbate levels and two common SNPs (rs6139591 and rs2681116) in intron 3 of SLC23A2 (9). The second study observed significantly lower plasma ascorbate levels in subjects with variants of an SNP in intron 1 (rs1279683) (97). Since this SNP lies in the intronic regions of the gene, it is difficult to explain why it affects circulating ascorbate levels. Because it lies on the 5′ end of the gene, it is possible that the rs1279683 polymorphism affects the transcriptional regulation of SVCT2 mRNA.
Studies are lacking on the possible effects of genetic variation in SLC23A2 on cellular vitamin C status. No studies have looked at SVCT2 protein levels in human cell lines expressing these SNPs, and no individuals carrying these variant alleles have been evaluated for tissue ascorbate levels. As indicated above, measuring plasma vitamin C may not be an accurate evaluation of the effect of SLC23A2 SNPs on the concentration of vitamin C in tissues. Future studies should assess ascorbate levels of circulating leukocytes or other cells, e.g., tissue biopsies or buccal cells, as a surrogate of tissues in subjects with genetic variations in SLC23A2.
Genetic Variation in SLC23A2 and Chronic Disease Risk
Since vitamin C concentrations in the eye are among the highest in the human body (Figure 2), changes in SVCT2 function may have a strong impact on eye health. Indeed, in a Mediterranean population, a polymorphism found in intron 1 of SLC23A2 (rs1279683) was strongly associated with an increased risk of developing glaucoma (97). Not only was this association statistically significant after adjusting for many confounding factors that could impact disease risk or alter vitamin C status, but glaucoma patients with the variant allele had the lowest plasma ascorbate levels of all subjects enrolled in the study. Severity of cataracts has also been associated with vitamin C levels in the eye lens in humans. Although some studies have observed increased dietary vitamin C intake and increased blood levels of vitamin C to be associated with decreased risk of cataracts (22, 74, 87), no studies have been performed in conjunction with genetic variation in SLC23A2. Hence, this may prove an interesting avenue for future research.
Vitamin C in tissues has been postulated to affect relative cancer risk, but the overall support of this association for SLC23A2 is limited (Table 2). A variant in SLC23A2 observed within intron 2 (rs12479919) correlated with a trend (p = 0.06) toward a decrease in risk of gastric cancer (94). In addition, genetic variation at this site modified risk for bladder cancer, but only in association with SNPs in 20 other “high-risk” genes (2). An SNP in intron 9 (rs4987129) was associated with a decreased incidence of head and neck cancer in individuals testing positive for human papilloma virus infection (15). Haplotypes with the rs4987129 SNP and a SNP in exon 11 (rs1110277 revealed that the combination of the two rare alleles reduced the risk of developing advanced colorectal cancer (28). Similarly, other haplotype analyses suggested the involvement of intronic SNPs in a reduced risk of developing gastric cancer and non-Hodgkin’s lymphoma (82). It should be noted that in each of these studies investigating different types of cancer, plasma or tissue ascorbate levels were not assessed in association with the SNPs in SLC23A2. Therefore, it is not known whether these genetic variants may affect cancer risk due to changes in vitamin C plasma or tissue levels. However, if these genetic variants indeed lead to lower tissue concentrations of vitamin C, a plausible mechanism for the increased cancer risk may be increased expression of hypoxia-inducible factor 1α (34).
Although not a disease state per se, the presence of SLC23A2 SNPs in mothers was associated with an increased risk of spontaneous preterm delivery (24–37 weeks gestational age), but the overall effects of the genetic variation were not robust (27).
As mentioned above for SLC23A1, a majority of the studies investigating disease risk did not assess plasma ascorbate levels. Since SVCT2 controls the transport of ascorbate from the bloodstream to tissues, circulating levels of ascorbate may influence the risk of disease in tissues. In light of the strong associations between vitamin C levels and vascular function mentioned above and the fact that the heart and vascular endothelium only express SVCT2, it is interesting to note that no studies have examined the impact of genetic variation in SLC23A2 on cardiovascular disease risk. In addition, no studies have been performed on SLC23A2 variation and brain function. Because vitamin C is critical for normal brain development and function and is thought to play a role in the prevention of neurodegenerative diseases, behavioral or functional associations with SLC23A2 SNPs should be addressed in future studies.
HAPTOGLOBIN
Unlike the SVCT proteins, the interaction between haptoglobin and vitamin C is believed to be indirect, with iron dysregulation and the generation of reactive oxygen species acting as intermediaries. Both increased iron levels and oxidative stress may lead to a decline in circulating ascorbate levels due to its antioxidant activity. Despite this indirect relationship, haptoglobin is the only circulating blood factor for which there is a known, strong relationship between genetic variation and vitamin C homeostasis.
Function of Haptoglobin
Haptoglobin is a hemoglobin-binding protein synthesized in the liver and released into the circulation. The primary role of haptoglobin is to bind free hemoglobin after it has been released from damaged or lysed erythrocytes. It is believed that this interaction allows haptoglobin to sequester the iron-porphyrin ring and prevent it from reducing molecular oxygen to superoxide radicals and other reactive oxygen species (36). Experimental animals without a functional copy of the haptoglobin gene (HP) suffer from extensive oxidative damage in tissues, especially the kidneys, where iron may be released from the porphyrin ring (55).
A majority of the haptoglobin-hemoglobin complex is cleared from the circulation by parenchymal tissue in the liver. To a lesser degree, circulating macrophages also remove this complex from the blood, as the CD136 receptor expressed by these cells can bind to conjugated haptoglobin. This may be an important mechanism for the clearance of haptoglobin-hemoglobin complexes trapped in capillaries of peripheral tissues. In either case, after cellular uptake the complex is subjected to lysosomal degradation and its iron atom is recycled for use in other heme proteins (36).
Characterization of Human Haptoglobin Genotypes
Found on chromosome 16, the human haptoglobin gene is polymorphic, with two alleles found in the population at relatively high frequency. The ancestral allele (HP1) consists of 5 exons, while the variant allele (HP2) contains 7 exons. The HP2 allele is thought to have emerged by a duplication of exons 3 and 4. The resulting protein has a larger α chain with a duplication of the cysteine residue that forms intermolecular disulfide bonds (36, 55).
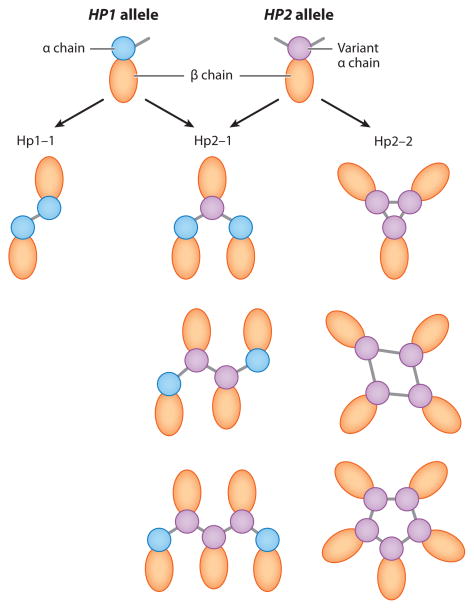
Individuals homozygous for the HP1 allele have traditionally been termed Hp1-1 phenotype. The haptoglobin protein encoded by HP1 is an (αβ)2-tetramer, as the cysteine in the α chain binds a cysteine residue in an adjacent α chain (Figure 4). On the other hand, the α chain encoded by the HP2 allele has an additional cysteine residue that binds two other α chain cysteine residues. Therefore, heterozygous individuals (Hp2-1) can form haptoglobin proteins with three or more α chains in a linear polymer: a core of one or more Hp2 proteins that are capped at each end by Hp1 subunits. In contrast, Hp2-2 individuals, homozygous for the HP2 allele, form larger haptoglobin protein complexes. The protein interactions between the cysteine residues in the HP2 α chains require that these multimeric proteins are cyclical in nature (Figure 4).
Figure 4.
Haptoglobin phenotypes. Haptoglobin is translated as a single polypeptide chain that undergoes posttranslational processing and cleavage to form a light chain (α subunit) and a heavy chain (β subunit). The α subunit of haptoglobin also binds to other α chains through disulfide bridges (55). The haptoglobin protein encoded by HP1 has a cysteine residue in the α chain that binds a cysteine residue in an adjacent α chain. The α chain encoded by the HP2 variant allele has an additional cysteine residue and hence binds two other α chain cysteine residues. The resulting phenotypes are a combination of those two α chains forming a tetrameric protein (Hp1-1), or different sizes of linear (Hp2-1) or cyclic (Hp2-2) haptoglobin polymers.
The molecular interactions of the different haptoglobin proteins distinguish their genotypes. The haptoglobin protein encoded by HP2 binds poorly to hemoglobin compared to HP1. Compared to Hp1-1 individuals, individuals expressing the Hp2-2 phenotype have increased circulating levels of iron, possibly free or still bound to the porphyrin ring of hemoglobin. It is believed that this increased redox-active iron can participate in the generation of superoxide and hydroxyl radicals and is associated with increased oxidative damage to lipoproteins in Hp2-2 individuals (36, 55). Thus, it would be expected that plasma vitamin C levels decline accordingly. The Hp2-2 phenotype is particularly detrimental in diabetics, where glycosylated hemoglobin reduces the ability of haptoglobin to bind to the protein. Thus, diabetic individuals carrying the HP2 allele have a significantly increased risk of cardiovascular disease and diabetes-related complications (55).
Beyond this more common allelic variation, there are reports of SNPs in the HP2 carriers that impact circulating haptoglobin levels (18). In addition, there are also individuals who have a genetic loss of haptoglobin (HP0 allele), but these are found in very low frequencies (67).
Haptoglobin Genetic Variation and Vitamin C Status
As mentioned above, diabetic Hp2-2 individuals demonstrate increased oxidative damage to lipoproteins, which is likely the result of increased redox-active iron levels. In vitro studies have shown that the rate of ascorbic acid oxidation in human plasma containing free hemoglobin is decreased by the addition of haptoglobin from either Hp1-1 or Hp2-2 individuals. This is presumably due to binding of the redox-active iron in the porphyrin ring of hemoglobin by haptoglobin. However, the rate of ascorbate oxidation in the presence of haptoglobin from Hp2-2 individuals is much higher than the oxidation rate observed with the protein isolated from Hp1-1 carriers (51), in agreement with the observation that the Hp2-2 protein binds hemoglobin with weaker affinity.
Several clinical studies have found a relationship between circulating levels of ascorbic acid and haptoglobin phenotype. In two studies, serum ascorbate levels were lower in healthy Hp2-2 subjects compared to both Hp2-1 and Hp1-1 individuals (21, 66). No significant differences in plasma vitamin C concentrations were noted between subjects with the Hp2-1 and Hp1-1 phenotype. The lower serum ascorbate levels in Hp2-2 subjects were correlated with increased serum iron and an overall decrease in circulating levels of the haptoglobin protein. This phenotype-specific decline in plasma ascorbic acid was also demonstrated in individuals with peripheral artery disease (20) and HIV infection (66).
An additional study on haptoglobin phenotype showed no overall difference in plasma ascorbate levels in Hp2-2 individuals versus those carrying the HP1 allele (10). However, Hp2-2 individuals were more likely to have plasma ascorbate levels in the deficient range (<11 μM) when consuming less than the U.S. recommended dietary allowance (RDA) of vitamin C compared to individuals with the Hp2-1 or Hp1-1 phenotype. The authors suggested that this is indicative of a significant diet-gene interaction between vitamin C and haptoglobin phenotype, with Hp2-2 individuals achieving lower steady-state plasma ascorbate concentrations than Hp2-1 or Hp1-1 individuals with the same dietary intake of vitamin C. However, as mentioned above, assessment of dietary intake of vitamin C is problematic, and more carefully controlled studies are needed to establish a relationship between dietary intake of vitamin C, steady-state levels of plasma ascorbate, and haptoglobin phenotype.
It should be noted that most of the health effects of haptoglobin polymorphisms have been determined in individuals with diabetes mellitus (55). However, phenotypic changes in plasma ascorbate levels have been determined primarily in healthy Hp2-2 individuals. It is possible that, under normal conditions, ascorbate provides adequate antioxidant protection to individuals carrying the HP2 allele against oxidative damage to lipoproteins and increased disease risk. It has been speculated that vitamin C supplementation may actually worsen iron-catalyzed oxidative damage in Hp2-2 individuals suffering from diabetes and should be avoided, but experimental evidence supporting this notion is lacking (3).
Furthermore, it is unclear whether the changes in vitamin C status associated with haptoglobin genetic variation are directly related to changes in iron regulation. For example, it is not known whether the haptoglobin phenotype is as pronounced in individuals with low dietary iron intake or chronic anemia. It is also possible that iron dysregulation exacerbates the loss of vitamin C seen with the Hp2-2 phenotype. One study of Hp2-2 individuals from Zimbabwe found that a variant allele in the TF gene, encoding the iron-binding protein transferrin, further reduced circulating levels of ascorbate (46). It is also possible that genetic variations in hemoglobin or the CD136 receptor may compound any effects of the haptoglobin phenotype, increasing the levels of circulating redox-active iron. Therefore, diet-gene interactions in Hp2-2 versus Hp1-1 individuals may extend beyond vitamin C regulation and should be examined with respect to iron status as well.
GLUTATHIONE S-TRANSFERASES
Similar to haptoglobin, most of the interactions between vitamin C and glutathione S-transferases (GSTs) are thought to be mediated by reactive oxygen species. The genetic variations in GST may also impact ascorbate levels through glutathione status, either by preventing the oxidation of ascorbate by reactive oxygen species—hence “sparing” it—or by recycling ascorbate from its oxidized form, dehydroascorbic acid (DHA).
Functions of Glutathione S-Transferase Isoforms
GSTs are phase II enzymes involved in the detoxification of harmful electrophilic compounds of endogenous or exogenous origin. This is accomplished by the conjugation of glutathione to a target molecule recognized by the GST enzyme. Several members of the GST family exist, each with different ranges of substrate targets. Based on sequence homology, eight distinct classes of soluble human glutathione S-transferases have been identified: alpha, kappa, mu, omega, pi, sigma, theta, and zeta. It is believed that the various members emerged over time as a result of a progressive series of gene duplication, recombination, and mutation (39, 60). Inactive GST pseudogenes and repeated DNA sequences lie on chromosomes near active GST members, suggesting the flexible nature of this genetic sequence. This flexibility is also seen in the protein activity, as substrate specificities overlap between isoforms of different GST families.
As part of their response to toxic environmental factors, including the detoxification of carcinogens that otherwise may cause oxidative damage, the GSTs are also induced under conditions of oxidative stress. Alpha, pi, mu, and theta-class GSTs have been implicated in detoxification of numerous reactive lipid oxidation products, such as α,β-unsaturated aldehydes (39). Therefore, it is conceivable that decreased GST activity results in lower levels of ascorbate due to its direct reaction with products of lipid peroxidation (14, 64). Furthermore, changes in glutathione S-transferase activity may also affect glutathione levels, which in turn may alter ascorbate levels through sparing or recycling in cells and plasma, as explained above (98).
Multiple isoforms exist in the GST mu (GSTM1-5) and theta (GSTT1-2) families (39). Both GSTM1 and GSTT1 have deletion variants occurring in relatively high frequencies in human populations from diverse ethnic backgrounds. These are found on chromosome 1 and chromosome 22, respectively. Other common variants are a missense mutation in GSTM1 and a deletion mutation in GSTM3. The GST pi class consists of a single member encoded by the gene GSTP1, found on chromosome 11. Several SNPs have been described in this gene, with the most commonly reported being a missense mutation at position 105, resulting in an isoleucine-to-valine (Ile105Val) amino acid substitution. This mutation reportedly affects the substrate specificity of the protein.
In general, subjects with GSTM1 and GSTT1 deletions are considered to be at high risk from exposure to environmental toxins. Since these two isoforms can also recognize lipid peroxidation products as substrates, a loss of activity may predispose the body to increased oxidative stress.
Human GST Polymorphisms and Vitamin C Status
The relationship between plasma vitamin C and GST genetic variation was first explored in 2001 in a study of middle-aged men in Slovakia, which found that the GSTM1-null genotype was associated with increased circulating levels of ascorbate (25). This was also observed in a more recent study in a U.S. cohort, where individuals with the highest vitamin C levels were more likely to be GSTM1-null than to have a functional copy of GSTM1 (6). However, this relationship between vitamin C and the presence of GSTM1 has not been universally observed. Two studies, one in a Chinese cohort (96) and another with a mixed population in Canada (11), showed no differences in vitamin C status associated with the GSTM1 genotype. In addition, a study of factory workers in Slovakia reported an overall decline in plasma vitamin C levels associated with the GSTM1-null genotype (42).
Although difficult to explain, these disparate results may reflect a more complex relationship of the GSTM1 genotype with vitamin C homeostasis, involving, e.g., dietary vitamin C intake and environmental exposures. The GSTM1-null genotype appeared to be associated with increased plasma levels of ascorbate when the dietary intake of vitamin C also was high (6, 25). Conversely, the populations with low dietary intake of vitamin C and the GSTM1 deletion showed lower plasma ascorbate levels than did individuals with functional GSTM1 at the same intake level (11, 42). This suggests a heretofore uncharacterized interaction between dietary vitamin C, plasma ascorbate, and the activity of GSTs, possibly involving lipid peroxidation products or environmental toxins. There were many different types of environmental exposure in these studies, from cigarette smoking to hazardous working conditions. Together, these data would suggest that further studies on genetic variation in GSTM1 and vitamin C levels in plasma, tissues, and diet are warranted, with special attention to known substrates for GST enzymes.
GSTP1 variation has a poorly defined relationship with vitamin C status. One report in middle-aged men from Slovakia showed that individuals heterozygous for the Ile105Val variant had lower circulating ascorbate levels than did those homozygous for the valine substitution (25). However, this relationship with GSTP1 was not observed in a study of other factory workers in Slovakia (42). A more detailed study of bioavailability of vitamin C in Japanese women showed that individuals heterozygous for the GSTP1 variant had lower absorption after a single oral dose of ascorbic acid, despite having nearly identical plasma ascorbate levels before the supplementation began (41). This would suggest that GSTP1 variations may alter vitamin C bioavailability.
An additional study on vitamin C intake in pregnant women showed that GSTP1 genotype and dietary vitamin C appeared to interact with fetal development and exposure to environmental carcinogens. Although circulating levels of ascorbic acid were not measured in this study, GSTP1 variation and vitamin C intake were associated with differential effects of benzo(a)pyrene exposure (24). Overall, women with the Ile/Val or Val/Val variants of GSTP1 exposed to high levels of benzo(a)pyrene were at significantly higher risk of having children with a low birth weight when their vitamin C intakes were low. However, high vitamin C intake negated the increased susceptibility of those carrying the GSTP1 polymorphism.
In contrast to other GSTs, a loss of GSTT1 does not appear to impact circulating vitamin C levels in the general population. Although an early study of individuals with the GSTT1-null genotype showed a decline in serum ascorbate (25), this was not seen in four other study populations (6, 11, 42, 96). One study reported that in individuals who consumed less than the RDA of vitamin C, GSTT1 gene deletion may contribute to lower circulating levels of ascorbate (11), but the same was not observed in two other studies (6, 96). On the other hand, isolated lymphocytes from individuals missing a functional GSTT1 gene were more susceptible to hydrogen peroxide-induced DNA damage (92), suggesting that cellular ascorbate levels, rather than those in the plasma, should be measured as a functional consequence of GSTT1 disruption.
In general, it appears that interactions between vitamin C levels and genetic variation of GSTs exist, but overall conclusions are difficult to reach. Future studies need to focus on the interactions of GSTs not only with vitamin C bioavailability, but also the interaction of these polymorphisms with each other and with exposure to environmental toxins. Other classes of GSTs should also be examined besides deletions in GSTM1 and GSTT1, or variations in GSTP1. Genetic variation in other members of the mu and theta classes of the enzyme might provide further insight into vitamin C interactions. GST omega has recently been postulated to have DHA reductase activity (98), and future work may address the effect of variation in the GSTO gene family on vitamin C recycling.
OTHER GENETIC INTERACTIONS WITH VITAMIN C
Manganese Superoxide Dismutase Polymorphisms and Vitamin C Status
Superoxide dismutase (SOD) is responsible for the removal of superoxide radicals by conversion to hydrogen peroxide and molecular oxygen and is thus considered one of the major antioxidant enzymes in the body. Three SOD isoforms exist in humans. The isoform found in mitochondria contains manganese as a cofactor (MnSOD) and is encoded by the gene SOD2 (16). A common polymorphism exists in SOD2 that causes a missense mutation at codon 16, causing an alanine-to-valine substitution. The valine-containing isoform of MnSOD is thought to affect the mitochondrial targeting sequence (84) and, hence, resistance of mitochondria to oxidative stress.
Although many studies have found associations between vitamin C intake, MnSOD polymorphisms, and cancer risk (1, 37), only one has measured the effect of MnSOD variants on plasma vitamin C levels (23). Interestingly, healthy individuals homozygous for the valine isoform had higher levels of serum ascorbate. Conversely, those individuals with hypercholesterolemia and at least one copy of the valine-containing MnSOD showed a significant decline in serum ascorbate levels compared to subjects homozygous for the alanine variant. Further work is needed to confirm and expand these observations before a definite conclusion on the effect of SOD2 polymorphisms on vitamin C status can be drawn.
Glucose Transporters and Vitamin C Transport
In addition to the SVCTs, vitamin C accumulation in cells may occur through the transport of DHA by glucose transporter (GLUT) proteins. Specifically, GLUT1, GLUT3, and GLUT10, the gene products of SLC2A1, SLC2A3, and SLC2A10, respectively, have been shown to transport DHA (52, 78). Other GLUT proteins, such as GLUT2 and GLUT4, also have been shown to transport DHA, albeit at a lower rate (77, 89). In many cell types, the rate of transport of DHA through GLUTs can exceed the transport of ascorbic acid through the SVCTs (93). Once brought into the intracellular environment, DHA is rapidly reduced by a variety of nicotinamide adenine dinucleotide phosphate (NADPH)- or glutathione-dependent mechanisms to ascorbic acid. Therefore, supplementing cells with DHA can lead to increased intracellular ascorbate. However, DHA is chemically unstable, with a half-life of only a few minutes in aqueous solutions, and usually is not detectable in tissues or plasma except in times of oxidative stress. In addition, animal models unable to express SVCT1 or SVCT2 have severe phenotypes due to ascorbate deficiency (17, 83), demonstrating that GLUT transporters cannot compensate for the lack of SVCT-mediated vitamin C transport. Thus, it is unclear what, if any, contribution GLUT expression makes to cell and tissue vitamin C levels under normal physiological conditions.
Human genetic variation in glucose transport follows a broad range of gene changes in the SLC2 family. With respect to GLUT variation and vitamin C homeostasis, the evidence is scant. Erythrocytes from individuals with GLUT1 deficiency syndrome demonstrate a decreased ability to accumulate DHA ex vivo (48, 49), but whether this affects the concentration of ascorbate in erythrocytes in vivo is doubtful. Arterial tortuosity syndrome is a genetic disorder that results in the development of elongated and twisted arteries, including the aorta, and is caused by several loss-of-function mutations in SLC2A10 (GLUT10). It has been postulated that GLUT10 is a mitochrondrial DHA transport protein since mitochondria isolated from murine models expressing one of these SLC2A10 genetic variants exhibit decreased DHA uptake in vitro and increased oxidative damage (52). However, there is no evidence to date that these genetic changes in GLUT10 result in altered circulating or tissue vitamin C levels in humans. Overall, genetic variation in the various members of the SLC2 family may affect vitamin C status, but more studies are needed to support this notion.
CONSEQUENCES OF GENETIC VARIATION
Pharmacokinetic studies have demonstrated that plasma ascorbate rises in a linear fashion with increasing doses of vitamin C until plasma and tissues become saturated and a maximum steady-state level is reached (53, 54) (Figure 3d). However, a key aspect to understanding the impact of human genetic variation on vitamin C homeostasis is that the relationship between dietary and plasma vitamin C levels may be altered. It is possible that those individuals expressing particular variant gene products might require more vitamin C in the diet to achieve the same plasma and tissue ascorbate levels as those seen in individuals without these genetic variants. Another possibility is that these individuals simply may not achieve the same maximum steady-state level of plasma ascorbate even when consuming large doses of vitamin C, as described for SVCT1 variants (Figure 3d).
It is likely that individuals with the lowest dietary intake of vitamin C are the most susceptible to the effects of genetic variation. The differences in plasma vitamin C levels are relatively small between those considered severely deficient (<11 μM) and marginally deficient (11–28 μM). Severe vitamin C deficiency is associated with clinical symptoms of scurvy, such as impaired wound healing and gingivitis, whereas marginally deficient plasma ascorbate levels are associated with early signs of scurvy, including malaise and fatigue, gingival inflammation, and bone abnormalities (30). In the presence of a genetic polymorphism, the number of individuals in the marginally and severely deficient vitamin C categories might be increased compared to other members of the population without this polymorphism, as postulated for GST variations (11).
Numerous epidemiological studies have observed that individuals with the highest plasma ascorbate levels display a reduced risk of cardiovascular diseases and cancer compared to individuals with the lowest plasma ascorbate levels (30). For example, the EPIC-Norfolk study concluded that each 20 μM increase in plasma ascorbate level was associated with a 20% decreased risk of all-cause mortality (47) and a 9% relative reduction in risk of heart failure (68). To achieve these benefits, it is commonly recommended that dietary consumption of vitamin C–rich fruits and vegetables should be increased. Individuals with genetic variants might require even higher dietary vitamin C intakes or vitamin C supplements to achieve maximal plasma and tissue ascorbate levels or may be unable to reach the same levels as individuals without these genetic variants, even at very high vitamin C intakes (see Figure 3d).
Human genetic variation is a confounding factor in epidemiological studies looking at vitamin C because it increases the variability in response to dietary intake or supplementation of vitamin C and consequently reduces the statistical power to detect significant changes with an intervention. In order to overcome these differences, a further subgrouping of the study population(s) by the polymorphisms described in this review may be necessary to detect specific effects on vitamin C homeostasis or disease risk (30, 56). This has already been observed with haptoglobin, where a beneficial effect of vitamin E supplementation is observed only in diabetics with the Hp2-2 phenotype, who are under increased oxidative stress, but not the Hp1-1 or Hp2-1 phenotypes (88). Future studies conducted without any regard to genetic variation in the population are likely to result in a poor association of vitamin C supplementation with disease prevention.
SUMMARY
Understanding the complex network of interactions involved in the regulation of vitamin C homeostasis in humans has proven difficult because data from animal and cell culture studies cannot be easily extrapolated to humans. However, studies into the normal genetic variation in the human population have proven to be a useful guide and, as evidenced by this review, are starting to provide meaningful information about the importance of several factors controlling vitamin C levels in the human body.
Due to the specific and direct interaction with ascorbic acid, genetic variation in the SLC23 family provides the best information about how alterations in SVCT-mediated vitamin C transport affect vitamin C homeostasis. The SNPs present in the protein-coding region of SLC23A1, in particular, reveal the critical relationship of SVCT1 function with plasma ascorbate levels. The profound nature of this genetic effect cannot be overemphasized. Despite contributions from diet, environmental exposure, health status, and lifestyle, a statistically significant decline in steady-state plasma ascorbate levels has been demonstrated for one of these SLC23A1 polymorphisms (rs33972313), and two others (rs4257763 and rs6596473) showed nonsignificant but still notable reductions in plasma vitamin C levels. It also should be noted that rs4257763 is not a rare allele found only in very specific populations but rather has been found in many study populations. Therefore, there is potential for SLC23A1 genetic changes to have a widespread impact on human vitamin C homeostasis. In addition, studies on variation in the gene coding SVCT2 strongly suggest an important role of vitamin C in disease prevention, especially in certain cancers and glaucoma. It is likely that the effect of SNPs in SLC23A2 will be evident in more tissues once more exacting studies are performed.
Although many of these genetic factors have been examined for their influence on vitamin C homeostasis because of their known role in lowering oxidative stress in the body, the observed interactions with vitamin C reinforce the role of ascorbic acid as a critical biological antioxidant in tissues and extracellular fluids. Genetic variation in the haptoglobin gene demonstrates this relationship directly, as variants in the HP gene show direct correlation with iron dysregulation and oxidative stress. The loss of ascorbate from the plasma reflects the increased demands for antioxidant protection the variant proteins have placed on the system. Genetic variation in SOD2, leading to changes in MnSOD activity, likely will show similar, nonspecific interactions with vitamin C due its nature as an antioxidant enzyme.
Similar antioxidant-related interactions between vitamin C and glutathione S-transferases may occur, but, due to the pluripotent nature of glutathione, the relationship is more complex and currently incompletely understood. This uncertainty reflects the complex interactions that govern vitamin C homeostasis, as a wide range of dietary, environmental, and lifestyle factors affect the interaction between ascorbate, glutathione, and GST activity.
One topic only briefly touched on in this review is the impact that epigenetic regulation of these genes may have on vitamin C status. MicroRNA regulation and methylation-dependent transcription of SLC23A2 were mentioned above, but other types of epigenetic regulation may also apply to this gene and other genes examined in this review. This type of genetic regulation of vitamin C status goes beyond base-pair changes in DNA sequence and may play an important role in vitamin C homeostasis in development and aging.
FUTURE DIRECTIONS
Early studies of the human SLC23 family investigated variations in these genes and chronic disease risk or health outcomes. However, genetic variation is only a single variable among many factors affecting vitamin C homeostasis. We now know that these studies were limited by the nature of these interactions that affect the interplay between dietary, circulating, and tissue levels of vitamin C. Without assessment of vitamin C status, either by measuring plasma, serum, or cellular ascorbic acid concentrations, such genetic studies are likely to result in a null outcome. Measuring dietary intake of vitamin C is an inaccurate measure of vitamin C body status, due to human recall error, questionnaire validity, and variations in food sources, storage, and preparation that affect vitamin C content (4, 19, 40). Future studies must rely only on direct measurement of circulating or cellular vitamin C levels to accurately reflect vitamin C status. Only when held to this rigorous standard can we expect to find robust interactions between vitamin C–related genetic variation and disease risk.
Furthermore, the need for more mechanistic studies is apparent. For example, changes in GST isoforms appear to have an effect on vitamin C levels in some studies, but whether this is due to increased oxidative stress and, hence, depletion of ascorbate by free-radical scavenging is not clear. More mechanistic studies are needed to better understand the interaction between GST activity and ascorbate function. Although genetic changes in SLC23A1 present in the coding region of the SVCT1 protein have been linked to a decline in transport activity, we know little about the effects of changes in the noncoding regions of these genes. This is particularly true for SNPs in SLC23A2, which show only synonymous codon mutations, intronic polymorphisms, and changes in the untranslated regions of the gene.
The human genetic variations covered in this review is by no means an exhaustive list of factors that can influence vitamin C homeostasis. Partially, this is due to the manner in which these genes were discovered. In the various studies reviewed, genetic variants in HP, GSTs, and SOD2 were not associated a priori with ascorbic acid but rather were examined because of their known role in oxidative stress. Because SNPs in each of these genes were found to be related to ascorbate status, these findings not only confirm the role of vitamin C as a powerful antioxidant in the human body but also suggest that genetic variations of other antioxidant or oxidative stress–related genes may affect vitamin C status. For example, genes other than GSTs involved in glutathione homeostasis, such as glutathione peroxidase (GPX), glutathione reductase (GR), glutamate-cysteine ligase (GCLC or GCLM), or the transcription factor Nrf2 (NFE2L2), should be examined. Interactions of polymorphisms in some of these genes with vitamin C (12, 81) have already been proposed to affect lung function. Other obvious candidates to explore for a possible role in vitamin C homeostasis include SOD1 and SOD3 and genes involved in iron and heme metabolism, such as transferrin (TF), ferritin (FT), and heme oxygenase (HO). Perhaps with a focus on changes in vitamin C homeostasis, an exploration of human genetic variation will reveal more genes and enzymes that may influence ascorbate levels in the body.
Along these lines, we have only scratched the surface of the protein-gene or RNA interactions for many of the genes discussed. We do not know if polymorphisms in the untranslated regions of the SLC23 genes lead to a change in gene transcription due to changes in transcription factor binding, for instance. On the other hand, polymorphisms in the transcription factors themselves might lead to a change in expression. A genetic alteration in HNF1α, found in patients with MODY3, would be expected to lead to a substantial decline in SVCT1 synthesis and the renal threshold for vitamin C reabsorption (63). As HNF4α is an upstream regulating factor of HNF1α, the former is also a potential target for SVCT1 regulation. If indeed genetic variants of HNF1A and HNF4A are found to play a role in vitamin C homeostasis, this would suggest that vitamin C is regulated in the body as a carbohydrate in addition to an antioxidant. This intriguing approach to vitamin C genetic regulation has never been examined.
What is the overall impact of human genetic variation in light of the myriad of factors that affect vitamin C homeostasis? The answer to this question is not immediately forthcoming. However, it is becoming increasingly clear that the optimum intake of vitamin C to support normal physiological function and promote good health differs widely between individuals and hence needs to be addressed on an individual basis. Not only are vitamin C levels influenced by age, diet, lifestyle, and various environmental exposures, but also—as evident from this review—by multiple genetic variations. These genetic variations may modify the relationships between ascorbic acid and each of the other variables, raising intriguing new questions. For example, do GST deletions affect cellular ascorbate levels differently in smokers and nonsmokers? Are haptoglobin polymorphisms an important determinant of vitamin C levels in the presence of iron deficiency? Do changes in SLC23A2 result in an altered relationship between dietary intake and plasma and tissue levels of vitamin C? Only as our knowledge of vitamin C homeostasis grows can we make these more complex associations to determine their effects on human health and disease.
ACRONYMS/TERMS
- Vitamin C
includes ascorbic acid and its one- and two-electron oxidation products, ascorbyl radical and dehydroascorbic acid, respectively
- Solute carrier family 23 (SLC23)
includes both SLC23A1 (SVCT1) and SLC23A2 (SVCT2). A gene identified as SLC23A3 has an undefined function
- Apical
in renal physiology, refers to the side of the cell facing the lumen (interior) of the proximal tubule
- Hepatic nuclear factor 1a (HNF1a)
encoded by the gene HNF1A, it is a transcription factor highly expressed in the liver
- Mature onset diabetes of the young (MODY)
a hereditary form of diabetes mellitus. MODY3 is associated with mutations in HNF1A
- Single-nucleotide polymorphism (SNP)
changes in genetic structure between individuals differing by a single nucleotide
- Nonsynonymous
DNA change in the coding region of the protein that results in an amino acid change
- Haplotype
a combination of SNPs; haplotype analysis is used to determine additive effects of separate polymorphisms on an individual’s phenotype
- microRNA
short, noncoding RNAs involved in the regulation of messenger RNA levels
- CD136
also known as macrophage stimulating 1 receptor, involved in response to inflammatory signals, injury, and invasive growth
- Recommended dietary allowance (RDA)
in the United States the RDA for vitamin C is currently 90 mg/day for men and 75 mg/day for women
- Porphyrin
a carbon- and nitrogen-based ring that can bind metals; heme (such as found in hemoglobin) is an iron-containing porphyrin
- Glutathione
a cellular tripeptide with a reactive sulfhydryl group; it is a cellular antioxidant capable of reducing dehydroascorbic acid to ascorbic acid
- Environmental exposure
both man-made and natural toxins present in the environment (smoke, asbestos, heavy metals, etc.)
- Superoxide
reduced form of molecular oxygen (O2•−); a free radical and reactive oxygen species
- Transferrin
an iron-carrying protein found in extracellular fluids
- Solute carrier family 2 (SLC2)
includes SLC2A1 and SLC2A14, encoding glucose transporters GLUT1 and GLUT14, respectively
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Contributor Information
Alexander J. Michels, Email: michelsa@onid.orst.edu.
Tory M. Hagen, Email: tory.hagen@oregonstate.edu.
Balz Frei, Email: balz.frei@oregonstate.edu.
LITERATURE CITED
- 1.Ambrosone CB, Freudenheim JL, Thompson PA, Bowman E, Vena JE, et al. Manganese superoxide dismutase (MnSOD) genetic polymorphisms, dietary antioxidants, and risk of breast cancer. Cancer Res. 1999;59:602–6. [PubMed] [Google Scholar]
- 2.Andrew AS, Gui J, Sanderson AC, Mason RA, Morlock EV, et al. Bladder cancer SNP panel predicts susceptibility and survival. Hum Genet. 2009;125:527–39. doi: 10.1007/s00439-009-0645-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asleh R, Levy AP. Divergent effects of alpha-tocopherol and vitamin C on the generation of dysfunctional HDL associated with diabetes and the Hp 2-2 genotype. Antioxid Redox Signal. 2010;12:209–17. doi: 10.1089/ars.2009.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birlouez-Aragon I, Saavedra G, Tessier FJ, Galinier A, Ait-Ameur L, et al. A diet based on high-heat-treated foods promotes risk factors for diabetes mellitus and cardiovascular diseases. Am J Clin Nutr. 2010;91:1220–26. doi: 10.3945/ajcn.2009.28737. [DOI] [PubMed] [Google Scholar]
- 5.Block G, Jensen CD, Dalvi TB, Norkus EP, Hudes M, et al. Vitamin C treatment reduces elevated C-reactive protein. Free Radic Biol Med. 2009;46:70–77. doi: 10.1016/j.freeradbiomed.2008.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Block G, Shaikh N, Jensen CD, Volberg V, Holland N. Serum vitamin C and other biomarkers differ by genotype of phase 2 enzyme genes GSTM1 and GSTT1. Am J Clin Nutr. 2011;94:929–37. doi: 10.3945/ajcn.111.011460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boekholdt SM, Meuwese MC, Day NE, Luben R, Welch A, et al. Plasma concentrations of ascorbic acid and C-reactive protein, and risk of future coronary artery disease, in apparently healthy men and women: the EPIC-Norfolk prospective population study. Br J Nutr. 2006;96:516–22. [PubMed] [Google Scholar]
- 8.Boyer JC, Campbell CE, Sigurdson WJ, Kuo SM. Polarized localization of vitamin C transporters, SVCT1 and SVCT2, in epithelial cells. Biochem Biophys Res Commun. 2005;334:150–56. doi: 10.1016/j.bbrc.2005.06.069. [DOI] [PubMed] [Google Scholar]
- 9.Cahill LE, El-Sohemy A. Vitamin C transporter gene polymorphisms, dietary vitamin C and serum ascorbic acid. J Nutrigenet Nutrigenomics. 2009;2:292–301. doi: 10.1159/000314597. [DOI] [PubMed] [Google Scholar]
- 10.Cahill LE, El-Sohemy A. Haptoglobin genotype modifies the association between dietary vitamin C and serum ascorbic acid deficiency. Am J Clin Nutr. 2010;92:1494–500. doi: 10.3945/ajcn.2010.29306. [DOI] [PubMed] [Google Scholar]
- 11.Cahill LE, Fontaine-Bisson B, El-Sohemy A. Functional genetic variants of glutathione S-transferase protect against serum ascorbic acid deficiency. Am J Clin Nutr. 2009;90:1411–17. doi: 10.3945/ajcn.2009.28327. [DOI] [PubMed] [Google Scholar]
- 12.Canova C, Dunster C, Kelly FJ, Minelli C, Shah PL, et al. PM10-induced hospital admissions for asthma and chronic obstructive pulmonary disease: the modifying effect of individual characteristics. Epidemiology. 2012;23:607–15. doi: 10.1097/EDE.0b013e3182572563. [DOI] [PubMed] [Google Scholar]
- 13.Carr AC, Frei B. Toward a new recommended dietary allowance for vitamin C based on antioxidant and health effects in humans. Am J Clin Nutr. 1999;69:1086–107. doi: 10.1093/ajcn/69.6.1086. [DOI] [PubMed] [Google Scholar]
- 14.Chavez J, Chung WG, Miranda CL, Singhal M, Stevens JF, Maier CS. Site-specific protein adducts of 4-hydroxy-2(E)-nonenal in human THP-1 monocytic cells: Protein carbonylation is diminished by ascorbic acid. Chem Res Toxicol. 2010;23:37–47. doi: 10.1021/tx9002462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen AA, Marsit CJ, Christensen BC, Houseman EA, McClean MD, et al. Genetic variation in the vitamin C transporter, SLC23A2, modifies the risk of HPV16-associated head and neck cancer. Carcinogenesis. 2009;30:977–81. doi: 10.1093/carcin/bgp076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Church SL, Grant JW, Meese EU, Trent JM. Sublocalization of the gene encoding manganese superoxide dismutase (MnSOD/SOD2) to 6q25 by fluorescence in situ hybridization and somatic cell hybrid mapping. Genomics. 1992;14:823–25. doi: 10.1016/s0888-7543(05)80202-2. [DOI] [PubMed] [Google Scholar]
- 17.Corpe CP, Tu H, Eck P, Wang J, Faulhaber-Walter R, et al. Vitamin C transporter Slc23a1 links renal reabsorption, vitamin C tissue accumulation, and perinatal survival in mice. J Clin Invest. 2010;120:1069–83. doi: 10.1172/JCI39191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cox SE, Doherty C, Atkinson SH, Nweneka CV, Fulford AJ, et al. Haplotype association between haptoglobin (Hp2) and Hp promoter SNP (A-61C) may explain previous controversy of haptoglobin and malaria protection. PLoS ONE. 2007;2:e362. doi: 10.1371/journal.pone.0000362. Creation of the SLC23A1 knockout mouse, linking renal reabsorption to SVCT1 function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dehghan M, Akhtar-Danesh N, McMillan CR, Thabane L. Is plasma vitamin C an appropriate biomarker of vitamin C intake? A systematic review and meta-analysis. Nutr J. 2007;6:41. doi: 10.1186/1475-2891-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delanghe J, Langlois M, Duprez D, De Buyzere M, Clement D. Haptoglobin polymorphism and peripheral arterial occlusive disease. Atherosclerosis. 1999;145:287–92. doi: 10.1016/s0021-9150(99)00079-9. [DOI] [PubMed] [Google Scholar]
- 21.Delanghe JR, Langlois MR, Boelaert JR, Van Acker J, Van Wanzeele F, et al. Haptoglobin polymorphism, iron metabolism and mortality in HIV infection. AIDS. 1998;12:1027–32. [PubMed] [Google Scholar]
- 22.Dherani M, Murthy GV, Gupta SK, Young IS, Maraini G, et al. Blood levels of vitamin C, carotenoids and retinol are inversely associated with cataract in a North Indian population. Invest Ophthalmol Vis Sci. 2008;49:3328–35. doi: 10.1167/iovs.07-1202. [DOI] [PubMed] [Google Scholar]
- 23.Duarte MM, Moresco RN, Duarte T, Santi A, Bagatini MD, et al. Oxidative stress in hypercholesterolemia and its association with Ala16Val superoxide dismutase gene polymorphism. Clin Biochem. 2010;43:1118–23. doi: 10.1016/j.clinbiochem.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Duarte-Salles T, Mendez MA, Morales E, Bustamante M, Rodriguez-Vicente A, et al. Dietary benzo(a)pyrene and fetal growth: effect modification by vitamin C intake and glutathione S-transferase P1 polymorphism. Environ Int. 2012;45:1–8. doi: 10.1016/j.envint.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dusinska M, Ficek A, Horska A, Raslova K, Petrovska H, et al. Glutathione S-transferase polymorphisms influence the level of oxidative DNA damage and antioxidant protection in humans. Mutat Res. 2001;482:47–55. doi: 10.1016/s0027-5107(01)00209-3. [DOI] [PubMed] [Google Scholar]
- 26.Eck P, Erichsen HC, Taylor JG, Yeager M, Hughes AL, et al. Comparison of the genomic structure and variation in the two human sodium-dependent vitamin C transporters, SLC23A1 and SLC23A2. Hum Genet. 2004;115:285–94. doi: 10.1007/s00439-004-1167-x. First association of GST polymorphisms with ascorbic acid levels in humans. [DOI] [PubMed] [Google Scholar]
- 27.Erichsen HC, Engel SA, Eck PK, Welch R, Yeager M, et al. Genetic variation in the sodium-dependent vitamin C transporters, SLC23A1, and SLC23A2 and risk for preterm delivery. Am J Epidemiol. 2006;163:245–54. doi: 10.1093/aje/kwj035. An in-depth genetic characterization of SLC23A1 and SLC23A2, including the first mention of genetic variants. [DOI] [PubMed] [Google Scholar]
- 28.Erichsen HC, Peters U, Eck P, Welch R, Schoen RE, et al. Genetic variation in sodium-dependent vitamin C transporters SLC23A1 and SLC23A2 and risk of advanced colorectal adenoma. Nutr Cancer. 2008;60:652–59. doi: 10.1080/01635580802033110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fischer H, Schwarzer C, Illek B. Vitamin C controls the cystic fibrosis transmembrane conductance regulator chloride channel. Proc Natl Acad Sci USA. 2004;101:3691–96. doi: 10.1073/pnas.0308393100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frei B, Birlouez-Aragon I, Lykkesfeldt J. Authors’ perspective: What is the optimum intake of vitamin C in humans? Crit Rev Food Sci Nutr. 2012;52:815–29. doi: 10.1080/10408398.2011.649149. [DOI] [PubMed] [Google Scholar]
- 31.Frikke-Schmidt H, Lykkesfeldt J. Role of marginal vitamin C deficiency in atherogenesis: in vivo models and clinical studies. Basic Clin Pharmacol Toxicol. 2009;104:419–33. doi: 10.1111/j.1742-7843.2009.00420.x. Extensive review of the factors involved in vitamin C research in humans, including evaluation of current RDAs. [DOI] [PubMed] [Google Scholar]
- 32.Gale CR, Martyn CN, Winter PD, Cooper C. Vitamin C and risk of death from stroke and coronary heart disease in cohort of elderly people. BMJ. 1995;310:1563–66. doi: 10.1136/bmj.310.6994.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gamazon ER, Ziliak D, Im HK, LaCroix B, Park DS, et al. Genetic architecture of microRNA expression: implications for the transcriptome and complex traits. Am J Hum Genet. 2012;90:1046–63. doi: 10.1016/j.ajhg.2012.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao P, Zhang H, Dinavahi R, Li F, Xiang Y, et al. HIF-dependent antitumorigenic effect of antioxidants in vivo. Cancer Cell. 2007;12:230–38. doi: 10.1016/j.ccr.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldenberg H, Schweinzer E. Transport of vitamin C in animal and human cells. J Bioenerg Biomembr. 1994;26:359–67. doi: 10.1007/BF00762776. [DOI] [PubMed] [Google Scholar]
- 36.Goldenstein H, Levy NS, Levy AP. Haptoglobin genotype and its role in determining heme-iron mediated vascular disease. Pharmacol Res. 2012;66:1–6. doi: 10.1016/j.phrs.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Han J, Colditz GA, Hunter DJ. Manganese superoxide dismutase polymorphism and risk of skin cancer (United States) Cancer Causes Control. 2007;18:79–89. doi: 10.1007/s10552-006-0079-6. [DOI] [PubMed] [Google Scholar]
- 38.Haque R, Chun E, Howell JC, Sengupta T, Chen D, Kim H. MicroRNA-30b-mediated regulation of catalase expression in human ARPE-19 cells. PLoS ONE. 2012;7:e42542. doi: 10.1371/journal.pone.0042542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annu Rev Pharmacol Toxicol. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- 40.Henriquez-Sanchez P, Sanchez-Villegas A, Doreste-Alonso J, Ortiz-Andrellucchi A, Pfrimer K, Serra-Majem L. Dietary assessment methods for micronutrient intake: a systematic review on vitamins. Br J Nutr. 2009;102(Suppl 1):S10–37. doi: 10.1017/S0007114509993126. [DOI] [PubMed] [Google Scholar]
- 41.Higasa S, Tsujimura M, Hiraoka M, Nakayama K, Yanagisawa Y, et al. Polymorphism of glutathione S-transferase P1 gene affects human vitamin C metabolism. Biochem Biophys Res Commun. 2007;364:708–13. doi: 10.1016/j.bbrc.2007.10.076. [DOI] [PubMed] [Google Scholar]
- 42.Horska A, Mislanova C, Bonassi S, Ceppi M, Volkovova K, Dusinska M. Vitamin C levels in blood are influenced by polymorphisms in glutathione S-transferases. Eur J Nutr. 2011;50:437–46. doi: 10.1007/s00394-010-0147-2. [DOI] [PubMed] [Google Scholar]
- 43.Jenab M, Riboli E, Ferrari P, Sabate J, Slimani N, et al. Plasma and dietary vitamin C levels and risk of gastric cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST) Carcinogenesis. 2006;27:2250–57. doi: 10.1093/carcin/bgl096. [DOI] [PubMed] [Google Scholar]
- 44.Juraschek SP, Guallar E, Appel LJ, Miller ER., 3rd Effects of vitamin C supplementation on blood pressure: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2012;95:1079–88. doi: 10.3945/ajcn.111.027995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kang JS, Kim HN, Jung da J, Kim JE, Mun GH, et al. Regulation of UVB-induced IL-8 and MCP-1 production in skin keratinocytes by increasing vitamin C uptake via the redistribution of SVCT-1 from the cytosol to the membrane. J Invest Dermatol. 2007;127:698–706. doi: 10.1038/sj.jid.5700572. [DOI] [PubMed] [Google Scholar]
- 46.Kasvosve I, Delanghe JR, Gomo ZA, Gangaidzo IT, Khumalo H, et al. Effect of transferrin polymorphism on the metabolism of vitamin C in Zimbabwean adults. Am J Clin Nutr. 2002;75:321–25. doi: 10.1093/ajcn/75.2.321. [DOI] [PubMed] [Google Scholar]
- 47.Khaw KT, Bingham S, Welch A, Luben R, Wareham N, et al. Relation between plasma ascorbic acid and mortality in men and women in EPIC-Norfolk prospective study: a prospective population study. European Prospective Investigation into Cancer and Nutrition. Lancet. 2001;357:657–63. doi: 10.1016/s0140-6736(00)04128-3. [DOI] [PubMed] [Google Scholar]
- 48.Klepper J, Vera JC, De Vivo DC. Deficient transport of dehydroascorbic acid in the glucose transporter protein syndrome. Ann Neurol. 1998;44:286–87. doi: 10.1002/ana.410440225. [DOI] [PubMed] [Google Scholar]
- 49.Klepper J, Wang D, Fischbarg J, Vera JC, Jarjour IT, et al. Defective glucose transport across brain tissue barriers: a newly recognized neurological syndrome. Neurochem Res. 1999;24:587–94. doi: 10.1023/a:1022544131826. [DOI] [PubMed] [Google Scholar]
- 50.Kumar M, Lu Z, Takwi AA, Chen W, Callander NS, et al. Negative regulation of the tumor suppressor p53 gene by microRNAs. Oncogene. 2011;30:843–53. doi: 10.1038/onc.2010.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Langlois MR, Delanghe JR, De Buyzere ML, Bernard DR, Ouyang J. Effect of haptoglobin on the metabolism of vitamin C. Am J Clin Nutr. 1997;66:606–10. doi: 10.1093/ajcn/66.3.606. [DOI] [PubMed] [Google Scholar]
- 52.Lee YC, Huang HY, Chang CJ, Cheng CH, Chen YT. Mitochondrial GLUT10 facilitates dehydroascorbic acid import and protects cells against oxidative stress: mechanistic insight into arterial tortuosity syndrome. Hum Mol Genet. 2010;19:3721–33. doi: 10.1093/hmg/ddq286. [DOI] [PubMed] [Google Scholar]
- 53.Levine M, Conry-Cantilena C, Wang Y, Welch RW, Washko PW, et al. Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc Natl Acad Sci USA. 1996;93:3704–9. doi: 10.1073/pnas.93.8.3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Levine M, Wang Y, Padayatty SJ, Morrow J. A new recommended dietary allowance of vitamin C for healthy young women. Proc Natl Acad Sci USA. 2001;98:9842–46. doi: 10.1073/pnas.171318198. First association with the Hp2-2 phenotype and decreased levels of plasma ascorbate. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Levy AP, Asleh R, Blum S, Levy NS, Miller-Lotan R, et al. Haptoglobin: basic and clinical aspects. Antioxid Redox Signal. 2010;12:293–304. doi: 10.1089/ars.2009.2793. [DOI] [PubMed] [Google Scholar]
- 56.Lykkesfeldt J, Poulsen HE. Is vitamin C supplementation beneficial? Lessons learned from randomised controlled trials. Br J Nutr. 2010;103:1251–59. doi: 10.1017/S0007114509993229. [DOI] [PubMed] [Google Scholar]
- 57.Macias RI, Hierro C, de Juan SC, Jimenez F, Gonzalez-San Martin F, Marin JJ. Hepatic expression of sodium-dependent vitamin C transporters: ontogeny, subtissular distribution and effect of chronic liver diseases. Br J Nutr. 2011;106:1814–25. doi: 10.1017/S0007114511002273. [DOI] [PubMed] [Google Scholar]
- 58.Maulen NP, Henriquez EA, Kempe S, Carcamo JG, Schmid-Kotsas A, et al. Up-regulation and polarized expression of the sodium-ascorbic acid transporter SVCT1 in post-confluent differentiated CaCo-2 cells. J Biol Chem. 2003;278:9035–41. doi: 10.1074/jbc.M205119200. [DOI] [PubMed] [Google Scholar]
- 59.May JM, Qu ZC, Qiao H, Koury MJ. Maturational loss of the vitamin C transporter in erythrocytes. Biochem Biophys Res Commun. 2007;360:295–98. doi: 10.1016/j.bbrc.2007.06.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McIlwain CC, Townsend DM, Tew KD. Glutathione S-transferase polymorphisms: cancer incidence and therapy. Oncogene. 2006;25:1639–48. doi: 10.1038/sj.onc.1209373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meredith ME, Harrison FE, May JM. Differential regulation of the ascorbic acid transporter SVCT2 during development and in response to ascorbic acid depletion. Biochem Biophys Res Commun. 2011;414:737–42. doi: 10.1016/j.bbrc.2011.09.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Michels AJ, Frei BB. Vitamin C. In: Stipanuk MH, Caudill MA, editors. Biochemical, Physiological, and Molecular Aspects of Human Nutrition. St. Louis, Mo: Elsevier/Saunders; 2012. pp. 626–54. [Google Scholar]
- 63.Michels AJ, Hagen TM. Hepatocyte nuclear factor 1 is essential for transcription of sodium-dependent vitamin C transporter protein 1. Am J Physiol Cell Physiol. 2009;297:C1220–27. doi: 10.1152/ajpcell.00348.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miranda CL, Reed RL, Kuiper HC, Alber S, Stevens JF. Ascorbic acid promotes detoxification and elimination of 4-hydroxy-2(E)-nonenal in human monocytic THP-1 cells. Chem Res Toxicol. 2009;22:863–74. doi: 10.1021/tx900042u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Myint PK, Luben RN, Welch AA, Bingham SA, Wareham NJ, Khaw KT. Plasma vitamin C concentrations predict risk of incident stroke over 10 y in 20 649 participants of the European Prospective Investigation into Cancer Norfolk prospective population study. Am J Clin Nutr. 2008;87:64–69. doi: 10.1093/ajcn/87.1.64. [DOI] [PubMed] [Google Scholar]
- 66.Na N, Delanghe JR, Taes YE, Torck M, Baeyens WR, Ouyang J. Serum vitamin C concentration is influenced by haptoglobin polymorphism and iron status in Chinese. Clin Chim Acta. 2006;365:319–24. doi: 10.1016/j.cca.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 67.Park KU, Song J, Kim JQ. Haptoglobin genotypic distribution (including Hp0 allele) and associated serum haptoglobin concentrations in Koreans. J Clin Pathol. 2004;57:1094–95. doi: 10.1136/jcp.2004.017582. A broad overview of vitamin C, with a review of ascorbate chemistry, biology, nutrition, and health effects of supplementation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pfister R, Sharp SJ, Luben R, Wareham NJ, Khaw KT. Plasma vitamin C predicts incident heart failure in men and women in European Prospective Investigation into Cancer and Nutrition—Norfolk prospective study. Am Heart J. 2011;162:246–53. doi: 10.1016/j.ahj.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 69.Portugal CC, da Encarnacao TG, Socodato R, Moreira SR, Brudzewsky D, et al. Nitric oxide modulates sodium vitamin C transporter 2 (SVCT-2) protein expression via protein kinase G (PKG) and nuclear factor-κB (NF-κB) J Biol Chem. 2012;287:3860–72. doi: 10.1074/jbc.M111.260166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Qiao H, May JM. Macrophage differentiation increases expression of the ascorbate transporter (SVCT2) Free Radic Biol Med. 2009;46:1221–32. doi: 10.1016/j.freeradbiomed.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Qiao H, May JM. CpG methylation at the USF-binding site mediates cell-specific transcription of human ascorbate transporter SVCT2 exon 1a. Biochem J. 2011;440:73–84. doi: 10.1042/BJ20110392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Qiao H, May JM. Regulation of the human ascorbate transporter SVCT2 exon 1b gene by zinc-finger transcription factors. Free Radic Biol Med. 2011;50:1196–209. doi: 10.1016/j.freeradbiomed.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rajan DP, Huang W, Dutta B, Devoe LD, Leibach FH, et al. Human placental sodium-dependent vitamin C transporter (SVCT2): molecular cloning and transport function. Biochem Biophys Res Commun. 1999;262:762–68. doi: 10.1006/bbrc.1999.1272. [DOI] [PubMed] [Google Scholar]
- 74.Ravindran RD, Vashist P, Gupta SK, Young IS, Maraini G, et al. Inverse association of vitamin C with cataract in older people in India. Ophthalmology. 2011;118:1958–65. e2. doi: 10.1016/j.ophtha.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reidling JC, Rubin SA. Promoter analysis of the human ascorbic acid transporters SVCT1 and 2: mechanisms of adaptive regulation in liver epithelial cells. J Nutr Biochem. 2011;22:344–50. doi: 10.1016/j.jnutbio.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rubin SA, Dey S, Reidling JC. Functional analysis of two regulatory regions of the human Na+-dependent vitamin C transporter 2, SLC23A2, in human vascular smooth muscle cells. Biochim Biophys Acta. 2005;1732:76–81. doi: 10.1016/j.bbaexp.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 77.Rumsey SC, Daruwala R, Al-Hasani H, Zarnowski MJ, Simpson IA, Levine M. Dehydroascorbic acid transport by GLUT4 in Xenopus oocytes and isolated rat adipocytes. J Biol Chem. 2000;275:28246–53. doi: 10.1074/jbc.M000988200. [DOI] [PubMed] [Google Scholar]
- 78.Rumsey SC, Kwon O, Xu GW, Burant CF, Simpson I, Levine M. Glucose transporter isoforms GLUT1 and GLUT3 transport dehydroascorbic acid. J Biol Chem. 1997;272:18982–89. doi: 10.1074/jbc.272.30.18982. [DOI] [PubMed] [Google Scholar]
- 79.Savini I, Catani MV, Arnone R, Rossi A, Frega G, et al. Translational control of the ascorbic acid transporter SVCT2 in human platelets. Free Radic Biol Med. 2007;42:608–16. doi: 10.1016/j.freeradbiomed.2006.11.028. [DOI] [PubMed] [Google Scholar]
- 80.Savini I, Rossi A, Catani MV, Ceci R, Avigliano L. Redox regulation of vitamin C transporter SVCT2 in C2C12 myotubes. Biochem Biophys Res Commun. 2007;361:385–90. doi: 10.1016/j.bbrc.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 81.Siedlinski M, Postma DS, van Diemen CC, Blokstra A, Smit HA, Boezen HM. Lung function loss, smoking, vitamin C intake, and polymorphisms of the glutamate-cysteine ligase genes. Am J Respir Crit Care Med. 2008;178:13–19. doi: 10.1164/rccm.200711-1749OC. [DOI] [PubMed] [Google Scholar]
- 82.Skibola CF, Bracci PM, Halperin E, Nieters A, Hubbard A, et al. Polymorphisms in the estrogen receptor 1 and vitamin C and matrix metalloproteinase gene families are associated with susceptibility to lymphoma. PLoS ONE. 2008;3:e2816. doi: 10.1371/journal.pone.0002816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sotiriou S, Gispert S, Cheng J, Wang Y, Chen A, et al. Ascorbic-acid transporter Slc23a1 is essential for vitamin C transport into the brain and for perinatal survival. Nat Med. 2002;8:514–17. doi: 10.1038/0502-514. [DOI] [PubMed] [Google Scholar]
- 84.Sutton A, Imbert A, Igoudjil A, Descatoire V, Cazanave S, et al. The manganese superoxide dismutase Ala16Val dimorphism modulates both mitochondrial import and mRNA stability. Pharmacogenet Genomics. 2005;15:311–19. doi: 10.1097/01213011-200505000-00006. [DOI] [PubMed] [Google Scholar]
- 85.Timpson NJ, Forouhi NG, Brion MJ, Harbord RM, Cook DG, et al. Genetic variation at the SLC23A1 locus is associated with circulating concentrations of L-ascorbic acid (vitamin C): evidence from 5 independent studies with >15,000 participants. Am J Clin Nutr. 2010;92:375–82. doi: 10.3945/ajcn.2010.29438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tsukaguchi H, Tokui T, Mackenzie B, Berger UV, Chen XZ, et al. A family of mammalian Na+-dependent L-ascorbic acid transporters. Nature. 1999;399:70–75. doi: 10.1038/19986. [DOI] [PubMed] [Google Scholar]
- 87.Valero MP, Fletcher AE, De Stavola BL, Vioque J, Alepuz VC. Vitamin C is associated with reduced risk of cataract in a Mediterranean population. J Nutr. 2002;132:1299–306. doi: 10.1093/jn/132.6.1299. [DOI] [PubMed] [Google Scholar]
- 88.Vardi M, Levy AP. Is it time to screen for the haptoglobin genotype to assess the cardiovascular risk profile and vitamin E therapy responsiveness in patients with diabetes? Curr Diab Rep. 2012;12:274–79. doi: 10.1007/s11892-012-0265-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vera JC, Rivas CI, Fischbarg J, Golde DW. Mammalian facilitative hexose transporters mediate the transport of dehydroascorbic acid. Nature. 1993;364:79–82. doi: 10.1038/364079a0. [DOI] [PubMed] [Google Scholar]
- 90.Voko Z, Hollander M, Hofman A, Koudstaal PJ, Breteler MM. Dietary antioxidants and the risk of ischemic stroke: the Rotterdam Study. Neurology. 2003;61:1273–75. doi: 10.1212/01.wnl.0000090458.67821.a3. [DOI] [PubMed] [Google Scholar]
- 91.Wang H, Dutta B, Huang W, Devoe LD, Leibach FH, et al. Human Na(+)-dependent vitamin C transporter 1 (hSVCT1): primary structure, functional characteristics and evidence for a non-functional splice variant. Biochim Biophys Acta. 1999;1461:1–9. doi: 10.1016/s0005-2736(99)00182-0. Development of the SVCT2 knockout mouse, originally named SLC23A1, linking perinatal survival with ascorbate levels. [DOI] [PubMed] [Google Scholar]
- 92.Wilms LC, Claughton TA, de Kok TM, Kleinjans JC. GSTM1 and GSTT1 polymorphism influences protection against induced oxidative DNA damage by quercetin and ascorbic acid in human lymphocytes in vitro. Food Chem Toxicol. 2007;45:2592–96. doi: 10.1016/j.fct.2007.06.031. [DOI] [PubMed] [Google Scholar]
- 93.Wilson JX. Regulation of vitamin C transport. Annu Rev Nutr. 2005;25:105–25. doi: 10.1146/annurev.nutr.25.050304.092647. First association of SLC23A1 polymorphisms with plasma or serum ascorbate levels in human volunteers. [DOI] [PubMed] [Google Scholar]
- 94.Wright ME, Andreotti G, Lissowska J, Yeager M, Zatonski W, et al. Genetic variation in sodium-dependent ascorbic acid transporters and risk of gastric cancer in Poland. Eur J Cancer. 2009;45:1824–30. doi: 10.1016/j.ejca.2009.01.027. The first characterization of the genes SLC23A1 and SLC23A2 and their function as vitamin C transporters. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ye Z, Song H. Antioxidant vitamins intake and the risk of coronary heart disease: meta-analysis of cohort studies. Eur J Cardiovasc Prev Rehabil. 2008;15:26–34. doi: 10.1097/HJR.0b013e3282f11f95. [DOI] [PubMed] [Google Scholar]
- 96.Yuan LH, Meng LP, Ma WW, Li S, Feng JF, et al. The role of glutathione S-transferase M1 and T1 gene polymorphisms and fruit and vegetable consumption in antioxidant parameters in healthy subjects. Br J Nutr. 2012;107:928–33. doi: 10.1017/S0007114511003746. [DOI] [PubMed] [Google Scholar]
- 97.Zanon-Moreno V, Ciancotti-Olivares L, Asencio J, Sanz P, Ortega-Azorin C, et al. Association between a SLC23A2 gene variation, plasma vitamin C levels, and risk of glaucoma in a Mediterranean population. Mol Vis. 2011;17:2997–3004. [PMC free article] [PubMed] [Google Scholar]
- 98.Zhou H, Brock J, Liu D, Board PG, Oakley AJ. Structural insights into the dehydroascorbate reductase activity of human omega-class glutathione transferases. J Mol Biol. 2012;420:190–203. doi: 10.1016/j.jmb.2012.04.014. [DOI] [PubMed] [Google Scholar]