Preface
Atherosclerosis is a chronic inflammatory disease arising from an imbalance in lipid metabolism and a maladaptive immune response driven by the accumulation of cholesterol-laden macrophages in the artery wall. Through the analysis of animal models of atherosclerosis progression and regression, there is a growing understanding that the balance of macrophages in the plaque is dynamic, with both macrophage numbers and an inflammatory phenotype influencing plaque fate. Here we summarize recently identified pro- and anti-inflammatory pathways linking lipid and inflammation biology with the retention of macrophages in plaques, as well as factors with the potential to promote their egress from these sites.
Introduction
Atherosclerosis results from a maladaptive inflammatory response set in motion by the intramural retention of cholesterol-rich, apolipoprotein B-containing lipoproteins in susceptible areas of the arterial vasculature (reviewed in 1). Lipoproteins sequestered in the arterial wall are susceptible to various modifications (such as oxidation, enzymatic and non-enzymatic cleavage and aggregation), which render these particles pro-inflammatory and triggers of activation of the overlying endothelium. The ensuing immune response is mediated by the recruitment of monocyte-derived cells into the subendothelial space, where they differentiate into mononuclear phagocytes that ingest the accumulated normal and modified lipoproteins, transforming them into the cholesterol-laden “foam cells”. Foam cells, typically classified as a type of macrophage, persist in plaques, promoting disease progression. While macrophage clearance of lipoproteins is likely to be beneficial at the outset of this immune response, there is little negative feedback of uptake and thus these cells become grossly engorged with lipids. The resultant dysregulation of lipid metabolism alters the macrophage phenotype and compromises critical immune functions.
Notably, macrophages that accumulate in atherosclerotic plaques appear to have a diminished capacity to migrate, which contributes to failure to resolve inflammation and to progression of these lesions to more advanced, complex plaques in which other immune cell subsets and vascular smooth muscle cells participate in the inflammatory process2. In these advanced plaques, macrophages continue to be major contributors to the inflammatory response through their secretion of pro-inflammatory mediators (chemokines, cytokines and reactive oxygen and nitrogen species) and matrix-degrading proteases, and through their eventual death by necrosis or apoptosis. Dying macrophages release their lipid contents and tissue factor, leading to the formation of a pro-thrombotic necrotic core, a key component of unstable plaques that contributes to their rupture and the ensuing intravascular blood clot that underlies myocardial infarction and stroke.
Though many cell types contribute to the formation of atherosclerotic plaques, including endothelial cells, monocytes, dendritic cells (DCs), lymphocytes, eosinophils, mast cells and smooth muscle cells, foam cells are so central in the pathophysiology of atherosclerosis that emphasis has long been placed on understanding the mechanisms of monocyte recruitment into plaques and identifying strategies to reduce monocyte influx and thereby retard plaque progression. However, it has become apparent that the recruitment of monocytes and other leukocytes into the artery may also be critical to promoting disease regression and inflammation resolution3. In addition, studies in some models of atherosclerosis regression have shown that macrophage retention can be reversed4–6, leading to the identification of pathways that promote macrophage accumulation in, or egress from, the inflamed plaque. These advances have revealed that both the quantity and phenotype of macrophages influence the inflammatory state of the plaque, and have identified potentially new targets for plaque intervention. Here we review the key roles of macrophages in the initiation, progression and resolution of atherosclerotic inflammation, with a focus on how the dynamics of macrophage recruitment, egress and death alter the fate of the plaque.
Circulating monocytes and their recruitment in atherosclerosis
Hypercholesterolemia and monocytosis
Hypercholesterolemia is associated with increased numbers of circulating monocytes in mice, swine and rabbits7,8. In apolipoprotein E-deficient (Apoe−/−) mice, circulating monocytes are ∼50% higher than in wild-type mice9,10. How does hypercholesterolemia cause monocytosis? Studies using mice have shown that cholesterol enrichment of hematopoietic stem and progenitor cells (HSPCs; precursors of monocytes and neutrophils) increases their expression of the common β-subunit of the interleukin-3 (IL-3) and the granulocyte/macrophage colony-stimulating factor (GM-CSF) receptor, and thus HSPC proliferation11. Notably, the expression of factors that promote cholesterol efflux (high-density lipoprotein (HDL) and APOE) in hypercholesterolemic mouse models corrected HSPC proliferation.
Circulating monocytes in mice consist of two major subsets, LY6Chi and LY6Clow (Box 1). Interestingly, the monocytosis in hypercholesterolemic mice derives primarily from an increase in the more inflammatory LY6Chi subset, which makes up the majority of cells recruited to progressing atherosclerotic plaques and which is thought to be the source of the M1 (classically activated) macrophages found in the plaques 9–11. The basis for this bias has been postulated to be due to an impairment by hypercholesterolemia of a process in which LY6Chi cells are converted to LY6Clow cells9, however this remains an area of active investigation.
Box 1. Characteristics of monocyte and macrophage subsets.
LY6Chi monocytes
Express high levels of the chemokine receptor CCR2
Thought to be proinflammatory by virtue of their recruitment to sites of inflammation, including atherosclerotic plaques
Normally represent 50% of monocytes in mouse, but are increased with hyperlipidemia
Thought to correspond to CD14++CD16− monocyte subset in humans, which represent 95% of monocytes in humans
Proposed to be precursors of M1 macrophages
LY6Clow monocytes
Express high levels of the chemokine receptor CX3CR1
Thought to patrol the vasculature in a homeostatic function
Proposed to be precursors of M2 macrophages
Thought to correspond to CD14+/lowCD16+ monocyte subset in humans
M1 macrophages
Classical activation by lipopolysaccharide (or other Toll-like receptor ligands) and interferon-γ
Enriched in progressing plaques
Secrete pro-inflammatory cytokines, such as interleukin-1 β (IL-1β), IL-12 and tumour necrosis factor (TNF)
Produce high levels of nitric oxide synthase 2 (Nos2) and nitric oxide
Express the pro-inflammatory transcription factors nuclear factor-κB (NF-κB), activator protein 1 (AP1) and hypoxia-inducible factor 1α (HIF1α)
Express MHC class II molecules and the co-stimulatory molecules CD80 and CD86
M2 macrophages
Alternative activation by IL-4 and IL-13
Enriched in regressing plaques
High endocytic activity
Take up and oxidize fatty acids
Secrete anti-inflammatory cytokines, such as IL-1 receptor antagonist and IL-10
M2 macrophages express high levels of Arginase 1 and have increased secretion collagen, which promotes tissue repair. Express the transcription factors Krüppel-like factor 4 (KLF4), peroxisome proliferator activated receptor-γ (PPARγ) and signal transducer and activator of transcription 6 (STAT6)
Express CD163, mannose receptor and FIZZ1
Mox macrophages
Induced by oxidized phospholipids and nitrosylated fatty acids
Enriched in progressing plaques
Express high levels of reactive oxygen species and haem oxygenase 1, and the transcription factor nuclear respiratory factor 2 (NRF2)
Recruitment of monocytes into athero-prone arterial sites
The early steps of atherogenesis have been subjects of numerous reviews (for example 12–14) and will only be briefly covered here. Atherosclerotic plaques are not randomly distributed, but tend to form at the inner curvatures and branch points of arteries, where laminar flow is either disturbed or insufficient to support the normal, quiescent state of the endothelium. This activation of endothelium leads to increased permeability to lipoproteins and an accumulation of extracellular matrix proteins that cause a poorly understood diffuse intimal thickening and the retention of the atherogenic APOB lipoproteins. The arterial intima at these athero-prone sites contains an increase in myeloid cells with features of DCs15.
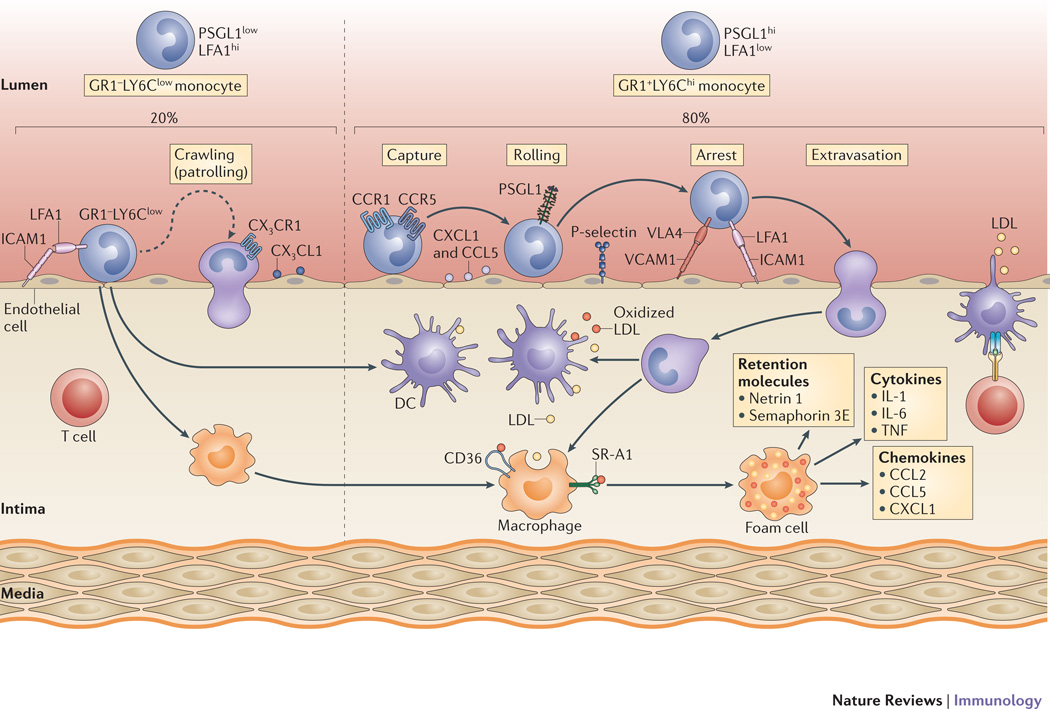
The activation of the endothelium also promotes the recruitment of circulating monocytes (Figure 1). In addition to a bone marrow origin of these monocytes, it has been recently recognized that splenic HSPCs can be a extramedullary myelopoietic source of monocytes that is mobilized to inflammatory sites, including atherosclerotic plaques16. Apparently, the steps regulating monocyte entry into the arterial intima are similar independent of the source of the cells and depend on the upregulation on activated endothelial cells of molecules that mediate the arrest of circulating monocytes by the leukocyte adhesion cascade (reviewed in 17). The capture and rolling phases of this cascade depend on the immobilization of chemokines, particularly CC-chemokine ligand 5 (CCL5) and CXC-chemokine ligand 1 (CXCL1), to endothelial cell glycosaminoglycans, and on P-selectin, which is expressed on the luminal side of endothelial cells. Very recent results have shown that the arrest of LY6Chi monocytes through CCL5 depends not only on its interaction with CC-chemokine receptor 5 (CCR5), but also with CCR118. Vascular cell adhesion molecule 1 (VCAM1) and intercellular adhesion molecule 1 (ICAM1), which bind to the integrins VLA4 and lymphocyte function-associated antigen 1 (LFA1), respectively, are important for the firm adhesion of monocytes to the luminal surface of the endothelium, with comparatively more LFA1 expressed by LY6Clow cells, which may underlie their greater tendency to adhere to, but not enter, the vasculature19.
Figure 1. Mechanisms regulating monocyte recruitment and accumulation in plaques.
Hyperlipidemia increases the number of GR1+LY6Chi monocytes, which constitute 80% of the monocytes recruited to mouse atherosclerotic plaques, with the remainder being the GR1–LY6Clow patrolling monocytes. These monocyte subsets use different chemokine—chemokine receptor pairs to infiltrate the intima, which is facilitated by endothelial adhesion molecules, including selectin, intercellular adhesion molecule 1 (ICAM1) and vascular adhesion molecule 1 (VCAM1). The recruited monocytes differentiate into macrophages or dendritic cells (DCs) in the intima, where they interact with atherogenic lipoproteins. Macrophages avidly take up native and modified low-density lipoprotein (LDL) via macropinocytosis or scavenger receptor-mediated pathways (including scavenger receptor A (SRA and CD36), resulting in the formation of the foam cells that are a hallmark of the atherosclerotic plaque. These foam cells secrete pro-inflammatory cytokines (including interleukin-1 (IL-1), IL-6, and tumor necrosis factor (TNF)) and chemokines (such as CC-chemokine ligand 2 (CCL2), CCL5 and CXC-chemokine ligand 1 (CXCL1)), as well as macrophage retention factors (such as netrin 1 and semaphorin 3E) that amplify the inflammatory response. CX3CL1, CX3C–chemokine ligand 1; CX3CR1, CX3C–chemokine receptor 1; LFA1, lymphocyte function-associated antigen 1; PSGL1, P-selectin glycoprotein ligand 1;
Transmigration of monocytes across the endothelium into plaques is mediated by chemokines secreted by endothelial cells, intimal macrophages and smooth muscle cells. Although several chemokines have been implicated in atherosclerosis20, the three major chemokine receptor-chemokine pairs involved in monocyte transmigration are CCR2–CCL2, CX3CR1–CX3CRL1 and CCR5-CCL510. Indeed, elimination of these three chemokine axes led to a ∼90% reduction in atherosclerosis in Apoe−/− mice21. In addition to these chemokines, CD31 (also known von Willebrand factor; an endothelial cell surface immunoglobulin-like adhesion molecule) and VCAM1 may also have a role in monocyte transmigration into atherosclerotic plaques. It should also be noted that CCR2 and CX3CR1, in addition to their effects on transmigration, have indirect roles in influencing the number of monocytes that enter plaques: In particular CCR2 is also required for the extravasation of LY6Chi cells from the bone marrow and CX3CR1 promotes their survival by inhibiting apoptosis22,23.
In addition to the factors described above, emerging evidence suggests that neuronal guidance cues are involved in monocyte recruitment in atherosclerosis. We recently reported that members of the netrin, semaphorin and ephrin families are expressed by arterial endothelial cells and are differentially regulated under conditions that promote or protect from atherosclerosis24. For example, the expression of ephrin B2 is up-regulated under pro-atherosclerotic conditions and functions as a chemoattractant, increasing leukocyte recruitment to athero-prone arterial sites in the absence of additional chemokines24. In contrast, the expression of netrin 1 and semaphorin 3A, which inhibit chemokine-directed migration of human and murine monocytes in vitro, is decreased in athero-prone regions and their inhibition by blocking peptides in wild-type mice increased leukocyte adhesion to the endothelium24. While further studies in hyperlipidemic mouse models are needed, the data suggest that the coordinate regulation of positive and negative guidance cues facilitates leukocyte infiltration of the endothelium. Notably, these neuronal guidance cues have additional roles in atherosclerosis by regulating the chemostasis of plaque macrophages25,26 (see below).
Overall, then, recruitment of circulating monocytes into plaques requires the integration of at least 3 discrete processes, namely, their capture, rolling and transmigration, with each step regulated by multiple, and sometimes overlapping, molecular factors. The fates of these recruited monocytes in the plaques are addressed in the next sections.
Foam cell formation in atherosclerosis
Lipoprotein uptake
Lipoprotein uptake by monocyte-derived macrophages is thought to be one of the earliest pathogenic events in the nascent plaque and results in the development of foam cells (Figure 2). The mechanisms of foam cell formation have been intensely studied (reviewed in 27). Although macrophages can clear APOB-containing lipoproteins through the low-density lipoprotein (LDL) receptor, expression of this receptor is downregulated early during foam cell formation by the increased cellular cholesterol levels. These observations lead to the early hypothesis that lipoproteins must become modified in the artery wall and be taken up by other mechanisms. Multiple means of LDL modification have now been identified that facilitate cholesterol loading of macrophages in vitro (Figure 2); however, the physiologically relevant in vivo pathways of foam cell formation remain an area of open debate.
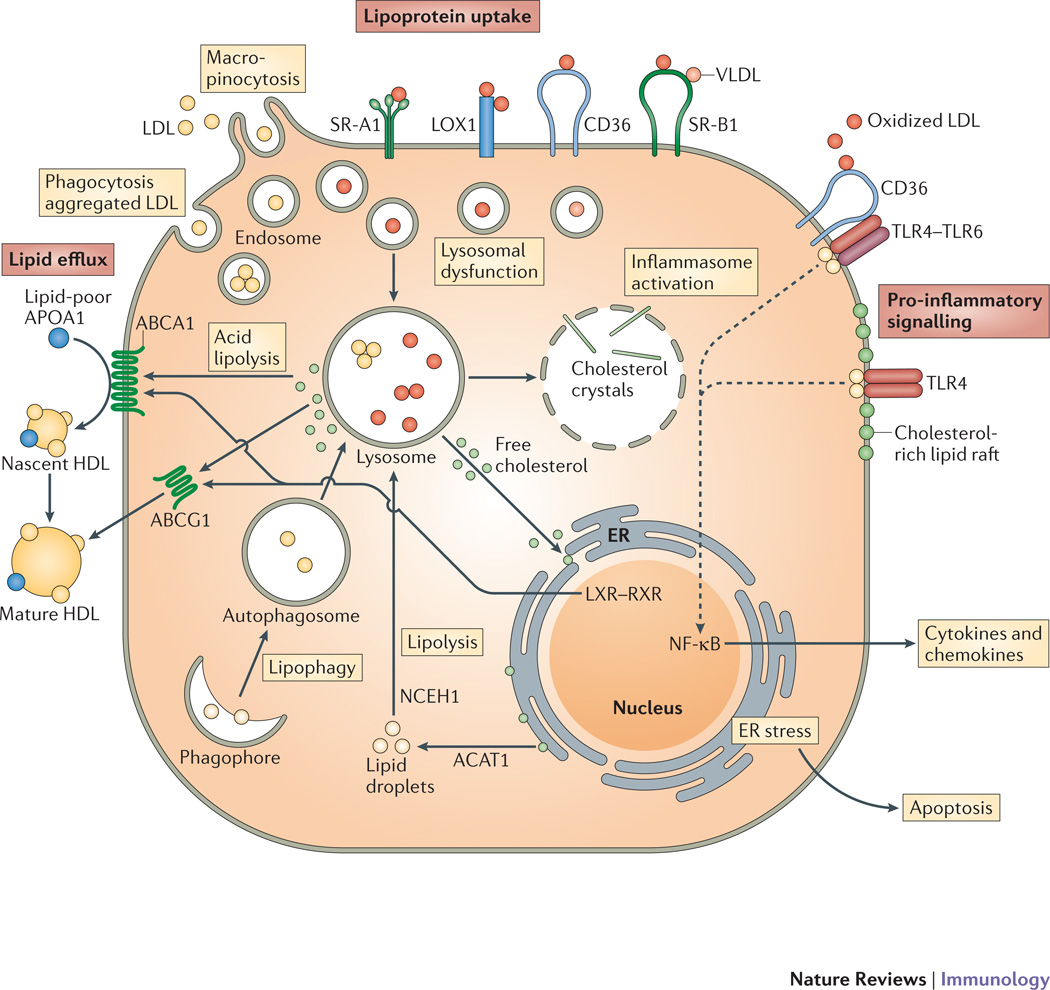
Figure 2. Mechanisms governing macrophage lipoprotein uptake and efflux.
Macrophages internalize native (low density lipoprotein, LDL; very low density lipoprotein, VLDL) and oxidized lipoproteins in the plaque via macropinocytosis, phagocytosis of aggregated LDL and scavenger receptor-mediated uptake (including by scavenger receptor A (SRA), LOX1, SRB1 and CD36). The internalized lipoproteins and their associated lipids are digested in the lysosome resulting in the release of free cholesterol that can travel to the plasma membrane and be effluxed from the cell or to the endoplasmic reticulum (ER) membrane and be esterified by acyl-coA cholesterol acyltransferase (ACAT) and ultimately stored in this form in cytosolic lipid droplets. These stored lipids can be mobilized for efflux via either lipolysis by neutral cholesterol ester hydrolases (nCEH) or lipophagy, a form of autophagy, which results in delivery of lipid droplets to lysosomes. The accumulation of cellular cholesterol activates the liver-X-receptor (LXR)/retinoid X receptor (RXR) heterodimeric transcription factor that upregulates expression of the ABC transporters ABCA1 and ABCG1, that mediate the transfer of free cholesterol to lipid poor APOA-I to form nascent high-density lipoprotein (HDL) or more lipidated HDL particles in which free cholesterol has been esterified and stored the core of the particle (mature HDL),. Excessive free cholesterol accumulation can induce cholesterol crystal formation in the lysosome to activate the NLRP3 inflammasome, and may also interfere with the function of the ER (ER stress), which if prolonged results in cell death by apoptosis, and Lipid rafts are enriched in sphingomyelin, which forms a complex with free cholesterol. As the cholesterol content of lipid rafts increases, pro-inflammatory Toll-like receptor 4 (TLR4) signalling is promoted, which can also be induced by oxidized low-density lipoprotein (LDL) through a heterotrimeric complex composed of CD36–TLR4–TLR6. This signalling resulted in the activation of nuclear factor-κB (NF-κB) and production of pro-inflammatory cytokines and chemokines.
A prevailing paradigm has been that heightened oxidative stress in the artery wall promotes modifications of LDL, generating “damage” signals that are recognized by pattern recognition receptors (PRRs) on cells of the innate immune system. This thesis is supported by the finding of oxidized LDL in both human and mouse atheroma, and of natural antibodies (predominantly IgM) that recognize oxidation epitopes of LDL (reviewed in 28). A variety of mechanisms mediated by enzymes (such as 12/15-lipoxygenase and myeloperoxidase) and free-radicals (such as superoxide, hydrogen peroxide and nitric oxide) have been identified that could promote LDL oxidation in the artery wall28 and in vitro preparations of such modified LDLs are avidly endocytosed by macrophages29,30.
Scavenger receptors, which are a type of PRR expressed by macrophages, have an important role in atherosclerosis and were originally described by their ability to recognize and process modified LDL (reviewed in 27). Numerous scavenger receptor family members — including scavenger receptor A (SRA; encoded by MSR), MARCO, CD36, scavenger receptor class B member 1 (SRB1), lectin-type oxidized low-density lipoprotein receptor 1 (LOX1), scavenger receptor class E member 1 (SREC1) and scavenger receptor for phosphatidylserine and oxidized low-density lipoprotein (SRPSOX; also known as CXCL16) — can bind oxidized LDL and promote foam cell formation31. SRA and CD36 mediate 75–90% of the degradation of LDL modified by acetylation or oxidation by macrophages in vitro29. These receptors internalize the lipoproteins, and in the late endolysosomal compartment, the cholesteryl esters of the lipoproteins are hydrolyzed to free cholesterol and fatty acids. Free cholesterol in the endolysosomal compartment is then trafficked to the endoplasmic reticulum (ER), where it undergoes re-esterification by acyl-CoA:cholesterol ester transferase (ACAT) to cholesteryl fatty acid esters that provide the “foam” of foam cells32.
Combined deficiency of SRA and CD36 reduced foam cell formation in Apoe−/− mice; however, this effect was incomplete, suggesting the existence of additional mechanisms of macrophage cholesterol uptake in vivo33,34. Despite this redundancy in cholesterol uptake mechanisms, plaques in Cd36−/−Apoe−/− and Msr−/− Cd36−/−Apoe−/− mice have reduced signs of inflammation, macrophage apoptosis and secondary necrosis, suggesting roles for these scavenger receptors beyond lipid uptake33,34. Nevertheless, the in vivo relevance of oxidative processes in atherosclerosis remains speculative. Several well-powered human clinical trials of antioxidant vitamins, such as vitamin E and vitamin C, have failed to show a reduction of cardiovascular events35, pushing the field to consider alternative bases for foam cell formation.
Modification by various proteases and lipases present in the intima can also mediate LDL modifications, particularly its aggregation. The extracellular matrix glycoproteins contribute to this process by retaining the lipoproteins and by modulating the activity of various enzymes, including group IIA secretory phospholipase A2 secretory phospholipase A2 (GIIA sPLA2), GV sPLA2 and GX sPLA2), as well as secretory sphingomylinase (reviewed in 27). These lipolytic enzymes produce modified forms of LDL that are taken up via pathways independent of scavenger receptors36. Evidence from mouse models support a role for sPLA2 family members in atherosclerosis progression37, and circulating sPLA2 levels in humans correlate with coronary artery disease risk38,39, identifying it as a promising therapeutic target, although further validation is required.
Finally, although a role for native LDL in foam cell formation was initially discounted, recent studies have shown that at concentrations in the arterial intima similar to those under hyperlipidemic conditions, LDL undergoes pinocytosis by macrophages resulting in foam cell formation40. This receptor-independent endocytic pathway also delivers cholesterol into the endolysosomal compartment and stimulates cholesterol esterification. Thus, rather than the originally envisioned “single modification model” — by which LDL and other APOB-containing lipoproteins would be rendered atherogenic — it is probable that multiple, simultaneous pathways contribute to foam cell formation in vivo.
Defective cholesterol trafficking
Macrophage cholesterol metabolism can become overwhelmed in the face of excessive cholesterol uptake, resulting in pathological processes. When stored in the cell as cholesteryl ester, cholesterol is relatively inert, however free cholesterol can be toxic to cells. Enrichment of ER membranes with free cholesterol can result in defective cholesterol esterification by ACAT in macrophages favoring further accumulation of free cholesterol. Also, free cholesterol enrichment of cell membranes can enhance inflammatory signalling from lipid rafts, particularly TLR signalling and activation of nuclear factor-κB (NF-κB) 41–43. Furthermore, trafficking of free cholesterol out of lysosomes may also become defective in these macrophages, constituting a barrier to cholesterol efflux and further amplifying inflammation44. Such dysregulation in lipid metabolism contributes to ER stress in macrophages, which if prolonged and combined with other insults, can ultimately result in apoptotic cell death45. Efficient clearance of apoptotic cells by surrounding macrophages (the process of efferocytosis), requires intact lipid metabolism pathways (such as cholesterol esterification and efflux) in the engulfing cell to deal with the ingested lipids of the apoptotic bodies. Thus, as macrophage lipid metabolism becomes dysregulated, the increase in macrophage apoptosis combined with defective efferocytosis results in secondary necrosis and the release of cellular components and lipids that form the necrotic core46. This feature of advanced atherosclerotic plaques, along with thinning of the fibrous cap, may increase the vulnerability of plaques to rupture.
Lipid efflux
Cells attempt to respond to excessive lipid accumulation by pathways that promote the removal of cholesterol and other lipids from the cell. In foam cells several macrophage transporters facilitate this efflux of lipids — including ATP-binding cassette transporter A family member 1 (ABCA1), ABCG1 and SRB1 (Figure 2) — although passive diffusion from the plasma membrane also occurs47. ABCA1 promotes cholesterol efflux to lipid-poor APOA1, which is the building block of HDL, whereas ABCG1 promotes efflux to mature HDL particles. The genes encoding ABCA1 and ABCG1 are transcriptionally upregulated in response to elevated cellular cholesterol levels by liver X receptors (LXRs), which are ligand-activated nuclear receptors that act as sterol sensors. For example, LXR activation by cholesterol derivatives (such as oxysterols) or by desmosterol (a molecule similar to cholesterol)48, promotes macrophage cholesterol efflux via ABCA1 and ABCG1 and also has anti-inflammatory actions49. Thus, synthetic LXR agonists have been actively investigated for the treatment of atherosclerosis.
Autophagy also has a critical role in macrophage cholesterol efflux: lipid droplets in macrophages and other cell types are targeted to and hydrolyzed by the autophagy machinery in a process known as lipophagy50. Fusion of the autophagosome with the lysosome degrades cholesteryl esters and makes free and modified cholesterol available for efflux through an ABCA1-dependent pathway51 (Figure 2). The protective role of autophagy has been demonstrated in studies in Apoe−/− mice in which the deletion of components of the autophagy machinery enhanced atherosclerosis52,53. Furthermore, autophagy regulates innate and adaptive immune responses (discussed below), including inflammasome activation, antigen presentation, and T cell activation53–55. Thus, pathways that stimulate the efflux of cholesterol from the macrophage serve dual atheroprotective functions by promoting lipid homeostasis and protecting from inflammation.
Innate immune activation
Evidence supports innate immune activation as a central process in the pathogenesis of atherosclerosis. As reviewed above, dysregulated lipid metabolism contributes to the development of foam cells. Such aberrations and the resultant endogenous danger ligands that accumulate in atherosclerotic plaques trigger PRRs expressed by macrophages, including the NOD-like receptors (NLRs), scavenger receptors and TLRs, thereby activating the inflammatory response.
NLRs and inflammasome activation
Cholesterol crystals are present in atherosclerotic plaques in both extracellular spaces and within macrophages. Although previously thought to be a feature of advance plaques, a recent study using combined confocal-reflection microscopy demonstrated the presence of cholesterol crystals in early lesions in Apoe−/− mice 56,57 and showed that macrophage engulfment of cholesterol crystals triggers the NLRP3 inflammasome (Figure 2). Uptake of pre-formed crystals by human and mouse macrophages induces lysosomal destabilization and the release of proteases and/or reactive oxygen species into the cytosol that activate NLRP3, leading to the processing and secretion of the cytokine IL-1β56,58,59,60,61. A study using low-density lipoprotein receptor (Ldlr)−/− mice in which bone marrow cells are deficient in IL-1β or components of the NLRP3 inflammasome complex have shown reduced plaque formation, demonstrating the potential importance of this pathway in atherogenesis56. A subsequent study of Apoe−/− mice with somatic deficiency of Nlrp3 failed to show protection from atherosclerosis62. While the reasons for this discrepancy will need to be investigated, a potential confounding factor may have been the different cholesterol contents of the western diet used in the two studies (0.3% versus 1.25%).
In addition to pre-formed cholesterol crystals, recent work indicates that loading of macrophages with cholesterol can lead to the de novo formation of intracellular cholesterol crystals that trigger NLRP363. CD36 plays a critical role in the accrual and nucleation of cholesterol crystals within macrophages treated with oxidized LDL, as well as the ensuing lysosomal disruption and NLRP3 inflammasome activation63. Consequently, macrophages lacking CD36 failed to elicit IL-1β production in response to oxidized LDL, and targeting CD36 in atherosclerotic mice reduced serum IL-1β levels and plaque cholesterol crystal accumulation. Notably, CD36-mediated uptake of amyloid-forming peptides implicated in Alzheimer’s disease and type 2 diabetes also activates NLRP3, suggesting a common pathway of lysosomal-mediated activation of NLRP3 that occurs from within the cell after new aggregation and transformation of these soluble ligands into their pathogenic form63. Although not yet investigated, other crystalline or amyloid substances in atherosclerotic plaques, such as calcium phosphate crystals or serum amyloid A64, may also represent damage-associated molecular patterns (DAMPs) that could trigger the inflammasome and IL-1β secretion.
TLR signaling
The participation of TLR signaling pathways in the promotion of atherosclerosis is supported by mouse studies in which whole body deletion of Tlr2 or Tlr465–68 or of the adaptor proteins used by these TLRs, including IL-1R-associated kinase 4 (IRAK4)69,70, TNFR-associated factor 6 (TRAF6)71, TIR-domain-containing adaptor protein inducing IFNβ (TRIF)72 and myeloid differentiation primary-response protein 88 (MYD88)65,73, confers protection from atherosclerosis. This has spurred investigations of the endogenous ligands that accumulate during hypercholesterolemia and in plaques that may trigger these microbial sensing pathways in macrophages. Among the candidates proposed, oxidized LDL species have been extensively studied as ligands for both the scavenger receptors and TLRs, and the extent of oxidation influences their recognition by these receptors (Figure 2). For example, minimally oxidized LDL is recognized by CD14–TLR4–MD2 and initiates cytoskeletal rearrangements, as well as tumour necrosis factor (TNF), interleukin-6 (IL-6) and IL-10 production74. Moderately oxidized LDL recognized by CD36 triggers signaling via a heterodimer of TLR4 and TLR6 resulting in nuclear factor-κB (NF-κB) activation and the expression of chemokines that promote monocyte recruitment to atherosclerotic lesions67. Finally, oxidized phospholipids and saturated fatty acids induce cooperative signaling of CD36 and TLR2 that promotes apoptosis in macrophages undergoing prolonged ER stress75. However, in addition to these ligand-initiated signaling pathways, enrichment of macrophage plasma membranes with free cholesterol can also lead to sustained activation of various TLRs, including TLR4 and TLR343,76. Thus, numerous pathways may contribute to triggering and sustaining TLR-induced macrophage inflammation in atherosclerotic plaques.
Macrophage polarization and plasticity in atherosclerosis
One consequence of TLR-dependent activation of monocyte-derived cells entering the plaque may be their polarization to M1-type macrophages. These inflammatory macrophages secrete pro-atherosclerotic cytokines (such as IL-6 and IL-12), as well as reactive oxygen and nitrogen species that would exacerbate oxidative stress in the plaque77 (Box 1). Histological analysis of human plaques showed M1 macrophages to be enriched in lipid and localized to areas distinct from their less inflammatory M2 (alternatively activated) macrophages78. Studies of M1 and M2 macrophages polarized in vitro and in mouse models of atherosclerosis have led to a simplified view that M1 macrophages promote plaque inflammation and M2 macrophages resolve plaque inflammation. However, the phenotypic spectrum of macrophages in vivo is likely to be complex, as macrophages encounter a microenvironment of diverse, and even opposing, signals. For example, in addition to triggering TLR signaling that can lead to M1 polarization, oxidized LDL has also been reported to induce the expression of the M2 macrophage phenotypic marker arginase 1 via activation of peroxisome proliferator activated receptor-γ (PPARγ)79, and oxidized phospholipids present in oxidized LDL induce a macrophage phenotype distinct from M1 or M2 that has been termed Mox, which is characterized by increased expression of nuclear respiratory factor 2 (NRF2)-dependent genes and reactive oxygen species80. It is likely that T helper 1 (Th1) and Th2 cells in plaques secrete macrophage-polarizing factors81 that also contribute to the balance of M1 and M2 macrophages. Nonetheless, the factors in the plaque microenvironment that promote the polarization of these cells in vivo remain incompletely defined.
The recent identification of transcriptional programs that regulate macrophage polarization has provided some insights into the effects of M1 and M2 macrophages on atherogenesis. Whole body or bone marrow-specific deletion of the transcription factor NR4A1 (also known as NUR77), which has been suggested to control the LY6Clow patrolling monocyte phenotype and favor M2 macrophage differentiation82, resulted in increased polarization of macrophages toward an M1 macrophage phenotype and acceleration of atherosclerosis in Apoe−/− and Ldlr−/− mouse83,84, though this result has been inconsistent85. Similarly, targeted deletion of the transcription factor Krüppel-like factor 4 (KLF4), which promotes M2 and inhibits M1 macrophage polarization86, enhanced both pro-inflammatory M1 macrophage activation and foam cell formation, and accelerated atherosclerosis in Apoe−/− mice87. Notably, the expression of macrophage KLF4 is reduced by pro-inflammatory cytokines and oxidized phospholipids found in plaques87, suggesting that the KLF4-driven M2 phenotype may be repressed during atherogenesis , contributing to disease progression when such signals predominate. Indeed, administration of the M2-polarizing cytokine IL-13 to Ldlr−/− mice was shown to drive plaque macrophages to M2-like cells and inhibit atherosclerosis progression88. Moreover, an enrichment of M2 macrophages has been shown in plaques in which regression of atherosclerosis in mice (table 1) is induced by aggressive lipid lowering or raising of HDL levels4,5,89 (discussed further below). Collectively, these studies suggest that pathways that promote M2-polarization of macrophages protect against atherosclerosis.
Table 1.
Selected mouse models of atherosclerosis progression and regression
| Mouse model | Important Features | Lipoprotein profile |
Ref |
|---|---|---|---|
| Progression | |||
| Apoe−/− mice |
|
Intestinally derived remnant particles | |
| Ldlr−/− mice |
|
VLDL; LDL | |
| Regression | |||
| Aortic transplant Mice |
|
||
| REVERSA mice |
|
All revert to near wild-type mouse levels |
|
| Reconstitution of APOE in Apoe−/− |
|
||
The origin of M1 and M2 macrophages in plaques remains an area of open debate. Although it has been suggested that Ly6Chi monocytes are the precursors of M1 macrophages, studies of Apoe−/− mice have shown that M2 macrophages populate early, fatty streak-like lesions, a stage in which Ly6Chi monocytes are thought to be the predominant monocyte subset recruited into plaques. Yet, as plaques progress to more complex, inflammatory lesions, the M1 macrophage phenotype became more frequent90. Further studies are needed to address the origins of M1 and M2 macrophages in atherosclerosis, particularly whether recruitment of LY6Clow monocytes, thought to preferentially become M2 macrophages, predominates in the earliest lesions, whether there is inter-conversion between M1 and M2 macrophage phenotypes in vivo, or whether M2 macrophages are derived from the proliferation of a small population of tissue-resident M2 macrophages, as recently described in other disease models91,92. A better understanding of the regulation of macrophage polarization is likely to offer insights into pathways for potential manipulation of macrophage behavior towards an athero-protective state.
Macrophage retention in and emigration from plaques
The number of macrophages in the plaque is kinetically determined by monocyte recruitment, counterbalanced by the emigration, local proliferation, and death of macrophages. Recruitment factors were discussed above. With regard to local proliferation of monocyte-derived macrophages, this likely occurs in the plaque, as suggested by the assessment of proliferation markers in lesional macrophages and DCs93. Nevertheless, the quantitative importance of macrophage proliferation in atherosclerosis progression remains to be determined, but is likely to be variable in different stages of disease.
Macrophage emigration has been shown to occur in early atherosclerotic plaques, however the rate of macrophage egress was reported to decrease with atherosclerosis progression94 (Figure 3). It is likely that plaque macrophages are subject to both retention and emigration signals, and the balance of these forces contributing to plaque macrophage net accumulation. These signals are only beginning to be defined. Cholesterol loading of macrophages has been shown to increase expression of the neuro-immune guidance cues netrin 1 and semaphorin 3E, which both function to induce macrophage chemostasis in vitro25,26. Macrophage expression of these migration inhibitory molecules are also induced during hypoxia, which is intimately linked to atherosclerosis26,95, including in mice96, and has become recognized as a primary impetus of plaque inflammation. Studies of Ldlr−/− mice with bone marrow deficiency of netrin 1 demonstrated reduced atherosclerosis progression and increased macrophage emigration from lesions, suggesting that netrin 1 may function to retain macrophages in plaques25. Similar experiments with mice lacking semaphorin 3E in macrophages will be needed to extend these findings, and are in progress. Other factors that inhibit cell movement (such as adhesion molecules97) or resolution of inflammation are also likely contribute to the retention of macrophages in the plaque, and studies comparing mouse models of atherosclerosis progression and regression are beginning to elucidate these signals (see below).
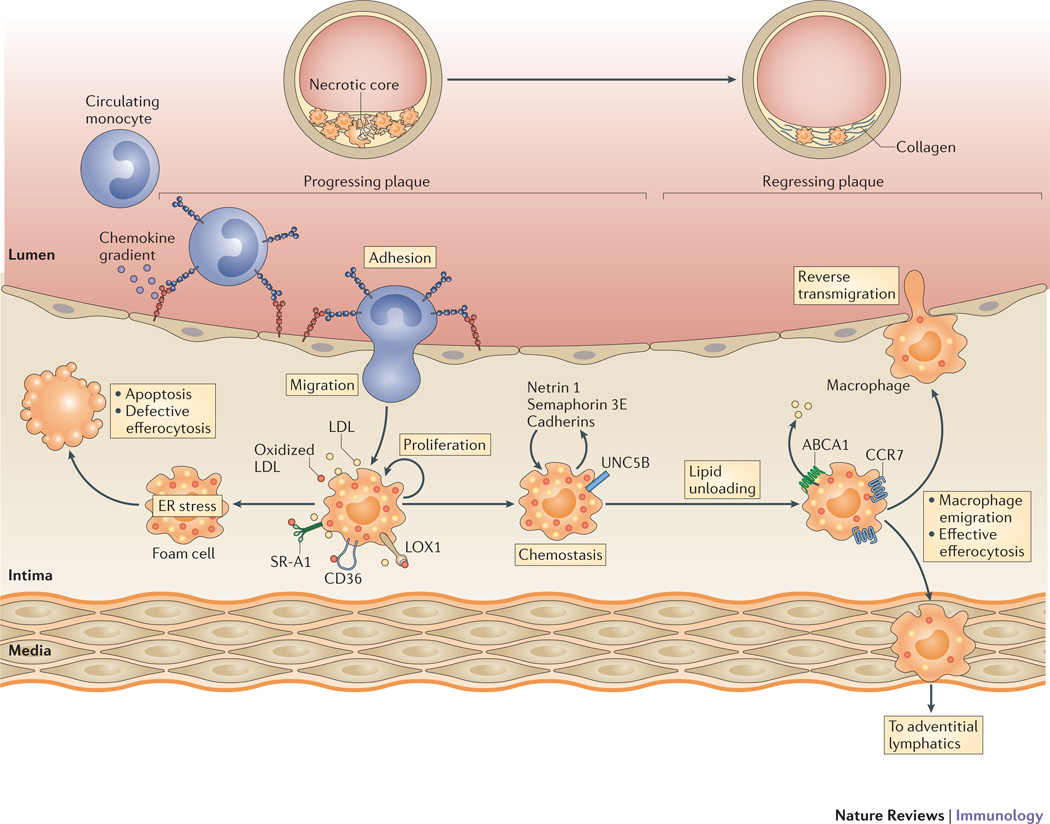
Figure 3. Pathways regulating macrophage retention and emigration in plaques.
Imbalances in macrophage lipid metabolism in the progressing plaque lead to the retention of macrophages and chronic inflammation. The accumulating lipid-laden macrophages express retention molecules (such as netrin 1 and its receptor Unc5b, semaphorin 3E and cadherins) that promote macrophage chemostasis. In this inflammatory milieu, these accumulating macrophages experience endoplasmic reticulum (ER) stress, which if prolonged, results in apoptosis. This cell death, coupled with defective efferocytosis, results in the formation of the necrotic core characteristic of advance plaques. Mechanisms that promote lipid unloading of the foam cell, including factors that upregulate ABCA1 expression on plaque macrophages and cholesterol efflux, reverse the accumulation of these foam cells. This plaque regression is characterized by an upregulation of CC-chemokine receptor 7 (CCR7) on myeloid-derived cells and a decrease in the expression of retention factors. Accumulating evidence summarized in this review supports that the regulation of these macrophage migration factors contributes to macrophage emigration from the plaque through reverse transmigration to the lumen or trafficking to the adventitial lymphatics.
The signals that guide macrophages to exit from plaques, either by reverse transmigration through the endothelium to the lumen or by migrating through the media to the adventitial lymphatics, remain poorly defined. In studies in which macrophage emigration from plaques was induced by normalizing the hyperlipidemic plasma profile of mice in an aortic transplant model, the cells that emigrated expressed several markers that are characteristic of both macrophages and DCs98. For example, the expression of CCR7, which is the receptor for the chemokines CCL19 and CCL21 that regulate DC homing to lymph nodes, is upregulated in the emigrating CD68+ cells and blocking this pathway led to their substantial retention in the plaque98. Further studies are needed to define other such factors in this and other models of regression.
Turning to apoptosis, the continued presence of macrophage foam cells in the inflammatory, lipid-rich environment of the plaque can eventually lead to cytotoxicity from ER and oxidative stress1. Activation of ER stress responses occurs as a result of free cholesterol accumulation in macrophages and by saturated fatty acids signaling via SRA, TLR2 and TLR475. Prolonged ER stress leads to macrophage apoptosis, which is observed in 2–4% of cells in mouse plaques, with the highest levels in advanced plaques. In these late-stage plaques, the ability of macrophages to clear their dying counterparts through such receptors as tyrosine protein kinase MER (MERTK) and low-density lipoprotein receptor-related protein 1 (LRP1) becomes compromised, and this has been attributed in part to cholesterol accumulation in the engulfing cells99. This defective efferocytosis contributes to secondary necrosis and the formation and expansion of the lipid cores, which in turn contribute to plaque vulnerability to rupture46. It is possible, then, that apoptosis, especially in the context of efficient efferocytosis, also contributes to net changes in macrophage or foam cell content, as suggested in a recent study of regression100, though a mathematical analysis of those data suggest that a rate 10-fold higher than usual would be required for the changes observed (S. Russell and E. A. F., unpublished observations). In summary, monocyte recruitment and cell retention, emigration, and death are all potential kinetic contributors to the net plaque contents of macrophages and foam cells. The quantitative impact of each of these processes will likely vary in the stages of disease and in different models of progression and regression, as well as in co-morbid states, such as insulin resistance/diabetes and chronic kidney disease.
Lessons learnt from models of atherosclerosis regression
The historical focus on atherosclerosis in both human and animal studies has been on its progression, with the prevailing view that except for early lesions dominated by foam cells (“fatty streaks”), atherosclerosis was essentially irreversible, though the mechanisms by which even an immature plaque regressed remained undefined. More recent discoveries, including finding that macrophages can emigrate from plaques in some animal models and that tissue-remodeling M2 macrophages are present in human and animal plaques, there is optimism that clinical atherosclerosis regression can be achieved. Nevertheless, understanding the biology of atherosclerosis regression, and the discovery of therapeutic targets to achieve it, require robust pre-clinical models. Toward this end, several mouse models of atherosclerosis, such as Apoe−/− and Ldlr−/− mice, have been adapted for studies of regression (Table I). Common to all models has been the demonstration that in the regressing plaque there is a decline in the number of macrophages, and in some, a change in their phenotypic characteristics, with an enrichment in M2 macrophage characteristics (e.g. 4–6,89,101–104), suggesting that this is a common signature of regressing plaques.
Transcriptomic profiling of macrophages isolated by laser capture microdissection98 of progressing and regressing plaques in an aortic transplantation mouse model revealed >700 differentially regulated genes97, including the recently described macrophage retention factors semaphorin 3E and netrin 1. Other genes that are downregulated in macrophages of regressing plaques include adhesion molecules, such as members of the cadherin family97. In contrast, cellular motility factors were upregulated. In addition, CCR7 was expressed at low levels in plaque macrophages likely suppressed by hypercholesterolemia due to an SRE element in its promoter105. Notably, the transcription of Ccr7 was upregulated in macrophages when plaques were placed in a regression environment, thereby increasing the migratory capacity of the cells. Together, the transcriptomic data from the aortic transplantation model indicate that the emigration of macrophages from plaques is a highly regulated process, reflecting coordinated changes in macrophage retention and movement. Transcriptome analyses from other models of regression will be needed to determine how conserved these changes are.
Therapeutic targeting of plaque macrophages
Therapies that alter macrophage content by reducing macrophage recruitment to atherosclerotic plaques, or by promoting macrophage apoptosis, efferocytosis or emigration have been proposed to have beneficial clinical effects on plaques. However, the quantitative impact of each of these processes on disease progression likely depends on the stage of disease. For example, macrophage recruitment dominates over emigration in disease progression, whereas macrophage emigration is increased in several models of regression, and the low level of macrophage apoptosis that is seen in early atherosclerosis (typically ∼2–4% of cells) increases as plaques become more complex, with secondary necrosis also becoming prominent as efferocytosis of apoptotic cells falters1. As also seen in models of progression and regression (for exmaple 5,90,97), the inflammatory phenotype of the macrophages (using the simplified scheme of M1 versus M2 macrophages) is not fixed, most likely reflecting the well-known plasticity of monocyte-derived cells in response to microenvironmental changes. Therapies that alter macrophage inflammation by increasing polarization to an M2 macrophage phenotype, efferocytosis, or macrophage emigration would be predicted to be beneficial based on the pre-clinical models.
The fact that new clinical targets are needed is obvious from the failure of conventional risk factor management to effectively eliminate the risk of cardiovascular disease, with well over half of patients in controlled trials having heart attacks or strokes despite aggressive treatments. A recent example of the discovery of a potential target from mouse studies is our demonstration that neuronal guidance molecules serve as macrophage retention factors in plaque progression, with their expression in macrophages falling in regressing plaques 25,26,97. Thus, it may be desirable to selectively deliver small interfering RNAs (siRNAs) or other therapeutics directed against these and other factors that facilitate emigration of macrophages. There is considerable optimism that the goal of the specific targeting of agents to modify any of the factors discussed above in plaques is achievable, based on recent studies using nanoparticles, including reconstituted lipoproteins, to deliver siRNAs, imaging agents and small molecules to plaques (e.g., 106–108).
One obvious impediment in treating macrophages in plaques is that targeting a specific process, such as monocyte recruitment, may be advantageous locally, but not desirable systemically. In addition, even if monocyte recruitment to plaques could be specifically blocked, the timing of inhibition may be crucial. For example, blocking monocyte recruitment via CCR2 may be an effective strategy in atherosclerosis progression, but recent studies (Y. Vengrenyuk and E.A.F., unpublished observations) suggest that the shift in phenotypic balance to the M2 macrophage phenotype during atherosclerosis regression in the aortic transplant model requires the recruitment of LY6Chi monocytes via CCR2, similar to what has been observed in autoimmune encephalomyelitis and allergic skin reactions109,110. Thus, inhibition of CCR2 may impair atherosclerosis regression. Similarly, timing may also be an issue for targeting other chemokine receptors, such as CX3CR1 and CCR5, which together with CCR2 control over 90% of monocyte entry into progressing plaques 21.
The timing of strategies that therapeutically target macrophage death is also an important issue — it is expected that in early plaque development, where efferocytosis is efficient, increasing apoptosis would be beneficial. In contrast, efferocytosis is impaired in more complex plaques, which is the type relevant to clinical events, and therefore increasing apoptosis would lead to augmented releases of macrophage lipid content and tissue factor that would expand the necrotic core and enhance its thrombogenicity. Current efforts are focused on maintaining levels of efferocytosis throughout plaque progression through the use of agents such as IL-10 or LXR agonists, which also have additional plaque benefits, such as reducing inflammation (IL-10 and LXR agonists) or promoting cholesterol efflux (LXR agonists). In addition to reducing plaque lipid content, increased lipid efflux would be expected to favorably affect the inflammatory state of macrophages43,76 and their ability to emigrate5. In addition to LXR agonists, increasing autophagy51–53,111 or ABCA1 and ABCG1 expression levels by inhibiting the microRNA miR-3389, may help to target macrophage cholesterol efflux.
Another therapeutic strategy would be to favorably modify the inflammatory state of plaque macrophages. One approach would be to polarize macrophages to the M2 phenotype, as these cells may be particularly important in the regressing atherosclerotic plaque by: one, secreting anti-inflammatory factors and promoting tissue remodeling and repair through the induction of collagen formation and the clearance of dying cells and debris; two, secreting potent anti-inflammatory factors such as IL-10 and reducing the production of damaging reactive nitrogen species; and three, expressing high levels of MERTK and thereby increasing efferocytosis of dying macrophages112,113. Thus, promoting the M2 macrophage phenotype in plaques would be expected to be beneficial in both atherosclerosis progression and regression, consistent with recent studies showing that Ldlr−/− mice treated with IL-13 were protected from atherosclerosis88 and that M2 macrophages are required for disease regression in the aortic transplant model (Y. Vengrenyuk and E.A. F., unpublished observations). The manipulation of other factors that inhibit M1 macrophage polarization may be similarly successful in progression or regression settings.
Conclusions and future perspective
Macrophages are the central cells in atherosclerosis, with the quantity and phenotype of these cells in plaques influencing both disease progression and regression. Both aspects of the disease are dynamic processes representing a confluence of diverse metabolic and inflammatory pathways, working in part through the entry of monocytes to plaques and the retention, emigration, and death of lesional macrophages. Important areas of future investigation include the regulation and quantitative impact of each of these kinetic factors, the effects of other immune cells in the atheroma on the properties of macrophages, and the therapeutic manipulation of existing and newly discovered factors affecting the lipid content of macrophages, their number, and inflammatory phenotype. Despite the necessity of performing mechanistic studies in preclinical models, it will be important to relate these findings to human pathophysiology. This is starting to become a possibility, in part by mining human genetic studies (such as genome wide association studies) and various “omic” characterizations of human tissues. A remaining challenge will be to use the present and ongoing research to design clinical interventions that reduce the unacceptably high risk of cardiovascular disease.
Key points.
Macrophages are key integrators of inflammatory and metabolic signals in atherosclerotic plaques.
The macrophage content of the plaque and their activation state changes during both the progression and regression of atherosclerosis.
The macrophage content of the plaque represents the kinetic balance between the recruitment of blood monocytes, their differentiation into tissue macrophages and proliferation in situ, and their emigration or death.
The lipid content of macrophages promotes innate immune responses and inflammation by both increasing the sensitivity of Toll-like receptors to their ligands and activating the NLRP3 inflammasome.
The study of new mouse models of atherosclerosis regression has established the reversibility of macrophage accumulation and activation in plaques, challenging the long-held belief that failing to resolve chronic inflammation is an inevitable feature of atherosclerosis.
Acknowledgements
Work in the authors’ laboratories related to this review are supported by the National Institutes of Health (R01 HL084312 and R01 HL098055 to EAF; R01 R01HL117334 and R01HL108182 to KJM).
Glossary
- Foam cell
A macrophage in the arterial wall that ingests oxidized low-density lipoprotein and assumes a foamy appearance. These cells secrete various substances involved in plaque growth
- Myocardial infarction
An episode of acute cardiac ischaemia that leads to death of heart muscle cells. It is usually caused by a thrombotic atherosclerotic plaque
- Atherosclerosis regression
A decrease in atherosclerotic plaque size that is typically accompanied by a reduction in lipid levels, immune cells and inflammatory gene expression
- M1 macrophage
A macrophage that is activated by Toll-like receptor ligands (such as lipopolysaccharide) and interferon-γ and that expresses, among others, inducible nitric oxide synthase and nitric oxide
- ATP-binding cassette (ABC) transporter family
A family of proteins that transport various molecules across extracellular and intracellular membranes by coupling ATP hydrolysis to the transport. Eukaryotic ABC genes are classified in seven families, from ABCA to ABCG, based on gene organization and primary sequence homology. Functional characterization can be made, in part, by differential sensitivity to inhibitory drugs
- Leukocyte adhesion cascade
The key steps are involved in leukocyte adhesion to the endothelium: rolling, which is mediated by selectins, activation, which is mediated by chemokines, and arrest, which is mediated by integrins. Recent additional steps have been defined: capture (or tethering), slow rolling, adhesion strengthening and spreading, intravascular crawling, and paracellular and transcellular transmigration
- Firm adhesion
The interactions of rolling leukocytes with chemokines or lipid mediators, such as leukotriene B4, at the endothelial surface leads to the activation of leukocyte integrins — another family of adhesion molecules. When activated. integrins mediate high-affinity adhesive interactions between leukocytes and endothelial cells, resulting in the arrest and firm adhesion of rolling leukocytes
- M2 macrophage
A macrophage that is stimulated by interleukin-4 (IL-4) or IL-13 and that expresses arginase-1, the mannose receptor CD206 and the IL-4 receptor α-chain
- Pattern recognition receptor
(PRR). A host receptor (such as Toll-like receptors) that can sense pathogen-associated or damage-associated molecular patterns and initiate signalling cascades (which involve activation of nuclear factor-κB) that lead to an innate immune response
- Pinocytosis
Also known as fluid-phase endocytosis. A process of engulfment of extracellular fluid and its solutes. It can be mediated by an actin-dependent mecha nism that can engulf large volumes (macropinocytosis) or by other mechanisms that result in engulfment of smaller volumes (micropinocytosis)
- Autophagy
An evolutionary conserved process in which acidic double-membrane vacuoles sequester intracellular contents (such as damaged organelles and macromolecules) and target them for degradation, through fusion to secondary lysosomes
- Efferocytosis
The process of macrophage clearance of apoptotic cells
- NLRP3 inflammasome
A molecular complex containing NLRP3 and the adaptor molecule ASC that controls the activity of caspase 1. Formation of this complex results in the cleavage of the highly pro-inflammatory cytokines pro-IL-1β and pro-IL-18, thereby producing active IL-1β and IL-18
- MicroRNAs
Single-stranded RNA molecules of approximately 21–23 nucleotides in length that regulate the expression of other genes
Biographies
Author biographies
Kathryn J Moore received her Ph.D. from McGill University in Montreal, Quebec, Canada, and her postdoctoral training from Harvard Medical School in Boston, USA. After serving as an assistant professor at Harvard Medical School, USA, she joined the New York University (NYU) School of Medicine, where she is currently a Professor in the Departments of Medicine and Cell Biology, and a member of the Marc and Ruti Bell Program in Vascular Biology. Dr. Moore’s research focuses on the mechanisms of innate immune activation and macrophage retention in atherosclerosis, as well as the pathways that regulate cholesterol homeostasis.
Frederick J Sheedy received his PhD from Trinity College, Dublin, Ireland, under the supervision of Prof. Luke A.J. O’Neill. His graduate work examined the impact of TLR activation on miRNA expression in DCs and macrophages. He is currently pursuing postdoctoral training on the control of inflammation in atherosclerosis in the Moore laboratory at New York University School of Medicine, New York, USA.
Edward A Fisher received his MD degree from the NYU School of Medicine and his PhD from MIT. He did clinical training at Duke and Harvard, and completed a post-doctoral fellowship at the NIH. In 2003, he joined the NYU School of Medicine where he is the Leon H. Charney Profesor of Cardiovascular Medicine and Professor of Cell Biology. He also directs the Marc and Ruti Bell Program in Vascular Biology and the Center for the Prevention and Treatment of Cardiovascular Disease. Dr. Fisher’s research focuses on the cell biology of apolipoprotein B (the major protein component of the atherogenic lipoproteins) and on atherosclerosis, especially its regression and non-invasive imaging.
References
- 1.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Randolph GJ. Emigration of monocyte-derived cells to lymph nodes during resolution of inflammation and its failure in atherosclerosis. Curr Opin Lipidol. 2008;19:462–468. doi: 10.1097/MOL.0b013e32830d5f09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140:871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 4.Feig JE, et al. Reversal of hyperlipidemia with a genetic switch favorably affects the content and inflammatory state of macrophages in atherosclerotic plaques. Circulation. 2011;123:989–998. doi: 10.1161/CIRCULATIONAHA.110.984146. • This study describes the use of REVERSA mice as a model of atherosclerosis regression.
- 5.Feig JE, et al. HDL promotes rapid atherosclerosis regression in mice and alters inflammatory properties of plaque monocyte-derived cells. Proc Natl Acad Sci U S A. 2011;108:7166–7171. doi: 10.1073/pnas.1016086108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Llodra J, et al. Emigration of monocyte-derived cells from atherosclerotic lesions characterizes regressive, but not progressive, plaques. Proc Natl Acad Sci U S A. 2004;101:11779–11784. doi: 10.1073/pnas.0403259101. • This study demonstrated the emigration of CD68+ cells from regressing atherosclerotic plaques.
- 7.Averill LE, Meagher RC, Gerrity RG. Enhanced monocyte progenitor cell proliferation in bone marrow of hyperlipemic swine. Am J Pathol. 1989;135:369–377. [PMC free article] [PubMed] [Google Scholar]
- 8.Feldman DL, Mogelesky TC, Liptak BF, Gerrity RG. Leukocytosis in rabbits with diet-induced atherosclerosis. Arterioscler Thromb. 1991;11:985–994. doi: 10.1161/01.atv.11.4.985. [DOI] [PubMed] [Google Scholar]
- 9.Swirski FK, et al. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. • This study demonstrated that Apoe−/− mice have a monocytosis that is due to an increase in the LY6Chi monocyte population.
- 10.Tacke F, et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–194. doi: 10.1172/JCI28549. • This study demonstrated that Apoe−/− mice have a monocytosis and describes the chemokine receptors that contribute to monocyte recruitment in progressing plaques.
- 11.Yvan-Charvet L, et al. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science. 2010;328:1689–1693. doi: 10.1126/science.1189731. • This study established the essential role of cellular cholesterol efflux in suppressing haematopoietic stem cell proliferation.
- 12.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–1126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 13.Tabas I, Williams KJ, Boren J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 2007;116:1832–1844. doi: 10.1161/CIRCULATIONAHA.106.676890. [DOI] [PubMed] [Google Scholar]
- 14.Glass CK, Witztum JL. Atherosclerosis the road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 15.Paulson KE, et al. Resident intimal dendritic cells accumulate lipid and contribute to the initiation of atherosclerosis. Circ Res. 2010;106:383–390. doi: 10.1161/CIRCRESAHA.109.210781. [DOI] [PubMed] [Google Scholar]
- 16.Dutta P, et al. Myocardial infarction accelerates atherosclerosis. Nature. 2012;487:325–329. doi: 10.1038/nature11260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 18.Soehnlein O, et al. Distinct functions of chemokine receptor axes in the atherogenic mobilization and recruitment of classical monocytes. EMBO Mol Med. 2013;5:471–481. doi: 10.1002/emmm.201201717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woollard KJ, Geissmann F. Monocytes in atherosclerosis: subsets and functions. Nat Rev Cardiol. 2010;7:77–86. doi: 10.1038/nrcardio.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weber C, Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nat Med. 2011;17:1410–1422. doi: 10.1038/nm.2538. [DOI] [PubMed] [Google Scholar]
- 21.Combadiere C, et al. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6C(hi) and Ly6C(lo) monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation. 2008;117:1649–1657. doi: 10.1161/CIRCULATIONAHA.107.745091. [DOI] [PubMed] [Google Scholar]
- 22.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- 23.Landsman L, et al. CX3CR1 is required for monocyte homeostasis and atherogenesis by promoting cell survival. Blood. 2009;113:963–972. doi: 10.1182/blood-2008-07-170787. [DOI] [PubMed] [Google Scholar]
- 24.van Gils JM, et al. Endothelial expression of guidance cues in vessel wall homeostasis dysregulation under proatherosclerotic conditions. Arterioscler Thromb Vasc Biol. 2013;33:911–919. doi: 10.1161/ATVBAHA.112.301155. • This study demonstrated that neuronal guidance molecules are differentially expressed on endothelium in athero-prone and athero-protected regions of the vasculature.
- 25.van Gils JM, et al. The neuroimmune guidance cue netrin-1 promotes atherosclerosis by inhibiting the emigration of macrophages from plaques. Nat Immunol. 2012;13:136–143. doi: 10.1038/ni.2205. • This work identifies netrin 1 as a retention signal that blocks macrophage egress from inflamed vessel walls in the presence of hypercholesterolemia, leading to chronic vessel-wall inflammation and plaque progression.
- 26.Wanschel A, et al. Neuroimmune Guidance Cue Semaphorin 3E Is Expressed in Atherosclerotic Plaques and Regulates Macrophage Retention. Arterioscler Thromb Vasc Biol. 2013 doi: 10.1161/ATVBAHA.112.300941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moore KJ, Freeman MW. Scavenger receptors in atherosclerosis: beyond lipid uptake. Arterioscler Thromb Vasc Biol. 2006;26:1702–1711. doi: 10.1161/01.ATV.0000229218.97976.43. [DOI] [PubMed] [Google Scholar]
- 28.Miller YI, et al. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ Res. 2011;108:235–248. doi: 10.1161/CIRCRESAHA.110.223875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kunjathoor VV, et al. Scavenger receptors class A-I/II and CD36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J Biol Chem. 2002;277:49982–49988. doi: 10.1074/jbc.M209649200. [DOI] [PubMed] [Google Scholar]
- 30.Podrez EA, Schmitt D, Hoff HF, Hazen SL. Myeloperoxidase-generated reactive nitrogen species convert LDL into an atherogenic form in vitro. J Clin Invest. 1999;103:1547–1560. doi: 10.1172/JCI5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kzhyshkowska J, Neyen C, Gordon S. Role of macrophage scavenger receptors in atherosclerosis. Immunobiology. 2012;217:492–502. doi: 10.1016/j.imbio.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 32.Maxfield FR, Tabas I. Role of cholesterol and lipid organization in disease. Nature. 2005;438:612–621. doi: 10.1038/nature04399. [DOI] [PubMed] [Google Scholar]
- 33.Kuchibhotla S, et al. Absence of CD36 protects against atherosclerosis in ApoE knock-out mice with no additional protection provided by absence of scavenger receptor A I/II. Cardiovasc Res. 2008;78:185–196. doi: 10.1093/cvr/cvm093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manning-Tobin JJ, et al. Loss of SR-A and CD36 activity reduces atherosclerotic lesion complexity without abrogating foam cell formation in hyperlipidemic mice. Arterioscler Thromb Vasc Biol. 2009;29:19–26. doi: 10.1161/ATVBAHA.108.176644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tardif JC. Antioxidants: the good, the bad and the ugly. Can J Cardiol. 2006;(22 Suppl B):61B–65B. doi: 10.1016/s0828-282x(06)70988-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boyanovsky BB, van der Westhuyzen DR, Webb NR. Group V secretory phospholipase A2-modified low density lipoprotein promotes foam cell formation by a SR-A- and CD36-independent process that involves cellular proteoglycans. J Biol Chem. 2005;280:32746–32752. doi: 10.1074/jbc.M502067200. [DOI] [PubMed] [Google Scholar]
- 37.Oorni K, Kovanen PT. Lipoprotein modification by secretory phospholipase A(2) enzymes contributes to the initiation and progression of atherosclerosis. Curr Opin Lipidol. 2009;20:421–427. doi: 10.1097/MOL.0b013e32832fa14d. [DOI] [PubMed] [Google Scholar]
- 38.Lind L, et al. Circulating levels of secretory- and lipoprotein-associated phospholipase A2 activities: relation to atherosclerotic plaques and future all-cause mortality. Eur Heart J. 2012;33:2946–2954. doi: 10.1093/eurheartj/ehs132. [DOI] [PubMed] [Google Scholar]
- 39.Kugiyama K, et al. Circulating levels of secretory type II phospholipase A(2) predict coronary events in patients with coronary artery disease. Circulation. 1999;100:1280–1284. doi: 10.1161/01.cir.100.12.1280. [DOI] [PubMed] [Google Scholar]
- 40.Kruth HS. Receptor-independent fluid-phase pinocytosis mechanisms for induction of foam cell formation with native low-density lipoprotein particles. Curr Opin Lipidol. 2011;22:386–393. doi: 10.1097/MOL.0b013e32834adadb. • Discussion of studies that established that native LDL can contribute to foam cell formation through its uptake by macrophage fluid-phase pinocytosis.
- 41.Zhu X, et al. Macrophage ABCA1 reduces MyD88-dependent Toll-like receptor trafficking to lipid rafts by reduction of lipid raft cholesterol. J Lipid Res. 2010;51:3196–3206. doi: 10.1194/jlr.M006486. • This study demonstrated that cholesterol enrichment of lipid rafts promotes signaling via TLRs.
- 42.Mogilenko DA, et al. Endogenous apolipoprotein A-I stabilizes ATP-binding cassette transporter A1 and modulates Toll-like receptor 4 signaling in human macrophages. FASEB J. 2012;26:2019–2030. doi: 10.1096/fj.11-193946. [DOI] [PubMed] [Google Scholar]
- 43.Yvan-Charvet L, et al. Increased inflammatory gene expression in ABC transporter-deficient macrophages: free cholesterol accumulation, increased signaling via toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation. 2008;118:1837–1847. doi: 10.1161/CIRCULATIONAHA.108.793869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jerome WG. Advanced atherosclerotic foam cell formation has features of an acquired lysosomal storage disorder. Rejuvenation Res. 2006;9:245–255. doi: 10.1089/rej.2006.9.245. [DOI] [PubMed] [Google Scholar]
- 45.Feng B, et al. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat Cell Biol. 2003;5:781–792. doi: 10.1038/ncb1035. [DOI] [PubMed] [Google Scholar]
- 46.Tabas I. Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis: the importance of lesion stage and phagocytic efficiency. Arterioscler Thromb Vasc Biol. 2005;25:2255–2264. doi: 10.1161/01.ATV.0000184783.04864.9f. [DOI] [PubMed] [Google Scholar]
- 47.Yvan-Charvet L, Wang N, Tall AR. Role of HDL, ABCA1, and ABCG1 transporters in cholesterol efflux and immune responses. Arterioscler Thromb Vasc Biol. 2010;30:139–143. doi: 10.1161/ATVBAHA.108.179283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spann NJ, et al. Regulated accumulation of desmosterol integrates macrophage lipid metabolism and inflammatory responses. Cell. 2012;151:138–152. doi: 10.1016/j.cell.2012.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Calkin AC, Tontonoz P. Transcriptional integration of metabolism by the nuclear sterol-activated receptors LXR and FXR. Nat Rev Mol Cell Biol. 2012;13:213–224. doi: 10.1038/nrm3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singh R, et al. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. doi: 10.1038/nature07976. • This study was the first to demonstrate a role for autophagy in regulating lipid metabolism.
- 51.Ouimet M, et al. Autophagy regulates cholesterol efflux from macrophage foam cells via lysosomal acid lipase. Cell Metab. 2011;13:655–667. doi: 10.1016/j.cmet.2011.03.023. • This study demonstrated that autophagy regulates cholesterol efflux in macrophage foam cells.
- 52.Liao X, et al. Macrophage autophagy plays a protective role in advanced atherosclerosis. Cell Metab. 2012;15:545–553. doi: 10.1016/j.cmet.2012.01.022. • This study uncovered a protective role for the autophagy process in atherosclerosis through regulation of plaque necrosis.
- 53.Razani B, et al. Autophagy links inflammasomes to atherosclerotic progression. Cell Metab. 2012;15:534–544. doi: 10.1016/j.cmet.2012.02.011. • This study uncovered a protective role for the autophagy process in atherosclerosis through regulation of inflammasome activation.
- 54.Nakahira K, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12:222–230. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saitoh T, et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature. 2008;456:264–268. doi: 10.1038/nature07383. [DOI] [PubMed] [Google Scholar]
- 56.Duewell P, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. • This study described a role for the NLRP3 inflammasome in atherogenesis thereby uncovering a previously unappreciated role of cholesterol crystals as key early initiators of vascular inflammation.
- 57.Lim RS, et al. Identification of cholesterol crystals in plaques of atherosclerotic mice using hyperspectral CARS imaging. J Lipid Res. 2011;52:2177–2186. doi: 10.1194/jlr.M018077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gage J, Hasu M, Thabet M, Whitman SC. Caspase-1 deficiency decreases atherosclerosis in apolipoprotein E-null mice. Can J Cardiol. 2012;28:222–229. doi: 10.1016/j.cjca.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 59.Usui F, et al. Critical Role of Caspase-1 in Vascular Inflammation and Development of Atherosclerosis in Western Diet-fed Apolipoprotein E-deficient Mice. Biochem Biophys Res Commun. 2012;425:162–168. doi: 10.1016/j.bbrc.2012.07.058. [DOI] [PubMed] [Google Scholar]
- 60.Hornung V, et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Freigang S, et al. Nrf2 is essential for cholesterol crystal-induced inflammasome activation and exacerbation of atherosclerosis. Eur J Immunol. 2011;41:2040–2051. doi: 10.1002/eji.201041316. [DOI] [PubMed] [Google Scholar]
- 62.Menu P, et al. Atherosclerosis in ApoE-deficient mice progresses independently of the NLRP3 inflammasome. Cell Death Dis. 2011;2:e137. doi: 10.1038/cddis.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sheedy FJ, et al. CD36 coordinates activation of the NLRP3 inflammasome by facilitating the intracellular nucleation of soluble to particulate ligands in sterile inflammation. Nature Immunology. 2013;14:812–820. doi: 10.1038/ni.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Niemi K, et al. Serum amyloid A activates the NLRP3 inflammasome via P2×7 receptor and a cathepsin B-sensitive pathway. J Immunol. 2011;186:6119–6128. doi: 10.4049/jimmunol.1002843. [DOI] [PubMed] [Google Scholar]
- 65.Michelsen KS, et al. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci U S A. 2004;101:10679–10684. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mullick AE, Tobias PS, Curtiss LK. Modulation of atherosclerosis in mice by Toll-like receptor 2. J Clin Invest. 2005;115:3149–3156. doi: 10.1172/JCI25482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stewart CR, et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010;11:155–161. doi: 10.1038/ni.1836. • This study identifies a new TLR heterodimer, TLR4–TLR6, that is triggered by oxidized LDL through CD36 and promotes proinflammatory signaling in macrophages.
- 68.Ding Y, et al. Toll-like receptor 4 deficiency decreases atherosclerosis but does not protect against inflammation in obese low-density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2012;32:1596–1604. doi: 10.1161/ATVBAHA.112.249847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim TW, et al. The critical role of IL-1 receptor-associated kinase 4-mediated NF-kappaB activation in modified low-density lipoprotein-induced inflammatory gene expression and atherosclerosis. J Immunol. 2011;186:2871–2880. doi: 10.4049/jimmunol.1002242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rekhter M, et al. Genetic ablation of IRAK4 kinase activity inhibits vascular lesion formation. Biochem Biophys Res Commun. 2008;367:642–648. doi: 10.1016/j.bbrc.2007.12.186. [DOI] [PubMed] [Google Scholar]
- 71.Lutgens E, et al. Deficient CD40-TRAF6 signaling in leukocytes prevents atherosclerosis by skewing the immune response toward an antiinflammatory profile. J Exp Med. 2010;207:391–404. doi: 10.1084/jem.20091293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Richards MR, et al. The LPS2 mutation in TRIF is atheroprotective in hyperlipidemic low density lipoprotein receptor knockout mice. Innate Immun. 2013;19:20–29. doi: 10.1177/1753425912447130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bjorkbacka H, et al. Reduced atherosclerosis in MyD88-null mice links elevated serum cholesterol levels to activation of innate immunity signaling pathways. Nat Med. 2004;10:416–421. doi: 10.1038/nm1008. [DOI] [PubMed] [Google Scholar]
- 74.Bae YS, et al. Macrophages generate reactive oxygen species in response to minimally oxidized low-density lipoprotein: toll-like receptor 4- and spleen tyrosine kinase-dependent activation of NADPH oxidase 2. Circ Res. 2009;104:210–218. doi: 10.1161/CIRCRESAHA.108.181040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Seimon TA, et al. Atherogenic lipids and lipoproteins trigger CD36-TLR2-dependent apoptosis in macrophages undergoing endoplasmic reticulum stress. Cell Metab. 2010;12:467–482. doi: 10.1016/j.cmet.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhu X, et al. Increased cellular free cholesterol in macrophage-specific Abca1 knock-out mice enhances pro-inflammatory response of macrophages. J Biol Chem. 2008;283:22930–22941. doi: 10.1074/jbc.M801408200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Adamson S, Leitinger N. Phenotypic modulation of macrophages in response to plaque lipids. Curr Opin Lipidol. 2011;22:335–342. doi: 10.1097/MOL.0b013e32834a97e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chinetti-Gbaguidi G, et al. Human atherosclerotic plaque alternative macrophages display low cholesterol handling but high phagocytosis because of distinct activities of the PPARgamma and LXRalpha pathways. Circ Res. 2011;108:985–995. doi: 10.1161/CIRCRESAHA.110.233775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gallardo-Soler A, et al. Arginase I induction by modified lipoproteins in macrophages: a peroxisome proliferator-activated receptor-gamma/delta-mediated effect that links lipid metabolism and immunity. Mol Endocrinol. 2008;22:1394–1402. doi: 10.1210/me.2007-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kadl A, et al. Identification of a novel macrophage phenotype that develops in response to atherogenic phospholipids via Nrf2. Circ Res. 2010;107:737–746. doi: 10.1161/CIRCRESAHA.109.215715. • This study identified a new macrophage phenotype called “Mox” that occurs in macrophages exposed to atherogenic phospholipids.
- 81.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12:204–212. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 82.Hanna RN, et al. The transcription factor NR4A1 (Nur77) controls bone marrow differentiation and the survival of Ly6C- monocytes. Nat Immunol. 2011;12:778–785. doi: 10.1038/ni.2063. • This study identifies an important role for Nur77/NR4A1 in macrophage M2 polarization.
- 83.Hanna RN, et al. NR4A1 (Nur77) deletion polarizes macrophages toward an inflammatory phenotype and increases atherosclerosis. Circ Res. 2012;110:416–427. doi: 10.1161/CIRCRESAHA.111.253377. • This study reported that deletion of NUR77 (also known as NR4A1) worsens atherosclerosis by increasing macrophage polarization to the M1 phenotype.
- 84.Hamers AA, et al. Bone marrow-specific deficiency of nuclear receptor Nur77 enhances atherosclerosis. Circ Res. 2012;110:428–438. doi: 10.1161/CIRCRESAHA.111.260760. • This study reported that deletion of NUR77 worsens atherosclerosis and inflammation.
- 85.Chao LC, et al. Bone marrow NR4A expression is not a dominant factor in the development of atherosclerosis or macrophage polarization in mice. J Lipid Res. 2013;54:806–815. doi: 10.1194/jlr.M034157. • In contrast to references 83 and 84, this study found no role for NUR77 in macrophage polarization or atherogenesis.
- 86.Liao X, et al. Kruppel-like factor 4 regulates macrophage polarization. J Clin Invest. 2011;121:2736–2749. doi: 10.1172/JCI45444. • This study identifies a key role for the transcription factor KLF4 in directing macrophage M2 polarization.
- 87.Sharma N, et al. Myeloid Kruppel-Like Factor 4 Deficiency Augments Atherogenesis in ApoE−/−Mice--Brief Report. Arterioscler Thromb Vasc Biol. 2012;32:2836–2838. doi: 10.1161/ATVBAHA.112.300471. • This study showed that deletion of KLF4 lead to enhanced atherosclerosis in both chow-fed and western diet fed Apoe−/− mice.
- 88.Cardilo-Reis L, et al. Interleukin-13 protects from atherosclerosis and modulates plaque composition by skewing the macrophage phenotype. EMBO Mol Med. 2012;4:1072–1086. doi: 10.1002/emmm.201201374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rayner KJ, et al. Antagonism of miR-33 in mice promotes reverse cholesterol transport and regression of atherosclerosis. J Clin Invest. 2011;121:2921–2931. doi: 10.1172/JCI57275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Khallou-Laschet J, et al. Macrophage plasticity in experimental atherosclerosis. PLoS One. 2010;5:e8852. doi: 10.1371/journal.pone.0008852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jenkins SJ, et al. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011;332:1284–1288. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang MZ, et al. CSF-1 signaling mediates recovery from acute kidney injury. J Clin Invest. 2012;122:4519–4532. doi: 10.1172/JCI60363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhu SN, Chen M, Jongstra-Bilen J, Cybulsky MI. GM-CSF regulates intimal cell proliferation in nascent atherosclerotic lesions. J Exp Med. 2009;206:2141–2149. doi: 10.1084/jem.20090866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gerrity RG, Naito HK. Lipid clearance from fatty streak lesions by foam cell migration. Artery. 1980;8:215–219. [PubMed] [Google Scholar]
- 95.Ramkhelawon B, et al. Hypoxia induces netrin-1 and unc5b in atherosclerotic plaques: mechanism for macrophage retention and survival. Arterioscler Thromb Vasc Biol. 2013;33:1180–1188. doi: 10.1161/ATVBAHA.112.301008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Parathath S, et al. Hypoxia is present in murine atherosclerotic plaques and has multiple adverse effects on macrophage lipid metabolism. Circ Res. 2011;109:1141–1152. doi: 10.1161/CIRCRESAHA.111.246363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Feig JE, et al. Regression of atherosclerosis is characterized by broad changes in the plaque macrophage transcriptome. PLoS One. 2012;7:e39790. doi: 10.1371/journal.pone.0039790. • Through transcriptome analysis of macrophages in progressing and regressing plaques, this work identifies the gene signature of macrophages during the resolution of atherosclerotic inflammation.
- 98.Trogan E, et al. Laser capture microdissection analysis of gene expression in macrophages from atherosclerotic lesions of apolipoprotein E-deficient mice. Proc Natl Acad Sci U S A. 2002;99:2234–2239. doi: 10.1073/pnas.042683999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yvan-Charvet L, et al. ABCA1 and ABCG1 protect against oxidative stress-induced macrophage apoptosis during efferocytosis. Circ Res. 2010;106:1861–1869. doi: 10.1161/CIRCRESAHA.110.217281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Potteaux S, et al. Suppressed monocyte recruitment drives macrophage removal from atherosclerotic plaques of Apoe−/− mice during disease regression. J Clin Invest. 2011;121:2025–2036. doi: 10.1172/JCI43802. • In this model of atherosclerosis regression, achieved through complementation of APOE in Apoe−/− mice, the authors demonstrate that reduced monocyte recruitment and increased apoptotic turnover of macrophages are critical components of atherosclerosis resolution.
- 101.Nagareddy PR, et al. Hyperglycemia promotes myelopoiesis and impairs the resolution of atherosclerosis. Cell Metab. 2013;17:695–708. doi: 10.1016/j.cmet.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Raffai RL, Weisgraber KH. Hypomorphic apolipoprotein E mice: a new model of conditional gene repair to examine apolipoprotein E-mediated metabolism. J Biol Chem. 2002;277:11064–11068. doi: 10.1074/jbc.M111222200. [DOI] [PubMed] [Google Scholar]
- 103.Reis ED, et al. Dramatic remodeling of advanced atherosclerotic plaques of the apolipoprotein E-deficient mouse in a novel transplantation model. J Vasc Surg. 2001;34:541–547. doi: 10.1067/mva.2001.115963. [DOI] [PubMed] [Google Scholar]
- 104.Rong JX, et al. Elevating high-density lipoprotein cholesterol in apolipoprotein E-deficient mice remodels advanced atherosclerotic lesions by decreasing macrophage and increasing smooth muscle cell content. Circulation. 2001;104:2447–2452. doi: 10.1161/hc4501.098952. • This study reports that atherosclerosis regression induced by raising HDL is characterized by decreasing macrophage content and increasing smooth muscle cell content.
- 105.Feig JE, et al. Statins promote the regression of atherosclerosis via activation of the CCR7-dependent emigration pathway in macrophages. PLoS One. 2011;6:e28534. doi: 10.1371/journal.pone.0028534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Frias JC, Ma Y, Williams KJ, Fayad ZA, Fisher EA. Properties of a versatile nanoparticle platform contrast agent to image and characterize atherosclerotic plaques by magnetic resonance imaging. Nano Lett. 2006;6:2220–2224. doi: 10.1021/nl061498r. [DOI] [PubMed] [Google Scholar]
- 107.Leuschner F, et al. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat Biotechnol. 2011;29:1005–1010. doi: 10.1038/nbt.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pan H, et al. Programmable nanoparticle functionalization for in vivo targeting. FASEB J. 2013;27:255–264. doi: 10.1096/fj.12-218081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Egawa M, et al. Inflammatory Monocytes Recruited to Allergic Skin Acquire an Anti-inflammatory M2 Phenotype via Basophil-Derived Interleukin-4. Immunity. 2013;38:570–580. doi: 10.1016/j.immuni.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 110.Denney L, et al. Activation of invariant NKT cells in early phase of experimental autoimmune encephalomyelitis results in differentiation of Ly6Chi inflammatory monocyte to M2 macrophages and improved outcome. J Immunol. 2012;189:551–557. doi: 10.4049/jimmunol.1103608. [DOI] [PubMed] [Google Scholar]
- 111.Schrijvers DM, De Meyer GR, Martinet W. Autophagy in atherosclerosis: a potential drug target for plaque stabilization. Arterioscler Thromb Vasc Biol. 2011;31:2787–2791. doi: 10.1161/ATVBAHA.111.224899. [DOI] [PubMed] [Google Scholar]
- 112.Thorp E, Cui D, Schrijvers DM, Kuriakose G, Tabas I. Mertk receptor mutation reduces efferocytosis efficiency and promotes apoptotic cell accumulation and plaque necrosis in atherosclerotic lesions of apoe−/− mice. Arterioscler Thromb Vasc Biol. 2008;28:1421–1428. doi: 10.1161/ATVBAHA.108.167197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zizzo G, Hilliard BA, Monestier M, Cohen PL. Efficient clearance of early apoptotic cells by human macrophages requires M2c polarization and MerTK induction. J Immunol. 2012;189:3508–3520. doi: 10.4049/jimmunol.1200662. [DOI] [PMC free article] [PubMed] [Google Scholar]