Abstract
Objectives
The most common etiology for acute pancreatitis results from the impaction of gallstones or sludge in the distal common bile duct (CBD). The result is pancreatic duct obstruction, diversion of bile into the pancreas, or cholestasis. In the current study, we examined whether combining both aspects, that is, infusion of the bile acid taurocholate (TC) followed by bile duct ligation (BDL), could yield a more severe form of pancreatitis that mimics biliary pancreatitis.
Methods
In mice, following laparotomy, the CBD was infused with either normal saline (NS) or TC. Subsequently, the CBD was ligated at the ampulla.
Results
Mice receiving TC infusion followed by BDL (TC+BDL) had higher mortality compared to animals receiving intra-ductal NS with BDL (NS+BDL). The TC+BDL arm developed more severe and diffuse pancreatic necrosis. In addition, serum amylase, IL-6, and bilirubin, were significantly higher. However, pancreatic edema as well as lung and liver injury were unchanged between TC+BDL and NS+BDL.
Conclusions
In summary, the combination of bile infusion into the pancreas followed by BDL causes a more severe, necrotizing pancreatitis. We believe this novel model of pancreatitis is useful because it can be employed in transgenic mice and recapitulates several aspects of biliary pancreatitis.
Keywords: biliary pancreatitis, bile acid infusion, bile duct ligation, pancreatic necrosis, experimental pancreatitis
INTRODUCTION
Acute pancreatitis is a debilitating inflammatory disease of the pancreas that contributes to nearly 6,000 deaths in the United States annually1. It is the most common reason for gastrointestinal-related hospitalization and costs an estimated 2.6 billion dollars per year1. For this reason, mechanistic studies in experimental models are necessary to understand the pathogenesis of the problem.
Although the current experimental models of pancreatitis have several strengths, many lack clinical relevance. Most of the commonly used mouse models of acute pancreatitis, such as caerulein hyperstimulation: (1) cause only mild disease or (2) fail to correspond to a clinically relevant etiology of pancreatitis2. Consequently, there is a need to develop animal models that: (1) are more severe and (2) closely relate to the clinical scenario.
Biliary pancreatitis is the most common cause of acute pancreatitis in both children and adults and accounts for approximately 30 – 60% of all cases3,4. The primary problem in biliary pancreatitis is the impaction of gallstones or sludge in the distal common bile duct (CBD)5. The stone impaction leads to: (1) pancreatic duct hypertension, (2) diversion of bile into the pancreatic duct, or (3) back-up of bile into the liver (i.e. cholestasis), or a combination of the three. In the current study, we sought to examine whether a model that integrates the three factors could yield a more severe form of pancreatitis.
To do this, we employed a previously described method of retrograde infusion of bile acids6–14 into the CBD of mice and added to it a subsequent ligation of the distal CBD at the ampulla. We found that bile acid infusion followed by CBD ligation (BDL) could produce a more severe pancreatitis as an experimental model in mice.
METHODS
Reagents and animals
All reagents were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise stated. Male Swiss Webster mice weighing 22–28 g (Charles River; Wilmington, MA) were fed standard laboratory chow and given free access to water. All animal experiments were performed using a protocol approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
Intra-ductal bile acid infusion and BDL in mice
The procedure for retrograde infusion of bile acids into the distal CBD and pancreatic duct has been described by Perides et al.6. Briefly, Swiss Webster mice were anesthetized with isoflurane. A midline incision was made to reveal the abdominal cavity. The duodenum was flipped to reveal its distal side and held in place by ligatures. The bile duct was identified, and a 30G needle was inserted through the anti-mesenteric aspect of the duodenum to cannulate the CBD. A small bull dog clamp was applied to the distal CBD (near the duodenum) to prevent back flow of the infusate into the duodenal lumen and to hold the cannula in place. A larger bull dog clamp was applied to the proximal CBD (near the liver) to prevent infusion into the liver and also to divert flow to the pancreatic duct. Sodium taurocholate (TC; 2.5%-5%) or normal saline (NS) was infused at 10 µl per min for 5 min using a P33 perfusion pump (Harvard Apparatus, Holliston, MA). Following infusion, the CBD was ligated just proximal to the ampulla of Vater using a 7-0 proline suture. Upon completion of the infusion, the bull dog clamps were released. The exterior abdominal wound was closed using 7 mm wound clips, and a single injection of buprenorphine (0.075 mg/kg) was given immediately after the surgery. Mice recovered on a heating pad for 30 min after the procedure. They were given free access to food and water after the surgery and euthanized 24 hr later via CO2 asphyxiation.
Pancreas tissue preparation and histological grading
The pancreas, duodenum, and spleen were excised en bloc and fixed at room temperature for 24 hr in 4% paraformaldehyde. Paraffin-embedded sections were stained with hematoxylin and eosin (HE) and graded using a 20X objective over 10 separate fields in a semiquantitative fashion using a scoring system described by Wildi et al.15. A score of 0–3 was used to evaluate the following histological features:
Inflammatory cell infiltrate
-
0)
Absent or around ductal margins and lobules
-
1)
In the parenchyma (≤ 25% of the lobules)
-
2)
In the parenchyma (25% < and ≤ 50% of the lobules)
-
3)
In the parenchyma (> 50% of the lobules)
Edema
-
0)
Absent or focally increased between lobules
-
1)
Diffusely increased in acini and lobules, but acini intact
-
2)
Diffusely increased and acini focally disrupted
-
3)
Acini disrupted and separated
Necrosis
-
0)
Absent or ≤ 5%
-
1)
5%< and ≤ 10%
-
2)
10%< and ≤ 20%
-
3)
> 20% (diffuse)
Digital analysis to quantify pancreatic edema
HE pancreatic sections were scanned on an Aperio slide scanner using a 40X objective. Whole pancreatic sections were then analyzed in Adobe Photoshop, and all non-pancreatic tissue (i.e. duodenum, spleen, lymph nodes, blood vessels, ducts) was excluded. Total pancreas tissue (e.g. pancreatic parenchyma without peri-pancreatic fat or large vessels) for each region was determined. Intra-lobular or intra-acinus separation denoted by white space (i.e. edema) was identified and marked using black fill. The amount of black area (i.e. number of pixels) was measured as a percent of the total tissue area, and data were represented as percent edema.
Serum amylase, IL-6, and hepatic indices
Whole blood samples were centrifuged at 1500×g for 5 min at 4°C. Serum amylase and IL-6 were measured using a Phadebas kit (Amersham Pharmacia; Rochester, NY) and an enzyme-linked immunoadsorbent assay (ELISA) kit (BioLegend; San Diego, CA), respectively. Serum alanine aminotransferase (ALT) and total bilirubin were analyzed by the clinical pathology lab at the Children’s Hospital of Pittsburgh.
Lung histology and bronchoalveolar lavage (BAL) analysis
To collect BAL fluid, the neck of the euthanized mouse was dissected, and the trachea was cannulated using a 21G lavage catheter. A 1 ml syringe was connected and 1 ml of PBS was gently infused into the lung. The fluid was suctioned back into the syringe, spun at 500×g for 5 min, and the supernatant containing BAL was stored at −80°C.
To fix the lung in situ, the chest was dissected and 1 ml of 10% formalin was gently infused into the trachea through the lavage catheter. Once infused with fixative, the lung and surrounding organs were removed from the thoracic cavity and immediately placed in 15 ml of 10% formalin overnight at room temperature. The next day, the lung was separated from the adjacent organs and submitted for paraffin embedding and HE staining.
Lung tissue sections were graded using a 40X objective over 20 separate fields in a semiquantitative fashion using a scoring system described by Matute-Bello et al.16. A score of 0, 1, or 2 was used to evaluate the following histological features: alveolar macrophages (none, 1–5 cells, >5 cells, respectively), hyaline membranes (none, 1–5 cells, >5 cells, respectively), proteinaceous debris filling the airspace (none, 1 airspace, >1 airspace, respectively), and alveolar septal thickening (< 2× normal, 2× – 4× normal, >4× normal, respectively).
Lung injury was further assessed by the presence of the cell injury marker lactate dehydrogenase (LDH) in the BAL fluid. LDH was measured using a colorimetric cytotoxicity assay kit (Promega; Madison, WI).
Statistical analysis
Data were expressed as mean ± standard deviation unless otherwise stated. Statistical analysis was performed using a Student’s t-test or log-ranked test (for the Kaplan-Meier survival curves). Statistical significance was defined as a P value ≤ 0.05.
RESULTS
Intra-ductal infusion of taurocholate followed by BDL reduces mouse survival
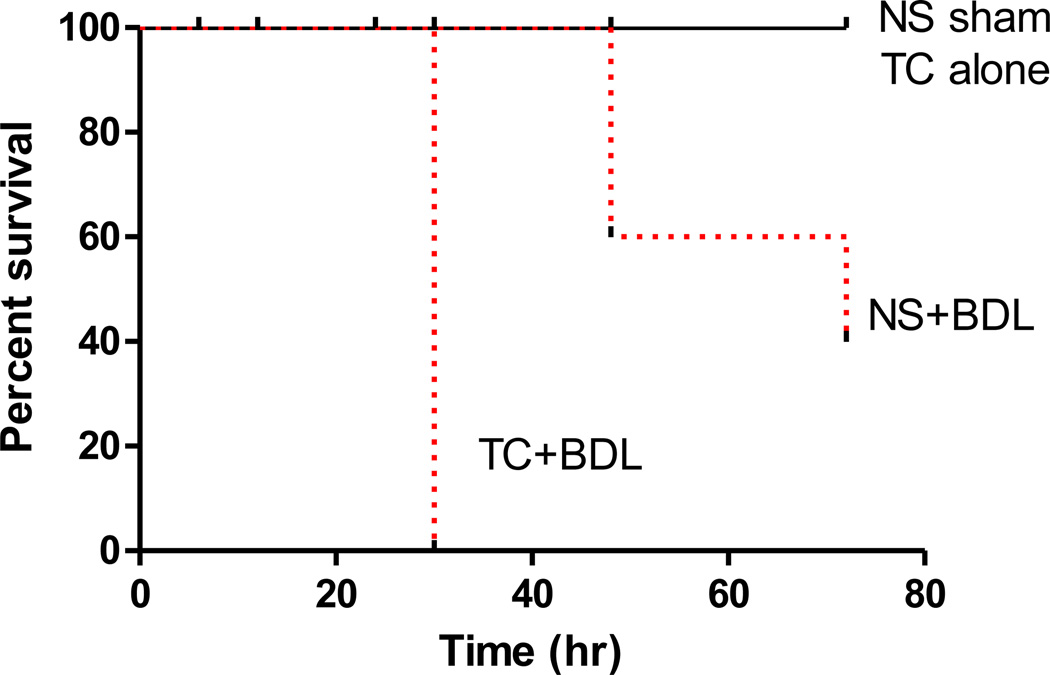
We began the current study with intra-ductal infusions of 5% TC, a concentration which has been used in most models of retrograde infusion8,11–13,17–22. However, all of the mice receiving the experimental arm of 5% TC followed by BDL died within 24 hr (data not shown). For this reason, we reduced the TC concentration to 2.5%. There were 4 discrete conditions: (1) intra-ductal infusion of NS alone (NS sham); (2) 2.5% TC alone; (3) NS infusion followed by BDL (NS+BDL); and (4) 2.5% TC infusion followed by BDL (TC+BDL). Serum was collected 6 hr after surgery and tissues were collected starting at 24 hr as depicted in Figure 1. An important strength of the set-up is that the experimental condition (i.e. TC+BDL) was directly compared with the other 3 “control” arms, whereas previous studies have compared the experimental to a control sham animal that underwent a laparotomy but did not undergo manipulation of the CBD or pancreatic duct23. In addition these studies had not characterized the relative contribution of each intervention, that is the role of bile acid infusion along with BDL. We observed that in comparison to the non-ligated animals, mice receiving BDL (whether with NS or TC infusion) had massive CBD dilatation, and the pancreas appeared grossly edematous. All of the mice receiving NS or 2.5% TC alone survived up to 3 days after the surgical procedure (Figure 2). Only 60% of mice in the NS+BDL group survived after 2 days, and 40% of mice in this group survived for up to 3 days. All of the mice receiving 2.5% TC followed by BDL died within 36 hr after the procedure, demonstrating that the TC+BDL model causes a high degree of mortality.
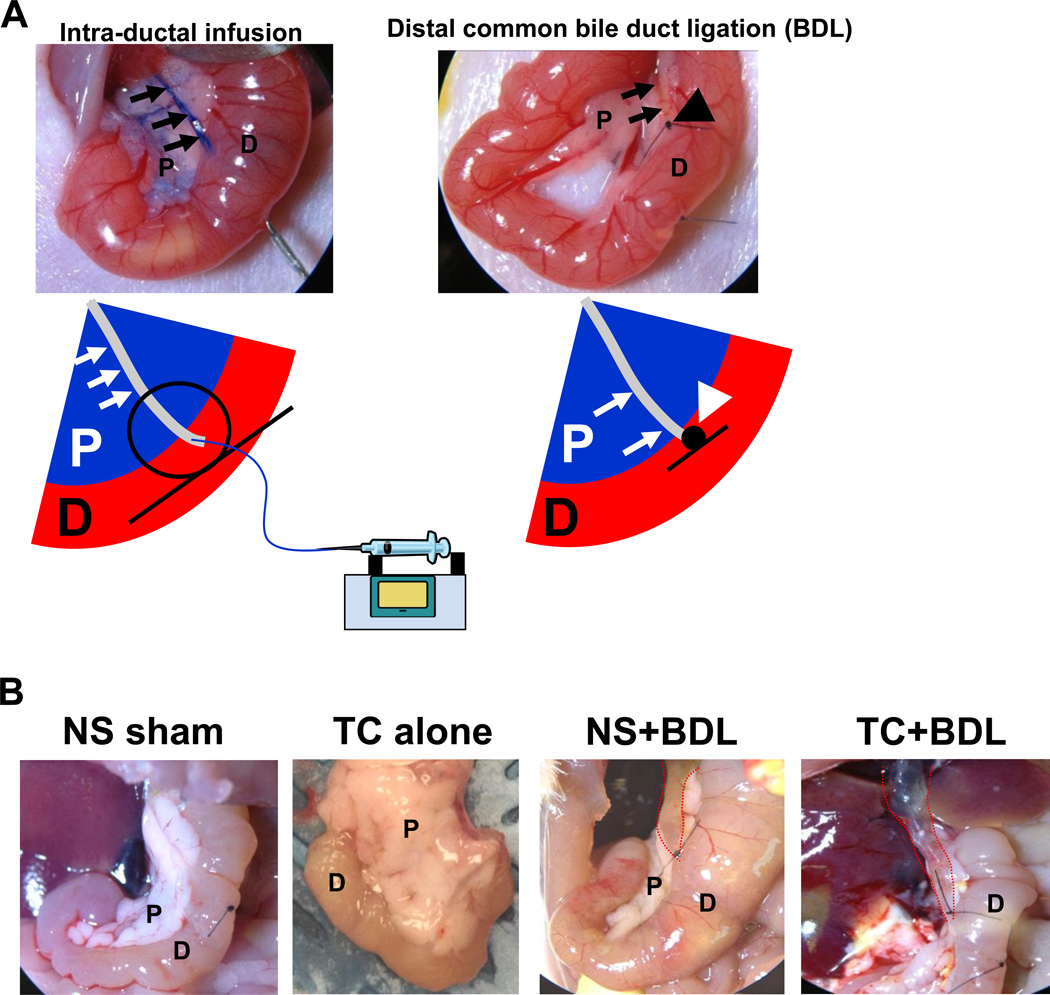
Figure 1. Schematic of the surgical procedure.
(A) The distal common bile duct (CBD; arrows) was cannulated via a trans-duodenal puncture, and methylene blue was added to the infusate. The CBD was ligated close to the ampulla (large arrow head). (B) Images of the pancreas at laparotomy 24 hr after the surgical procedure show that distal CBD ligation, or BDL, causes massive CBD dilation (dotted red lines). NS, normal saline; TC, sodium taurocholate; P, pancreas; D, duodenum.
Figure 2. Intra-ductal infusion of taurocholate followed by BDL leads to greater mortality over BDL alone.
Kaplan-Meier curves over a 72 hr time course. P<0.05, comparing NS+BDL or TC+BDL with the NS sham and also between the 2 BDL groups.
Intra-ductal infusion of taurocholate followed by BDL causes severe and diffuse pancreatic necrosis
The pancreas, duodenum, and spleen were excised en bloc 24 hr after the surgical procedure. Using the orientation of adjacent organs, we were able to approximate the regions containing the head, body, and tail of the pancreas into 3 roughly equal portions, denoted as Regions# 1, 2, and 3, respectively (Figure 3). HE-stained sections of pancreas were graded for edema, inflammatory infiltrate, and necrosis from all 3 regions. Compared to the NS sham, there was no significant increase in histological severity in pancreatic sections from the TC alone group. However, there was a 2- and 2.5-fold increase above the NS sham in the NS+BDL and TC+BDL groups, respectively in Region# 1 (P<0.05). Regions# 2 and 3 had a lower magnitude of severity than Region# 1 for each of the 4 conditions. Nonetheless, there was a similar fold increase in histological severity in NS+BDL and TC+BDL groups among the 3 regions, compared to the NS sham (P<0.05). Among the BDL groups, there was a 22–35% increase in severity with TC+BDL compared to NS+BDL in each of the regions. The primary contributor to overall severity was the necrosis index. There was a 9.1- and 16-fold increase in necrosis in NS+BDL and TC+BDL above the NS sham in Region #1, respectively (P<0.05). These data demonstrate that TC+BDL causes a more severe and diffuse pancreatic necrosis than either TC or BDL alone. Edema was calculated in a subjective fashion using a semiquantitative grading scale, as well as from an objective method using intensity thresholding, as described in the Methods. Edema was significantly increased in the NS+BDL and TC+BDL groups by 2.7- and 3.1-fold, respectively, in Region# 1 above the NS sham (P<0.05; Figure 4). In Regions# 2 and 3, there was only a 1.7-fold increase above the NS sham (P<0.05). There was no significant difference in edema or inflammatory cell infiltrate between the 2 BDL groups.
Figure 3. Intra-ductal infusion of taurocholate followed by BDL causes a more severe and diffuse pancreatic necrosis over BDL alone.
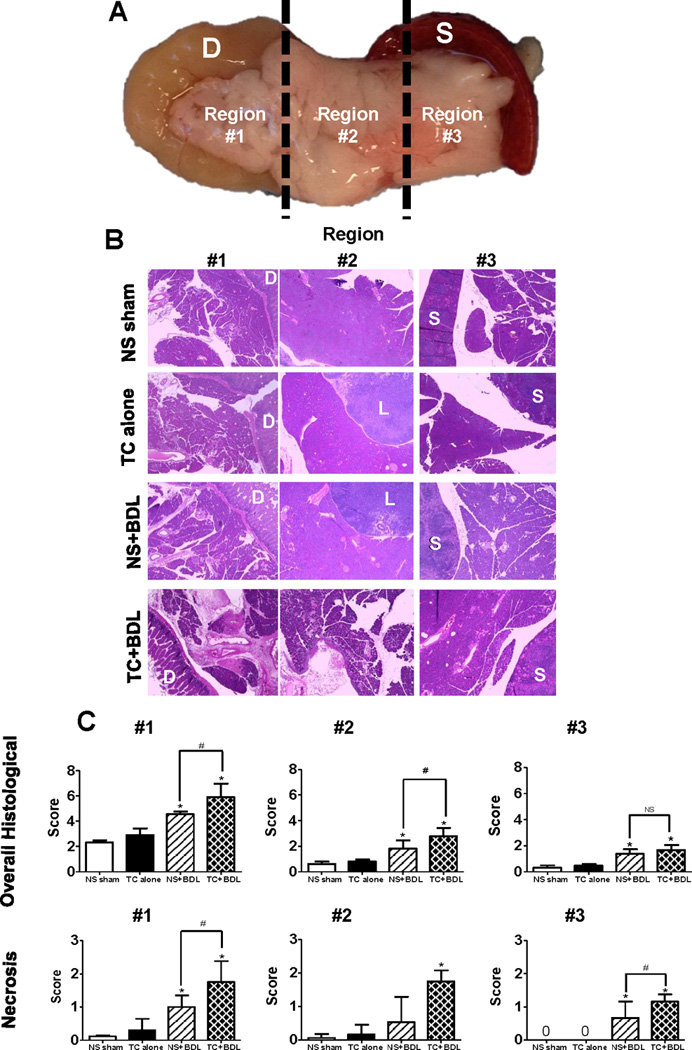
(A) For proper orientation, the pancreas was removed en bloc along with the (D)uodenum and (S)pleen. It was then divided into 3 equal parts. Notably, the head of the pancreas was contained within Region# 1. (B) Representative HE sections at 20X for each region. L, lymph node; S, spleen. (C) Semiquantitative scoring of overall histological severity and, specifically, pancreatic necrosis (n=5 animals per group and 10 fields at 20X for each region). *, P<0.05, relative to the NS sham. #, P<0.05.
Figure 4. BDL is the primary contributor to pancreatic edema.
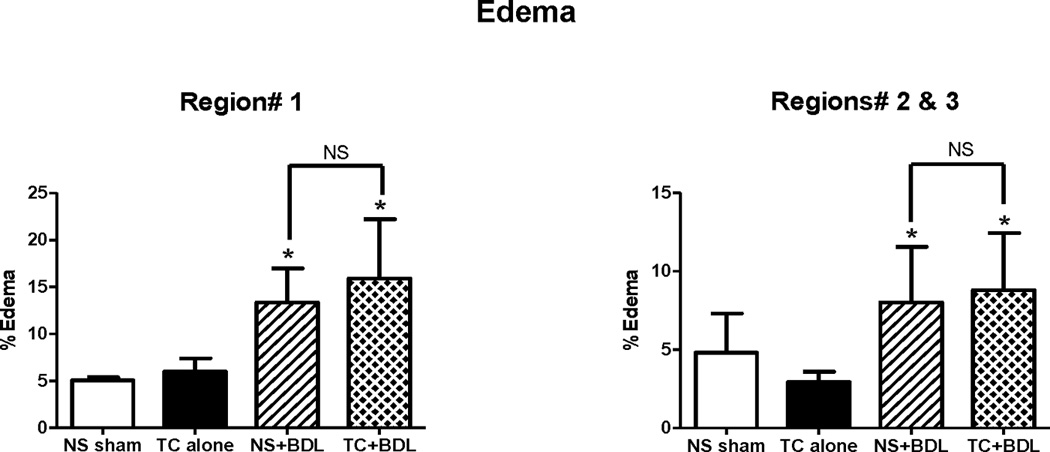
As described in the Methods, edema was quantified within (A) Region# 1 and (B) Regions# 2 and 3 combined. (n=5 animals per group over each whole pancreatic section). *, P<0.05, relative to the NS sham.
Serum markers and lung and liver injury
To further compare the severity among the 4 experimental models, we measured serum amylase and IL-6 levels 6 hr after the surgical procedure (Figure 5). There was no significant increase in the 2.5% TC alone group above the NS sham for each of the 2 indices. However, there was a 27.7- and 36.7-fold increase in serum amylase and a 2.5- and 4.4-fold increase in serum IL-6 among the NS+BDL and TC+BDL groups relative to the NS sham, respectively (P<0.05 for each comparison). Serum amylase and IL-6 from the TC+BDL arm was increased 24% and 44% above NS+BDL, respectively (P<0.05 for each comparison). These data complement the pancreatic histological grading and further support the finding that TC+BDL induces a more severe pancreatitis than TC alone or BDL alone.
Figure 5. Intra-ductal infusion of taurocholate followed by BDL causes higher elevations in (A) serum amylase and (B) IL-6 compared with BDL alone.
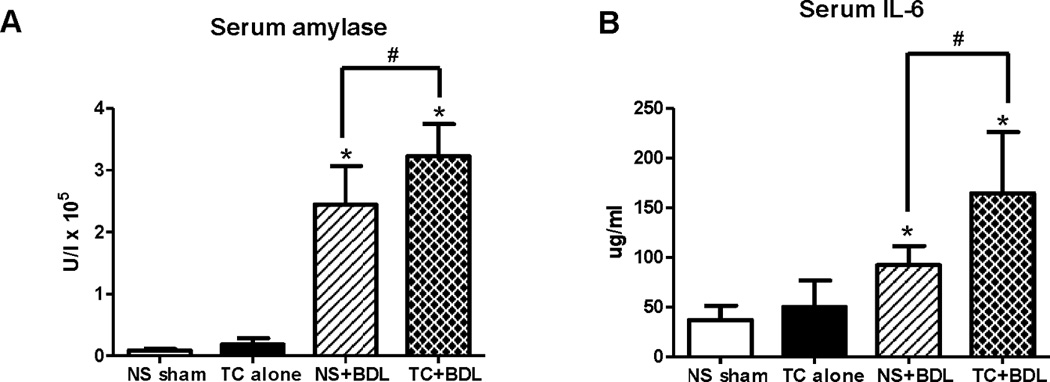
Serum was collected 6 hr after surgery. *, P<0.05, relative to the NS sham. #, P<0.05.
The lung is a primary site of extra-organ involvement in severe acute pancreatitis24,25. To assess whether the combination of bile acid infusion and BDL worsens lung injury, lung tissue was obtained 24 hr after the surgical procedure and graded in a semiquantitative fashion for the degree of alveolar macrophage cell infiltrate, hyaline membranes, proteinaceous debris, and alveolar septal thickening (Figure 6). BAL fluid was also assessed for leakage of the cell injury marker LDH. There was no significant increase in the lung histological severity score or LDH leakage with TC alone compared to the NS sham. However, there was a roughly equal increase by 34% in the severity score and a 4.6-fold increase in LDH leakage from the BAL fluid among both BDL groups (P<0.05 for each comparison). The results suggest that, at 24 hr, BDL alone is the primary factor in inducing lung injury. Besides obstruction of the pancreatic duct, BDL also causes biliary obstruction and resulting hepatitis. In the NS+BDL group, serum ALT, a marker of hepatocyte injury, was 43.4-fold higher than the NS sham (Figure 7; P<0.05). However, TC in addition to BDL did not further increase those levels. On the other hand, NS+BDL caused a 9.1-fold increase in serum bilirubin, which is a surrogate marker of cholestasis, and TC+BDL caused a 26% increase above NS+BDL (P<0.05). Although the percent increase is relatively modest, taken together, the findings suggest that compared to BDL alone, the TC+BDL model provokes the same level of hepatocyte injury but a greater degree of cholestasis.
Figure 6. Lung injury in the surgical models.
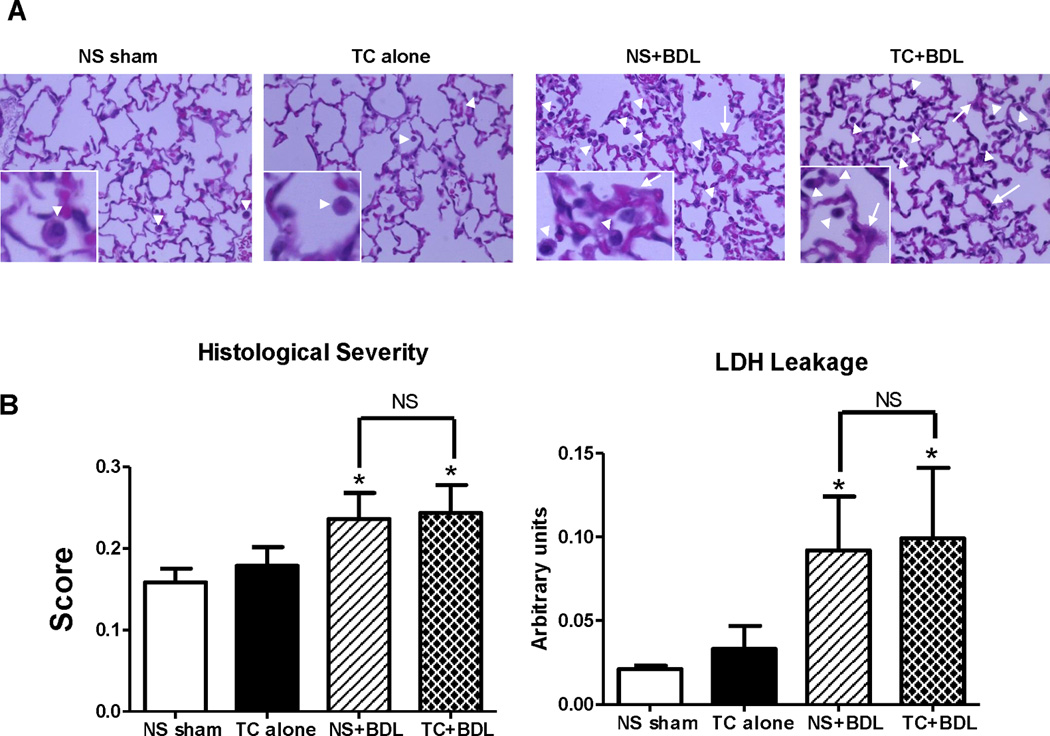
(A) Representative HE sections at 40X from lung tissue obtained 24 hr after the surgical procedure. The insets zoom in on macrophages in the alveolar space (arrow heads) and alveolar septal wall thickening (arrows). (B) Overall histological severity (left) and lactate dehydrogenase (LDH) from bronchoavleolar lavage (BAL) fluid (right). (n=5 animals per group; lung tissue was graded over 20 fields at 40X). *, P<0.05, relative to the NS sham.
Figure 7. Hepatocyte injury and cholestasis in the surgical models.
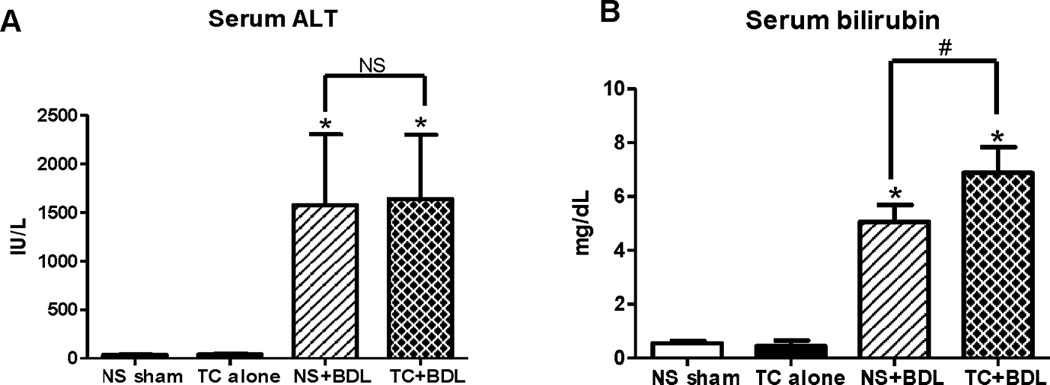
Serum (A) alanine aminotransferase (ALT) and (B) bilirubin 24 hr after the surgical procedure. (n=5 animals per group). *, P<0.05, relative to the NS sham.
DISCUSSION
In the current study, we report a new model of severe acute pancreatitis in mice that mimics 3 postulated aspects of biliary pancreatitis: (1) pancreatic duct hypertension via BDL; (2) exposure of the pancreas to bile via TC infusion; and (3) increased cholestasis. Previous studies have demonstrated that, by themselves, each of these aspects either fails to induce pancreatitis in most situations or causes a focal, limited pancreatitis. Most experimental models that ligate the pancreatic duct cause pancreatic atrophy, rather than inflammation26,27. The American opossum, however, seems to be the only animal model in which pancreatic duct ligation induces severe acute pancreatitis28,29 with mortality rates approaching 100% within 2 weeks. Pancreatitis models employing the intra-ductal infusion of bile acids have been reported in dogs30, rabbits23,31, rats14,32,33, and more recently, in mice6. A major limitation of the bile acid infusion model of pancreatitis, however, is that pancreatic necrosis is restricted to the head of the pancreas. Further, the question of whether bile reflux into the pancreas can occur in the clinical setting of persistent distal CBD obstruction is still unanswered34. Regarding the scenario of cholestasis alone, patients who develop cholestasis due to more proximal CBD obstruction or from intra-hepatic causes do not appear to be predisposed to the development of pancreatitis. Yet, many patients with biliary pancreatitis have some degree of cholestasis35. We found that intra-ductal bile infusion followed by BDL could combine all three aspects of pancreatic duct obstruction, bile exposure, and cholestasis. In addition we demonstrate that using three unique control arms—(1) normal saline infusion sham, (2) bile acid infusion only, (3) BDL preceded by NS infusion—and a final experimental arm—bile acid infusion followed by BDL—there were in the combination arm worsened pancreatitis outcomes compared to each modality alone. The pancreatitis induced by TC+BDL had a more severe and diffuse pancreatic histology, primarily due to the presence of pancreatic necrosis, and the levels of serum amylase and IL-6 were higher than BDL alone.
A similar methodology of bile acid infusion followed by BDL was reported in rabbits using human bile or taurochenodeoxycholic acid (TCDC), and the model manifested a similar degree of pancreatic necrosis and mortality as our mouse model23,31. TCDC as well as TC, which we have used, each make up roughly 35% of the bile acid composition in bile36. Although the mouse model requires stereoscopic micro-cannulation of the CBD and a slow, regulated infusion via a peristaltic pump, we believe the mouse offers a key advantage. Specifically, this model will be useful for a multitude of future studies to examine signaling or inflammatory pathways involved in biliary pancreatitis, specifically using transgenic mice. A limitation of the combination model is that there were no differences in lung injury compared with BDL alone. A possible reason for the limited lung injury is the timing of the analysis. We examined the parameter 24 hr after ligation, and it is, therefore, possible that if the animals survived for a longer time there might have been greater pulmonary manifestations in the TC+BDL group compared with BDL alone.
In summary, the combination of bile infusion into the pancreas, CBD obstruction, and cholestasis that is mimicked by intra-ductal infusion of TC, followed by BDL causes a more severe, necrotizing pancreatitis. We believe this novel experimental mouse model of pancreatitis is useful because (1) it can be employed in transgenic mice and (2) it recapitulates several aspects of biliary pancreatitis.
Acknowledgments
Financial Support:
This work was supported by National Institutes of Health Grants DK083327, DK093491 and DK03002 (to SZH).
Footnotes
Disclosures:
None
References
- 1.Peery AF, Dellon ES, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143:1179–1187. e1171–e1173. doi: 10.1053/j.gastro.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saluja AK, Dudeja V. Relevance of animal models of pancreatic cancer and pancreatitis to human disease. Gastroenterology. 2013;144:1194–1198. doi: 10.1053/j.gastro.2013.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bai HX, Lowe ME, Husain SZ. What have we learned about acute pancreatitis in children? J Pediatr Gastroenterol Nutr. 2011;52:262–270. doi: 10.1097/MPG.0b013e3182061d75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144:1252–1261. doi: 10.1053/j.gastro.2013.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Geenen EJ, van der Peet DL, Bhagirath P, et al. Etiology and diagnosis of acute biliary pancreatitis. Nat Rev Gastroenterol Hepatol. 2010;7:495–502. doi: 10.1038/nrgastro.2010.114. [DOI] [PubMed] [Google Scholar]
- 6.Perides G, van Acker GJ, Laukkarinen JM, et al. Experimental acute biliary pancreatitis induced by retrograde infusion of bile acids into the mouse pancreatic duct. Nat Protoc. 2010;5:335–341. doi: 10.1038/nprot.2009.243. [DOI] [PubMed] [Google Scholar]
- 7.Fetaud-Lapierre V, Pastor CM, Jorge-Costa M, et al. Time-course proteomic analysis of taurocholate-induced necrotizing acute pancreatitis. J Proteomics. 2013 doi: 10.1016/j.jprot.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 8.Awla D, Zetterqvist AV, Abdulla A, et al. NFATc3 Regulates Trypsinogen Activation, Neutrophil Recruitment, and Tissue Damage in Acute Pancreatitis in Mice. Gastroenterology. 2012 doi: 10.1053/j.gastro.2012.07.098. [DOI] [PubMed] [Google Scholar]
- 9.Sandoval J, Escobar J, Pereda J, et al. Pentoxifylline prevents loss of PP2A phosphatase activity and recruitment of histone acetyltransferases to proinflammatory genes in acute pancreatitis. J Pharmacol Exp Ther. 2009;331:609–617. doi: 10.1124/jpet.109.157537. [DOI] [PubMed] [Google Scholar]
- 10.Moretti AI, Rios EC, Soriano FG, et al. Acute pancreatitis: hypertonic saline increases heat shock proteins 70 and 90 and reduces neutrophil infiltration in lung injury. Pancreas. 2009;38:507–514. doi: 10.1097/MPA.0b013e31819fef75. [DOI] [PubMed] [Google Scholar]
- 11.Wittel UA, Wiech T, Chakraborty S, et al. Taurocholate-induced pancreatitis: a model of severe necrotizing pancreatitis in mice. Pancreas. 2008;36:e9–e21. doi: 10.1097/MPA.0b013e3181575103. [DOI] [PubMed] [Google Scholar]
- 12.Gutierrez PT, Folch-Puy E, Bulbena O, et al. Oxidised lipids present in ascitic fluid interfere with the regulation of the macrophages during acute pancreatitis, promoting an exacerbation of the inflammatory response. Gut. 2008;57:642–648. doi: 10.1136/gut.2007.127472. [DOI] [PubMed] [Google Scholar]
- 13.Long J, Song N, Liu XP, et al. Nuclear factor-kappaB activation on the reactive oxygen species in acute necrotizing pancreatitic rats. World J Gastroenterol. 2005;11:4277–4280. doi: 10.3748/wjg.v11.i27.4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aho HJ, Nevalainen TJ, Aho AJ. Experimental pancreatitis in the rat. Development of pancreatic necrosis, ischemia and edema after intraductal sodium taurocholate injection. Eur Surg Res. 1983;15:28–36. doi: 10.1159/000128330. [DOI] [PubMed] [Google Scholar]
- 15.Wildi S, Kleeff J, Mayerle J, et al. Suppression of transforming growth factor {beta} signalling aborts caerulein induced pancreatitis and eliminates restricted stimulation at high caerulein concentrations. Gut. 2007;56:685–692. doi: 10.1136/gut.2006.105833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matute-Bello G, Downey G, Moore BB, et al. An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals. Am J Respir Cell Mol Biol. 2011;44:725–738. doi: 10.1165/rcmb.2009-0210ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muili KA, Wang D, Orabi AI, et al. Bile acids induce pancreatic acinar cell injury and pancreatitis by activating calcineurin. J Biol Chem. 2012 doi: 10.1074/jbc.M112.428896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Awla D, Abdulla A, Regner S, et al. TLR4 but not TLR2 regulates inflammation and tissue damage in acute pancreatitis induced by retrograde infusion of taurocholate. Inflamm Res. 2011;60:1093–1098. doi: 10.1007/s00011-011-0370-1. [DOI] [PubMed] [Google Scholar]
- 19.Abdulla A, Awla D, Thorlacius H, et al. Role of neutrophils in the activation of trypsinogen in severe acute pancreatitis. J Leukoc Biol. 2011;90:975–982. doi: 10.1189/jlb.0411195. [DOI] [PubMed] [Google Scholar]
- 20.Kim KH, Lee MG, Kim DG. The cholecystokinin receptor antagonist L-364,718 reduces taurocholate-induced pancreatitis in rats. Int J Pancreatol. 1996;20:205–211. doi: 10.1007/BF02803770. [DOI] [PubMed] [Google Scholar]
- 21.Satoh AA, Shimosegawa TT, Satoh KK, et al. Activation of adenosine A1-receptor pathway induces edema formation in the pancreas of rats. Gastroenterology. 2000;119:829–836. doi: 10.1053/gast.2000.16502. [DOI] [PubMed] [Google Scholar]
- 22.Folch EE, Closa DD, Prats NN, et al. Leukotriene generation and neutrophil infiltration after experimental acute pancreatitis. Inflammation. 1998;22:83–93. doi: 10.1023/a:1022399824880. [DOI] [PubMed] [Google Scholar]
- 23.Osman MO, Lausten SB, Jakobsen NO, et al. Graded experimental acute pancreatitis: monitoring of a renewed rabbit model focusing on the production of interleukin-8 (IL-8) and CD11b/CD18. Eur J Gastroenterol Hepatol. 1999;11:137–149. [PubMed] [Google Scholar]
- 24.Zhou MT, Chen CS, Chen BC, et al. Acute lung injury and ARDS in acute pancreatitis: mechanisms and potential intervention. World J Gastroenterol. 2010;16:2094–2099. doi: 10.3748/wjg.v16.i17.2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andersson R, Sward A, Tingstedt B, et al. Treatment of acute pancreatitis: focus on medical care. Drugs. 2009;69:505–514. doi: 10.2165/00003495-200969050-00001. [DOI] [PubMed] [Google Scholar]
- 26.Walker NI. Ultrastructure of the rat pancreas after experimental duct ligation. I. The role of apoptosis and intraepithelial macrophages in acinar cell deletion. Am J Pathol. 1987;126:439–451. [PMC free article] [PubMed] [Google Scholar]
- 27.Lerch MM, Adler G. Experimental animal models of acute pancreatitis. Int J Pancreatol. 1994;15:159–170. [PubMed] [Google Scholar]
- 28.Acosta JM, Ledesma CL. Gallstone migration as a cause of acute pancreatitis. N Engl J Med. 1974;290:484–487. doi: 10.1056/NEJM197402282900904. [DOI] [PubMed] [Google Scholar]
- 29.Lerch MM, Saluja AK, Dawra R, et al. Acute necrotizing pancreatitis in the opossum: earliest morphological changes involve acinar cells. Gastroenterology. 1992;103:205–213. doi: 10.1016/0016-5085(92)91114-j. [DOI] [PubMed] [Google Scholar]
- 30.Musa BE, Nelson AW, Gillette EL, et al. A model to study acute pancreatitis in the dog. J Surg Res. 1976;21:51–56. doi: 10.1016/0022-4804(76)90009-3. [DOI] [PubMed] [Google Scholar]
- 31.Hong SS, Chin DS, Cho TS, et al. Experimental pancreatitis induced by alcohol and bile in rabbits: studies on changes of serum enzymes and histopathologic findings. Ann Surg. 1962;156:929–939. doi: 10.1097/00000658-196212000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aho HJ, Koskensalo SM, Nevalainen TJ. Experimental pancreatitis in the rat. Sodium taurocholate-induced acute haemorrhagic pancreatitis. Scand J Gastroenterol. 1980;15:411–416. doi: 10.3109/00365528009181493. [DOI] [PubMed] [Google Scholar]
- 33.Aho HJ, Nevalainen TJ. Experimental pancreatitis in the rat. Ultrastructure of sodium taurocholate-induced pancreatic lesions. Scand J Gastroenterol. 1980;15:417–424. doi: 10.3109/00365528009181494. [DOI] [PubMed] [Google Scholar]
- 34.Lerch MM, Aghdassi AA. The role of bile acids in gallstone-induced pancreatitis. Gastroenterology. 2010;138:429–433. doi: 10.1053/j.gastro.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 35.Santos JS, Kemp R, Ardengh JC, et al. Conservative management of cholestasis with and without fever in acute biliary pancreatitis. World J Gastrointest Surg. 2012;4:55–61. doi: 10.4240/wjgs.v4.i3.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ridlon JM, Kang DJ, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res. 2006;47:241–259. doi: 10.1194/jlr.R500013-JLR200. [DOI] [PubMed] [Google Scholar]