Abstract
The present study tested whether rats release more accumbens dopamine (DA) during a sugar binge when they are underweight vs. normal weight. Since acetylcholine (ACh) in the nucleus accumbens (NAc) normally increases as a meal progresses and satiety ensues, we also tested whether ACh release is altered when an animal has lost weight. Rats were maintained on daily 8-h access to chow, with 10% sucrose solution available for the first 2 h. Microdialysis performed on day 21, at normal body weight, revealed an increase in extracellular DA to 122% of baseline in response to drinking sucrose. Extracellular ACh peaked at the end of the meal. Next, the rats were food and sucrose restricted so that by day 28 they were at 85% body weight. When retested, these animals released significantly more DA when drinking sucrose (179%), but ACh release failed to rise. A control group was tested in the same manner but given sugar only on days 1, 21 and 28. At normal body weight, control animals showed a non-significant rise in DA when drinking sucrose on day 21. On day 28, at 85% body weight, the controls showed a small increase (124%) in DA release; however, this was significantly lower than the 179% observed in the underweight rats with daily sugar access. These findings suggest that when an animal binges on sugar and then loses weight, the binge releases significantly more DA and less ACh than when animals are at a normal body weight.
Keywords: sugar, food restriction, microdialysis, eating disorders
Drugs of abuse produce their reinforcing effects by over-stimulating neural pathways activated during naturally rewarding experiences (Hoebel et al., 1999; Kelley and Berridge, 2002). Therefore, it is logical that behavioral and neurochemical links between drug abuse and aberrant eating have been reported. In particular, a relationship between food deprivation or restriction and the reinforcing effects of drugs has been well documented (Carroll, 1985; Bell et al., 1997; Carr, 2002). Underweight animals that have been maintained on a restricted diet will more readily seek and self-administer drugs of abuse compared with normal weight counterparts. This phenomenon has been shown across drug classes, having been observed with alcohol, opiates, and psychostimulants (Carroll and Meisch, 1979; Carroll and Stotz, 1983; Oei, 1983; Carroll, 1985; Papasava and Singer, 1985; Pfeffer and Samson, 1985; Papasava et al., 1986). Moreover, the rewarding effects of drugs, such as alcohol, morphine and cocaine, are increased in food-restricted animals, as measured by a downward shift in lateral-hypothalamic self-stimulation threshold (Cabeza de Vaca and Carr, 1998; Carr et al., 2000).
One possible neurochemical basis for this phenomenon arises from work showing that the reinforcing value of both food and drug consumption is associated with activity in the mesolimbic dopamine (DA) system (Hoebel, 1985; Kelley and Berridge, 2002; Wise, 2006; Di Chiara and Bassareo, 2007). In rats reduced 20–30% below normal weight, basal extracellular DA in the nucleus accumbens (NAc) decreases as much as 50% (Pothos et al., 1995a,b). There are no observed differences in basal DA levels in the NAc in rats with less severe weight loss (10–20%) (Rouge-Pont et al., 1995; Cadoni et al., 2003). Underweight animals show an increase in DA release in the NAc in response to accumbens infusion of amphetamine (Pothos et al., 1995a), and they also display enhanced locomotor sensitization in response to accumbens or intraventricular infusion of amphetamine (Deroche et al., 1995; Cabeza de Vaca and Carr, 1998).
Similar to the effects of some drugs of abuse, repeated daily bingeing on a sugar solution (10% sucrose or 25% glucose) can result in behavioral signs of dependence (Avena et al., 2008). Bingeing is defined as the consumption of a large amount of food, more than would normally be consumed in a discrete period of time (American Psychiatric Association, 2000). The signs of dependence induced by sugar bingeing include opiate-like withdrawal signs, enhanced amphetamine-induced hyperactivity and increased alcohol consumption (Avena et al., 2008). Sugar-bingeing rats also release DA in the NAc in response to tasting sugar each day (Rada et al., 2005; Avena et al., 2006), an effect that is qualitatively similar to most drugs of abuse (Di Chiara and Imperato, 1988), and unlike the diminishing effect of repeated, palatable food consumption (Bassareo and Di Chiara, 1999). For these reasons, we hypothesized underweight rats would show an enhanced DA response in the NAc after bingeing on sugar, when compared with normal body weight controls. It was also predicted that acetylcholine (ACh), which in the accumbens has been shown to increase with satiety (Mark et al., 1992; Avena et al., 2006), would be attenuated or delayed in underweight rats due to reduced or slower satiation. Some of these data have been discussed in a previous review paper (Avena, 2007).
EXPERIMENTAL PROCEDURES
Subjects and surgery
Male Sprague–Dawley rats (300–325 g) were obtained from Taconic Farms (Germantown, NY, USA) and housed individually on a reversed 12-h light/dark cycle. All procedures were approved by the Princeton University Institutional Animal Care and Use Committee and conformed to the National Institutes of Health guidelines on the ethical use of animals. Efforts were made to minimize the use of animals and their suffering. Water was continuously available except during the microdialysis tests.
All rats underwent surgery to implant guide cannulas for microdialysis. They were anesthetized with 20 mg/kg xylazine and 100 mg/kg ketamine (i.p.), supplemented with ketamine as needed. Bilateral 21 gauge stainless steel guide shafts were aimed at the posterior medial accumbens shell (anterior: +1.2 mm, lateral: 0.8 mm and ventral: 4.0 mm, with reference to bregma, midsagittal sinus, and surface of the level skull, respectively). Microdialysis probes were inserted later (see below) and extended another 5 mm ventrally.
Behavioral procedures
Following approximately 1 week of surgical recovery, the experimental group (n=7) was maintained on 16-h daily food-restriction (12 h of light and 4 h into the dark, no food available) followed by 2-h access to a 10% sucrose solution (from 4th–6th h of the dark) and 8-h access to rodent chow (from 4th h of dark onset). This limited access procedure is slightly different from, but in many ways similar to, what we have used in the past to elicit signs of dependence (Avena et al., 2008). The control group (n=7) was maintained on this schedule on day 1 and day 21 and had chow available ad libitum in the interim. On day 21, microdialysis was performed, as described below.
Beginning on day 22, all rats were gradually reduced in body weight to 85% of their starting weight over the course of the next week. The experimental group was limited to 5 g of chow per day and access to the sucrose solution for 2 h, but the amount of sucrose given was limited to the mean amount that each animal had been consuming during days 19–21. This was done to ensure that the animals would lose weight and not compensate for the lack of calories available by consuming excessive amounts of sucrose. The control group was similarly weight-reduced, but did not have access to sucrose during this period, except on day 28 during the microdialysis session (described below). Body weights were recorded daily during the weight-reduction period, and if the animals were not losing weight at a steady rate, to be at 85% of their body weight by day 28, they were given slightly less chow the next day.
Microdialysis procedures
In vivo microdialysis was used to measure extracellular DA and ACh release in the NAc shell. Microdialysis probes were constructed of silica glass tubing (37 μm inner diameter, Polymicro Technologies Inc., Phoenix, AZ, USA) inside a 26 gauge stainless steel tube with a microdialysis tip of cellulose tubing sealed at the end with epoxy (Spectrum Medical Co., Los Angeles, CA, USA, 6000 molecular weight, 0.2 mm outer diameter×2.0 mm long) (Hernandez et al., 1986). On day 20, microdialysis probes were inserted and cemented in place for at least 18 h prior to collections to allow neurotransmitter recovery to stabilize. Probes were perfused with a buffered Ringer’s solution (142 mM NaCl, 3.9 mM KCl, 1.2 mM CaCl2, 1.0 mM MgCl2, 1.35 mM Na2HPO4, 0.3 mM NaH2PO4, pH 7.35) at a flow rate of 0.5 μl/min overnight and 1.3 μl/min starting 2 h before the experiment began on day 21. Neostigmine (0.3 μM) was added to the perfusion fluid to improve basal recovery of ACh by hindering enzymatic degradation.
On day 21 at normal body weight, three consecutive 30-min baseline samples were collected prior to sucrose access. All rats were then given ad libitum access only to sucrose for 2 h, with samples collected every 30 min. Post-samples were collected following sucrose access, during which time the rats had no access to sucrose or chow. Each sample was split; half for DA analysis and half for ACh.
Following the experiment on day 21, animals were weight-reduced as described above. On day 27 they were returned to the dialysis cages. A new microdialysis probe was inserted in the NAc on the contralateral side (counterbalanced between rats), and perfused for stabilization overnight. On day 28, the same microdialysis procedures were followed as on day 21, except this time the animals were in a weight-reduced state, and the amount of sucrose that they were allowed to consume was clamped at the mean intake for each animal on days 19–21.
DA and ACh assays
DA and its metabolites, 3,4-dihydroxy-phenylacetic acid (DOPAC) and homovanillic acid (HVA), were analyzed by reverse phase, high performance liquid chromatography with electrochemical detection (HPLC-EC). Samples were injected into a 20-μl sample loop leading to a 10-cm column with 3.2-mm bore and 3 μm C18 packing (Brownlee Co. Model 6213, San Jose, CA, USA). The mobile phase contained 60 mM NaH2PO4, 100 μM EDTA, 1.24 mM CH3(CH2)6SO3Na·H2O, and 5% vol/vol MeOH. DA, DOPAC and HVA were measured with a coulometric detector (ESA Co. Model 5100A, Chelmsford, MA, USA) with the conditioning potential set at +500 mV and working cell potential at −400 mV.
ACh was measured by reverse phase HPLC-EC using a 20-μl sample loop with a 10-cm C18 analytical column (Chrompack Inc., Palo Alto, CA, USA). ACh was converted to betaine and hydrogen peroxide (H2O2) by an immobilized enzyme reactor (acetylcholinesterase and choline oxidase from Sigma, St Louis, MO, USA). The mobile phase was 200 mM K3PO4 at pH 8.0. An amperometric detector was used (EG&G Princeton Applied Research, Law-renceville, NJ, USA). The H2O2 was oxidized on a platinum electrode (BAS, West Lafayette, IN, USA) set at 500 mV with respect to an Ag–AgCl reference electrode (EG&G Princeton Applied Research).
Histology
At the end of the experiment histology was performed to verify microdialysis probe placement. Rats received an overdose of sodium pentobarbital and when deeply anesthetized were intracardially perfused with 0.9% saline followed by 10% formaldehyde. The brains were removed, frozen, and cut into 40 μm sections, starting anterior to the accumbens until the sites of probe tips were located and plotted using the atlas of Paxinos and Watson (2005).
Data analysis
Sucrose intake was recorded to the nearest ml, and between-group intake was analyzed by an unpaired t-test comparing the intakes on day 21 between the daily sugar-bingeing group and the sugar-twice group. Daily sugar intake and basal DA levels were analyzed by a one-way repeated measures analysis of variance (ANOVA). Body weights during the weight-restriction phase were compared between groups by two-way repeated measures ANOVA. Microdialysis data were normalized to percent of base-line and analyzed by one- or two-way repeated measures ANOVA. Post hoc Tukey’s Honestly Significantly Difference Tests were utilized when justified.
RESULTS
DA release is enhanced by body-weight reduction in sugar-bingeing rats
At a normal body weight, rats with 2-h access to sugar every day increased their intake during the 21 days (F(20,230)=6.02, P<0.001, Fig. 1), and by day 21 they consumed significantly more than the control group that had access only on days 1 and 21 (t(16)=4.84, P<0.001; 16.2±1.5 kcal vs. 3.9±1 kcal, respectively).
Fig. 1.
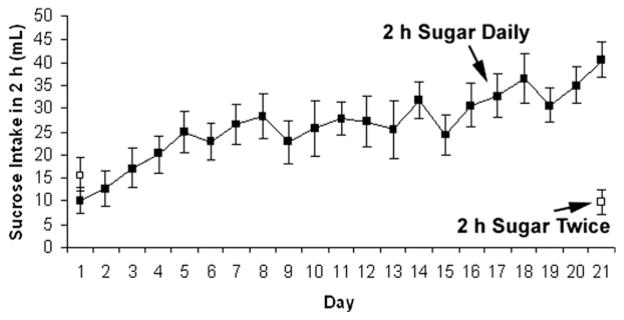
Daily sugar intake during the 21 days at a normal body weight. Intake increased significantly over time for the rats with 2 h of access to sugar each day. The control group drank approximately the same amount on days 1 and 21.
Basal DA levels were as follows: 2-h daily sugar group at normal body weight (day 21)=0.75±0.18 fmol; 2-h daily sugar group at reduced body weight (day 28)=0.88±0.35 fmol; 2-h sugar twice control group at normal body weight (day 21)=1.03±0.17 fmol; 2-h sugar twice control group at reduced body weight (day 28)=0.78±0.24 fmol, with no significant differences among groups.
For the experimental group that binged on sucrose daily, microdialysis performed on day 21, at normal body weight, revealed an increase in extracellular DA to 122±4% in response to drinking sucrose (day 21:F(6,48)=8.23, P<0.001, Fig. 2A). Control animals showed no significant rise in DA on day 21, when drinking sucrose for the second time.
Fig. 2.
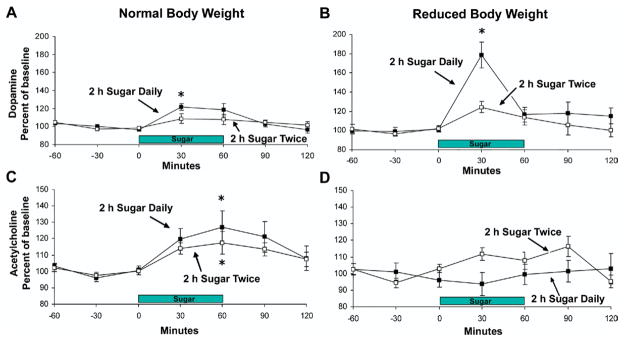
Accumbens DA and ACh release when rats binge on sugar at a normal body weight and then again at 85% body weight. (A) DA is released in response to drinking sugar on day 21 of access at a normal body weight, and (B) this release is enhanced (to 179% of baseline) when animals binge on sugar at a reduced body weight. Rats with access to sugar just two times do not show this neurochemical effect. (C) ACh rises as the sugar meal progresses for both groups when at normal body weight. (D) This effect is blunted for the daily bingeing group when at a reduced body weight. Error bars represent standard error of the mean. * P<0.05 from baseline. White squares represent the 2-h sugar twice group, and black squares represent the 2-h sugar daily group. Figure reprinted with permission (Avena, 2007).
During the weight-reduction phase, the body weights of the rats in both groups steadily fell to approximately 85% over the course of 7 days (86±1.5% and 82±1.2%, experimental and control groups, respectively). On day 28, at 85% body weight, rats that had been bingeing released more DA in the NAc when drinking sugar (179±14% of baseline) compared with the control group (124±6%; F(6,72)=3.98, P<0.002, Fig. 2B).
When comparing each group over time, DA release was significantly greater for the 2-h daily sugar group when they were at a reduced body weight compared with at normal body weight (F(1,7)=19.93, P<0.005). This effect was not observed in the 2-h sugar twice control group, which showed a similar rise in DA at normal and reduced body weight.
Analysis of data for DOPAC and HVA is presented in Table 1. Levels of the metabolites were generally greater for the daily binge group compared with the control group and were not significantly changed by food restriction.
Table 1.
DA metabolite levels (DOPAC and HVA) in animals that were binge eating each day at normal and reduced body weight, and controls with access to sugar only a few times, at normal and reduced body weight
| Treatment | DOPAC | HVA |
|---|---|---|
| Normal body weight | ||
| 2-h Daily sugar | 110% F(6,48)=3.4, P<0.01 |
115% F(6,48)=3.8, P<0.01 |
| Control | 108% F(6,48)=3.0, P<0.05 |
112% F(6,48)=3.1, P<0.01 |
| Reduced body weight | ||
| 2-h Daily sugar | 114% F(6,48)=4.9, P<0.001 |
115% F(6,48)=3.8, P<0.001 |
| Control | 108% F(6,48)=2.3, P<0.05 |
115% F(6,48)=8.4, P<0.0001 |
P values based on results from one-way ANOVAs for each group compared to baseline.
ACh release is attenuated in sugar-bingeing rats when they are underweight
On day 21, at normal body weight, extracellular ACh increased during the sugar meal and peaked at the end for the binge group (day 21: 127±10%, F(6,48)=3.11, P<0.005, Fig. 2C); however, on day 28 the ACh effect disappeared when rats were underweight (100±6% of baseline). Control animals, on the other hand, showed a significant increase in ACh release at the end of the meal at both normal weight (177±7%, F(6,36)=4.59, P<0.005; Fig. 2C) and reduced body weight (116±6%, F(6,36)=3.94, P<0.005; Fig. 2D).
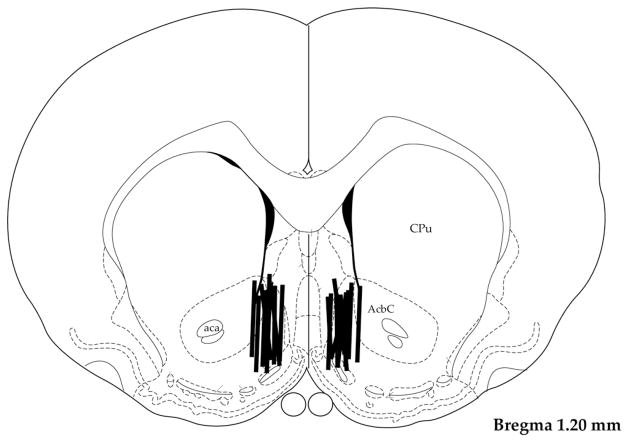
Microdialysis probes were located primarily in the medial shell region of the NAc (Fig. 3).
Fig. 3.
Histology revealed that microdialysis samples were drawn primarily from the medial NAc shell. AcbC=accumbens core, CPu=caudate, aca=anterior commissure.
DISCUSSION
Sugar-induced DA release is enhanced in bingeing rats at a low body weight
The findings suggest that animals that binge eat a sugar solution, and then lose weight, show a greater percent increase in DA release in the NAc than at normal body weight, and more than non-bingeing animals at a low weight. In a prior study, when underweight rats were fed ordinary chow or given systemic amphetamine or morphine, enhanced DA release was not observed; however, when amphetamine was administered directly into the NAc, it did release significantly more DA, suggesting that vesicular DA had accumulated (Pothos et al., 1995a). Changes in basal level, the amount released and receptor binding may all bear on the fact that drugs are more reinforcing when animals are at a low weight (Carroll and Meisch, 1979; Carroll and Stotz, 1983; Carroll, 1985; Carr et al., 2000; Carr, 2002; Cadoni et al., 2003). The present data suggest that increased release is a factor in sugar bingeing when food restricted.
The enhanced DA increase in the NAc is coupled with an attenuation of ACh release. We have previously shown that ACh levels in the NAc normally increase during a meal when feeding slows down (Mark et al., 1992) and may peak when feeding stops (Rada et al., 2005; Hoebel et al., 2007). Pratt and Kelley (2005) also suggested a role for accumbens ACh in satiety by showing that antagonism of the muscarinic receptors with scopolamine inhibits feeding. This drug may act, in part, indirectly by increasing extracellular ACh levels (Chau et al., 2001). In the present study, ACh release was attenuated when animals were at a low body weight. This blunted ACh release occurred independent of caloric intake, since both the 2-h daily and the control rats consumed similar amounts of sugar at normal and reduced body weights. Thus, the attenuated ACh release may play a role in mitigating sugar satiation. Together with the results obtained with DA, it may be that bingeing is more reinforcing in food-restricted animals due to both the increased percent rise in DA and attenuated ACh satiation factor.
Binge eating at a low body weight
The present experiment uses a modified version of the sugar binge-eating model that we have previously shown to produce behaviors and neurochemical changes qualitatively like those seen with drugs of abuse (Avena, 2007; Avena et al., 2008). The main differences are a more limited period of access to sucrose (2 h vs. 12 h) and food-restriction to decrease body weight to 85%. Weight-reduction to 85% or more over the course of a week, as in the present study, has been used by others (Pothos et al., 1995a; Cadoni et al., 2003). These modifications to the model were incorporated 1) to facilitate weight loss, 2) highlight that binge-eating behavior can also be modeled with shorter periods of access, and 3) to test the suggestion that sugar bingeing may be more reinforcing, as measured by DA release, at a reduced body weight.
In addition to the model described in this manuscript, other models of binge eating have been described (Hagan and Moss, 1997; Corwin and Buda-Levin, 2004; Boggiano et al., 2005), some of which have shown that bingeing behavior is enhanced when animals are chronically food restricted (Hagan and Moss, 1997; Boggiano et al., 2005). Other models have also used short (e.g. 1 or 2 h) limited-access periods to palatable foods, such as sugars, fats, and/or sweet–fat mixtures (Corwin et al., 1998; Boggiano et al., 2005; Berner et al., 2008).
This report extends the literature by showing enhanced DA release in the NAc in response to repeated binge eating of a sugar solution while at a reduced body weight. Wilson et al. (1995) showed that 20-h food restriction increased release of accumbens DA in response to drinking a palatable solution. Bassareo and Di Chiara (1999) found that acute food restriction could reinstate DA release in the NAc after the response has habituated due to lack of novelty. We reported that daily 12-h food restriction followed by sugar bingeing released DA in the NAc, even after 3 weeks on this diet (Rada et al., 2005). The present results support all these findings, and further suggest that repeated exposure to a palatable solution in the form of binge eating can produce an enhancement of DA release when rats are underweight. It is expected that the palatability of the sucrose solution used in the present study is partially responsible for the results. Since fat (Liang et al., 2006), sucrose (Rada et al., 2005), and the taste of sucrose (Avena et al., 2006) have all been shown to repeatedly release DA in the NAc in normal weight, binge-eating animals, it is predicted that these foods and other palatable tastes would all produce an enhancement in DA release in underweight animals, as shown with sugar in the present study.
A gateway to eating disorders?
Short periods of access may model binge eating in humans, which is defined by the DSM-IV-TR as a bout of approximately 2 h of excessive eating (American Psychiatric Association, 2000). The shorter periods of access are particularly relevant when discussing binge eating at a low body weight as a model of some restrictive-type eating disorders. These binge-feeding episodes are accompanied by a lack of control, such as the feeling that one cannot stop eating. Clinically, binge-eating episodes are associated with three or more of the following: 1) eating until feeling uncomfortably full, 2) eating large amounts of food when not physically hungry, 3) eating much more rapidly than normal, 4) eating alone because one is embarrassed by how much they are eating, 4) feeling disgusted, depressed, or guilty after overeating, or 5) marked distress or anxiety regarding binge eating. To meet diagnostic criteria for binge-eating disorder, bingeing must occur, on average, at least 2 days per week for 6 months. A role for DA has been suggested by studies demonstrating that patients who binge eat have a polymorphism in the DA transporter gene (Shinohara et al., 2004). Also, patients with binge-eating disorder show changes in the brain indicative of an altered reward sensitivity, including the presence of the A1 allele, which is associated with decreased D2 receptor density (Davis et al., 2008). Together, these gene changes may cause a dysregulation of DA reuptake that contributes to the altered hedonic responses to food reported by patients who binge eat (Davis et al., 2008).
Similar results have been found in patients with bulimia nervosa. With this eating disorder, patients binge eat and then engage in compensatory actions to purge ingested calories via excessive exercise or food deprivation. These patients show changes in brain areas that participate in reinforcement. In particular, recovering bulimics have blunted activation of the anterior cingulate cortex, a brain area that has a role in the anticipation of reward in response to glucose ingestion (Frank et al., 2006). This finding suggests that such individuals may have a reduced response to the reinforcing aspects of foods, thus causing vulnerability to overeating. In the present experiment, binge eating at a low body weight resulted in an increase in accumbens DA release. This further supports the role of DA in the rewarding effects observed with bulimics with self-inflicted food restriction followed by bingeing episodes.
CONCLUSION
As reviewed elsewhere, it has been previously shown that sugar bingeing results in behaviors and neurochemical changes that are similar to those observed with drugs of abuse (Avena et al., 2008). The present findings suggest that in rats with a history of binge eating, access to a palatable food (sucrose) at a low body weight is associated with a simultaneous increase in DA and attenuated ACh release in the NAc. This may make the effect of sugar more like a substance of abuse. Binge eating on sugar can result in a state that is like an “addiction” (Avena et al., 2008). The resulting enhanced release of DA without the opposing rise in ACh that occurs when bingeing at a low body weight, as shown here, might perpetuate binge eating and contribute to addictive-like behavior characteristic of some eating disorders.
Acknowledgments
This research was supported by MH-65024 (to B. T. Walsh at NY Psychiatric Inst./Columbia Univ. and B.G.H. et al.), DA-10608 (to B.G.H.) and DA-16458 and DK-79793 (fellowships to N.M.A.). We thank Miriam Bocarsly and Jacqueline Sullivan for their assistance preparing the manuscript. The data presented here have been discussed in a review paper (Avena, 2007).
Abbreviations
- ACh
acetylcholine
- ANOVA
analysis of variance
- DA
dopamine
- DOPAC
3,4-dihydroxy-phenylacetic acid
- HPLC-EC
high performance liquid chromatography with electrochemical detection
- HVA
homovanillic acid
- NAc
nucleus accumbens
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders fourth edition text revision (DSM-IV-TR) Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Avena NM. Examining the addictive-like properties of binge eating using an animal model of sugar dependence. Exp Clin Psychopharmacol. 2007;15:481–491. doi: 10.1037/1064-1297.15.5.481. [DOI] [PubMed] [Google Scholar]
- Avena NM, Rada P, Hoebel BG. Evidence of sugar addiction: Behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev. 2008;32:20–39. doi: 10.1016/j.neubiorev.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avena NM, Rada P, Moise N, Hoebel BG. Sucrose sham feeding on a binge schedule releases accumbens dopamine repeatedly and eliminates the acetylcholine satiety response. Neuroscience. 2006;139:813–820. doi: 10.1016/j.neuroscience.2005.12.037. [DOI] [PubMed] [Google Scholar]
- Bassareo V, Di Chiara G. Modulation of feeding-induced activation of mesolimbic dopamine transmission by appetitive stimuli and its relation to motivational state. Eur J Neurosci. 1999;11:4389–4397. doi: 10.1046/j.1460-9568.1999.00843.x. [DOI] [PubMed] [Google Scholar]
- Bell SM, Stewart RB, Thompson SC, Meisch RA. Food-deprivation increases cocaine-induced conditioned place preference and locomotor activity in rats. Psychopharmacology (Berl) 1997;131:1–8. doi: 10.1007/s002130050258. [DOI] [PubMed] [Google Scholar]
- Berner LA, Avena NM, Hoebel BG. Bingeing, self-restriction, and increased body weight in rats with access to a sweet-fat diet. Obesity. 2008 doi: 10.1038/oby.2008.328. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Boggiano MM, Chandler PC, Viana JB, Oswald KD, Maldonado CR, Wauford PK. Combined dieting and stress evoke exaggerated responses to opioids in binge-eating rats. Behav Neurosci. 2005;119:1207–1214. doi: 10.1037/0735-7044.119.5.1207. [DOI] [PubMed] [Google Scholar]
- Cabeza de Vaca S, Carr KD. Food restriction enhances the central rewarding effect of abused drugs. J Neurosci. 1998;18:7502–7510. doi: 10.1523/JNEUROSCI.18-18-07502.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadoni C, Solinas M, Valentini V, Di Chiara G. Selective psychostimulant sensitization by food restriction: differential changes in accumbens shell and core dopamine. Eur J Neurosci. 2003;18:2326–2334. doi: 10.1046/j.1460-9568.2003.02941.x. [DOI] [PubMed] [Google Scholar]
- Carr KD. Augmentation of drug reward by chronic food restriction: behavioral evidence and underlying mechanisms. Physiol Behav. 2002;76:353–364. doi: 10.1016/s0031-9384(02)00759-x. [DOI] [PubMed] [Google Scholar]
- Carr KD, Kim GY, Cabeza de Vaca S. Chronic food restriction in rats augments the central rewarding effect of cocaine and the delta1 opioid agonist, DPDPE, but not the delta2 agonist, deltorphin-II. Psychopharmacology (Berl) 2000;152:200–207. doi: 10.1007/s002130000523. [DOI] [PubMed] [Google Scholar]
- Carroll ME. The role of food deprivation in the maintenance and reinstatement of cocaine-seeking behavior in rats. Drug Alcohol Depend. 1985;16:95–109. doi: 10.1016/0376-8716(85)90109-7. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Meisch RA. Effects of food deprivation on etonitazene consumption in rats. Pharmacol Biochem Behav. 1979;10:155–159. doi: 10.1016/0091-3057(79)90182-5. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Stotz DC. Oral d-amphetamine and ketamine self-administration by rhesus monkeys: effects of food deprivation. J Pharmacol Exp Ther. 1983;227:28–34. [PubMed] [Google Scholar]
- Chau DT, Rada P, Kosloff RA, Taylor JL, Hoebel BG. Nucleus accumbens muscarinic receptors in the control of behavioral depression: antidepressant-like effects of local M1 antagonist in the Porsolt swim test. Neuroscience. 2001;104:791–798. doi: 10.1016/s0306-4522(01)00133-6. [DOI] [PubMed] [Google Scholar]
- Corwin RL, Buda-Levin A. Behavioral models of binge-type eating. Physiol Behav. 2004;82:123–130. doi: 10.1016/j.physbeh.2004.04.036. [DOI] [PubMed] [Google Scholar]
- Corwin RL, Wojnicki FH, Fisher JO, Dimitriou SG, Rice HB, Young MA. Limited access to a dietary fat option affects ingestive behavior but not body composition in male rats. Physiol Behav. 1998;65:545–553. doi: 10.1016/s0031-9384(98)00201-7. [DOI] [PubMed] [Google Scholar]
- Davis C, Levitan RD, Kaplan AS, Carter J, Reid C, Curtis C, Patte K, Hwang R, Kennedy JL. Reward sensitivity and the D2 dopamine receptor gene: a case-control study of binge eating disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:620–628. doi: 10.1016/j.pnpbp.2007.09.024. [DOI] [PubMed] [Google Scholar]
- Deroche V, Marinelli M, Maccari S, Le Moal M, Simon H, Piazza PV. Stress-induced sensitization and glucocorticoids. I. Sensitization of dopamine-dependent locomotor effects of amphetamine and morphine depends on stress-induced corticosterone secretion. J Neurosci. 1995;15:7181–7188. doi: 10.1523/JNEUROSCI.15-11-07181.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V. Reward system and addiction: what dopamine does and doesn’t do. Curr Opin Pharmacol. 2007;7:69–76. doi: 10.1016/j.coph.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank GK, Wagner A, Achenbach S, McConaha C, Skovira K, Aizenstein H, Carter CS, Kaye WH. Altered brain activity in women recovered from bulimic-type eating disorders after a glucose challenge: a pilot study. Int J Eat Disord. 2006;39:76–79. doi: 10.1002/eat.20210. [DOI] [PubMed] [Google Scholar]
- Hagan MM, Moss DE. Persistence of binge-eating patterns after a history of restriction with intermittent bouts of refeeding on palatable food in rats: implications for bulimia nervosa. Int J Eat Disord. 1997;22:411–420. doi: 10.1002/(sici)1098-108x(199712)22:4<411::aid-eat6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Hernandez L, Stanley BG, Hoebel BG. A small, removable microdialysis probe. Life Sci. 1986;39:2629–2637. doi: 10.1016/0024-3205(86)90119-0. [DOI] [PubMed] [Google Scholar]
- Hoebel BG. Brain neurotransmitters in food and drug reward. Am J Clin Nutr. 1985;42:1133–1150. doi: 10.1093/ajcn/42.5.1133. [DOI] [PubMed] [Google Scholar]
- Hoebel BG, Avena NM, Rada P. Accumbens dopamine-acetylcholine balance in approach and avoidance. Curr Opin Pharmacol. 2007;7:617–627. doi: 10.1016/j.coph.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoebel BG, Rada P, Mark GP, Pothos E. Neural systems for reinforcement and inhibition of behavior: Relevance to eating, addiction, and depression. In: Kahneman D, et al., editors. Well-being: the foundations of hedonic psychology. New York: Russell Sage Foundation; 1999. pp. 558–572. [Google Scholar]
- Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang NC, Hajnal A, Norgren R. Sham feeding corn oil increases accumbens dopamine in the rat. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1236–R1239. doi: 10.1152/ajpregu.00226.2006. [DOI] [PubMed] [Google Scholar]
- Mark GP, Rada P, Pothos E, Hoebel BG. Effects of feeding and drinking on acetylcholine release in the nucleus accumbens, striatum, and hippocampus of freely behaving rats. J Neurochem. 1992;58:2269–2274. doi: 10.1111/j.1471-4159.1992.tb10973.x. [DOI] [PubMed] [Google Scholar]
- Oei TP. Effects of body weight reduction and food deprivation on cocaine self-administration. Pharmacol Biochem Behav. 1983;19:453–455. doi: 10.1016/0091-3057(83)90119-3. [DOI] [PubMed] [Google Scholar]
- Papasava M, Singer G. Self-administration of low-dose cocaine by rats at reduced and recovered body weight. Psychopharmacology (Berl) 1985;85:419–425. doi: 10.1007/BF00429657. [DOI] [PubMed] [Google Scholar]
- Papasava M, Singer G, Papasava CL. Intravenous self-administration of phentermine in food-deprived rats: effects of abrupt refeeding and saline substitution. Pharmacol Biochem Behav. 1986;25:623–627. doi: 10.1016/0091-3057(86)90151-6. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. New York: Academic Press; 2005. [DOI] [PubMed] [Google Scholar]
- Pfeffer AO, Samson HH. Oral ethanol reinforcement: interactive effects of amphetamine, pimozide and food-restriction. Alcohol Drug Res. 1985;6:37–48. [PubMed] [Google Scholar]
- Pothos EN, Creese I, Hoebel BG. Restricted eating with weight loss selectively decreases extracellular dopamine in the nucleus accumbens and alters dopamine response to amphetamine, morphine, and food intake. J Neurosci. 1995a;15:6640–6650. doi: 10.1523/JNEUROSCI.15-10-06640.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pothos EN, Hernandez L, Hoebel BG. Chronic food deprivation decreases extracellular dopamine in the nucleus accumbens: implications for a possible neurochemical link between weight loss and drug abuse. Obes Res. 1995b;3 (Suppl 4):525S–529S. doi: 10.1002/j.1550-8528.1995.tb00222.x. [DOI] [PubMed] [Google Scholar]
- Pratt WE, Kelley AE. Striatal muscarinic receptor antagonism reduces 24-h food intake in association with decreased preproenkephalin gene expression. Eur J Neurosci. 2005;22:3229–3240. doi: 10.1111/j.1460-9568.2005.04489.x. [DOI] [PubMed] [Google Scholar]
- Rada P, Avena NM, Hoebel BG. Daily bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience. 2005;134:737–744. doi: 10.1016/j.neuroscience.2005.04.043. [DOI] [PubMed] [Google Scholar]
- Rouge-Pont F, Marinelli M, Le Moal M, Simon H, Piazza PV. Stress-induced sensitization and glucocorticoids. II. Sensitization of the increase in extracellular dopamine induced by cocaine depends on stress-induced corticosterone secretion. J Neurosci. 1995;15:7189–7195. doi: 10.1523/JNEUROSCI.15-11-07189.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara M, Mizushima H, Hirano M, Shioe K, Nakazawa M, Hiejima Y, Ono Y, Kanba S. Eating disorders with binge-eating behaviour are associated with the s allele of the 3′-UTR VNTR polymorphism of the dopamine transporter gene. J Psychiatry Neurosci. 2004;29:134–137. [PMC free article] [PubMed] [Google Scholar]
- Wilson C, Nomikos GG, Collu M, Fibiger HC. Dopaminergic correlates of motivated behavior: importance of drive. J Neurosci. 1995;15:5169–5178. doi: 10.1523/JNEUROSCI.15-07-05169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Role of brain dopamine in food reward and reinforcement. Philos Trans R Soc Lond B Biol Sci. 2006;361:1149–1158. doi: 10.1098/rstb.2006.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]