GlcNAc activates two pathways in the fungal pathogen Candida albicans. In one pathway, GlcNAc induces hyphal morphology. In the other, GlcNAc metabolism raises the ambient pH, which activates pH signaling pathways to induce gene expression. The dual roles are likely important in other organisms in which GlcNAc is emerging as a key signaling molecule.
Abstract
Various stimuli, including N-acetylglucosamine (GlcNAc), induce the fungal pathogen Candida albicans to switch from budding to hyphal growth. Previous studies suggested that hyphal morphogenesis is stimulated by transcriptional induction of a set of genes that includes known virulence factors. To better understand hyphal development, we examined the role of GlcNAc metabolism using a triple mutant lacking the genes required to metabolize exogenous GlcNAc (hxk1Δ nag1Δ dac1Δ). Surprisingly, at low ambient pH (∼pH 4), GlcNAc stimulated this mutant to form hyphae without obvious induction of hyphal genes. This indicates that GlcNAc can stimulate a separate signal to induce hyphae that is independent of transcriptional responses. Of interest, GlcNAc could induce the triple mutant to express hyphal genes when the medium was buffered to a higher pH (>pH 5), which normally occurs after GlcNAc catabolism. Catabolism of GlcNAc raises the ambient pH rather than acidifying it, as occurs after dextrose catabolism. This synergy between alkalinization and GlcNAc to induce hyphal genes involves the Rim101 pH-sensing pathway; GlcNAc induced rim101Δ and dfg16Δ mutants to form hyphae, but hyphal gene expression was partially defective. These results demonstrate that hyphal morphogenesis and gene expression can be regulated independently, which likely contributes to pathogenesis at different host sites.
INTRODUCTION
The human fungal pathogen Candida albicans can infect diverse niches, ranging from mucosa to life-threatening internal organ infections (Odds, 1988; Heitman et al., 2006). An underlying factor that promotes C. albicans virulence is the ability to respond to different environmental conditions. One important example of this is that various stimuli, including serum, alkaline pH, CO2, and N-acetylglucosamine (GlcNAc), induce C. albicans growing as budding cells to switch to forming filamentous chains of pseudohyphal and hyphal cells (Biswas et al., 2007; Whiteway and Bachewich, 2007; Davis, 2009; Sudbery, 2011; Wang, 2013). The transition to filamentous growth is important for biofilm formation and for invasive growth in vivo. Induction of the hyphal form also correlates with increased expression of virulence factors, including adhesin proteins that promote biofilm formation and attachment to human cells, secreted lytic enzymes that facilitate invasive growth and inactivate the host complement pathway, and antioxidant enzymes that counteract the immune system (Calderone and Fonzi, 2001; Whiteway and Oberholzer, 2004; Kumamoto and Vinces, 2005; Blankenship and Mitchell, 2006).
The ability of GlcNAc to induce C. albicans is interesting because this amino sugar has been recognized as an important cell-signaling molecule in a wide range of organisms, from bacteria to humans (Konopka, 2012). The source of GlcNAc for cell signaling likely comes from remodeling or degradation of cell surface molecules, as it is a component of bacterial cell wall peptidoglycan, fungal cell wall chitin, and the extracellular matrix glycosaminoglycans of mammalian cells (Moussian, 2008). In this regard, it is also significant that GlcNAc stimulates C. albicans to undergo an epigenetic switch from the White phase to a distinct morphological state known as the Opaque phase, which expresses genes that facilitate mucosal infections, an environment in which GlcNAc is likely to be present due to remodeling of bacterial cell walls (Huang et al., 2010). C. albicans is emerging as an important model for GlcNAc signaling because the commonly studied model yeasts Saccharomyces cerevisiae and Schizosaccharomyces pombe lack the genes needed to catabolize this sugar and do not appear to respond to it. In contrast, GlcNAc can induce a diverse group of other fungi to undergo filamentous growth, including Blastomyces dermatitidis, Candida lusitaniae, Histoplasma capsulatum, and Yarrowia lipolytica (Perez-Campo and Dominguez, 2001; Reedy et al., 2009; Gilmore et al., 2013). Thus studies on GlcNAc signaling in C. albicans are leading to new insights, such as the identification of the first eukaryotic GlcNAc transporter (Alvarez and Konopka, 2007; Gilmore et al., 2013).
The cAMP pathway plays a key role in inducing hyphae. Induction of hyphal-specific gene expression by this pathway is believed to promote the transition from budding to hyphal morphogenesis (Harcus et al., 2004; Carlisle et al., 2009; Lu et al., 2011, 2013; Sudbery, 2011). Part of the evidence for this is that cyr1Δ mutants that lack adenylyl cyclase and efg1Δ mutants that lack a key transcription factor fail to induce both hyphal-specific genes and hyphal morphogenesis (Stoldt et al., 1997; Rocha et al., 2001; Harcus et al., 2004). Furthermore, mutation of certain transcriptional repressors, such as NRG1, or increased expression of the transcription factor UME6 causes constitutive hyphal growth (Braun and Johnson, 1997; Liu, 2001; Harcus et al., 2004; Carlisle et al., 2009). However, the mechanisms by which changes in transcription mediate hyphal morphogenesis are not well understood. The common set of genes that were stimulated by a group of different hyphal inducers in C. albicans do not appear to mediate the transition to hyphal growth (Martin et al., 2013). Further, although the hyphal-induced gene HGC1 encodes a cyclin that acts with the Cdc28 cyclin-dependent kinase to phosphorylate proteins that promote filamentous hyphal growth, HGC1 overexpression is not sufficient to induce hyphae (Zheng and Wang, 2004; Zheng et al., 2007; Wang et al., 2009; Sinha et al., 2007; Bishop et al., 2010; Sudbery, 2011). This indicates that other signals contribute to hyphal growth.
The GlcNAc transporter Ngt1 is important for induction of responses in C. albicans, indicating that GlcNAc must be taken up into cells to induce signaling (Alvarez and Konopka, 2007). At least two pathways are induced by GlcNAc: a cAMP-dependent pathway that induces hyphal responses and the White–Opaque switch, and a cAMP-independent pathway that induces the genes needed for catabolism of GlcNAc (Gunasekera et al., 2010). GlcNAc that has been taken up into the cell is phosphorylated by Hxk1 to create GlcNAc-6-PO4, which is either converted into fructose-6-PO4 and catabolized for energy or converted into UDP-GlcNAc and used to synthesize chitin, N-linked glycosylation, and glycosylphosphatidylinositol anchors on proteins (Kumar et al., 2000; Yamada-Okabe et al., 2001; Milewski et al., 2006; Wendland et al., 2009). However, metabolism of GlcNAc is not required to induce signaling. Deletion of HXK1, which precludes phosphorylation of GlcNAc and its entry into the metabolic pathways, did not prevent an hxk1Δ mutant from being induced to form hyphae (Naseem et al., 2011). This indicates that cells can sense nonphosphorylated GlcNAc. This has advantages for cell signaling by permitting sensitive detection of exogenous nonphosphorylated GlcNAc, since cells synthesize only phosphorylated forms such as GlcNAc-6-PO4 (Milewski et al., 2006; Konopka, 2012).
To determine whether there are other effects of GlcNAc on C. albicans that are dependent on its metabolism, we analyzed an hxk1Δ dac1Δ nag1Δ mutant. This triple mutant fails to metabolize GlcNAc since it lacks the GlcNAc kinase Hxk1, as well as Dac1 and Nag1, which deacetylate and deaminate GlcNAc-6-PO4 to create fructose-6-PO4. As part of these studies, we found that GlcNAc metabolism affects the ambient pH. Whereas growth on dextrose acidifies the medium, growth on GlcNAc makes the medium more alkaline, likely due to release of excess nitrogen as ammonia (Vylkova et al., 2011). This is significant because hyphal genes are also induced by alkaline ambient pH through a mechanism that involves the Rim101 transcription factor, which also regulates the expression of genes needed for cells to grow at high pH (Davis, 2009). Of interest, the hxk1Δ dac1Δ nag1Δ mutant could be induced to form hyphae at low pH in the absence of significant induction of hyphal-specific genes, but these genes were induced when the pH of the medium was buffered to pH 7. The results indicate that GlcNAc acts synergistically with ambient pH to induce hyphal genes and that hyphal morphology can be regulated independently of the expression of hyphal genes.
RESULTS
GlcNAc catabolism is not needed to stimulate hyphal morphogenesis at pH 4 but is needed for hyphal cells to clump
The role of GlcNAc in inducing hyphal responses was examined in a mutant strain lacking the genes needed to metabolize GlcNAc (hxk1Δ nag1Δ dac1Δ). For simplicity, this triple-mutant strain will be referred to as the h-d mutant, since it removes the cluster of three adjacent GlcNAc genes: HXK1, NAG1, and DAC1 (Kumar et al., 2000; Naseem et al., 2011). This mutant can still take up GlcNAc and be induced to form filamentous cells (Naseem et al., 2011). In preliminary studies, different responses were observed for the h-d mutant when it was induced in standard synthetic medium (∼pH 4) versus medium that had been buffered to pH 7 with 1,4-piperazinediethanesulfonic acid (PIPES). To examine this further, we used two types of induction conditions. In one condition, cells were grown to log phase in medium containing dextrose, washed, and then resuspended in medium containing dextrose or GlcNAc for 2 h (Figure 1A). It was necessary to wash out the dextrose because it inhibits the expression of the GlcNAc transporter (Alvarez and Konopka, 2007). Cells reinoculated in dextrose medium continued to grow as budding cells, whereas cells grown in GlcNAc were induced to form hyphae. Under these conditions, the h-d mutant can continue to grow by metabolizing the amino acids in the medium. Although amino acids can induce hyphal growth after a long incubation (Vylkova et al., 2011), cells grown in medium containing amino acids but lacking a sugar did not form filamentous cells after a 2-h incubation (Figure 1A). Care was taken to avoid other conditions that induce filamentous growth, such as a temperature shift or rapid dilution of cell density, which causes a release from farnesol inhibition (Enjalbert and Whiteway, 2005). Thus the transition to hyphal growth required GlcNAc.
FIGURE 1:
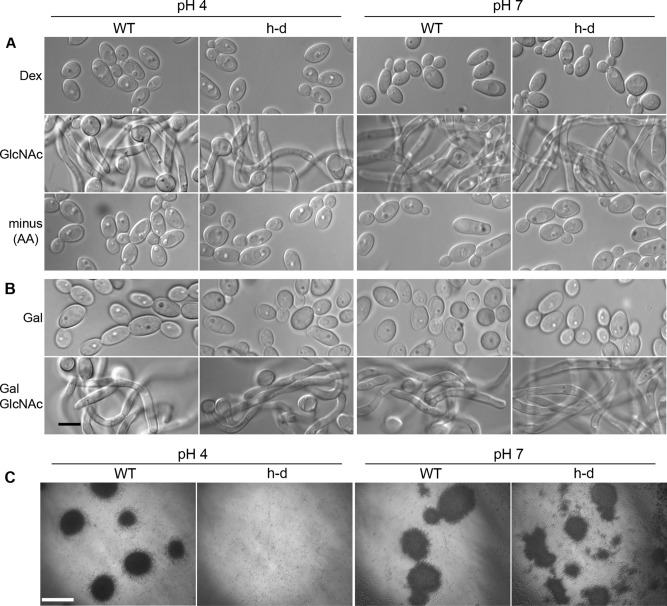
GlcNAc stimulates the h-d mutant to grow in a filamentous manner, but at pH 4, the cells do not clump. Wild-type control and h-d mutant cells were grown in synthetic medium with amino acids that was buffered to either pH 4 with citrate or pH 7 with PIPES. (A) Cells grown overnight at 37°C to log phase in dextrose were harvested, washed, and then resuspended in medium for 2 h with 50 mM dextrose, 50 mM GlcNAc, or no sugar added (minus). Cells in the minus sample lacking sugar could grow due to the presence of amino acids in the medium. (B) Cells were grown overnight to log phase at 37°C in buffered medium containing 50 mM galactose, and then one aliquot was induced by addition of GlcNAc to 50 mM and incubation for 2 h at 37°C (Gal GlcNAc). Black bar, 5 μm. (C) Low-resolution microscope images showing the lack of clumping in h-d mutant cultures grown in galactose medium at 37°C and then induced for 2 h with 50 mM GlcNAc at pH 4. Large dark areas correspond to clumps of cells. White bar, 500 μm. The wild-type control strain was DIC185, and the h-d mutant strain was AG738.
Cells were also induced by growing them to early log phase in galactose medium and then adding GlcNAc and incubating for 2 h (Figure 1B). Galactose was used because it does not repress the expression of the GlcNAc transporter and does not induce hyphal growth under these conditions (Figure 1B). Addition of GlcNAc to the galactose medium resulted in efficient induction of hyphal growth for both the wild-type control cells and the h-d mutant.
In the course of these studies, we discovered that the h-d mutant hyphae differed from wild-type control hyphae in that they were less clumped in liquid medium at pH 4 than at pH 7. This was readily apparent by visual inspection of cultures and was documented by photographing cells at low magnification (Figure 1C). This is significant, as it suggested that the h-d mutant is defective in inducing hyphal genes at pH 4. Clumping is due to induction of a set of hyphal-specific genes that encode cell-surface adhesin proteins, such as ALS3 and HWP1 (Nobile et al., 2008). Thus the h-d mutant forms hyphae at pH 4, albeit with reduced clumping.
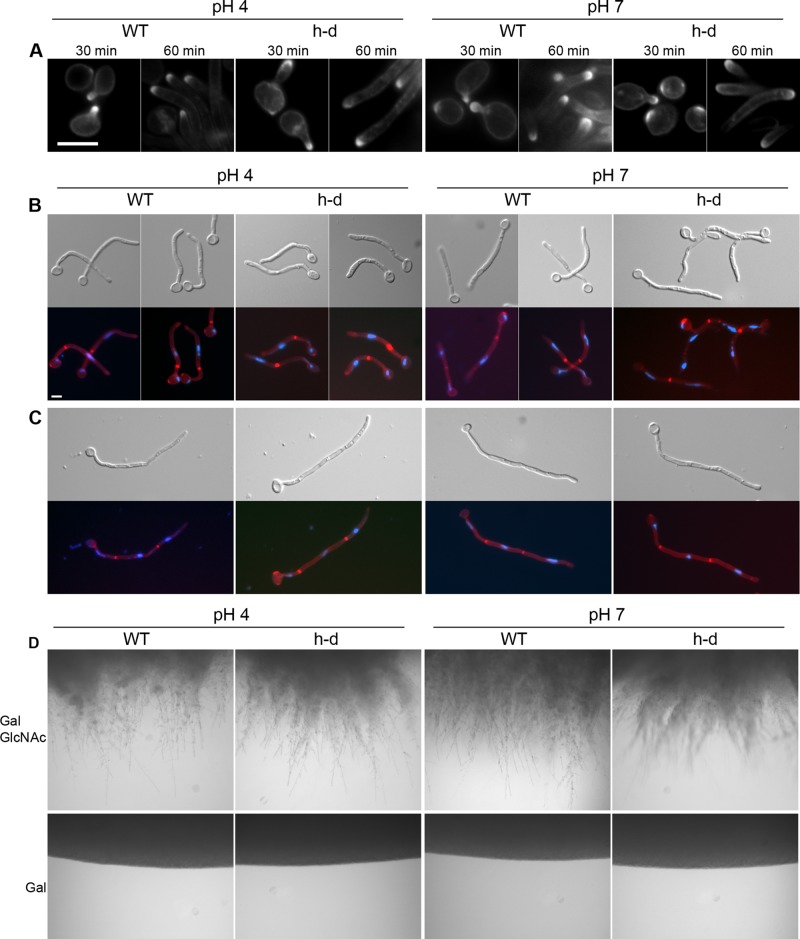
To examine the filamentous h-d cells in more detail, we induced cells in galactose plus GlcNAc medium and then stained them with filipin to detect polarization of lipids at the tips of emerging germ tubes. Filipin is a fluorescent compound that binds ergosterol in the plasma membrane (Alvarez et al., 2007). The tips of emerging germ tubes and hyphae stain more strongly with filipin, but not the growing tips of pseudohyphae (Martin and Konopka, 2004). Filipin staining was readily detected at the tips of the wild-type control and h-d mutant cells at 30 and 60 min of induction (Figure 2A). Similar results occurred at both pH 4 and 7. Additional studies showed that filipin staining was also detected after longer times of incubation (unpublished data). To examine whether the germ tubes elongated into true hyphae, we stained the cells that were induced for 2 h with Pontamine Scarlet 4B to detect the cell walls septae and Hoechst to detect nuclear DNA. Pontamine Scarlet 4B is a fluorescent stain that can detect septae similar to Calcofluor White (Hoch et al., 2005). Unlike Calcofluor White, however, it fluoresces at a distinct wavelength from Hoechst. This allows double staining to be used to examine whether cells are forming true hyphae. True hyphae form a septum at a site distal to the mother cell, whereas pseudohyphae form a septum at the junction with the mother cell (Sudbery et al., 2004). The results showed that at both pH 4 and 7, the h-d mutant formed true hyphae, with parallel walls and septae distal to the mother cell (red stain; Figure 2B). Hyphal growth was also evident after 4 h of induction, with the elongated cells showing multiple septae (Figure 2C). Hoechst staining of DNA (blue) demonstrated that each hyphal cell compartment contained a nucleus, as expected. To examine a later stage of incubation, we spotted cells onto agar plates buffered to either pH 4 or 7 and then incubated them at 37°C for 4 d (Figure 2D). The h-d mutant grew invasively into agar containing galactose plus GlcNAc medium at both pH 4 and 7, similar to the wild-type control (Figure 2D). In contrast, cells did not grow invasively in plates containing only galactose. These results indicate that the h-d mutant forms true hyphae at pH 4 similar to the wild-type control cells and similar to the cells grown at pH 7.
FIGURE 2:
The h-d mutant cells induced with GlcNAc display characteristics of true hyphae. Wild-type control and h-d mutant cells were grown at 37°C in synthetic medium with 50 mM galactose buffered to the indicated pH with citrate or PIPES and then induced by addition of GlcNAc to 50 mM. (A) Cells were induced with GlcNAc for 30 or 60 min as indicated, stained with filipin, and photographed with a fluorescence microscope. The tips of growing germ tubes showed strong staining with filipin for both h-d and wild-type control cells, as expected for true hyphae. Cells induced for 2 h (B) and 4 h (C) were stained with Pontamine Fast Scarlet 4B to detect chitin-rich septa and Hoechst to detect DNA. The results show that the h-d mutant forms true hyphae, as indicated by the placement of septa distal to the mother cells and because the filamentous cells comprise distinct cell compartments each containing a nucleus. (D) Invasive growth into agar medium after 4 d at 37°C. Cells were spotted onto agar medium containing 50 mM galactose or 50 mM galactose plus 50 mM GlcNAc. After growth for 4 d at 37°C, the edges of colonies were photographed. The results show that wild-type control and the h-d mutant colonies invaded into the agar at both pH 4 and 7. The wild-type control strain was DIC185, and the h-d mutant strain was AG738.
Hyphal-specific genes are not significantly induced at low ambient pH (pH 4) in the h-d mutant, which cannot metabolize GlcNAc
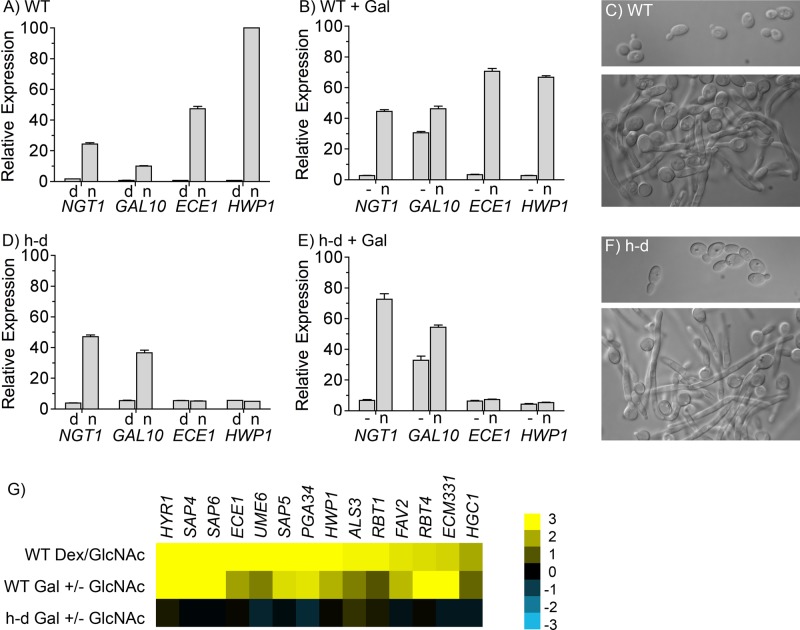
The ability of the h-d mutant to induce hyphal genes at pH 4 was analyzed by quantitative real time PCR (qRT-PCR). Of interest, when h-d cells grown in complete synthetic medium with amino acids were induced by a change from dextrose to GlcNAc medium for 2 h, they did not stimulate the hyphal-specific genes ECE1 or HWP1, which were highly induced by GlcNAc in the wild-type control strain (Figure 3, A and D). This is consistent with the failure of h-d mutant to clump at pH 4 (Figure 1C). Microscopic examination confirmed that the h-d mutant was still induced to form hyphae under these conditions (Figure 3, C and F). Control studies showed that GlcNAc still induced expression of both the NGT1 and GAL10 in the h-d mutant (Figure 3D), which are activated by a transcriptional mechanism that is distinct from the cAMP pathway that induces hyphal genes (Gunasekera et al., 2010).
FIGURE 3:
GlcNAc stimulates the h-d mutant to form hyphae but does not induce hyphal-specific genes at low ambient pH. qRT-PCR analysis of the relative expression of the indicated genes normalized to the expression of actin (ACT1) in each cell type. Cells were grown at 37°C at low ambient pH (∼pH 4) in synthetic medium containing amino acids. (A, D) wild-type control and h-d strains were grown to log phase in medium containing 50 mM dextrose and amino acids at 37°C, washed, and then incubated in similar medium containing 50 mM dextrose or 50 mM GlcNAc for 2 h. Cells grown in dextrose are labeled d, and those grown with GlcNAc are labeled n. (B, E) Wild-type control and h-d strains were grown in synthetic medium containing 50 mM galactose, and then 50 mM GlcNAc was added to one portion for 2 h. (C, F) Top, morphology of wild-type control and h-d cells grown in dextrose; bottom, cells grown in GlcNAc medium. (G) Summary of microarray analyses carried out in duplicate, showing the relative expression of the most highly induced hyphal-specific genes for the indicated strains and growth conditions. Scale bar on right, (log 2)-fold change in expression. The wild-type control strain was DIC185, and the h-d mutant strain was AG738. Cells were grown at 37°C at low pH (pH 4) in synthetic medium containing amino acids. Error bars on graphs indicate SD.
To examine the failure of the h-d mutant to induce hyphal-specific genes under another set of conditions, cells were first grown in galactose medium and then induced by addition of GlcNAc (Figure 3, B and E). As before, GlcNAc induced the h-d mutant to undergo hyphal morphogenesis and stimulate expression of NGT1 and GAL10, but the hyphal-specific genes ECE1 and HWP1 were not induced.
These results were surprising, since it had been suggested that induction of hyphal morphogenesis and hyphal-specific genes is linked, as they both require adenylyl cyclase and the transcription factor Efg1 (Stoldt et al., 1997; Rocha et al., 2001; Harcus et al., 2004). However, microarray analysis also indicated that there was no significant induction of other hyphal-specific genes by GlcNAc in the h-d mutant at low ambient pH (pH 4; Figure 3G and Supplemental Data). The induction of NGT1 in the h-d mutant was only moderate in these microarrays because there was a high basal level of expression. Previous studies found that this occurs as cultures of the h-d mutant grow to higher cell density, apparently because GlcNAc released during the remodeling of cell wall chitin accumulates in the medium since it cannot be metabolized by the h-d mutant (Naseem et al., 2011). In contrast, the hyphal-specific genes showed low basal levels that were not induced by GlcNAc in the h-d mutant (Supplemental Data). Thus the h-d mutant is broadly defective in inducing hyphal-specific genes at low ambient pH.
Catabolism of GlcNAc and dextrose has opposite effects on ambient pH
The role of GlcNAc metabolism on ambient pH was examined next as a possible difference between wild-type control and h-d mutant cells. Of interest, growth of wild-type control cells in the presence of dextrose or GlcNAc had opposite effects on ambient pH. Growth of wild-type control cells in GlcNAc medium raised the ambient pH, whereas growth in dextrose medium made it more acidic (Figure 4A). The pH of the medium for the h-d mutant remained stable in GlcNAc medium. Similar results were observed when cells were grown in medium containing galactose or galactose plus GlcNAc (Figure 4B). Catabolism of GlcNAc likely raises the pH because the excess ammonia generated by the deamination of glucosamine-6-PO4 will be excreted into the medium, similar to what was observed for growth of C. albicans on other nitrogen-rich media (Vylkova et al., 2011). This indicates that some effects on cells attributed to GlcNAc may be an indirect consequence of the distinct changes in ambient pH caused by catabolism of dextrose or GlcNAc.
FIGURE 4:
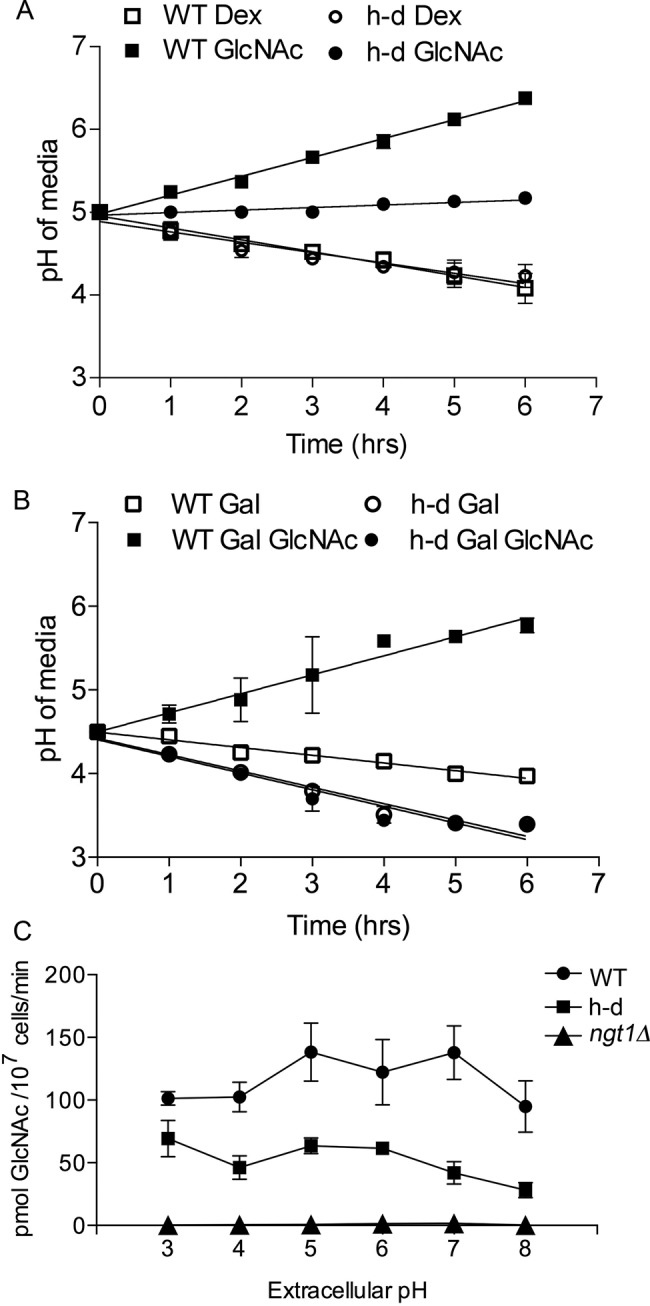
Growth of cells in GlcNAc medium raises the ambient pH, whereas growth in dextrose lowers it. Wild-type control strain DIC185 and h-d strain AG738 were grown in the indicated synthetic media, and then the pH of the culture was measured at the indicated times. Note that cells that can catabolize GlcNAc raise the ambient pH, whereas growth on dextrose lowers it. (A) Cells were grown in synthetic medium containing dextrose, washed, and then resuspended in medium containing dextrose or GlcNAc (50 mM). (B) Cells were grown in galactose and then split into two cultures, with one receiving GlcNAc (50 mM). (C) Uptake of [3H]GlcNAc in cells incubated at different pH levels. The indicated cell types were grown in the presence of 50 mM galactose and 50 mM GlcNAc for 2 h to induce the GlcNAc transporter Ngt1, washed, and resuspended in synthetic yeast medium lacking a sugar that was buffered to the indicated pH; then uptake of 3HGlcNAc was assayed. The wild-type control strain was DIC185, the h-d mutant strain was AG738, and the ngt1∆ strain was YJA3. Error bars, SD.
The ability of cells to take up GlcNAc at different levels of ambient pH was examined to determine whether this might influence signaling. The results showed that there was similar ability of h-d and wild-type control cells to take up [3H]GlcNAc at pH levels ranging from 3 to 8, although there was perhaps a slight drop-off at pH 8, which was beyond the range of pH levels used in our studies (Figure 4C). The h-d cells appeared to take up about twofold less GlcNAc than the wild-type control in these assays. However, both of these strains took up significantly more GlcNAc than the ngt1∆ cells, which lack the GlcNAc transporter. At all pH levels, the GlcNAc uptake by the ngt1∆ mutant was barely detectable above background.
Synergy between GlcNAc and ambient pH in the induction of hyphal-specific genes
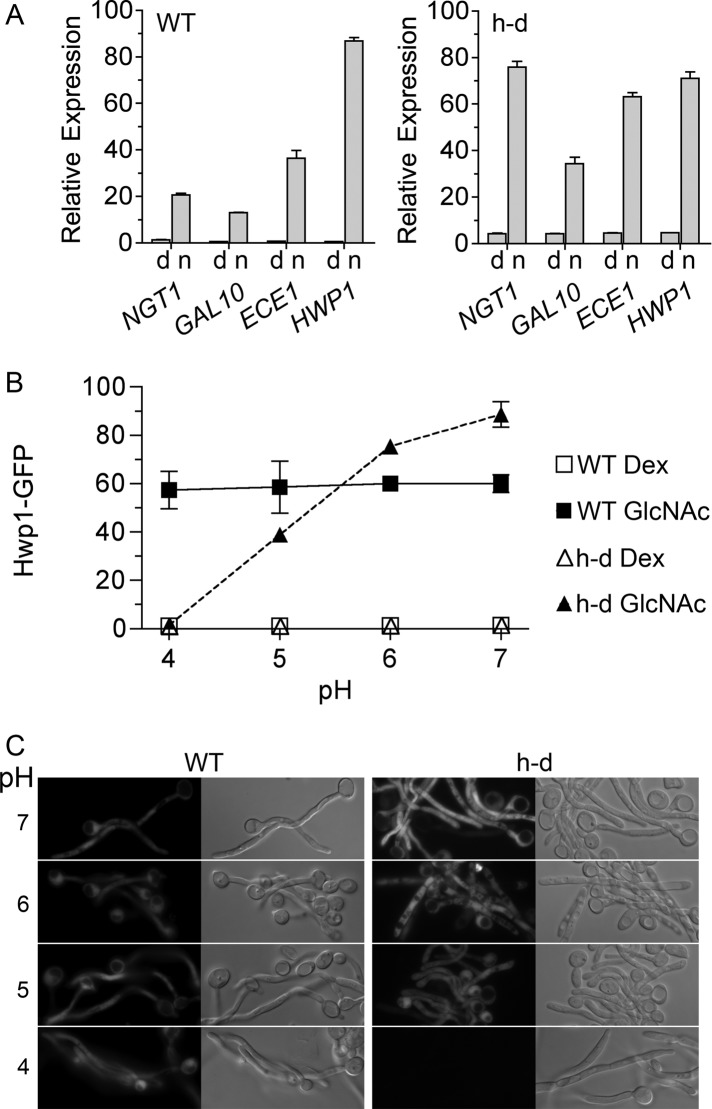
To test the role of ambient pH in the regulation of hyphal-specific genes, we grew h-d mutant cells in medium buffered to pH 7 to mimic the effects of GlcNAc catabolism. Under these conditions, qRT-PCR analysis showed that GlcNAc strongly stimulated the h-d cells to express the hyphal genes ECE1 and HWP1 similar to the wild-type control cells (Figure 5A). This indicates that ambient pH acts synergistically with GlcNAc to induce hyphal-specific genes. Consistent with this, the h-d hyphal cells were clumped at pH 7, indicating that the adhesin genes were induced (Figure 1C).
FIGURE 5:
GlcNAc induction of HWP1 in the h-d mutant is dependent on ambient pH. (A) Wild-type control and h-d strains were grown in synthetic medium buffered to pH 7 with PIPES and containing dextrose and amino acids. Cells were then resuspended in the same medium containing 50 mM dextrose (d) or GlcNAc (n) for 2 h. Samples were analyzed by qRT-PCR. (B) Wild-type control and h-d strains carrying HWP1-GFP were grown in synthetic medium with amino acids and 50 mM dextrose (Dex) or 50 mM GlcNAc at 37°C for 2 h, and then GFP fluorescence was quantified by fluorescence microscopy and shown as relative units. Culture medium was buffered to the indicated pH using pH 4 sodium citrate, pH 5 succinic acid, pH 6 MES, or pH 7 PIPES. (C) Fluorescence microscope images of the cells carrying the HWP1-GFP reporter gene. Cells were grown in 50 mM GlcNAc medium buffered to the pH indicated on the left. The wild-type control strain was SN969, and the h-d strain was SN971.
The effects of ambient pH on the induction of hyphal-specific genes were analyzed further by assaying the ability of GlcNAc to induce an h-d strain carrying a HWP1–green fluorescent protein (GFP) reporter gene in media buffered to different pH levels (Figure 5, B and C). Wild-type cells shifted from dextrose to GlcNAc medium showed strong induction of GFP and hyphae between pH 4 and 7, with perhaps a slight increase at the higher pH levels (Figure 5B). In contrast, GlcNAc did not detectably induce HWP1-GFP in the h-d mutant grown in medium buffered to pH 4. GFP was partially induced by GlcNAc in medium buffered to pH 5. At pH 6 or 7, GlcNAc induced the h-d mutant to produce higher levels of GFP than the wild-type control. This effect could be due to h-d mutant cells experiencing higher sustained intracellular levels of GlcNAc, since it lacks the enzymes to convert it to GlcNAc-6-PO4 and metabolize it. Unfortunately, the h-d mutant could not be readily tested for GlcNAc induction of HWP1-GFP when grown in galactose medium because it displayed a high basal level of GFP. This is likely due to a combination of the stability of GFP and an effect of long-term exposure to the GlcNAc that is released during cell wall remodeling but cannot be metabolized by the h-d mutant (Naseem et al., 2011). This effect is likely more pronounced for cells grown in galactose medium because the expression of the GlcNAc transporter (NGT1) is not repressed as it is in dextrose medium (Alvarez and Konopka, 2007).
Regulation of the hyphal-specific cyclin gene HGC1
The expression of HGC1 was examined because the Hgc1 cyclin associates with Cdc28 to help promote hyphal growth by phosphorylating morphogenesis proteins (Zheng and Wang, 2004; Sudbery, 2011). Induction of HGC1 expression is believed to be important for hyphal morphogenesis (Zheng and Wang, 2004). However, microarray analysis of the h-d mutant did not detect significant induction of HGC1 by GlcNAc at low pH (pH 4; Figure 3G). To verify this, we carried out qRT-PCR. As expected, GlcNAc induced HGC1 in wild-type control cells ∼8.6-fold (Figure 6). In contrast, qRT-PCR analysis of four independent RNA preparations showed that HGC1 was not significantly induced in h-d cells in medium buffered to pH 4. HGC1 appeared to only be induced ∼40%, but this was not statistically significant (p = 0.375). In contrast, in the same experiments, NGT1 was induced more highly in the h-d mutant than in the wild-type control strain (Figure 6). Significantly, the basal level of HGC1 was similar for the two strains and in fact was slightly higher in the h-d mutant. This is an important difference between the h-d mutant and mutants that fail to induce both hyphae and hyphal genes (e.g., adenylyl cyclase mutant cyr1Δ or efg1Δ). The latter mutants make low basal levels of HGC1 that are not further induced (Stoldt et al., 1997; Harcus et al., 2004). Thus the basal level of HGC1 in the h-d mutant appears to be sufficient to promote hyphal growth when GlcNAc is added as an inducer. Consistent with this, others have shown that overexpression of HGC1 is not sufficient to induce hyphal growth, indicating that a second type of hyphal signal is required (Zheng and Wang, 2004).
FIGURE 6:
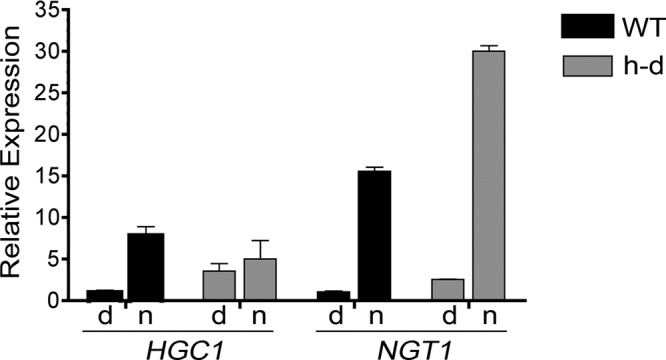
The basal level of HGC1 is higher in the h-d mutant but is not significantly induced by GlcNAc. Relative levels of HGC1 were determined by qRT-PCR analysis. Wild-type control (DIC185) and h-d mutant (AG738) cells were grown to log phase at 37°C in pH 4 synthetic medium containing amino acids and 50 mM dextrose, washed, and then resuspended in medium containing 50 mM dextrose (d) or GlcNAc (n) for 2 h.
The Rim101 alkaline pH response pathway contributes to GlcNAc induction of hyphal-specific genes
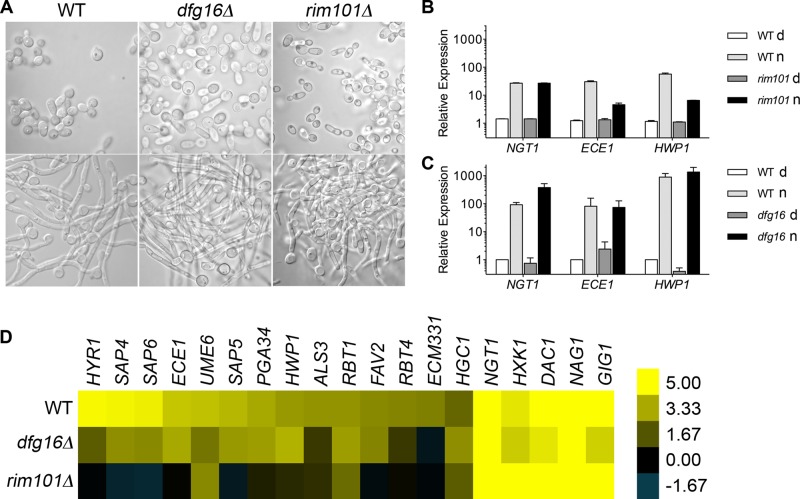
The synergy between ambient pH and GlcNAc was examined further by testing the rim101Δ and dfg16Δ mutants, which are defective in responding to alkaline pH (Davis, 2009). Rim101 encodes a transcription factor that regulates changes in the expression of genes needed to adapt to growth at alkaline pH, and it is also needed to promote hyphal morphogenesis in response to alkaline pH (Davis et al., 2000). Dfg16 is a plasma membrane protein that is believed to be part of a complex that senses ambient pH (Barwell et al., 2005). Both the rim101Δ and dfg16Δ mutants grew well in GlcNAc medium, as evidenced by their ability to be rapidly stimulated by GlcNAc to form hyphae and induce expression of NGT1 (Figure 7, A–C). However, microarray analysis showed that rim101Δ and dfg16Δ cells were partially defective in inducing the hyphal-specific genes (Figure 7D). The rim101Δ cells appeared to induce only 3 of the 14 hyphal-specific genes that are most highly induced by wild-type cells. The dfg16Δ mutant induced 11 of 14.
FIGURE 7:
The rim101Δ and dfg16Δ mutants are defective in inducing hyphal-specific genes in response to GlcNAc. (A) The indicated strains were grown in synthetic medium buffered to pH 7 with PIPES and also containing amino acids and 50 mM dextrose. Cells were washed and then resuspended in medium containing 50 mM dextrose (upper top) or 50 mM GlcNAc (bottom) for 2 h at 37°C, and then cells were photographed. (B, C) Relative expression of NGT1, ECE1, and HWP1 was determined by qRT-PCR for cells grown as described in A. Wild-type control, rim101Δ, and dfg16Δ strains were grown in medium containing 50 mM dextrose (d) or 50 mM GlcNAc (n) for 2 h, and then cells were harvested for analysis. (D) Summary of microarray results, displaying the relative expression of the most highly induced hyphal-specific genes and GlcNAc catabolic genes for the indicated strains grown in dextrose or GlcNAc media for 2 h. Scale bar on right, log 2 change in expression for cells grown in GlcNAc vs. dextrose. The wild-type control strain was DIC185, the rim101Δ strain was DAY25, and the dfg16Δ strain was KBC048.
Analysis by qRT-PCR revealed that GlcNAc only weakly induced the rim101Δ mutant to express ECE1 and HWP1 (Figure 7B), although both appeared to be stimulated more strongly by GlcNAc in the microarray studies (Figure 7D). The ECE1 and HWP1 genes were induced approximately three- and sixfold, respectively, in the rim101Δ mutant, whereas they were induced ∼25- and 48-fold in the wild-type control strain. In contrast, the rim101Δ mutant induced NGT1 to the same level as the wild type. Of interest, dfg16Δ was able to induce ECE1 and HWP1 efficiently in response to GlcNAc (Figure 7C). Perhaps other pH-sensing pathways can compensate for the lack of Dfg16, or there may be a lower requirement for Dfg16 in GlcNAc induction at neutral or slightly acidic pH, since the function of Dfg16 appears to be important at more-alkaline pH levels than used for our studies (Barwell et al., 2005). Nonetheless, it is significant that Rim101 and an alkaline ambient pH synergize with GlcNAc to induce hyphal genes.
Role of GlcNAc catabolism in virulence
Previous studies of GlcNAc catabolic mutants indicated that the ability to use exogenous GlcNAc is important for virulence in a mouse model of hematogenously disseminated candidiasis (Singh et al., 2001; Yamada-Okabe et al., 2001). GlcNAc could be present in vivo as a result of remodeling of fungal cell wall chitin or the mammalian extracellular matrix. However, the interpretation of these results is complicated because it was subsequently shown that the growth of the nag1Δ and dac1Δ mutants is strongly inhibited by the presence of exogenous GlcNAc, perhaps due to excess formation of UDP-GlcNAc (Naseem et al., 2011). In addition, these mutants did not show a competitive fitness defect in mice when infected as part of a pool of 48 bar-coded mutants (Noble et al., 2010). GlcNAc is not inhibitory to the h-d mutant, presumably because the lack of Hxk1 prevents phosphorylation of GlcNAc and its entry into the pathway that forms UDP-GlcNAc. Therefore, the h-d mutant was tested for virulence and found to be strongly defective in causing systemic infection in BALB/c mice (Figure 8A). Mice infected with 5 × 105 h-d mutant cells became moribund an average of ∼5 d later than mice infected with wild-type control C. albicans. Similarly, mice infected with 2.5 × 105 wild-type control cells became moribund in ∼ 9 d, but injection with 2.5 × 105 h-d cells resulted in 67% of mice (n = 6) becoming moribund at an average of ∼11 d, and 33% survived the infection. A control strain in which the HXK1, NAG1, and DAC1 genes were reintroduced into the h-d mutant showed a pattern of virulence that was similar to the wild-type control.
FIGURE 8:
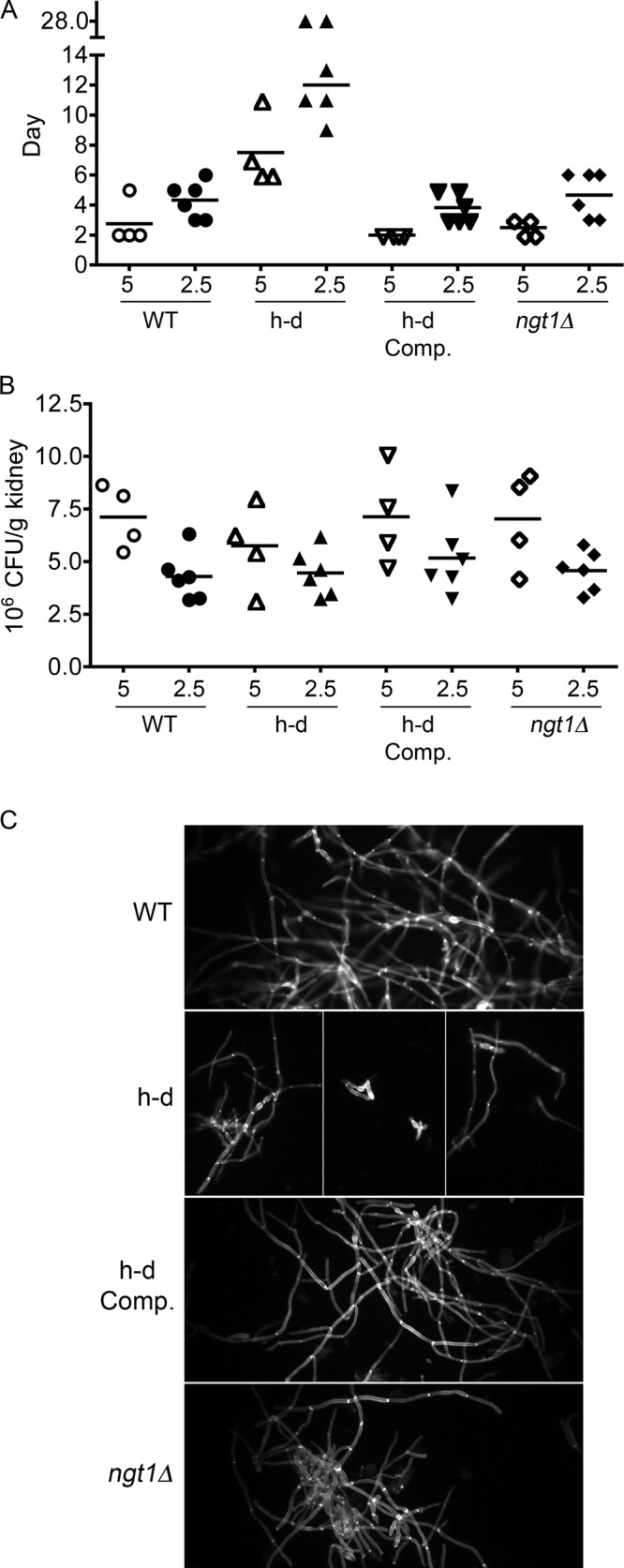
The h-d mutant is defective in virulence, but the ngt1Δ mutant is not. (A) BALB/c mice were injected via the tail vein with either 5 × 105 or 2.5 × 105 cells of the indicated C. albicans strain. The day at which the mice became moribund is shown in the scatter plot. Note the discontinuity in the y-axis, as two of the mice injected with 2.5 × 105 h-d mutant cells survived to the end of the experiment (day 28). p < 0.01 for wild-type control vs. the h-d mutant at the 5 × 105 dose, and p < 0.001 for the 2.5 × 105 dose by log-rank (Mantel–Cox) test. (B) The CFU/gram of kidney tissue was determined at the time the mice became moribund or on day 28 for those that survived to the end of the experiment. (C) Kidney homogenates were stained with Calcofluor White and then examined by fluorescence microscopy using a 100× objective. The wild-type control strain was DIC185, the ngt1Δ strain was YJA3, the h-d strain was SN973, and h-d Comp refers to an h-d strain (SN974) that was complemented by reintroduction of the HXK1, NAG1, and DAC1 genes.
There was no obvious difference in the colony-forming units in the kidneys of the moribund mice infected with either the wild-type control or the h-d mutant (Figure 8B). However, microscopic inspection of the kidney homogenates revealed that many of the h-d mutant cells were morphologically abnormal. Whereas the wild-type control and the complemented control strain formed almost exclusively chains of hyphal cells with parallel walls, some of the h-d mutant cells appeared aberrant or pseudohyphal due to rounded sidewalls (Figure 8C). This was surprising, since the h-d mutant showed a normal response in vitro to serum, and it is readily stimulated to form hyphae in response to GlcNAc.
To assess whether the virulence defect of the h-d mutant was due to an altered response to exogenous GlcNAc or whether it is important for cells to metabolize exogenous GlcNAc during an infection, we assessed the virulence of an ngt1Δ mutant that lacks the GlcNAc transporter (Alvarez and Konopka, 2007). This mutant is defective in transporting GlcNAc into the cell, so it does not show the altered behavior that is seen for the h-d mutant. When tested for virulence in the mouse model system, the ngt1Δ mutant caused disease very similar to the wild- type control C. albicans (Figure 8A). This indicates that it is the altered responses of the h-d mutant to GlcNAc, and not the ability to metabolize GlcNAc, that causes a defect in systemic infection.
DISCUSSION
Differential regulation of hyphal morphology and hyphal-specific genes by GlcNAc
GlcNAc was identified as an inducer of hyphal growth in C. albicans more than 40 yr ago (Simonetti et al., 1974). Since then, it has been widely used as a potent inducer of the transition from budding to filamentous growth (Biswas et al., 2007; Davis-Hanna et al., 2008; Midkiff et al., 2011; Konopka, 2012). Previous studies concluded that induction of hyphal morphogenesis and transcriptional responses are interconnected through the cAMP pathway (Biswas et al., 2007; Sudbery, 2011; Wang, 2013). Consistent with this, GlcNAc does not induce hyphal formation in mutants lacking either adenylyl cyclase (cyr1Δ) or a downstream transcription factor (efg1Δ) needed to induce hyphal genes (Lo et al., 1997; Leberer et al., 2001; Gunasekera et al., 2010; Naseem et al., 2011). Thus it was surprising that GlcNAc could induce the h-d mutant to form hyphae at low ambient pH without obviously stimulating the hyphal-specific genes. Failure to detect significant induction of the hyphal-specific genes in the h-d mutant was supported by four independent microarrays, qRT-PCR assays of three hyphal-specific genes (ECE1, HWP1, HGC1) and the analysis of an HWP1-GFP reporter gene (Figures 3, 5, and 6). Furthermore, the h-d mutant hyphae induced at low pH did not clump, consistent with the failure to induce expression of the adhesins (Figure 1). Analysis of the filamentous cells formed by the h-d mutant at pH 4 indicated that they were true hyphae, as evidenced by the enriched filipin staining at the tips of emerging germ tubes, parallel sidewalls, and the placement of septae distal from the mother cell (Figure 2).
These results indicate that high levels of hyphal-specific gene induction are not required to promote hyphal growth. A key difference between the h-d mutant and the cyr1Δ or efg1Δ mutant is that the latter mutants express very low basal levels of hyphal-specific genes, including the HGC1 cyclin that is important for hyphal growth (Stoldt et al., 1997; Harcus et al., 2004; Zheng and Wang, 2004). In contrast, the basal level of HGC1 expression in the h-d mutant was similar to that in the wild-type control cells (Figure 6). This basal level of HGC1 expression was not sufficient to promote hyphal growth; the h-d mutant cells still needed a signal from GlcNAc. This is consistent with previous results that overexpression of HGC1 is not sufficient to promote hyphal growth (Zheng and Wang, 2004). These data suggest that GlcNAc can transduce a signal to induce hyphal morphogenesis that is distinct from transcriptional regulation.
Other data also indicate that hyphal morphogenesis and hyphal-specific gene expression can be regulated independently. Comparison of a set of hyphal inducers found that they varied in their ability to stimulate different hyphal-specific genes (Martin et al., 2013). In addition, although efg1Δ cph1Δ mutants lacking the key transcription factors needed to induce hyphal genes are defective in forming hyphae under most conditions, they can still be induced to form hyphae on the surface of the tongue or when imbedded in a matrix (Riggle et al., 1999). In addition, the filamentous growth that occurs during chlamydospore formation is promoted by a distinct set of conditions, further indicating that there are independent mechanisms to regulate hyphal morphology (Staib and Morschhauser, 2007). The ability of cells to independently regulate hyphal morphogenesis and hyphal-specific genes under different conditions likely has special advantages for C. albicans pathogenesis. For example, because induction of the adhesin genes promotes adherence and biofilm formation (Blankenship and Mitchell, 2006), failure to induce the adhesins could help to disseminate an infection by releasing cells from a biofilm. Similarly, it may be advantageous to regulate independently hyphal morphology and production of virulence factors at different body sites that vary in the factors that induce hyphal growth, including pH, CO2, temperature, and oxidative stress (Biswas et al., 2007; Sudbery, 2011).
The differential regulation of hyphal growth and gene expression by GlcNAc contrasts with strong data showing that hyphal morphogenesis can be induced by increased transcription of hyphal-specific genes. For example, deletion of the NRG1 repressor or overexpression of the UME6 transcriptional activator promotes hyphal growth, clearly demonstrating that transcriptional changes can induce this morphological switch (Braun and Johnson, 1997; Liu, 2001; Harcus et al., 2004; Carlisle et al., 2009). One possibility is that there are distinct pathways for inducing hyphae, including those that are transcription dependent and independent. However, the mechanisms by which transcriptional regulation induces hyphal morphology are not known. Although numerous studies have examined hyphal-induced genes, it is not clear that any of the induced genes are directly responsible for the changes in actin and cell polarity proteins that initiate hyphal morphogenesis (Sudbery, 2011; Martin et al., 2013). It is possible that the key genes are difficult to identify because they are only induced weakly or transiently. Alternatively, the induction of hyphal-specific genes may act indirectly to promote hyphal growth, perhaps by altering metabolism.
GlcNAc synergizes with ambient pH signaling to induce hyphal genes
The ability of the h-d mutant to induce hyphal-specific genes at pH 7 suggested that GlcNAc synergizes with pH signaling. This synergy also has physiological significance for wild type C. albicans because growth on GlcNAc alkalinizes the extracellular medium rather than acidifying it as occurs during growth in dextrose (Figure 4). It is not surprising that GlcNAc catabolism alkalinizes the ambient pH. Previous studies showed that growth of C. albicans on other nitrogen-rich media alkalinized the ambient environment due to excretion of the excess nitrogen in the form of ammonia (Vylkova et al., 2011).
The synergy with GlcNAc involves the conserved pathway for sensing ambient pH that includes the transcription factor Rim101. Ambient pH appears to be sensed by a complex of plasma membrane proteins Dfg16, Rim9, and Rim21 (Barwell et al., 2005; Cornet et al., 2009; Obara et al., 2012). They transduce a signal to Rim8, which then promotes the Rim13 protease to cleave Rim101 and liberate an N-terminal domain with novel transcription factor activities (Li et al., 2004). Rim101 induces the genes needed to adapt to growth at higher pH and is also needed to stimulate hyphal growth in response to pH (Davis, 2009). Of interest, the rim101Δ mutant behaves similarly to the h-d mutant at low ambient pH, in that it forms hyphae efficiently but is partially defective in inducing the hyphal-specific genes (Figure 7). A similar, albeit weaker, defect was seen for the dfg16Δ mutant. This indicates that wild-type control cells take advantage of two signals generated by GlcNAc: one that is activated by GlcNAc, and another that results from catabolism of GlcNAc and increases the extracellular pH.
Virulence
Previous studies with GlcNAc catabolic mutants suggested that the ability to use exogenous GlcNAc is important for a systemic infection in mice (Singh et al., 2001; Yamada-Okabe et al., 2001). Consistently, the h-d mutant was defective in virulence (Figure 8). However, an ngt1Δ mutant that lacks the GlcNAc transporter did not show a virulence defect. A major difference between these mutants is that when the h-d mutant is grown to high cell density, it shows abnormal growth properties that are likely caused by the accumulation GlcNAc in the medium as a result of cell-wall chitin remodeling (Naseem et al., 2011). In contrast, the ngt1Δ mutant responds very poorly to GlcNAc, since it is defective in transporting this sugar into the cell (Alvarez and Konopka, 2007). Although this indicates that use of exogenous GlcNAc is not needed for a systemic infection, it may be important for growth of C. albicans in the GI tract. The ability to use GlcNAc is important for E. coli to colonize the gastrointestinal (GI) tract (Chang et al., 2004). GlcNAc is available in the GI tract due to its release during remodeling of bacterial peptidoglycan, which is composed in part of GlcNAc (Park and Uehara, 2008). Perhaps this explains why GlcNAc also induces C. albicans to undergo an epigenetic switch from the White phase to the Opaque phase, which shows increased expression of genes that facilitate mucosal infection (Huang et al., 2010).
Significance for GlcNAc signaling in other organisms
GlcNAc induces physiological changes in a wide range of organisms from bacteria to humans (Konopka, 2012). For example, GlcNAc stimulates a diverse set of fungi to switch from budding to hyphal growth, including Y. lipolytica, C. lusitaniae, H. capsulatum, and B. dermatitidis (Perez-Campo and Dominguez, 2001; Reedy et al., 2009; Gilmore et al., 2013). GlcNAc inhibits pathogenic E. coli from forming pili and CURLI fibers that promote adhesion to host cells (Barnhart et al., 2006), and it induces soil bacteria to undergo changes in morphogenesis and produce antibiotics (Rigali et al., 2008). Factors that increase UDP-GlcNAc levels in animal cells cause increased branching of N-linked glycans on cell surface proteins that affect cell signaling (Dennis et al., 2009). It also increases posttranslational attachment of GlcNAc onto Ser and Thr residues on intracellular proteins, which has been implicated in diseases such as cancer and diabetes (Slawson et al., 2010). The analysis of the h-d mutant shows that it will also be important in these systems to distinguish between direct effects of GlcNAc on cell signaling and effects that are induced as a result of GlcNAc metabolism. Growth of other organisms on GlcNAc is also likely to increase the ambient pH, since excess nitrogen is commonly released as ammonia. In addition, ammonia may also induce signaling, as it has been shown to promote differentiation of cells types in S. cerevisiae colonies (Cap et al., 2012). Thus GlcNAc can stimulate distinct signal pathways that are both dependent and independent of its metabolism.
MATERIALS AND METHODS
Strains and media
The C. albicans strains that were used in this study are described in Table 1. The strains described in Table 1 were grown in either rich yeast extract/peptone/dextrose (YPD) medium or complete synthetic medium containing yeast nitrogen base, amino acids, uridine, and the indicated sugars (Sherman, 1991). The h-d homozygous deletion mutant lacking the genes needed to metabolize GlcNAc (HXK1 NAG1 DAC1) was constructed by homologous recombination in C. albicans strain BWP17 (arg4Δ his1Δ ura3Δ; Wilson et al., 1999) as described previously (Naseem et al., 2011). The HXK1, NAG1, and DAC1 genes are adjacent to each other in the genome, so it was possible to delete all three in one step to create the homozygous hxk1Δ nag1Δ dac1Δ strain (AG738). For virulence studies, similar approaches were used to create an h-d mutant and a complemented strain in which URA3 was restored at its native locus (SN973 and SN974). The rim101Δ and dfg16Δ strains were kindly provided by Aaron Mitchell (Carnegie Mellon University, Pittsburgh, PA).
TABLE 1:
C. albicans strains used in this study.
| Strain | Genotype |
|---|---|
| BWP17 | ura3Δ::λimm434/ura3Δ::λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG |
| DIC185 | ura3Δ::λimm434/URA3 his1::hisG/HIS1 arg4::hisG/ARG4 |
| AG692 | Homozygous h-d strain; [hxk1Δ nag1Δ dac1Δ]::ARG4/[hxk1Δ nag1Δ dac1Δ]::URA3 his1::hisG/his1::hisG ura3Δ::λimm434/ura3Δ::λimm434 arg4::hisG/arg4::hisG |
| AG738 | His+ homozygous h-d strain; [hxk1 nag1 dac1]::ARG4/[hxk1 nag1Δ dac1Δ]::URA3 HIS1/his1::hisG ura3Δ::λimm434/ura3Δ::λimm434 arg4::hisG/arg4::hisG |
| SN778 | Complemented h-d strain; [hxk1Δ nag1Δ dac1Δ]::ARG4/[hxk1Δ nag1Δ dac1Δ]::URA3 [HXK1 NAG1 DAC1]::HIS1 his1::hisG/his1::hisG ura3Δ::λimm434/ura3Δ::λimm434 arg4::hisG/arg4::hisG |
| KBC048 | dfg16::ARG4/dfg16::URA3 HIS1/his1::hisG ura3Δ::λimm434/ura3Δ::λimm434 arg4::hisG/arg4::hisG |
| DAY25 | rim101::ARG4/rim101::URA3 HIS1/his1::hisG ura3Δ::λimm434/ura3Δ::λimm434 arg4::hisG/arg4::hisG |
| SN969 | BWP17 except HWP1-GFP::HIS1 |
| SN971 | AG738 except HWP1-GFP::HIS1 |
| SN973 | Homozygous h-d strain; [hxk1Δ nag1Δ dac1Δ]::ARG4/[hxk1Δ nag1Δ dac1Δ]::NAT1 HIS/his1::hisG URA3/ura3Δ::λimm434 arg4::hisG/arg4::hisG |
| SN974 | Complemented h-d strain; [hxk1Δ nag1Δ dac1Δ]::ARG4/[hxk1Δ nag1Δ dac1Δ]::NAT1 [HXK1 NAG1 DAC1]::HIS1 his1::hisG/his1::hisG URA3/ura3Δ::λimm434 arg4::hisG/arg4::hisG |
| YJA3 | ngt1∆::ARG4/ngt1∆::HIS1 ura3Δ::λimm434/URA3 his1::hisG/his1::hisG arg4::hisG/arg4::hisG |
An HWP1-GFP reporter gene was introduced into the indicated wild-type control and hxk1Δ nag1Δ dac1Δ triple-mutant strains by homologous recombination of GFP sequences to replace the open reading frame of one copy of the hyphal-induced gene HWP1, using previously described methods (Zhang and Konopka, 2010). PCR primers containing ∼70 base pairs of sequence homologous to the 5′ and 3′ ends of the HWP1 open reading frame were used to amplify a cassette containing a more photostable version of enhanced GFP (CaGFPγ) and a HIS1 selectable marker (Zhang and Konopka, 2010). After transformation into C. albicans, the resulting His+ colonies were confirmed by PCR to be carrying the HWP1-GFP reporter gene.
Microscopy
Cell morphology was assessed after growing cells overnight at 37°C to early log phase in synthetic medium with either dextrose or galactose. It was important to maintain cells at low density to avoid spontaneous filamentation of the h-d mutant, as described previously (Naseem et al., 2011). The cells were then resuspended at 1 × 106 cells/ml in synthetic medium containing GlcNAc or galactose plus GlcNAc, as indicated. The cells were grown for the indicated time at 37°C and then photographed using differential interference contrast microscopy. Cells were stained with Filipin III (10 μg/ml; Cayman Chemical Co., Ann Arbor, MI) for 5 min to detect polarization of lipids at the germ tube tips as described previously (Martin and Konopka, 2004). To colocalize cell septa and nuclear DNA, cells were first stained with Hoechst 33342 (2 μg/ml; Invitrogen, Grand Island, NY) to detect DNA and then stained with Pontamine Fast Scarlet 4B (0.3 μg/ml; gift from Charles Sprecht,University of Massachusetts Medical School, Worcester, MA) to detect cell wall and septae. Pontamine Fast Scarlet 4B stains cells similar to Calcofluor White (Hoch et al., 2005), but it fluoresces at a distinct wavelength from Calcofluor White, making it possible to carry out double-staining analyses with Hoechst stain. Induction of HWP1-GFP was detected in cells that were grown overnight to log phase in synthetic medium buffered to different pH levels with 100 mM sodium citrate (pH 4), 150 mM succinic acid (pH 5), 15 mM 2-(N-morpholino)ethanesulfonic acid (MES) buffer (pH 6), or 15 mM PIPES buffer (pH 7). Cells were viewed using an Olympus BH2 microscope, and images were captured with a Zeiss (Thornwood, NY) AxioCam digital camera. The relative fluorescence of the different cells was quantified using AxioVision software.
GlcNAc uptake
The ability of cells to take up GlcNAc was assayed essentially as described previously (Alvarez and Konopka, 2007). Cells were grown overnight in complete synthetic medium containing galactose, and then the GlcNAc transporter Ngt1 was induced by growing cells for 2 h in medium that also contained 50 mM GlcNAc. Cells were harvested, washed with yeast nitrogen base, and then resuspended at 1.1 × 108 cells/ml. Uptake assays were initiated by adding 10 μl of [3H]GlcNAc (200 μM; American Radiolabeled Chemicals, St. Louis, MO) to 90 μl of cells. After 2 min, cells were collected on Whatman GF/C glass microfiber filters (Whatman, Clifton, NJ). The radioactivity bound to the filters was then quantified using a scintillation counter.
Microarray analysis of gene expression
The cells were inoculated in synthetic medium containing either dextrose or galactose as indicated and kept in early log phase at 37°C for ∼24 h to avoid induction of hyphal signaling pathways due to a temperature shift or a release from farnesol inhibition (Enjalbert and Whiteway, 2005). In some experiments, the medium was buffered with 15 mM PIPES to maintain the cultures at pH 7. The cell cultures were periodically adjusted to keep the cells in log phase at 37°C for ∼24 h before shifting to medium containing GlcNAc or galactose plus GlcNAc. Dextrose-grown cells were harvested by centrifugation and then resuspended at a density of 2 × 106 cells/ml in prewarmed synthetic medium containing 50 mM of either dextrose or GlcNAc. Galactose-grown cells were resuspended at 2 × 106 cells/ml in 50 mM galactose, and then GlcNAc was added to one sample to a final concentration of 50 mM. The cultures were grown for 2 h, and then cell pellets were harvested for analysis.
Microarray analysis of gene expression was carried out with the help of the Spotted Array Facility at Stony Brook University, as described previously (Alvarez et al., 2007). RNA purified from 3 × 108 cells using a RiboPure-Yeast RNA isolation kit (Ambion, Grand Island, NY) was used as a template for cDNA synthesis using a Superscript II kit (Invitrogen) in a poly(T)-primed reaction containing a 3:2 mixture of aminoallyl-dUTP:dTTP plus dATP, dCTP, and dGTP. After treatment with RNase A, the cDNA was purified using a PCR clean-up column (Qiagen, Valencia, CA) and then coupled to NHS-functionalized Cy3 or Cy5 dyes (GE Biosciences, Piscataway, NJ). The efficiency of dye incorporation was determined spectrophotometrically. Microarrays containing 60-mer oligonucleotides representing the open reading frames in the C. albicans ORF19 database were synthesized by Agilent Technologies (Santa Clara, CA). Five or six independent oligonucleotides were included for each gene. Cy3- and Cy5-labeled samples were hybridized to the microarrays using a Tecan (Grand Island, NY) HS4300 Pro Hybridization Station, and the arrays were washed and then scanned using an Agilent G2505B scanner.
Real-time qRT-PCR analysis of mRNA levels
Cells were grown overnight in log phase and maintained at a low cell density to avoid high basal expression of the GlcNAc-induced genes in the h-d mutant, as described previously (Naseem et al., 2011). Cells were then grown in synthetic medium containing the indicated sugars for 2 h, and then the cell pellets were frozen at −80°C. RNA was extracted from a pellet of ∼3 × 108 cells using a RiboPure-Yeast RNA isolation kit (Ambion) and was tested by PCR to confirm the absence of DNA contamination. The RNA was used as a template for cDNA synthesis using an oligo-dT primer (Invitrogen) and Superscript III reverse transcriptase (Invitrogen). The cDNA was separated from RNA by treatment with RNase A (New England Biolabs, Beverly, MA), purified using a PCR clean-up column (Qiagen), and then used as a template for qRT-PCR in an Eppendorf (Hauppauge, NY) Mastercycler EP Realplex2. The 10-μl reaction mixture included 2× iQ SYBR Green Supermix (Bio-Rad, Hercules, CA), 1 μl of first-strand cDNA reaction mixture, and 0.1 μM primers. After a 95°C denaturation step for 5 min, the amplification program consisted of 40 cycles of 95°C for 15 s, 55°C for 15 s, and 72°C for 30 s. At the end of the program, a melting curve was carried out to verify the specificity of the PCR products. The data were analyzed using Mastercycler Realplex2 software to determine the relative differences in gene expression, which were normalized to the level of ACT1 mRNA in each sample. The results represent the average of at least two to four independent preparations of cells. Two sets of assays, each done in triplicate, were carried out on each RNA preparation. The primers used for qRT-PCR analysis were designed using Primer3 software (http://frodo.wi.mit.edu/) and custom synthesized by Invitrogen. The primer sequences used were as follows:
ACT1-F 5′-TCCAGAAGCTTTGTTCAGACCAGC-3′
ACT1-R 5′-TGCATACGTTCAGCAATACCTGGG-3′
NGT1-F 5′-TCGTGCCAAAATTGGTTGGGCT-3′
NGT1-R 5′-TGGACATGGCTCCCAATACCCA-3′
GIG1-F 5′-GCAAACCCCACCCACTTCACCA-3′
GIG1-R 5′-TGTTTGCTGTCGTGATCGAGCA-3′
GAL10-F 5′-AGGAGCAAACAACTTGCATGGTGG-3′
GAL10-R 5′-GCTTCAAGCTCACCTGGGAACC-3′
ECE1-F 5′-TGGCGTTCCAGATGTTGGCCT-3′
ECE1-R 5′-GCTAAGTGCTACTGAGCCGGCA-3′
HWP1-F 5′-GCTCCTGCCACTGAACCTTCCC-3′
HWP1-R 5′-ACTTGAGCCAGCTGGAGCGG-3′
Virulence assays
Cells were grown overnight at 30°C in YPD medium with 80 mg/l uridine, diluted into fresh medium, and incubated again overnight. Cells were washed with phosphate-buffered saline and then diluted so that the desired inoculum could be injected in 0.2 ml. Cell density was determined using a hemocytometer and confirmed by plating dilutions of cells onto YPD agar plates. Female BALB/c mice were injected with the indicated C. albicans strain into the lateral tail vein. The analysis was carried out in two independent experiments in which at least three mice were infected each time. Mice were considered to be moribund if they could no longer reach food and water and were then killed humanely. Fungal burden was quantified by disrupting kidneys for 30 s with a tissue homogenizer (Pro Scientific, Oxford, CT), plating serial dilutions of the homogenate onto YPD plates, and then determining the number of colony-forming units (CFU) per gram of kidney tissue. Samples of homogenized kidney tissue were also pelleted by centrifugation, resuspended in 20% KOH, incubated for 1 h at room temperature, stained with Calcofluor White (20 ng/ml) for 10 min (Pringle, 1991), and then examined by fluorescence microscopy.
Supplementary Material
Acknowledgments
We thank Charles Sprecht for the kind gift of Pontamine Scarlet 4B, Aaron Mitchell for sending strains, and Aaron Neiman and members of our lab for their helpful suggestions on the manuscript. We also thank Hong Wang and Sohail Kahn from the Stony Brook Spotted Array Facility and Jizu Zhi from the Stony Brook University Bioinformatics Facility for assistance with microarray analysis. This research was supported by Public Health Service grants awarded to J.B.K. from the National Institutes of Health (RO1 GM087368 and RO1 AI47837). S.N. was supported in part by a Training Grant from the National Cancer Institute of the National Institutes of Health (NIH T32 CA009176).
Abbreviations used:
- GFP
green fluorescent protein
- GlcNAc
N-acetylglucosamine
- MES
2-(N-morpholino)ethanesulfonic acid
- PIPES
1,4-piperazinediethanesulfonic acid
- qRT-PCR
quantitative real time PCR.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E14-08-1312) on January 21, 2015.
REFERENCES
- Alvarez FJ, Douglas LM, Konopka JB. Sterol-rich plasma membrane domains in fungi. Eukaryot Cell. 2007;6:755–763. doi: 10.1128/EC.00008-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez FJ, Konopka JB. Identification of an N-acetylglucosamine transporter that mediates hyphal induction in Candida albicans. Mol Biol Cell. 2007;18:965–975. doi: 10.1091/mbc.E06-10-0931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnhart MM, Lynem J, Chapman MR. GlcNAc-6P levels modulate the expression of Curli fibers by Escherichia coli. J Bacteriol. 2006;188:5212–5219. doi: 10.1128/JB.00234-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barwell KJ, Boysen JH, Xu W, Mitchell AP. Relationship of DFG16 to the Rim101p pH response pathway in Saccharomyces cerevisiae and Candida albicans. Eukaryot Cell. 2005;4:890–899. doi: 10.1128/EC.4.5.890-899.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop A, Lane R, Beniston R, Chapa-y-Lazo B, Smythe C, Sudbery P. Hyphal growth in Candida albicans requires the phosphorylation of Sec2 by the Cdc28-Ccn1/Hgc1 kinase. EMBO J. 2010;29:2930–2942. doi: 10.1038/emboj.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas S, Van Dijck P, Datta A. Environmental sensing and signal transduction pathways regulating morphopathogenic determinants of Candida albicans. Microbiol Mol Biol Rev. 2007;71:348–376. doi: 10.1128/MMBR.00009-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship JR, Mitchell AP. How to build a biofilm: a fungal perspective. Curr Opin Microbiol. 2006;9:588–594. doi: 10.1016/j.mib.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Braun BR, Johnson AD. Control of filament formation in Candida albicans by the transcriptional repressor TUP1. Science. 1997;277:105–109. doi: 10.1126/science.277.5322.105. [DOI] [PubMed] [Google Scholar]
- Calderone RA, Fonzi WA. Virulence factors of Candida albicans. Trends Microbiol. 2001;9:327–335. doi: 10.1016/s0966-842x(01)02094-7. [DOI] [PubMed] [Google Scholar]
- Cap M, Stepanek L, Harant K, Vachova L, Palkova Z. Cell differentiation within a yeast colony: metabolic and regulatory parallels with a tumor-affected organism. Mol Cell. 2012;46:436–448. doi: 10.1016/j.molcel.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Carlisle PL, Banerjee M, Lazzell A, Monteagudo C, Lopez-Ribot JL, Kadosh D. Expression levels of a filament-specific transcriptional regulator are sufficient to determine Candida albicans morphology and virulence. Proc Natl Acad Sci USA. 2009;106:599–604. doi: 10.1073/pnas.0804061106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang DE, Smalley DJ, Tucker DL, Leatham MP, Norris WE, Stevenson SJ, Anderson AB, Grissom JE, Laux DC, Cohen PS, Conway T. Carbon nutrition of Escherichia coli in the mouse intestine. Proc Natl Acad Sci USA. 2004;101:7427–7432. doi: 10.1073/pnas.0307888101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornet M, Richard ML, Gaillardin C. The homologue of the Saccharomyces cerevisiae RIM9 gene is required for ambient pH signalling in Candida albicans. Res Microbiol. 2009;160:219–223. doi: 10.1016/j.resmic.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Davis DA. How human pathogenic fungi sense and adapt to pH: the link to virulence. Curr Opin Microbiol. 2009;12:365–370. doi: 10.1016/j.mib.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Davis D, Edwards JE, Jr, Mitchell AP, Ibrahim AS. Candida albicans RIM101 pH response pathway is required for host- pathogen interactions. Infect Immun. 2000;68:5953–5959. doi: 10.1128/iai.68.10.5953-5959.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis-Hanna A, Piispanen AE, Stateva LI, Hogan DA. Farnesol and dodecanol effects on the Candida albicans Ras1-cAMP signalling pathway and the regulation of morphogenesis. Mol Microbiol. 2008;67:47–62. doi: 10.1111/j.1365-2958.2007.06013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis JW, Nabi IR, Demetriou M. Metabolism, cell surface organization, and disease. Cell. 2009;139:1229–1241. doi: 10.1016/j.cell.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enjalbert B, Whiteway M. Release from quorum-sensing molecules triggers hyphal formation during Candida albicans resumption of growth. Eukaryot Cell. 2005;4:1203–1210. doi: 10.1128/EC.4.7.1203-1210.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore SA, Naseem S, Konopka JB, Sil A. N-acetylglucosamine (GlcNAc) triggers a rapid, temperature-responsive morphogenetic program in thermally dimorphic fungi. PLoS Genet. 2013;9:e1003799. doi: 10.1371/journal.pgen.1003799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunasekera A, Alvarez FJ, Douglas LM, Wang HX, Rosebrock AP, Konopka JB. Identification of GIG1, a GlcNAc-induced gene in Candida albicans needed for normal sensitivity to the chitin synthase inhibitor nikkomycin Z. Eukaryot Cell. 2010;9:1476–1483. doi: 10.1128/EC.00178-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harcus D, Nantel A, Marcil A, Rigby T, Whiteway M. Transcription profiling of cyclic AMP signaling in Candida albicans. Mol Biol Cell. 2004;15:4490–4499. doi: 10.1091/mbc.E04-02-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitman J, Filler SG, Edwards JEJ, Mitchell AP. Molecular Principles of Fungal Pathogenesis. Washington, DC: ASM Press; 2006. [Google Scholar]
- Hoch HC, Galvani CD, Szarowski DH, Turner JN. Two new fluorescent dyes applicable for visualization of fungal cell walls. Mycologia. 2005;97:580–588. doi: 10.3852/mycologia.97.3.580. [DOI] [PubMed] [Google Scholar]
- Huang G, Yi S, Sahni N, Daniels KJ, Srikantha T, Soll DR. N-acetylglucosamine induces white to opaque switching, a mating prerequisite in Candida albicans. PLoS Pathog. 2010;6:e1000806. doi: 10.1371/journal.ppat.1000806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka JB. N-acetylglucosamine (GlcNAc) functions in cell signaling. Scientifica (Cairo) 2012;2012:489208. doi: 10.6064/2012/489208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamoto CA, Vinces MD. Contributions of hyphae and hypha-co-regulated genes to Candida albicans virulence. Cell Microbiol. 2005;7:1546–1554. doi: 10.1111/j.1462-5822.2005.00616.x. [DOI] [PubMed] [Google Scholar]
- Kumar MJ, Jamaluddin MS, Natarajan K, Kaur D, Datta A. The inducible N-acetylglucosamine catabolic pathway gene cluster in Candida albicans: discrete N-acetylglucosamine-inducible factors interact at the promoter of NAG1. Proc Natl Acad Sci USA. 2000;97:14218–14223. doi: 10.1073/pnas.250452997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leberer E, Harcus D, Dignard D, Johnson L, Ushinsky S, Thomas DY, Schroppel K. Ras links cellular morphogenesis to virulence by regulation of the MAP kinase and cAMP signalling pathways in the pathogenic fungus Candida albicans. Mol Microbiol. 2001;42:673–687. doi: 10.1046/j.1365-2958.2001.02672.x. [DOI] [PubMed] [Google Scholar]
- Li M, Martin SJ, Bruno VM, Mitchell AP, Davis DA. Rim13p, a protease required for Rim101p processing at acidic and alkaline pHs. Eukaryot Cell. 2004;3:741–751. doi: 10.1128/EC.3.3.741-751.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H. Transcriptional control of dimorphism in Candida albicans. Curr Opin Microbiol. 2001;4:728–735. doi: 10.1016/s1369-5274(01)00275-2. [DOI] [PubMed] [Google Scholar]
- Lo HJ, Kohler JR, DiDomenico B, Loebenberg D, Cacciapuoti A, Fink GR. Nonfilamentous C. albicans mutants are avirulent. Cell. 1997;90:939–949. doi: 10.1016/s0092-8674(00)80358-x. [DOI] [PubMed] [Google Scholar]
- Lu Y, Su C, Solis NV, Filler SG, Liu H. Synergistic regulation of hyphal elongation by hypoxia, CO2, and nutrient conditions controls the virulence of Candida albicans. Cell Host Microbe. 2013;14:499–509. doi: 10.1016/j.chom.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Su C, Wang A, Liu H. Hyphal development in Candida albicans requires two temporally linked changes in promoter chromatin for initiation and maintenance. PLoS Biol. 2011;9:e1001105. doi: 10.1371/journal.pbio.1001105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R, Albrecht-Eckardt D, Brunke S, Hube B, Hunniger K, Kurzai O. A core filamentation response network in Candida albicans is restricted to eight genes. PLoS One. 2013;8:e58613. doi: 10.1371/journal.pone.0058613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SW, Konopka JB. Lipid raft polarization contributes to hyphal growth in Candida albicans. Eukaryot Cell. 2004;3:675–684. doi: 10.1128/EC.3.3.675-684.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midkiff J, Borochoff-Porte N, White D, Johnson DI. Small molecule inhibitors of the Candida albicans budded-to-hyphal transition act through multiple signaling pathways. PLoS One. 2011;6:e25395. doi: 10.1371/journal.pone.0025395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milewski S, Gabriel I, Olchowy J. Enzymes of UDP-GlcNAc biosynthesis in yeast. Yeast. 2006;23:1–14. doi: 10.1002/yea.1337. [DOI] [PubMed] [Google Scholar]
- Moussian B. The role of GlcNAc in formation and function of extracellular matrices. Comp Biochem Physiol B Biochem Mol Biol. 2008;149:215–226. doi: 10.1016/j.cbpb.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Naseem S, Gunasekera A, Araya E, Konopka JB. N-acetylglucosamine (GlcNAc) induction of hyphal morphogenesis and transcriptional responses in Candida albicans are not dependent on its metabolism. J Biol Chem. 2011;286:28671–28680. doi: 10.1074/jbc.M111.249854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobile CJ, Schneider HA, Nett JE, Sheppard DC, Filler SG, Andes DR, Mitchell AP. Complementary adhesin function in C. albicans biofilm formation. Curr Biol. 2008;18:1017–1024. doi: 10.1016/j.cub.2008.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble SM, French S, Kohn LA, Chen V, Johnson AD. Systematic screens of a Candida albicans homozygous deletion library decouple morphogenetic switching and pathogenicity. Nat Genet. 2010;42:590–598. doi: 10.1038/ng.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obara K, Yamamoto H, Kihara A. Membrane protein Rim21 plays a central role in sensing ambient pH in Saccharomyces cerevisiae. J Biol Chem. 2012;287:38473–38481. doi: 10.1074/jbc.M112.394205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odds FC. Candida and Candidosis. Philadelphia, PA: Bailliere Tindall; 1988. [Google Scholar]
- Park JT, Uehara T. How bacteria consume their own exoskeletons (turnover and recycling of cell wall peptidoglycan) Microbiol Mol Biol Rev. 2008;72:211–227. doi: 10.1128/MMBR.00027-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Campo FM, Dominguez A. Factors affecting the morphogenetic switch in Yarrowia lipolytica. Curr Microbiol. 2001;43:429–433. doi: 10.1007/s002840010333. [DOI] [PubMed] [Google Scholar]
- Pringle J. Staining of bud scars and other cell wall chitin with Calcofluor. Methods Enzymol. 1991;194:732–735. doi: 10.1016/0076-6879(91)94055-h. [DOI] [PubMed] [Google Scholar]
- Reedy JL, Floyd AM, Heitman J. Mechanistic plasticity of sexual reproduction and meiosis in the Candida pathogenic species complex. Curr Biol. 2009;19:891–899. doi: 10.1016/j.cub.2009.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigali S, Titgemeyer F, Barends S, Mulder S, Thomae AW, Hopwood DA, van Wezel GP. Feast or famine: the global regulator DasR links nutrient stress to antibiotic production by Streptomyces. EMBO Rep. 2008;9:670–675. doi: 10.1038/embor.2008.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggle PJ, Andrutis KA, Chen X, Tzipori SR, Kumamoto CA. Invasive lesions containing filamentous forms produced by a Candida albicans mutant that is defective in filamentous growth in culture. Infect Immun. 1999;67:3649–3652. doi: 10.1128/iai.67.7.3649-3652.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha CR, Schroppel K, Harcus D, Marcil A, Dignard D, Taylor BN, Thomas DY, Whiteway M, Leberer E. Signaling through adenylyl cyclase is essential for hyphal growth and virulence in the pathogenic fungus Candida albicans. Mol Biol Cell. 2001;12:3631–3643. doi: 10.1091/mbc.12.11.3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman F. Getting started with yeast. Methods Enzymol. 1991;194:3–21. doi: 10.1016/0076-6879(91)94004-v. [DOI] [PubMed] [Google Scholar]
- Simonetti N, Strippoli V, Cassone A. Yeast-mycelial conversion induced by N-acetyl-D-glucosamine in Candida albicans. Nature. 1974;250:344–346. doi: 10.1038/250344a0. [DOI] [PubMed] [Google Scholar]
- Singh P, Ghosh S, Datta A. Attenuation of virulence and changes in morphology in Candida albicans by disruption of the N-acetylglucosamine catabolic pathway. Infect Immun. 2001;69:7898–7903. doi: 10.1128/IAI.69.12.7898-7903.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha I, Wang YM, Philp R, Li CR, Yap WH, Wang Y. Cyclin-dependent kinases control septin phosphorylation in Candida albicans hyphal development. Dev Cell. 2007;13:421–432. doi: 10.1016/j.devcel.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Slawson C, Copeland RJ, Hart GW. O-GlcNAc signaling: a metabolic link between diabetes and cancer. Trends Biochem Sci. 2010;35:547–555. doi: 10.1016/j.tibs.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staib P, Morschhauser J. Chlamydospore formation in Candida albicans and Candida dubliniensis—an enigmatic developmental programme. Mycoses. 2007;50:1–12. doi: 10.1111/j.1439-0507.2006.01308.x. [DOI] [PubMed] [Google Scholar]
- Stoldt VR, Sonneborn A, Leuker CE, Ernst JF. Efg1p, an essential regulator of morphogenesis of the human pathogen Candida albicans, is a member of a conserved class of bHLH proteins regulating morphogenetic processes in fungi. EMBO J. 1997;16:1982–1991. doi: 10.1093/emboj/16.8.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudbery PE. Growth of Candida albicans hyphae. Nat Rev Microbiol. 2011;9:737–748. doi: 10.1038/nrmicro2636. [DOI] [PubMed] [Google Scholar]
- Sudbery P, Gow N, Berman J. The distinct morphogenic states of Candida albicans. Trends Microbiol. 2004;12:317–324. doi: 10.1016/j.tim.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Vylkova S, Carman AJ, Danhof HA, Collette JR, Zhou H, Lorenz MC. The fungal pathogen Candida albicans autoinduces hyphal morphogenesis by raising extracellular pH. MBio. 2011;2:e00055–00011. doi: 10.1128/mBio.00055-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. Fungal adenylyl cyclase acts as a signal sensor and integrator and plays a central role in interaction with bacteria. PLoS Pathog. 2013;9:e1003612. doi: 10.1371/journal.ppat.1003612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A, Raniga PP, Lane S, Lu Y, Liu H. Hyphal chain formation in Candida albicans: Cdc28-Hgc1 phosphorylation of Efg1 represses cell separation genes. Mol Cell Biol. 2009;29:4406–4416. doi: 10.1128/MCB.01502-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendland J, Schaub Y, Walther A. N-acetylglucosamine utilization by Saccharomyces cerevisiae based on expression of Candida albicans NAG genes. Appl Environ Microbiol. 2009;75:5840–5845. doi: 10.1128/AEM.00053-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteway M, Bachewich C. Morphogenesis in Candida albicans. Annu Rev Microbiol. 2007;61:529–553. doi: 10.1146/annurev.micro.61.080706.093341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteway M, Oberholzer U. Candida morphogenesis and host-pathogen interactions. Curr Opin Microbiol. 2004;7:350–357. doi: 10.1016/j.mib.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Wilson RB, Davis D, Mitchell AP. Rapid hypothesis testing with Candida albicans through gene disruption with short homology regions. J Bacteriol. 1999;181:1868–1874. doi: 10.1128/jb.181.6.1868-1874.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada-Okabe T, Sakamori Y, Mio T, Yamada-Okabe H. Identification and characterization of the genes for N-acetylglucosamine kinase and N-acetylglucosamine-phosphate deacetylase in the pathogenic fungus Candida albicans. Eur J Biochem. 2001;268:2498–2505. doi: 10.1046/j.1432-1327.2001.02135.x. [DOI] [PubMed] [Google Scholar]
- Zhang C, Konopka JB. A photostable green fluorescent protein variant for analysis of protein localization in Candida albicans. Eukaryot Cell. 2010;9:224–226. doi: 10.1128/EC.00327-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Wang Y. Hgc1, a novel hypha-specific G1 cyclin-related protein regulates Candida albicans hyphal morphogenesis. EMBO J. 2004;23:1845–1856. doi: 10.1038/sj.emboj.7600195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng XD, Lee RT, Wang YM, Lin QS, Wang Y. Phosphorylation of Rga2, a Cdc42 GAP, by CDK/Hgc1 is crucial for Candida albicans hyphal growth. EMBO J. 2007;26:3760–3769. doi: 10.1038/sj.emboj.7601814. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.