Previous studies showed that morphogenic cell clustering depends on fibronectin fibrillar matrix assembly under procontractile conditions. The present study shows that disruption of fibronectin matrix necessary for dispersal of cell clusters under promigratory conditions requires matrix metalloproteinases, especially MMP-2.
Abstract
Formation of cell clusters is a common morphogenic cell behavior observed during tissue and organ development and homeostasis, as well as during pathological disorders. Dynamic regulation of cell clustering depends on the balance between contraction of cells into clusters and migration of cells as dispersed individuals. Previously we reported that under procontractile culture conditions, fibronectin fibrillar matrix assembly by human fibroblasts functioned as a nucleation center for cell clustering on three-dimensional collagen matrices. Here we report that switching preformed cell clusters from procontractile to promigratory culture conditions results in cell dispersal out of clusters and disruption of FN matrix. Experiments using small interfering RNA silencing and pharmacological inhibition demonstrated that matrix metalloproteinase activity involving MMP-2 was necessary for fibronectin matrix disruption and dispersal of cell clusters.
INTRODUCTION
Formation and maintenance of tissues depend on cell–cell and cell–extracellular matrix (ECM) interactions (Rozario and DeSimone, 2010). Disruption of these interactions can interfere with normal tissue homeostasis such as occurs in development and wound repair and plays an important role in pathological conditions such as tumor invasiveness and metastasis (Nelson and Bissell, 2006; Reinhart-King, 2011; Lu et al., 2012). Assembly and disassembly of multicellular complexes creates shape and functionality in tissues and organs, each of which has its unique composition and characteristics (Sasai, 2013). At the level of cell–cell interactions, cadherins provide tissue cohesion (Steinberg and Takeichi, 1994; Gumbiner, 1996; Duguay et al., 2003). Cell–matrix interactions mediated by integrins play equally important roles, not only by helping to stabilize multicellular structures, but also by regulating cell migration (Huttenlocher et al., 1998; Robinson et al., 2004; Sevilla et al., 2010; Wolf and Friedl, 2011).
Because of its biological relevance, tissue morphogenesis has become an important research topic and subject of bioengineering interest (Mironov et al., 2009). Although not well understood, microenvironmental cues such as ECM composition, stiffness, and porosity play pivotal roles in determining cell behavior (Discher et al., 2005; Grinnell and Petroll, 2010; Grinnell and Ho, 2013; Hakkinen et al., 2011; Petrie and Yamada, 2012; Trappmann et al., 2012; Gu et al., 2014). Formation of mesenchymal cell clusters is a common morphogenic cell behavior observed during tissue and organ development and homeostasis (reviewed in da Rocha-Azevedo and Grinnell, 2013) and may be related to stromatogenesis that occurs as part of invasiveness of tumor cells between fascial planes (Giatromanolaki et al., 2007). Fascial planes constitute paths of least resistance associated with tumor cell invasion (Cross, 2013).
The formation of cell cluster structures can be mimicked in vitro by diverse approaches, including nonadhesive substrates, rotary systems, and three-dimensional (3D) synthetic or biological scaffolds (reviewed in da Rocha-Azevedo and Grinnell, 2013). Using soft, collagen-coated polyacrylamide gels, the Wang laboratory showed that morphogenic cell clustering by fibroblasts occurred through a cell contractile–dependent mechanism requiring Rho and myosin II (Guo et al., 2006). Subsequent work using a similar model but with endothelial cells confirmed that substrate compliance was a key biomechanical parameter that regulated cell clustering (Reinhart-King et al., 2008).
Studies in our laboratory have focused on fibroblasts interacting with 3D collagen matrices (Grinnell and Petroll, 2010). We observed that fibroblasts incubated on 3D collagen matrices in serum-containing medium—a procontractile growth factor environment—also underwent Rho- and myosin II–dependent morphogenic cell clustering similar to cells on soft polyacrylamide. On the other hand, fibroblasts cultured in platelet-derived growth factor (PDGF)–containing medium—a promigratory growth factor environment—exhibited individual cell migration rather than cell clustering (Rhee et al., 2010).
Other research on morphogenic cell clustering on collagen matrices, carried out with fibronectin (FN)-null mouse fibroblasts, demonstrated a FN requirement for clustering and association of FN with the collagen matrix (Sevilla et al., 2010, 2013). We found that fibrillar FN matrix assembled by human fibroblasts functioned as a nucleation center for cell clustering on collagen matrices and that blocking Rho kinase and myosin II or interfering with α5β1 integrin receptors inhibited FN matrix assembly (da Rocha-Azevedo et al., 2013). These findings help explain how FN can function during mesenchymal condensation as a biological scaffold (Frenz et al., 1989; Jahoda et al., 1992; Singh and Schwarzbauer, 2012).
Dynamic regulation of morphogenic cell clustering depends on the balance between contraction of cells into clusters and migration of cells as dispersed individuals. In previous studies, we observed that cell clustering on 3D collagen matrices was a reversible process. That is, switching fibroblast clusters from a procontractile to promigratory growth factor environment resulted in cell dispersal (Rhee et al., 2010). We carried out the present studies to gain further insight into the mechanism and regulation of cell dispersal from clusters.
RESULTS
Growth factor dependence of fibroblast cluster dispersal and FN matrix disruption
As previously reported, fibroblasts incubated in fetal bovine serum (FBS)–containing medium (procontractile conditions) on 3D collagen matrices formed multicellular clusters concomitant with formation of a FN fibrillar matrix. In marked contrast, cells incubated in PDGF‑containing medium (promigratory conditions) remained dispersed without organizing FN (da Rocha-Azevedo et al., 2013). Figure 1A (results quantified in Figure 1B) shows the consequences of further incubating preformed cell clusters in FBS- and PDGF-containing media. In samples switched to PDGF- but not to FBS-containing medium, dispersal of cell clusters occurred. At the same time, disruption of the FN fibrillar matrix took place.
FIGURE 1:
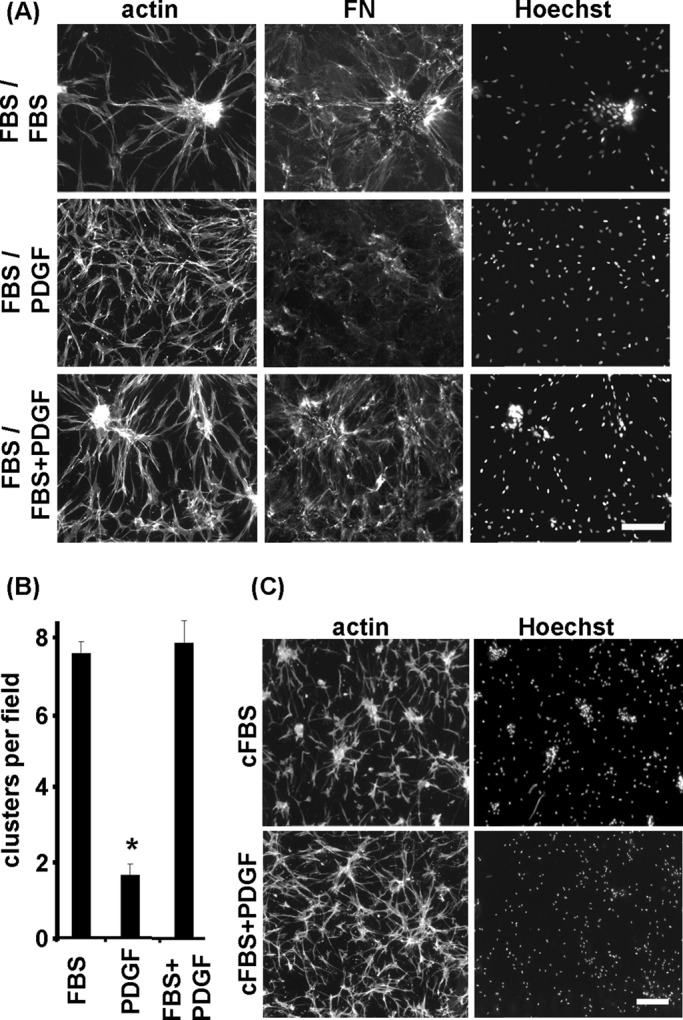
Dispersal of fibroblast clusters and disruption of FN fibrillar matrix disruption depends on switching from procontractile (FBS) to promigratory (PDGF) growth factor conditions. (A) Fluorescence microscopic images of previously formed cell clusters subsequently incubated 18 h in FBS-, PDGF-, or FBS/PDGF-containing medium. In PDGF-containing medium, cell clusters dispersed and FN matrix was disrupted, whereas under FBS/PDGF conditions, cells remained clustered similar to FBS alone. (B) Quantification of clusters in A; *p <0.05. (C) Fluorescence images of cell clusters incubated for 18 h in charcoal-treated FBS (cFBS) culture medium and cFBS/PDGF. Cluster dispersal was observed in cFBS/PDGF but not in cFBS alone. Bars, 100 μm.
To gain further insight into the mechanism of cluster dispersal, we carried out experiments varying procontractile and promigratory growth factor conditions. Figure 1 also shows the results of switching preformed cell clusters to medium containing both FBS and PDGF. FBS exerted a dominant effect. That is, neither FN matrix disruption nor cluster dispersal occurred in the FBS/PDGF samples. Therefore adding the promigratory stimulus by itself was not sufficient to cause cluster dispersal in the continued presence of serum.
Lipid agonists lysophosphatidic acid and sphingosine-1-phosphate are the growth factors responsible for serum procontractile activity necessary for cell clustering and can be removed from serum by treatment with activated charcoal without causing a major change in the overall serum protein composition (Rhee et al., 2010). Figure 1C shows that switching preclustered samples to serum that had been treated with activated charcoal (cFBS) did not result in cluster dispersal. Therefore removing procontractile growth factors also was not sufficient for cell dispersal to occur. On the other hand, samples switched to combined cFBS/PDGF underwent dispersal. Adding the promigratory stimulus in the presence of serum was able to cause cluster dispersal as long as serum procontractile growth factors had been removed.
The dynamics of cluster dispersal and FN matrix disruption was observed in real time by using samples in which clusters had been preformed in the presence of rhodamine‑conjugated FN (R-FN). Figure 2A shows combined phase contrast, fluorescence images from a time‑lapse experiment in which fibroblasts underwent cluster dispersal in PDGF medium during 20 h (see Supplemental Movie). Localized disruption of FN occurred, with residual clumps of R-FN seen remaining associated with cells. After cell dispersal, R-FN was mostly along cell margins rather than in a juxtanuclear location such as might have occurred if R-FN had been phagocytosed. In general, the timing and extent of cluster dispersal were inversely related to cluster size and extent of FN fibrillar matrix.
FIGURE 2:
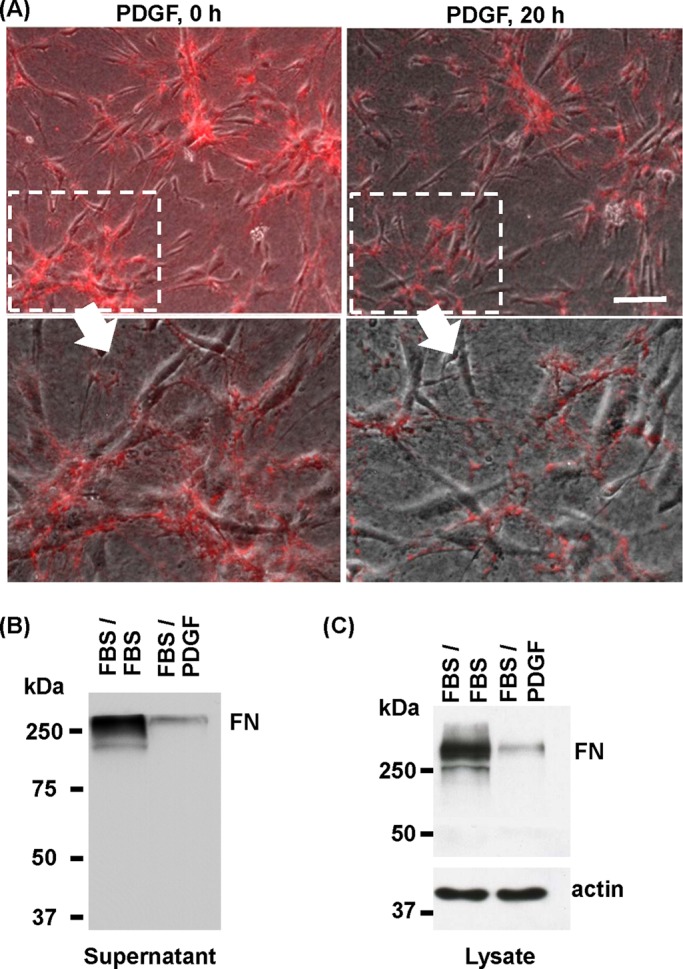
Dynamics of FN matrix disruption during dispersal of cell clusters. (A) Time-lapse phase contrast/fluorescence images of microscopic field (from Supplemental Movie) of cells that had been cultured for 18 h on collagen matrices in FBS-containing medium and 5 μg/ml R-FN to form cell clusters (PDGF, 0 h) and then switched for 20 h to PDGF-containing medium to permit cluster dispersal. Insets, close-up images of clusters and R-FN organization. After dispersal of cell clusters, residual clumps of R-FN were seen to remain associated with the cells, especially along cell margins. Bar, 100 μm. (B) FN analyzed in culture supernatants from experiments in which previously formed cell clusters subsequently were incubated 18 h in FBS- and PDGF- containing media. (C) FN analyzed in cell/matrix extracts from experiments in which previously formed cell clusters subsequently were incubated 18 h in FBS- and PDGF-containing media. Lower amounts of FN were detected in samples corresponding to cell cluster dispersal (FBS/PDGF), and most FN appeared to be intact.
Figure 2B shows immunoblotting analysis of FN in the medium after cluster dispersal. As expected, samples in the continued presence of FBS contained much more FN that samples that had been switched from FBS to PDGF. FN detected in the medium after cluster dispersal appeared to be mostly intact, since the polyclonal antibody we are using would have detected the presence of degraded FN polypeptides. Figure 2C shows immunoblotting analysis of other experiments to analyze FN associated with cells and the collagen matrix after samples were washed and extracted. Consistent with the findings using R-FN, less FN appeared to be associated with cells and matrix after dispersal. However, here again, FN appeared to be intact.
Fibroblast cluster dispersal depends on matrix metalloproteinase activity
Because dispersal of cell clusters was accompanied by disruption of FN fibrillar matrix, we tested whether proteolytic activity was required. Serine proteinases such as urokinase and tissue-type plasminogen activators can degrade FN (Chen and Chen, 1987; Marchina and Barlati, 1996). However, addition of the serine proteinase inhibitor aprotinin (20 μg/ml) to the incubations had no effect on cluster dispersal (unpublished data).
Matrix metalloproteinases (MMPs) also have been shown to degrade ECM and a play a role in cell migration (Robbins et al., 1999; Steffensen et al., 2011; Wolf and Friedl, 2011; Jiao et al., 2012). Figure 3 shows that adding broad-spectrum MMP inhibitors GM6001 (galardin) and BB-94 (batimastat; Galardy et al., 1992; Hidalgo and Eckhardt, 2001) inhibited cell cluster dispersal and prevented disruption of the FN fibrillar matrix. These inhibitors had no effect on initial formation of cell clusters (unpublished data).
FIGURE 3:
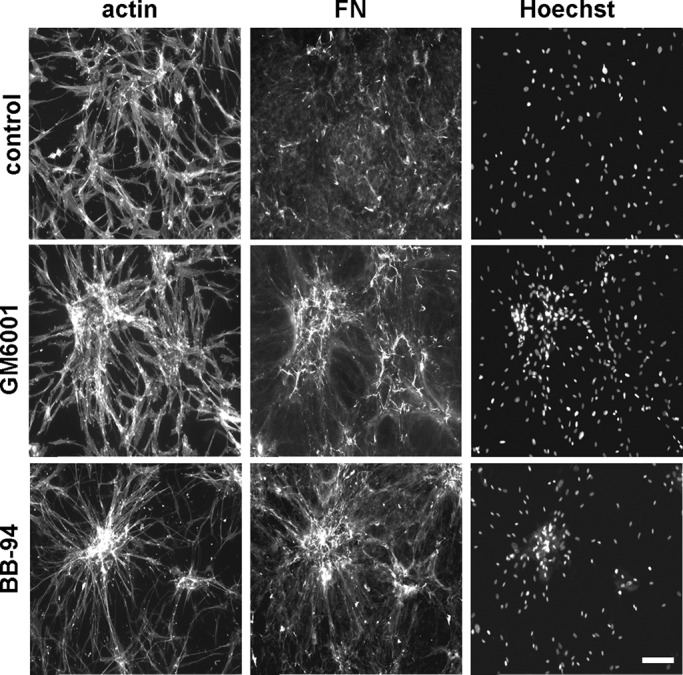
Blocking MMP activity inhibits fibroblast cluster dispersal. Previously formed cell clusters were switched for 18 h to PDGF-containing medium with or without MMP inhibitors BB-94 (10 μM) and GM6001 (50 μM). Fluorescence images show overall cell morphology (actin), organization of fibronectin (FN), and distribution of cell nuclei (Hoechst). Addition of the MMP inhibitors inhibited cluster dispersal and prevented disruption of the FN fibrillar matrix. Bars, 100 μm.
The results with broad-spectrum MMP inhibitors suggested that MMPs were involved in dispersal of cell clusters and disruption of fibronectin fibrillar matrix. Membrane type 1 MMP (MT1-MMP) frequently has been shown to play a role in degradation of ECM components in migrating cells (Kinoshita et al., 1998; Seiki, 2003; Takino et al., 2011). Therefore we tested whether MT1‑MMP was important in dispersal of cell clusters by using small interfering RNA (siRNA) to silence MT1‑MMP expression. Figure 4A shows that siRNA treatment effectively silenced MT1-MMP. However, dispersal of cell clusters occurred similarly with mock and MT1-MMP-silenced fibroblasts (Figure 5B). Silencing MT1-MMP did not prevent cells from forming clustering, and silencing persisted over the length of the experiments (unpublished data).
FIGURE 4:
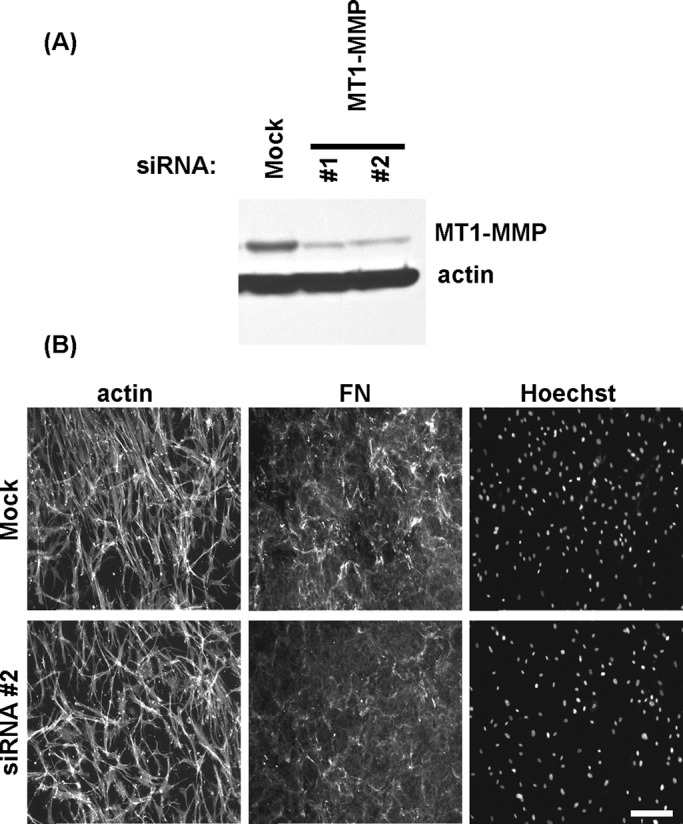
Silencing MT1-MMP does not inhibit cell cluster dispersal. (A) Immunoblotting results showing MT1-MMP and actin detection for cells after transfection with specific MT1-MMP or scrambled noncoding (mock) siRNA oligos for 4 d. (B) Mock and MT1-MMP–silenced fibroblasts were cultured 18 h on collagen matrices in FBS-containing medium to form cell clusters and then switched for 18 h to PDGF-containing medium. Fluorescence images show overall cell morphology (actin), organization of fibronectin (FN), and distribution of cell nuclei (Hoechst). Silencing MT1-MMP did not inhibit cluster dispersal or prevent disruption of the FN fibrillar matrix. Bar, 100 μm.
FIGURE 5:
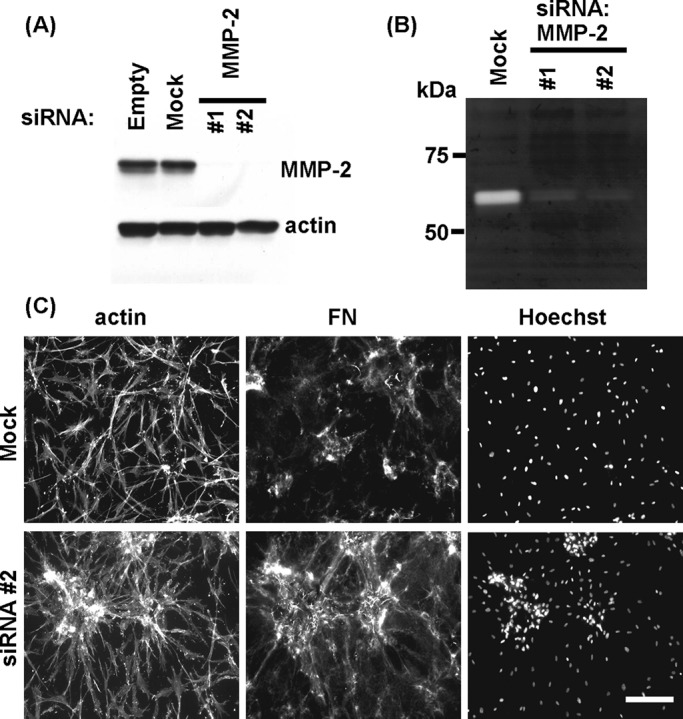
Silencing of MMP-2 inhibits cell cluster dispersal. Immunoblotting (A) and gelatin zymography (B) results showing the effect of MMP-2 silencing by specific MMP-2 but not scrambled noncoding (mock) siRNA oligos for 4 d. (C) Mock and MMP-2–silenced fibroblasts were cultured 18 h on collagen matrices in FBS-containing medium to form cell clusters and then switched for 18 h to PDGF-containing medium. Fluorescence images show overall cell morphology (actin), organization of fibronectin (FN), and distribution of cell nuclei (Hoechst). Silencing of MMP-2 inhibited cluster dispersal and prevented disruption of the FN fibrillar matrix. Bars, 100 μm.
Involvement of MMP-2 in fibroblast cluster dispersal
MMP-2 also has been shown play a role in degradation of ECM components and cell migration (Kinoshita et al., 1998; Xu et al., 2005; Kenny et al., 2008; Steffensen et al., 2011). Therefore we tested whether siRNA silencing of MMP-2 affected dispersal of cell clusters and disruption of fibronectin fibrillar matrix. Figure 5, A and B, shows effective siRNA silencing of MMP-2 expression detected by Western blotting and gelatin zymography. Figure 5C shows that cells with siRNA-silenced MMP-2 no longer exhibited cell cluster dispersal and FN matrix disruption, suggesting that MMP-2 was required for cell cluster dispersal and FN matrix disruption. In other experiments, we found that siRNA silencing of MMP-2 did not affect the ability of cells to undergo clustering (unpublished data).
We compared the expression levels of MMP-2 associated with cells and collagen matrix under dispersal conditions versus cultures maintained in a clustered state. Figure 6A shows that higher levels of MMP-2 were detected in extracted samples under conditions of cluster dispersal (FBS/PDGF). Figure 6B shows that higher levels of secreted MMP-2 were detected in the medium by enzyme-linked immunosorbent assay (ELISA). These data suggested that MMP-2 was up-regulated and secreted during cluster dispersal. We also analyzed cell lysates by zymography, which not only can detect both proenzyme and activated MMP forms, but also is more sensitive than immunoblotting (Troeberg and Nagase, 2004). Figure 6C shows the presence of two prominent gelatinolytic bands in the molecular range of MMP-2 (see Figure 5B) in samples undergoing cell dispersal (control), whereas samples from cultures that contained BB-94 to inhibit dispersal of cell clusters contained only the slower-moving band. This finding was consistent with the possibility that activation of proMMP-2 occurred under conditions of cell dispersal and that BB-94 blocked dispersal of cell clusters by preventing MMP-2 activation.
FIGURE 6:
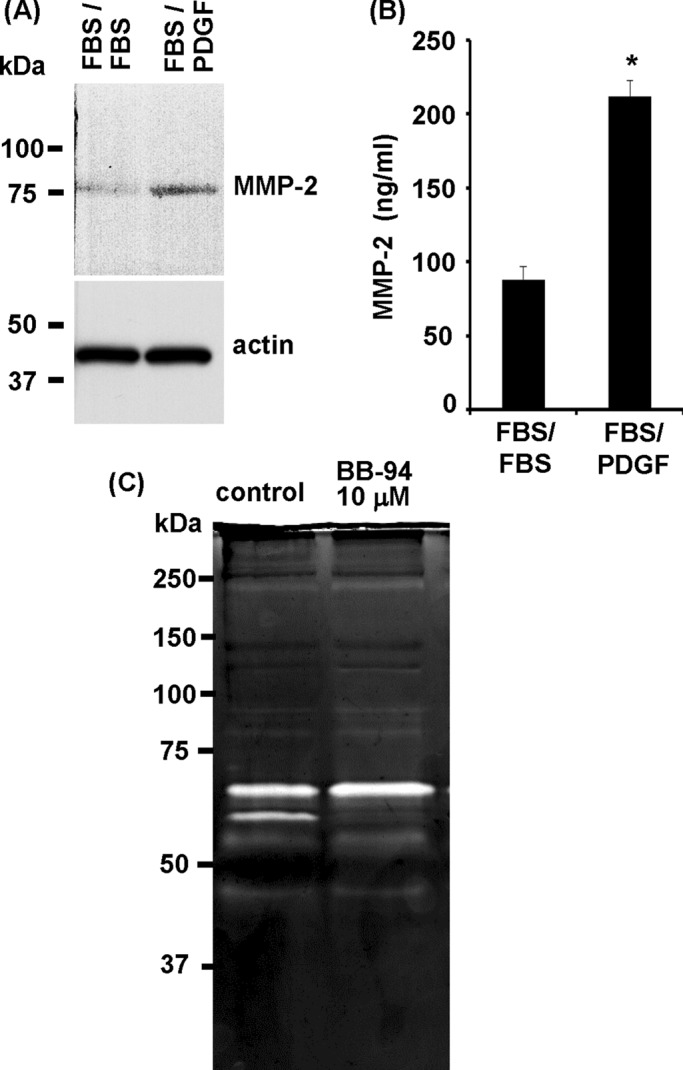
MMP-2 levels and cell cluster dispersal. (A) Previously formed cell clusters were incubated further for 18 h in FBS- or PDGF-containing medium. Cell/matrix extracts were analyzed by SDS–PAGE and immunoblotting for MMP-2 and actin. MMP-2 levels were higher in samples undergoing cell cluster dispersal (FBS/PDGF). (B) Same as A, except that culture supernatants were analyzed for MMP-2 by ELISA. MMP-2 levels were higher in samples undergoing cell cluster dispersal; *p < 0.05. (C) Previously formed cell clusters were incubated further for 18 h in PDGF-containing medium with or without 10 μM BB-94 as indicated. Cell/matrix extracts were analyzed by gelatin zymography. Two prominent bands were observed in the MMP-2 region in samples from cultures in which cell cluster dispersal occurred (control) but not if cell cluster dispersal was inhibited (BB-94).
Similar to other MMPs, MMP-2 enzyme activity requires activation of the proenzyme (Page-McCaw et al., 2007). To test further whether the requirement for MMP-2 in cluster dispersal depended on MMP activity, we carried out experiments using the MMP-2 inhibitor ARP101 (Jo et al., 2011). Figure 7A shows that ARP101 inhibited dispersal of cell clusters in a dose-dependent manner at concentrations others have reported to inhibit cellular MMP-2 activity (Tuccinardi et al., 2006). In addition, Figure 7B shows that FN matrix disruption was inhibited in the presence of the inhibitor. ARP101 had no effect on fibroblast clustering (unpublished data). These findings support the idea that MMP-2 enzymatic activity is required for dispersal of cell clusters and FN matrix disruption.
FIGURE 7:
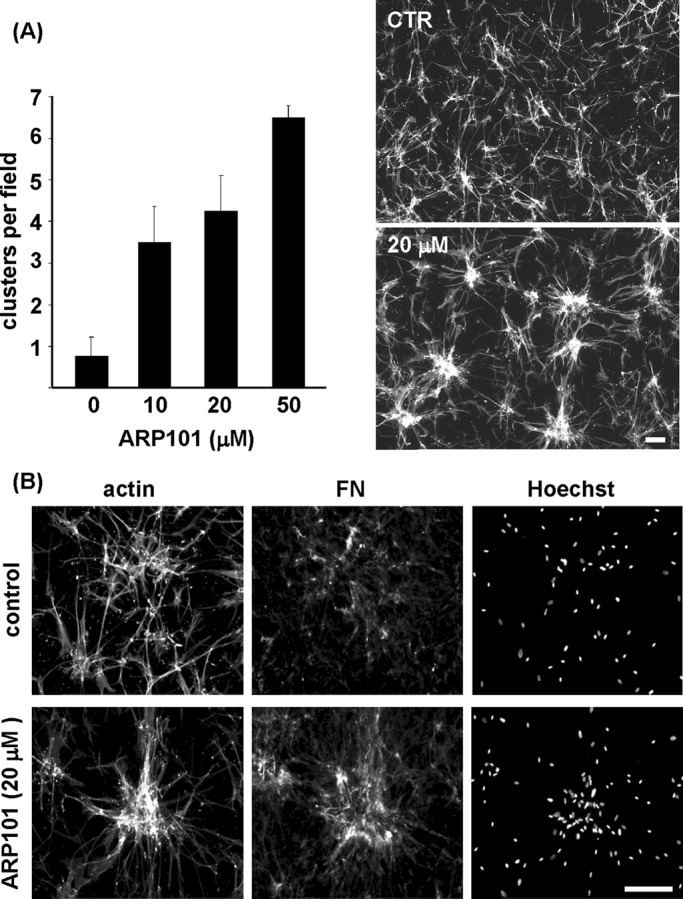
Inhibition of MMP-2 activity blocks cell cluster dispersal. (A) Previously formed cell clusters were incubated further for 18 h in PDGF-containing medium without and with the MMP-2–selective inhibitor ARP101 at the concentrations indicated. Increasing concentrations of ARP101 resulted in decreased cluster dispersal. (B) Fluorescence images show overall cell morphology (actin), organization of fibronectin (FN), and distribution of cell nuclei (Hoechst) in samples incubated with and without 20 μM ARP101. Bars, 100 μm.
DISCUSSION
Formation of mesenchymal cell clusters is a common morphogenic cell behavior observed during tissue and organ development and homeostasis (reviewed in da Rocha-Azevedo and Grinnell, 2013) and may be related to stromal formation that occurs between fascial planes (Giatromanolaki et al., 2007). Fascial planes provide paths of least resistance to tumor cell invasion (Rowe and Weiss, 2009). Previously we reported that fibroblast clustering on collagen matrices is a dynamic, reversible process that depends on the balance between contraction of cells into clusters and migration of cells as dispersed individuals (Rhee et al., 2010). The present research was carried out to gain further insight into the mechanism of cell dispersal. As will be discussed later in detail, our findings suggest that matrix metalloproteinases, especially MMP-2, are required for dispersal of cell clusters and disruption of FN fibrillar matrix upon switching from procontractile to promigratory conditions.
Our studies suggest that dispersal of cell clusters and disruption of the FN fibrillar matrix required two signals: removal of the procontractile stimulus and addition of the promigratory stimulus. This could be concluded since addition of PDGF and FBS together to preformed cell clusters did not cause cluster dispersal or FN matrix disruption, nor did switching preformed clusters to medium containing cFBS, that is, FBS that had been treated with activated charcoal to remove the procontractile serum lipid agonists required for cell clustering (Rhee et al., 2010). However, dispersal of preformed cell clusters did occur with the combination of PDGF and cFBS added to the incubations.
MMPs are known to play a role in cell migration, wound healing, tissue remodeling, and invasion (Stamenkovic, 2000; Page-McCaw et al., 2007; Martins et al., 2013), and broad-spectrum MMP inhibitors GM6001 and BB-94 (Galardy et al., 1992; Hidalgo and Eckhardt, 2001) inhibited cell cluster dispersal and FN matrix disruption. MT1-MMP has been shown to be important for cell migration (Sabeh et al., 2009; Kirmse et al., 2011) by causing pericellular proteolysis of the ECM (Hornebeck et al., 2002; Wolf and Friedl, 2011), but siRNA silencing of MT1-MMP did not block dispersal of cell clusters or FN matrix disruption. On the other hand, siRNA silencing of MMP-2 was as effective as the broad-spectrum MMP inhibitors in preventing cell cluster dispersal and fibronectin matrix disruption.
By immunoblotting, we observed an increase in MMP-2 levels in cell/matrix extracts prepared under conditions of cell cluster dispersal compared with controls. Albeit not detected in the immunoblots, cell/matrix extracts subjected to zymography exhibited two bands consistent with the presence of proMMP-2 and MMP-2. The faster-moving band was not observed if the dispersal incubations had contained BB-94 MMP inhibitor to inhibit cell cluster dispersal. These findings suggested that under promigratory conditions leading to dispersal of cell clusters, cellular proMMP-2 increased and MMP-2 activation occurred. Further evidence that MMP-2 activity was important for dispersal of cell clusters and disruption of FN fibrillar matrix came from pharmacological studies showing a robust inhibitory effect of the MMP-2 activity inhibitor ARP101.
Although our findings showed that MMP-2 activity is required for cell cluster dispersal and FN matrix disruption, the function of MMP-2 probably is not degradation of FN directly. During dispersal of cell clusters, residual clumps of FN fibrillar matrix remained associated with the cells. FN extracted from the cells and matrix and in the medium showed mostly intact FN and little evidence of smaller polypeptides. Therefore the residual clumps of fibronectin matrix probably contained intact FN. Although fibroblasts can phagocytose FN (Grinnell and Geiger, 1986; Salicioni et al., 2002; Sottile and Chandler, 2005), distribution of FN clumps mostly along cell margins rather than in a juxtanuclear location suggested that the FN had not been internalized. Instead of direct degradation of FN, disruption of the fibrillar matrix may be a secondary effect of MMP-2 activation, for instance, by disrupting FN–collagen interactions that have been implicated in microtissue organization (Sevilla et al., 2013).
Alternatively, cells might release FN fibrillar matrix from their surfaces by an as-yet-unknown, MMP-2–dependent regulatory mechanism. In this regard, it is worth noting that MMP‑2 can regulate other MMPs (Loffek et al., 2011), and that serum itself contains MMP-2 and other matrix metalloproteinases, as well as proteinase inhibitors (Wysocki et al., 1993; Grinnell and Zhu, 1996), that could become associated with the collagen matrix. The upstream mechanism of MMP activation in this model system will be an important subject of future research.
In summary, our studies demonstrate dynamic regulation of morphogenic cell structures that depends on the balance between cell clustering and individual cell migration. Our findings suggest that matrix metalloproteinases, especially MMP-2, play a key role in dispersal of cell clusters and disruption of fibronectin fibrillar matrix. These findings are consistent with the observation that PDGF stimulation and MMP-2 expression decrease mesenchymal cell condensation and increase cell migration in tissue explant models and during chondrogenesis (Robbins et al., 1999; Jin et al., 2007). Our experimental model should be useful to help understand better the effects of distinct growth factor signaling environments on cell behavior during normal tissue homeostasis such as occurs in development and wound repair and under pathological conditions such as tumor invasiveness and metastasis.
MATERIALS AND METHODS
Materials
High-concentration, rat-tail type I collagen was obtained from BD Biosciences (Bedford, MA). DMEM, CO2-independent DMEM, Opti-MEM, 0.25% trypsin-EDTA, and antibiotic-antimycotic solutions were purchased from GIBCO (Grand Island, NY). FBS was obtained from Atlanta Biologicals (Lawrenceville, GA). Human recombinant platelet-derived growth factor (PDGF-BB) was obtained from Upstate Biotechnology (Lake Placid, NY). Gelatin was purchased from Bio-Rad (Richmond, CA). Bovine serum albumin (BSA; fatty acid-free), activated charcoal, monoclonal anti-actin antibody, and batimastat (BB-94) were purchased from Sigma-Aldrich (St. Louis, MO). R-FN was purchased from Cytoskeleton (Denver, CO). Human recombinant MMP-2 and GM6001 were purchased from Calbiochem/EMD Millipore (Billerica, MA). ARP101 was obtained from TOCRIS Bioscience (Bristol, UK). Monoclonal antibodies against MMP-2 and MT1-MMP and polyclonal antibody against FN were obtained from Abcam (Cambridge, MA). BSA (fraction V) was obtained from Equitech (Kerrville, TX). Horseradish peroxidase (HRP)–conjugated goat anti-mouse and rabbit secondary antibodies were obtained from MP Biomedicals (Solon, OH), and Thermo Scientific (Pittsburgh, PA), respectively. Hoechst 33342, Alexa 488–phalloidin and Alexa 488– and 568–conjugated secondary antibodies against mouse and rabbit immunoglobulin Gs were obtained from Molecular Probes/Invitrogen (Eugene, OR). Fluoromount G was purchased from Southern Biotechnology (Birmingham, AL). Lipofectamine RNAiMAX was obtained from Life Technologies (Carlsbad, CA). Predesigned siRNA oligonucleotide sequences targeting MMP-2 were obtained from Sigma-Aldrich (Mission siRNA); specific oligonucleotides targeting MT1-MMP and a nontargeting siRNA were obtained from Thermo Scientific–Dharmacon (Lafayette, CO).
Cell culture
BR-5 cells (early passage, hTERT immortalized, human foreskin fibroblasts) were grown on DMEM supplemented with 10% FBS at 37°C and 5% CO2 in a humidified incubator (Rhee et al., 2007). In some experiments, FBS was treated with two rounds of activated charcoal (cFBS) to remove lipid agonists as described previously (Jiang et al., 2008). Experimental condition media consisted of incubating cells on DMEM supplemented with 10% FBS (DMEM-FBS), DMEM supplemented with 5 mg/ml fatty acid–free BSA, and 50 ng/ml PDGF-BB (DMEM-PDGF) or DMEM supplemented with activated charcoal-treated FBS (DMEM-cFBS). For time-lapse microscopy, CO2-independent basal DMEM replaces regular DMEM. The use of human fibroblasts was approved by the University Institutional Review Board (exemption #4).
Collagen matrix preparation, cell clustering, and cluster dispersal
Collagen matrices (1 mg/ml) were prepared as previously described (da Rocha-Azevedo et al., 2013). Cluster formation assays were performed by adding 2 × 104 cells on the top of collagen matrices for 18 h at 37°C in one of the aforementioned media. Cluster dispersal assays consist of incubating clusters premade on DMEM-FBS with DMEM-PDGF for 18 h at 37°C after one careful wash with phosphate-buffered saline (PBS). Matrix metalloproteinase inhibitors GM6001, BB-94, and ARP101 and recombinant MMP-2 (rMMP-2) were added on diverse media as indicated in the figures.
For quantification purposes, a cell cluster was defined as containing as ≥20 cell nuclei/100 μm2 area averaged over four low-magnification (4×) fields. Quantitative experiments were carried out in triplicate and repeated independently twice. Data shown are averages ± SE. Significance (p < 0.05) was determined by using Student's t test.
Microscopy
At the end of experiments, samples were fixed with 3% paraformaldehyde, diluted in PBS, and stained for actin, FN, and cell nuclei as described previously (da Rocha-Azevedo et al., 2013). Observations were made using an Eclipse Ti microscope (Nikon, Melville, NY), using 10×/0.45 PlanApo and 4×/0.13 PlanFluor infinity-corrected objectives. Images were acquired and processed with a CoolSNAP ES2 camera (Photometrics, Tucson, AZ) and NIS Elements imaging software. Final images were transferred to Photoshop (Adobe, San Jose, CA) for processing. Coupled phase contrast and fluorescence time-lapse microscopy of cluster dispersal was performed as previously described with images taken every 20 min for 20 h after addition of DMEM-PDGF (da Rocha-Azevedo et al., 2013).
Western immunoblotting
Immunoblotting was performed as before (da Rocha-Azevedo et al., 2013) using primary antibody dilutions of 1:1000 for FN, MMP-2, MT1-MMP, and actin and 1:5000 for HRP-conjugated goat anti-mouse and anti-rabbit secondary antibodies. For detection of FN in culture supernatants, medium samples were diluted in sample buffer, boiled, and submitted to SDS–PAGE and transferred to polyvinylidene fluoride membranes.
Extraction of cell-containing collagen matrices was accomplished similarly as described (Fringer and Grinnell, 2003). Briefly, for each SDS–PAGE sample, three collagen matrices were washed three times in PBS, combined, and centrifuged for 4 min at low speed and 4°C to remove excess medium. The samples were subjected to 50 strokes with a Dounce homogenizer (pestle B; Wheaton Scientific, Millville, NJ) in 200 μl of NP-40 lysis buffer containing protease and phosphatase inhibitor cocktails. Subsequently, samples were clarified by centrifugation at 14,000 rpm for 10 min at 4°C, and supernatants were dissolved in 4× sample buffer and boiled for 5 min.
siRNA transfection
Semiconfluent cell cultures on six-multiwell plates were washed twice with serum-free DMEM and incubated for 2 d in a mixture containing 250 μl of siRNA-lipid complex per well (final siRNA concentration of 25 pmol, 7.5 μl of Lipofectamine/well in Opti-MEM) in 1.75 ml of DMEM-FBS. After incubation, cells were trypsinized and added on collagen matrices in diverse experimental conditions as described. Mock experiments consist of control nontargeting siRNA sequences instead of MT1-MMP and MMP-2–specific siRNA.
Zymography
Proteolytic activity was assessed using gelatin zymography as described (Troeberg and Nagase, 2004). Briefly, samples prepared in SDS sample buffer under nonreducing conditions were subjected to SDS–PAGE using gels composed of 10% acrylamide copolymerized with 0.1% gelatin. After electrophoresis, gels were washed twice for 30 min with a 2.5% Triton X-100 solution at 4°C to remove SDS and incubated overnight at 37°C in development buffer (50 mM Tris base, 200 mM NaCl, and 5 mM CaCl2, pH 7.5) for protease activation. Areas of gelatinase activity appeared as clear bands against a dark blue background after being stained with Coomassie blue.
MMP-2 detection in culture supernatants
Culture supernatants were collected, neither concentrated nor diluted, and assayed for MMP-2 detection by using a Human MMP-2 ELISA Kit (Invitrogen, Camarillo, CA), following manufacturer's instructions.
Supplementary Material
Acknowledgments
We thank Zhenan Liu for helpful comments. This research was supported by National Institutes of Health Grant GM031321 and by department support provided by Sandra Schmid.
Abbreviations used:
- ECM
extracellular matrix
- FN
fibronectin
- MMP
matrix metalloproteinase
- MT1-MMP
membrane type 1 matrix metalloproteinase
- PDGF
platelet-derived growth factor
- R-FN
rhodamine-conjugated fibronectin.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E14-09-1396) on January 14, 2015.
B.R.A. raised the hypothesis, designed and performed experiments, interpreted results, and contributed to writing the manuscript. C.-H.H. performed experiments and provided helpful comments. F.G. supervised all aspects of the project and contributed to writing the manuscript.
REFERENCES
- Chen JM, Chen WT. Fibronectin-degrading proteases from the membranes of transformed cells. Cell. 1987;48:193-203. doi: 10.1016/0092-8674(87)90423-5. [DOI] [PubMed] [Google Scholar]
- Cross S. Underwood's Pathology: A Clinical Approach. London: Elsevier; 2013. [Google Scholar]
- da Rocha-Azevedo B, Grinnell F. Fibroblast morphogenesis on 3D collagen matrices: the balance between cell clustering and cell migration. Exp Cell Res. 2013;319:2440–2446. doi: 10.1016/j.yexcr.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Rocha-Azevedo B, Ho CH, Grinnell F. Fibroblast cluster formation on 3D collagen matrices requires cell contraction dependent fibronectin matrix organization. Exp Cell Res. 2013;319:546–555. doi: 10.1016/j.yexcr.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- Duguay D, Foty RA, Steinberg MS. Cadherin-mediated cell adhesion and tissue segregation: qualitative and quantitative determinants. Dev Biol. 2003;253:309–323. doi: 10.1016/s0012-1606(02)00016-7. [DOI] [PubMed] [Google Scholar]
- Fringer J, Grinnell F. Fibroblast quiescence in floating collagen matrices: decrease in serum activation of MEK and Raf but not Ras. J Biol Chem. 2003;278:20612–20617. doi: 10.1074/jbc.M212365200. [DOI] [PubMed] [Google Scholar]
- Frenz DA, Jaikaria NS, Newman SA. The mechanism of precartilage mesenchymal condensation: a major role for interaction of the cell surface with the amino-terminal heparin-binding domain of fibronectin. Dev Biol. 1989;136:97–103. doi: 10.1016/0012-1606(89)90133-4. [DOI] [PubMed] [Google Scholar]
- Galardy RE, Grobelny D, Kortylewicz ZP, Poncz L. Inhibition of human skin fibroblast collagenase by phosphorus-containing peptides. Matrix Suppl. 1992;1:259–262. [PubMed] [Google Scholar]
- Giatromanolaki A, Sivridis E, Koukourakis MI. The pathology of tumor stromatogenesis. Cancer Biol Ther. 2007;6:639–645. doi: 10.4161/cbt.6.5.4198. [DOI] [PubMed] [Google Scholar]
- Grinnell F, Geiger B. Interaction of fibronectin-coated beads with attached and spread fibroblasts. Binding, phagocytosis, and cytoskeletal reorganization. Exp Cell Res. 1986;162:449–461. doi: 10.1016/0014-4827(86)90349-6. [DOI] [PubMed] [Google Scholar]
- Grinnell F, Ho CH. The effect of growth factor environment on fibroblast morphological response to substrate stiffness. Biomaterials. 2013;34:965–974. doi: 10.1016/j.biomaterials.2012.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell F, Petroll WM. Cell motility and mechanics in three-dimensional collagen matrices. Annu Rev Cell Dev Biol. 2010;26:335–361. doi: 10.1146/annurev.cellbio.042308.113318. [DOI] [PubMed] [Google Scholar]
- Grinnell F, Zhu M. Fibronectin degradation in chronic wounds depends on the relative levels of elastase, alpha1-proteinase inhibitor, and alpha2-macroglobulin. J Invest Dermatol. 1996;106:335–341. doi: 10.1111/1523-1747.ep12342990. [DOI] [PubMed] [Google Scholar]
- Gu Z, Liu F, Tonkova EA, Lee SY, Tschumperlin DJ, Brenner MB. Soft matrix is a natural stimulator for cellular invasiveness. Mol Biol Cell. 2014;25:457–469. doi: 10.1091/mbc.E13-05-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- Guo WH, Frey MT, Burnham NA, Wang YL. Substrate rigidity regulates the formation and maintenance of tissues. Biophys J. 2006;90:2213–2220. doi: 10.1529/biophysj.105.070144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakkinen KM, Harunaga JS, Doyle AD, Yamada KM. Direct comparisons of the morphology, migration, cell adhesions, and actin cytoskeleton of fibroblasts in four different three-dimensional extracellular matrices. Tissue Eng Part A. 2011;17:713–724. doi: 10.1089/ten.tea.2010.0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo M, Eckhardt SG. Development of matrix metalloproteinase inhibitors in cancer therapy. J Natl Cancer Inst. 2001;93:178–193. doi: 10.1093/jnci/93.3.178. [DOI] [PubMed] [Google Scholar]
- Hornebeck W, Emonard H, Monboisse JC, Bellon G. Matrix-directed regulation of pericellular proteolysis and tumor progression. Semin Cancer Biol. 2002;12:231–241. doi: 10.1016/s1044-579x(02)00026-3. [DOI] [PubMed] [Google Scholar]
- Huttenlocher A, Lakonishok M, Kinder M, Wu S, Truong T, Knudsen KA, Horwitz AF. Integrin and cadherin synergy regulates contact inhibition of migration and motile activity. J Cell Biol. 1998;141:515–526. doi: 10.1083/jcb.141.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahoda CA, Mauger A, Bard S, Sengel P. Changes in fibronectin, laminin and type IV collagen distribution relate to basement membrane restructuring during the rat vibrissa follicle hair growth cycle. J Anat. 1992;181:47–60. [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Rhee S, Ho C-H, Grinnell F. Distinguishing fibroblast promigratory and procontractile growth factor environments in 3D collagen matrices. FASEB J. 2008;22:2151–2160. doi: 10.1096/fj.07-097014. [DOI] [PubMed] [Google Scholar]
- Jiao Y, Feng X, Zhan Y, Wang R, Zheng S, Liu W, Zeng X. Matrix metalloproteinase-2 promotes alphavbeta3 integrin-mediated adhesion and migration of human melanoma cells by cleaving fibronectin. PLoS One. 2012;7:e41591. doi: 10.1371/journal.pone.0041591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin EJ, Choi YA, Kyun Park E, Bang OS, Kang SS. MMP-2 functions as a negative regulator of chondrogenic cell condensation via down-regulation of the FAK-integrin beta1 interaction. Dev Biol. 2007;308:474–484. doi: 10.1016/j.ydbio.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Jo YK, Park SJ, Shin JH, Kim Y, Hwang JJ, Cho DH, Kim JC. ARP101, a selective MMP-2 inhibitor, induces autophagy-associated cell death in cancer cells. Biochem Biophys Res Commun. 2011;404:1039–1043. doi: 10.1016/j.bbrc.2010.12.106. [DOI] [PubMed] [Google Scholar]
- Kenny HA, Kaur S, Coussens LM, Lengyel E. The initial steps of ovarian cancer cell metastasis are mediated by MMP-2 cleavage of vitronectin and fibronectin. J Clin Invest. 2008;118:1367–1379. doi: 10.1172/JCI33775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T, Sato H, Okada A, Ohuchi E, Imai K, Okada Y, Seiki M. TIMP-2 promotes activation of progelatinase A by membrane-type 1 matrix metalloproteinase immobilized on agarose beads. J Biol Chem. 1998;273:16098–16103. doi: 10.1074/jbc.273.26.16098. [DOI] [PubMed] [Google Scholar]
- Kirmse R, Otto H, Ludwig T. Interdependency of cell adhesion, force generation and extracellular proteolysis in matrix remodeling. J Cell Sci. 2011;124:1857–1866. doi: 10.1242/jcs.079343. [DOI] [PubMed] [Google Scholar]
- Loffek S, Schilling O, Franzke CW. Series “matrix metalloproteinases in lung health and disease”: biological role of matrix metalloproteinases: a critical balance. Eur Respir J. 2011;38:191–208. doi: 10.1183/09031936.00146510. [DOI] [PubMed] [Google Scholar]
- Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol. 2012;196:395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchina E, Barlati S. Degradation of human plasma and extracellular matrix fibronectin by tissue type plasminogen activator and urokinase. Int J Biochem Cell Biol. 1996;28:1141–1150. doi: 10.1016/1357-2725(96)00055-6. [DOI] [PubMed] [Google Scholar]
- Martins VL, Caley M, O'Toole EA. Matrix metalloproteinases and epidermal wound repair. Cell Tissue Res. 2013;351:255–268. doi: 10.1007/s00441-012-1410-z. [DOI] [PubMed] [Google Scholar]
- Mironov V, Visconti RP, Kasyanov V, Forgacs G, Drake CJ, Markwald RR. Organ printing: tissue spheroids as building blocks. Biomaterials. 2009;30:2164–2174. doi: 10.1016/j.biomaterials.2008.12.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CM, Bissell MJ. Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2006;22:287–309. doi: 10.1146/annurev.cellbio.22.010305.104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie RJ, Yamada KM. At the leading edge of three-dimensional cell migration. J Cell Sci. 2012;125:5917–5926. doi: 10.1242/jcs.093732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart-King CA. How matrix properties control the self-assembly and maintenance of tissues. Ann Biomed Eng. 2011;39:1849–1856. doi: 10.1007/s10439-011-0310-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart-King CA, Dembo M, Hammer DA. Cell-cell mechanical communication through compliant substrates. Biophys J. 2008;95:6044–6051. doi: 10.1529/biophysj.107.127662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee S, Ho CH, Grinnell F. Promigratory and procontractile growth factor environments differentially regulate cell morphogenesis. Exp Cell Res. 2010;316:232–244. doi: 10.1016/j.yexcr.2009.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee S, Jiang H, Ho CH, Grinnell F. Microtubule function in fibroblast spreading is modulated according to the tension state of cell-matrix interactions. Proc Natl Acad Sci USA. 2007;104:5425–5430. doi: 10.1073/pnas.0608030104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins JR, McGuire PG, Wehrle-Haller B, Rogers SL. Diminished matrix metalloproteinase 2 (MMP-2) in ectomesenchyme-derived tissues of the Patch mutant mouse: regulation of MMP-2 by PDGF and effects on mesenchymal cell migration. Dev Biol. 1999;212:255–263. doi: 10.1006/dbio.1999.9373. [DOI] [PubMed] [Google Scholar]
- Robinson EE, Foty RA, Corbett SA. Fibronectin matrix assembly regulates alpha5beta1-mediated cell cohesion. Mol Biol Cell. 2004;15:973–981. doi: 10.1091/mbc.E03-07-0528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe RG, Weiss SJ. Navigating ECM barriers at the invasive front: the cancer cell-stroma interface. Annu Rev Cell Dev Biol. 2009;25:567–595. doi: 10.1146/annurev.cellbio.24.110707.175315. [DOI] [PubMed] [Google Scholar]
- Rozario T, DeSimone DW. The extracellular matrix in development and morphogenesis: a dynamic view. Dev Biol. 2010;341:126–140. doi: 10.1016/j.ydbio.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabeh F, Shimizu-Hirota R, Weiss SJ. Protease-dependent versus -independent cancer cell invasion programs: three-dimensional amoeboid movement revisited. J Cell Biol. 2009;185:11–19. doi: 10.1083/jcb.200807195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salicioni AM, Mizelle KS, Loukinova E, Mikhailenko I, Strickland DK, Gonias SL. The low density lipoprotein receptor-related protein mediates fibronectin catabolism and inhibits fibronectin accumulation on cell surfaces. J Biol Chem. 2002;277:16160–16166. doi: 10.1074/jbc.M201401200. [DOI] [PubMed] [Google Scholar]
- Sasai Y. Cytosystems dynamics in self-organization of tissue architecture. Nature. 2013;493:318–326. doi: 10.1038/nature11859. [DOI] [PubMed] [Google Scholar]
- Seiki M. Membrane-type 1 matrix metalloproteinase: a key enzyme for tumor invasion. Cancer Lett. 2003;194:1–11. doi: 10.1016/s0304-3835(02)00699-7. [DOI] [PubMed] [Google Scholar]
- Sevilla CA, Dalecki D, Hocking DC. Extracellular matrix fibronectin stimulates the self-assembly of microtissues on native collagen gels. Tissue Eng Part A. 2010;16:3805–3819. doi: 10.1089/ten.tea.2010.0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevilla CA, Dalecki D, Hocking DC. Regional fibronectin and collagen fibril co-assembly directs cell proliferation and microtissue morphology. PLoS One. 2013;8:e77316. doi: 10.1371/journal.pone.0077316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P, Schwarzbauer JE. Fibronectin and stem cell differentiation—lessons from chondrogenesis. J Cell Sci. 2012;125:3703–3712. doi: 10.1242/jcs.095786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sottile J, Chandler J. Fibronectin matrix turnover occurs through a caveolin-1-dependent process. Mol Biol Cell. 2005;16:757–768. doi: 10.1091/mbc.E04-08-0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamenkovic I. Matrix metalloproteinases in tumor invasion and metastasis. Semin Cancer Biol. 2000;10:415–433. doi: 10.1006/scbi.2000.0379. [DOI] [PubMed] [Google Scholar]
- Steffensen B, Chen Z, Pal S, Mikhailova M, Su J, Wang Y, Xu X. Fragmentation of fibronectin by inherent autolytic and matrix metalloproteinase activities. Matrix Biol. 2011;30:34–42. doi: 10.1016/j.matbio.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg MS, Takeichi M. Experimental specification of cell sorting, tissue spreading, and specific spatial patterning by quantitative differences in cadherin expression. Proc Natl Acad Sci USA. 1994;91:206–209. doi: 10.1073/pnas.91.1.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takino T, Nagao R, Manabe R, Domoto T, Sekiguchi K, Sato H. Membrane-type 1 matrix metalloproteinase regulates fibronectin assembly to promote cell motility. FEBS Lett. 2011;585:3378–3384. doi: 10.1016/j.febslet.2011.09.039. [DOI] [PubMed] [Google Scholar]
- Trappmann B, Gautrot JE, Connelly JT, Strange DG, Li Y, Oyen ML, Cohen Stuart MA, Boehm H, Li B, Vogel V, et al. Extracellular-matrix tethering regulates stem-cell fate. Nat Mater. 2012;11:642–649. doi: 10.1038/nmat3339. [DOI] [PubMed] [Google Scholar]
- Troeberg L, Nagase H. Zymography of metalloproteinases. Curr Protoc Protein Sci. 2004 doi: 10.1002/0471140864.ps2115s33. Chapter 21, Unit 21.15. [DOI] [PubMed] [Google Scholar]
- Tuccinardi T, Martinelli A, Nuti E, Carelli P, Balzano F, Uccello-Barretta G, Murphy G, Rossello A. Amber force field implementation, molecular modelling study, synthesis and MMP-1/MMP-2 inhibition profile of (R)- and (S)-N-hydroxy-2-(N-isopropoxybiphenyl-4-ylsulfonamido)-3-methylbutanamides. Bioorg Med Chem. 2006;14:4260–4276. doi: 10.1016/j.bmc.2006.01.056. [DOI] [PubMed] [Google Scholar]
- Wolf K, Friedl P. Extracellular matrix determinants of proteolytic and non-proteolytic cell migration. Trends Cell Biol. 2011;21:736–744. doi: 10.1016/j.tcb.2011.09.006. [DOI] [PubMed] [Google Scholar]
- Wysocki AB, Staiano-Coico L, Grinnell F. Wound fluid from chronic leg ulcers contains elevated levels of metalloproteinases MMP-2 and MMP-9. J Invest Dermatol. 1993;101:64–68. doi: 10.1111/1523-1747.ep12359590. [DOI] [PubMed] [Google Scholar]
- Xu X, Wang Y, Chen Z, Sternlicht MD, Hidalgo M, Steffensen B. Matrix metalloproteinase-2 contributes to cancer cell migration on collagen. Cancer Res. 2005;65:130–136. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


