Abstract
(−)-Epicatechin ((−)-EPI), a naturally occurring flavanol has emerged as a likely candidate for cocoa-based product reported reductions in cardiometabolic risk. The present study aimed to determine the safety, tolerability, pharmacokinetics and pharmacodynamics of purified (−)-EPI administered to healthy volunteers. In this phase I, open-label, two-part single- and multiple-dose study subjects received either a single dose (n=9) of 50, 100 or 200 mg or multiple doses (n=8) of 50 mg daily (q.d.) or twice daily (b.i.d) for 5 days. Blood was collected at 0, 0.5, 1, 2, 4 and 6 hrs after (−)-EPI administration in the single and multiple dose groups (blood collection repeated in day 5). Samples were analyzed by HPLC-HR-ESI-MS for EPI and metabolites quantification. In the q.d. and b.i.d. groups, blood samples were analyzed for NO surrogates, follistatin, platelet mitochondrial complex I, V and citrate synthase level determinations. (−)-EPI was well tolerated and readily absorbed with further phase 2 metabolism. On day 5, in the q.d. and b.i.d. groups, there were significant increases in plasma nitrite of 30 % and 17 %, respectively. In the q.d. group on day 5 vs. day 1, platelet mitochondria complexes I, IV and citrate synthase activities demonstrated a significant increase of ~ 92, 62 and 8 %, respectively. Average day 5 follistatin AUC levels were ~2.5 fold higher vs. day 1 AUC levels in the b.i.d. group. (−)-EPI was safe with no observed adverse effects and our findings suggest that increases in NO metabolites, mitochondrial enzyme function and plasma follistatin levels may underlie some of the beneficial effects of cocoa products or (−)-EPI as reported in other studies.
Keywords: (−)-epicatechin, flavanols, cocoa, chocolate, muscle, mitochondria
1. Introduction
Reports linking cardiovascular benefits to dark chocolate (i.e. cocoa) consumption have proliferated in recent years generating significant interest in both the medical and lay communities. Studies of Panamanian Kuna Indians living in the San Blas Islands, who regularly consume a natural cacao beverage, report less aging associated hypertension, loss of renal function and decreased cardiovascular mortality relative to non-islander Kuna populations 1. Epidemiological reports indicate significant associations between dark chocolate intake and improved hypertension as well as cardiovascular and all-cause mortality 1. A recent meta-analysis demonstrated a chocolate-associated reduction in cardiometabolic risk of 37% and a reduction in stroke risk of 29% 2. Clinical intervention studies using cocoa and/or dark chocolate in normal volunteers or subjects with cardiovascular diseases have reported on improvements in peripheral and coronary vascular endothelial dysfunction, hypertension, hyperlipidemia, insulin resistance and inflammatory markers 3.
(−)-Epicatechin ((−)-EPI), a flavanol found in grapes, green tea, apples and in particularly high concentrations in cacao seeds, has emerged as a prominent candidate for the active molecule in cocoa. A study in normal volunteers demonstrated that serum (−)-EPI levels increased after dark chocolate consumption and that the administration of pure (−)-EPI recapitulated the vasodilatory effects induced by dark chocolate 4. Studies using cocoa products or pure (−)-EPI have identified putative mechanisms that may underlie clinical benefits including increases in nitric oxide (NO) and reductions in endothelin-1, platelet activation, inflammatory cytokines and oxidative stress amongst others 5. A notable addition to this list is the enhancement of muscle mitochondrial density, structure and function as reported by others and us in rodents 6, 7. As mitochondria are the main site for production of ATP, the loss of organelle function (i.e. worsening of cellular bioenergetics/metabolism) has emerged as central to the pathophysiology of many diseases. Thus, findings on the effects of the flavanol on mitochondria suggest a possible role for (−)-EPI in the treatment of human diseases associated with skeletal muscle, metabolic and/or cardiovascular pathologies. In this regard, our research group recently completed a pilot study in heart failure and type 2 diabetes mellitus patients. Results indicate that the consumption of (−)-EPI rich cocoa can restore skeletal muscle mitochondria structure and stimulate multiple indicators (i.e. regulators) of biogenesis such as peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1-α) and function such as protein levels for oxidative phosphorylation complexes 8. We also documented improvements in multiple indicators/mediators of muscle growth and repair including tissue and plasma levels of follistatin 9.
So far, cocoa products have been the best means available to deliver (−)-EPI to humans. However, the use of cocoa products as a therapeutic tool is fraught with many limitations such as lack of standardized products and the presence of numerous other potentially active compounds. A suitable alternative would be the use of pure (−)-EPI as it is devoid of calories and can be provided orally. Pharmacokinetic (PK) and pharmacodynamics (PD) studies would be required as an initial step to fulfill this goal given the limited information available for (−)-EPI PK (mostly from cocoa products studies) in humans.
2. Methods
2.1 Subjects
Volunteers were recruited to participate in the study through word of mouth and posted flyers. Prior to enrollment, a physician obtained detailed health information and performed a brief physical examination. A CBC and electrocardiogram was performed in all volunteers. Electrolytes, renal and liver function tests were also performed in volunteers for the multi-dose group. Subjects were enrolled in the study if they were age 18 or older, healthy by history, non-smokers, took no daily prescription medications and had no abnormalities in laboratory tests and electrocardiogram. Exclusion criteria included pregnancy, breast feeding, abnormal BP, BMI >32 and a history of migraine headaches. The study was conducted at the San Francisco General Hospital Clinical Research Center in accordance with the guidelines on Good Clinical Practice and within ethical standards for human experimentation as established by the Declaration of Helsinki. The protocol and amendments were approved by the Committee on Human Research of the University of California, San Francisco. Each subject provided written informed consent before participating in the study.
2.2 Study compound
(−)-EPI extracted was obtained from Sigma-Aldrich Inc. To prepare for administration, (−)-EPI was re-purified in a Good Manufacturing Practices (GMP) facility. First, (−)-EPI was dissolved in ethanol then treated with charcoal and filtered to remove insoluble materials. The solvent was exchanged to purified water and dried by lyophilization. The re-purified compound was tested in a GMP certified analytical lab using HPLC methodology. Specifications required >90% purity, <5% of the enantiomer and 5% catechin. It was also tested for other characteristics typical in GMP materials (identity by 1H nuclear magnetic resonance spectroscopy, infra-red; water content by Karl Fischer titration; ethanol content by gas chromatography; and the general USP tests of residue on ignition and heavy metals). Based on test results, a certificate of analysis was generated and a percent content by weight calculated. (−)-EPI was supplied pre-weighed in single-use, polypropylene bottles manufactured under current GMP. This powder-in-bottle supply was stored in a refrigerator at −4°C until use. (−)-EPI demonstrated to be stable in the lyophilized state for at least 3 months under refrigeration. Microbiological tests for aerobic microorganisms, yeast and molds were negative (< 10 cfu/g). Tests for E. coli, Pseudomona aeruginosa, Salmonella species and Staphylococcus aureus were also negative. Cardero Therapeutics Inc (Los Altos Hills, CA) conducted the purification and formulation of (−) EPI and provided it as a gift to support the study.
2.3 Study design
The general outline of the study protocol is noted in Supplemental Table S3. No formal statistical analysis was performed for sample size calculations. This single center, non-randomized, open label study was designed to investigate the PK, safety and tolerability of a single dose of 50, 100 or 200 mg of (−)-EPI. The study also examined the PK, safety, tolerability, and PD related endpoints after 5 days of 50 mg (−)-EPI q.d. or b.i.d. For each of the single dosing levels, 3 subjects were enrolled. For the 50 mg q.d. or b.i.d., 4 subjects were enrolled for each of the dosing schemes. The subjects were required to abstain from foods high in (−)-EPI including, chocolate, tea and wine at least 12 h and to be fasting for 2 h before the study began. All study procedures for subjects receiving a single dose of (−)-EPI were performed in the outpatient unit of the clinical research center at San Francisco General Hospital. The first and final doses of (−)-EPI on day 1 and 5 were administered to the subjects receiving multiple doses, in the clinical research center and all remaining doses were self administered at home. Subjects were instructed to dissolve the powder in approximately 100 ml of water and ingest it as a bolus.
2.4 Pharmacokinetics
(−)-EPI and metabolite levels. Blood sample collection was pursued as stated in Supplemental Table S3. Samples were immediately centrifuged at 1,500 rpm for 10 min. Plasma was separated, aliquoted in 250 μL tubes and frozen at −80°C until used. Plasma (200 μL) samples were thawed on ice and 20 μL of vitamin C-EDTA solution (200 mg vitamin C, 1 mg EDTA in 1 ml water) was added. Samples were spiked with known amount of internal standard (Taxifolin). To precipitate plasma proteins, 600 μL of 0.1% phosphoric acid in acetonitrile was added and samples were vortexed for 1 min. Samples were centrifuged at 4° C for 10 min at 13,000 rpm and the supernatant was transferred to a glass tube and evaporated under high vacuum at room temperature. The dried down sample was dissolved in 200 uL of 5% acetonitrile in water with 0.1% formic acid. Either 5.0 uL or 10.0 uL of the solution was injected into the Liquid Chromatography column. In the absence of available standards, we implemented a modification of a published method by Roura et al. to identify and quantify each (−)-EPI metabolite based on their exact mass and on their fragmentation pattern by using HPLC-HR-Mass Spectrometry (MS) and HPLC-MS/MS, respectively. The metabolites were identified by both HPLC-MS/MS (daughter ion peak at m/z 289 and 303) using a Thermo LCQdeca mass spectrometer, and HPLC coupled with high resolution Electrospray Ionization (ESI)-Time-of-Flight Mass Spectrometry (TOFMS) (accurate mass measurement) using an Agilent 6230 high resolution ESI-TOFMS. Both instruments were operated under negative ion mode. HPLC-ESI-TOFMS was used for metabolites detection and quantification since it provided better detection sensitivity. Details of the HPLC method are as follows: mobile phase A: 2.5% Acetonitrile in water with 0.1% formic acid; mobile phase B: Acetonitrile with 0.1% formic acid. LC gradient: 5% B to 95% B in 10 minutes, back to 5% B in one minute, and hold at 5% B for 7 minutes. A Shiseido CAPCELL PAK C-18 column (MGIII, 2.0mm × 50 mm, 3.0 um) with guard column was used for separation at a flow rate of 300 uL/minute. Agilent MassHunter workstation was used for data acquisition and analysis. Taxifolin and known amounts of (−)-EPI were spiked in blank plasma to quantify the concentration of identified (−)-EPI metabolites as described elsewhere 10. The performance of the method was monitored using quality control samples in the same matrix. The calibration curve was linear over the range 1.2 to 1,500 ng/mL. The method was highly reproducible. The recovery rate for (−)-EPI in plasma was 72 % (CV=5.9 %). The CV during (−)-EPI quantification were 7.9, 7 and 4.1 % at 2.5, 50 and 1500 ng/mL, respectively. The lower limit of detection and quantification for the HPLC-ESI-TOFMS were determined to be 0.36 and 1.21 ng/mL, respectively with a CV lower than 20 %.
2.5 Pharmacodynamics
Prior to (−)-EPI consumption on days 1 and 5 blood was collected for analysis of the biological effects of the flavanol on NO related metabolites (nitrite, nitrate and S-nitrosothiols), follistatin levels and platelet mitochondrial complex I, V and citrate synthase activities. Approximately 3 ml of blood was collected into a vacutainer tube containing EDTA. The sample was placed immediately on ice and centrifuged at 2,400 g for 15 min at 4° C, aliquoted in 250 μL tubes and frozen at −80° C. To assess plasma concentrations of nitrite, nitrate and SNO all species were measured after reduction chemistry in a vessel connected in-line to a NO Analyzer (Sievers). Nitrite and SNO were measured by tri-iodide-based reduction, while nitrate was measured in vanadium chloride. Briefly, for tri-iodide (I3−) based reductive chemiluminescence, samples were separated into 3 aliquots and left either untreated, treated with acidified sulfanilamide (16% in 2M HCl) or treated with mercuric chloride. Each aliquot was injected into I3− and the area under the curve (AUC) measured and concentration quantified using a standard curve of known nitrite concentrations. The concentration of nitrite was the difference between the aliquot left untreated and that treated with acidified sulfanilamide alone. The concentration of SNO was calculated by taking the difference between the acid sulfanilamide treated aliquot and the aliquot treated with mercuric chloride. Nitrate concentration was measured by injecting samples into a solution of vanadium chloride at 90° C connected inline to a NO analyzer. This method detects nitrite and nitrate. Nitrate concentration was quantified by subtracting the signal obtained in I3− from the signal obtained by injection into vanadium chloride.
For the analysis of platelet mitochondrial enzyme function, in the absence of a tourniquet, ~10 ml of blood was collected in a cell preparation tube containing citrate and immediately placed upside down on ice. Tubes were centrifuged at 1,500 g for 10 min and platelet rich plasma transferred to 15 ml conical tubes. 1 μL of 1 mM prostaglandin I2 was added, tube were mixed gently and frozen at −80° C. The rotenone sensitive rate of NADH oxidation in permeabilized platelets was spectrophotometrically monitored at 340 nm to determine the activity of mitochondrial enzyme complex I. Complex IV was measured by monitoring the oxidation of ferrocytochrome c at 550 nm. KCN was used to determine specificity of oxidation by complex IV. Activity of the mitochondrial matrix marker citrate synthase was measured spectrophotometrically by assessing the rate of coenzyme A production 11.
Plasma follistatin levels were determined using an ELISA kit (OmniKine human follistatin kit) per the manufacturers instructions. Briefly, 40 μL of subject EDTA plasma was added to 96-well plates for 2 h. Plates were then washed with buffer 4 times followed by the addition of detection antibody and incubation for 2 h. Next, plates were washed prior to addition of the avidin-horseradish peroxidase conjugate solution followed by incubation for 30 min. The solution was removed by washing and 100 μL of 3,3′,5,5′-Tetramethylbenzidine substrate solution was added. When color development ceased, 100 μL of stop solution was added. A microplate reader set at 450 nm with a wavelength correction at 540 nm was used to determine the optical density of each well and values derived from a standard curve to determine follistatin concentrations.
2.6 Safety and tolerability
Safety evaluation included all treatment emergent adverse events and their severity and relationship to study treatment.
2.7 Statistical analysis
Statistical analysis was performed using Prism Graph Pad version 6.0. Laboratory results from repeated dosing on study day 5 were compared using paired t-tests. Effects of (−)-EPI on BP were evaluated using a mixed models approach. PK parameters were calculated using non-compartmental analysis. For PK analysis, concentration time curves were constructed and the AUC determined. Maximal plasma concentration (Cmax) and time of maximal concentration (Tmax) was determined for (−)-EPI and each of the detected metabolites. Natural log transformed data and non-linear regression analysis was used to determine the plasma elimination constant (kel) and plasma half-life (T1/2). Dose proportionality was assessed by calculating the dose-normalized AUC. Metabolite to parent drug AUC ratios were calculated for each metabolite.
3. Results
3.1 Subjects
Baseline characteristics of subjects are shown in Supplemental Table S1. Subjects in the single dose group were 44% male with a median age of 33 years (23–68 years) and body mass index (BMI) of 27.2 (SE 3.7) kg/m2. Subjects in the multidose group were 50% male with a median age of 25 years (22–45 years) and BMI of 23.2 (SE 2.6) kg/m2.
3.2 Pharmacokinetics
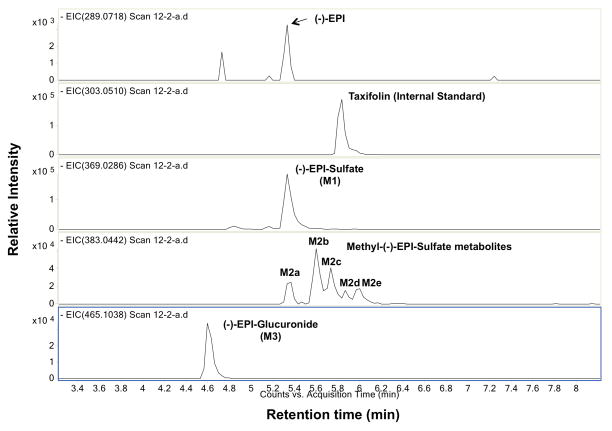
Figure 1 shows a representative extracted ion chromatogram (EIC) of a plasma sample obtained from the scan of ESI-TOFMS experiment where [M-H]− peaks of (−)-EPI and seven different (−)-EPI metabolites were detected; (−)-EPI shows an exact mass of 289.0718 (tr = 5.38 min). M1 shows an exact mass of 369.0286 with a time of retention (tr) of 5.38 min corresponding to (−)-EPI-Sulfate. M2 shows an exact mass of 383.0442 corresponding to five Methyl-(−)-EPI-Sulfate metabolites M2a (tr = 5.38 min), M2b (tr = 5.6 min), M2c (tr = 5.78 min), M2d (tr = 5.9 min), and M2e (tr = 6 min). M3 shows an exact mass of 465.1038 (tr = 4.6 min) corresponding to (−)-EPI Glucuronide. The exact positions of the substituents in M1, M2 and M3 could not be assigned due to the lack of reference standards.
Fig. 1.
Representative chromatogram used to derive (−)-Epicatechin ((−)-EPI) metabolite PK profiles. Extracted ion chromatograms (EICs) for (−)-EPI and metabolites in a representative plasma sample obtained by using high-resolution negative ion mode Electrospray Ionization-Time-of-flight mass spectrometry. Peaks: (−)-EPI (289.0718); Taxifolin (303.0510); (−)-EPI-Glucuronide (465.1038); Methyl-(−)-EPI-Sulfate (383.0442) and (−)-EPI-Sulfate (369.0286). The exact mass of their [M-H]− peaks are in parenthesis.
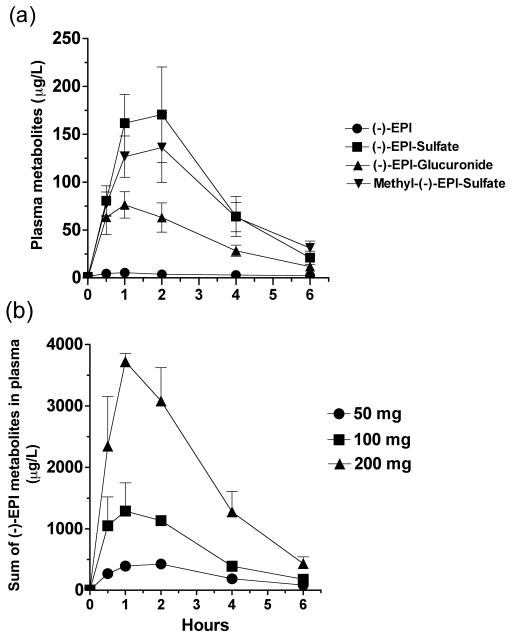
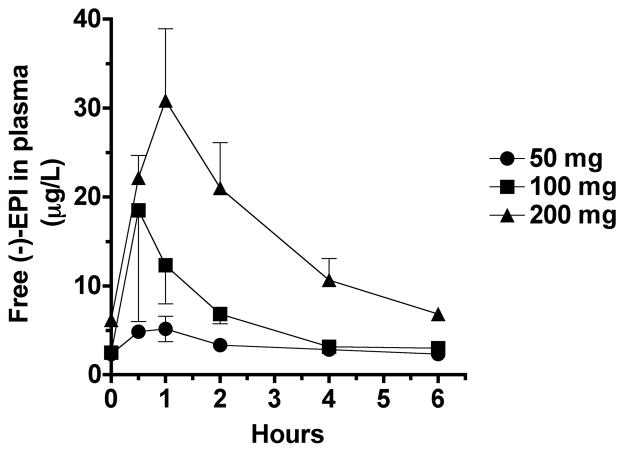
PK parameters are summarized in Tables 1, 2 and 3. In the absence of absolute bioavailability (F) an apparent volume of distribution (Vd/F) for (−)-EPI of ~50 L (Table 1) was estimated. Plasma concentration profiles of (−)-EPI and its metabolites were constructed (Figure 2a and b). The shape of the natural log transformed plasma concentration curves was consistent with first order kinetics. The concentration of (−)-EPI and its metabolites rose quickly following oral administration consistent with rapid absorption and phase 2 metabolism, as has been previously described (Figure 2a) 10, 12, 13. The maximum concentration of (−)-EPI and its metabolites was seen at 1–2 h following consumption and similar amongst them (Figure 2a). These findings were consistent for all of the study subjects. An area under the curve (AUC) was determined for (−)-EPI and each metabolite. The AUC increased out of proportion to the dose increase (Figure 2b and Table 1). The measured plasma concentrations of free (−)-EPI following the 50 mg dose were very low (below the limit of quantification) thus, an accurate and reliable half-life of free (−)-EPI could not be calculated. However, the half-life of (−)-EPI following the 100 and 200 mg doses was ~2.5 h (Figure 3 and Table 2). This was similar to the half-life observed for each of the (−)-EPI metabolites, which ranged from 1.2–3.1 h (Table 2). There were no significant differences in PK parameters on day 1 and day 5 in the repeat dosing groups suggesting that (−)-EPI absorption and metabolism is relatively unaffected by subchronic (5 days) consumption at the doses studied (Table 3).
Table 1.
Single Dose of (−)-Epicatechin ((−)-EPI) and sum of all metabolites PK parameters
| Single Dose of (−)-EPI
| ||||||
|---|---|---|---|---|---|---|
| Parameters | 50 mg
|
100 mg
|
200 mg
|
|||
| Mean | SEM | Mean | SEM | Mean | SEM | |
| Cmax (ug/L) | 427.66 | 76.49 | 1287.5 | 460.4 | 3720.16 | 131.48 |
| Tmax (hr)a | 2.0 | 1.0–2.0 | 2.0 | 1.0–2.0 | 1.0 | 1.0–2.0 |
| Kel (hr−1) | 0.405 | 0.015 | 0.474 | 0.013 | 0.481 | 0.018 |
| t ½ (hr) | 1.70 | 0.069 | 1.46 | 0.039 | 1.44 | 0.052 |
| AUC0–6 (ug·L−1·hr) | 1530 | 11.2 | 4154 | 660.5 | 11577 | 1845 |
| AUC0–inf (ug·L−1·hr) | 1741 | 244.1 | 4531 | 675.4 | 12499 | 2104 |
| Cl / F (L/hr) | 30.06 | 4.78 | 23.21 | 3.86 | 16.91 | 2.74 |
| Vd / F (L) | 73.38 | 9.5 | 49.31 | 8.9 | 34.82 | 4.4 |
Cmax, maximum plasma concentration; tmax, time to reach Cmax; AUC, area under the plasma concentration-time curve; Kel, terminal elimination constant; t1/2, terminal elimination half life; Cl/F, clearance; Vd/F, apparent volume of distribution.
tmax is median and range.
Table 2.
Single dose (−)-Epicatechin ((−)-EPI) and metabolite PK parameters
| 50 mg
|
100 mg
|
200 mg
|
||||
|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | Mean | SEM | |
| (−)-EPI | ||||||
| Cmax (ug /L) | 5.8 | 1.2 | 20.7 | 11.6 | 34.5 | 5.3 |
| T max (hr)a | 0.5 | 0.5–1.0 | 0.5 | 0.5–1.0 | 1.0 | 1.0–2.0 |
| Kel (hr−1) | ND | 0.277 | 0.048 | 0.277 | 0.041 | |
| t1/2 (hr) | ND | 2.5 | 0.5 | 2.5 | 0.4 | |
| AUC0–6 (ug·L−1·hr) | 19.88 | 1.1 | 38.7 | 10.8 | 95.4 | 11.2 |
| AUC0–inf (ug·L−1·hr) | ND | 58.1 | 13.6 | 122.4 | 9.0 | |
| (−)-EPI-Glucuronide | ||||||
| Cmax (ug /L) | 71.3 | 22.3 | 355.3 | 124 | 1092 | 194.1 |
| Tmax (hr)a | 1.0 | 0.5–2.0 | 1.0 | 1.0–2.0 | 1.0 | 0.5–1.0 |
| Kel (hr−1) | 0.362 | 0.013 | 0.43 | 0.013 | 0.509 | 0.018 |
| t1/2 (hr) | 1.9 | 0.07 | 1.5 | 0.04 | 1.3 | 0.04 |
| AUC0–6 (ug·L−1·hr) | 239.6 | 98.9 | 959.5 | 251.3 | 2972 | 505.6 |
| AUC0–inf (ug·L−1·hr) | 279.7 | 117.7 | 1054 | 267.9 | 3165 | 566.7 |
| Methyl-(−)-EPI-Sulfate – 1 | ||||||
| Cmax (ug /L) | 19.5 | 4.3 | 98.33 | 37.1 | 200.8 | 18.8 |
| Tmax (hr)a | 2.0 | 1.0–2.0 | 2.0 | 1.0–2.0 | 2.0 | 1.0–2.0 |
| Kel (hr−1) | 0.249 | 0.013 | 0.346 | 0.026 | 0.364 | 0.017 |
| t1/2 (hr) | 2.8 | 0.1 | 2 | 0.15 | 1.9 | 0.09 |
| AUC0–6 (ug·L−1·hr) | 78.3 | 17.3 | 296.3 | 78.4 | 719.1 | 127.4 |
| AUC0–inf (ug·L−1·hr) | 106.7 | 24.8 | 348.3 | 78.7 | 844 | 161.6 |
| Methyl-(−)-EPI-Sulfate – 2 | ||||||
| Cmax (ug /L) | 69.8 | 7.4 | 238.7 | 102.2 | 648.7 | 52 |
| Tmax (hr)a | 1.0 | 1.0–2.0 | 1.0 | 0.5–2.0 | 1.0 | 0.05–1.0 |
| Kel (hr−1) | 0.433 | 0.015 | 0.533 | 0.036 | 0.577 | 0.019 |
| t1/2 (hr) | 1.6 | 0.05 | 1.3 | 0.09 | 1.2 | 0.04 |
| AUC0–6 (ug·L−1·hr) | 229.1 | 42.6 | 625.6 | 144.8 | 1717 | 442.1 |
| AUC0–inf (ug·L−1·hr) | 255.7 | 40.5 | 661.7 | 144.2 | 1798 | 473.5 |
| Methyl-(−)-EPI-Sulfate – 3 | ||||||
| Cmax (ug /L) | 29.7 | 3.7 | 108.8 | 36.3 | 391.8 | 52.1 |
| Tmax (hr)a | 2.0 | 1.0–2.0 | 1.0 | 1.0–2.0 | 1.0 | 1.0–2.0 |
| Kel (hr−1) | 0.223 | 0.046 | 0.238 | 0.072 | 0.433 | 0.029 |
| t1/2 (hr) | 3.1 | 0.09 | 2.9 | 0.98 | 1.6 | 0.1 |
| AUC0–6 (ug·L−1·hr) | 123.5 | 370.6 | 304.4 | 92.2 | 1334 | 325.6 |
| AUC0–inf (ug·L−1·hr) | 176.3 | 25.4 | 396.5 | 82.7 | 1501 | 381.2 |
| Methyl-(−)-EPI-Sulfate – 4 | ||||||
| Cmax (ug /L) | 19.3 | 2.7 | 48.8 | 19.2 | 143.3 | 20.2 |
| Tmax (hr)a | 2.0 | 0.5–2.0 | 2.0 | 1.0–2.0 | 2.0 | 1.0–2.0 |
| Kel (hr−1) | 0.385 | 0.014 | 0.407 | 0.056 | 0.389 | 0.049 |
| t1/2 (hr) | 1.8 | 0.05 | 1.7 | 0.25 | 1.78 | 0.19 |
| AUC0–6 (ug·L−1·hr) | 58.3 | 10.4 | 150.9 | 38 | 476 | 126.5 |
| AUC0–inf (ug·L−1·hr) | 66.6 | 14.8 | 168.8 | 36 | 540.6 | 147.7 |
| Methyl-(−)-EPI-Sulfate – 5 | ||||||
| Cmax (ug /L) | 15.7 (3.1) | 3.1 | 54.7 (31.7) | 31.7 | 142.5 (25.3) | 25.3 |
| Tmax (hr)a | 2.0 | 1.0–2.0 | 1.0 | 1.0–2.0 | 1.0 | 1.0–2.0 |
| Kel (hr−1) | 0.277 | 0.05 | 0.28 | 0.036 | 0.346 | 0.036 |
| t1/2 (hr) | 2.5 | 1.0 | 2.47 | 0.28 | 2 | 0.23 |
| AUC0–6 (ug·L−1·hr) | 51.8 | 6.3 | 175 | 77 | 516.8 | 96.6 |
| AUC0–inf (ug·L−1·hr) | 68.0 | 3.8 | 225 | 80.7 | 612.2 | 101.1 |
| Methyl-(−)-EPI-Sulfate - total | ||||||
| Cmax (ug /L) | 156.5 | 22.9 | 557.3 | 207.6 | 1477 | 130 |
| Tmax (hr)a | 2.0 | 1.0–2.0 | 1.0 | 1.0–2.0 | 1.0 | 1.0–2.0 |
| Kel (hr−1) | 0.339 | 0.013 | 0.393 | 0.042 | 0.433 | 0.022 |
| t1/2 (hr) | 2.04 | 0.08 | 1.76 | 0.17 | 1.6 | 0.08 |
| AUC0–6 (ug·L−1·hr) | 540.9 | 97.1 | 1552 | 417.3 | 4763 | 1110 |
| AUC0–inf (ug·L−1·hr) | 652.1 | 117.8 | 1754 | 421.1 | 5258 | 1256 |
| (−)-EPI-Sulfate | ||||||
| Cmax (ug /L) | 240 | 49.5 | 586.5 | 93.9 | 1258 | 98.5 |
| Tmax (hr)a | 2.0 | 1.0–2.0 | 2.0 | 1.0–2.0 | 2.0 | 1.0–2.0 |
| Kel (hr−1) | 0.498 | 0.029 | 0.541 | 0.013 | 0.568 | 0.035 |
| t1/2 (hr) | 1.39 | 0.08 | 1.28 | 0.02 | 1.22 | 0.07 |
| AUC0–6 (ug·L−1·hr) | 729.3 | 147 | 1604 | 338.5 | 4081.1 | 590.99 |
| AUC0–inf (ug·L−1·hr) | 791.6 | 163.5 | 1705 | 370.1 | 4296 | 653.8 |
Data is expressed as mean and SEM.
Cmax, maximum plasma concentration; tmax, time to reach Cmax; AUC, area under the plasma concentration-time curve; Kel, terminal elimination constant; t1/2, terminal elimination half life. ND, not determined.
Tmax is median and range.
Table 3.
Multiple dose (−)-Epicatechin ((−)-EPI) and metabolite PK parameters
| 50 mg q.d.
|
50 mg b.i.d.
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Day 1
|
Day 5
|
Day 1
|
Day 5
|
|||||
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | |
| (−)-EPI | ||||||||
| Cmax (ug /L) | 10.6 | 5.3 | 3.3 | 2.3 | 5.5 | 1.7 | 3.9 | 1.4 |
| Tmax (hr)a | 1.0 | 0.0–1.0 | 0.5 | 0.5–2.0 | 0.75 | 0.5–6.0 | 0.5 | 0.5–1.0 |
| Kel (hr−1) | ND | ND | ND | ND | ||||
| t1/2 (hr) | ND | ND | ND | ND | ||||
| AUC0–6 (ug·L−1·hr) | 35.5 | 18.0 | 8.0 | 4.4 | 21.67 | 7.0 | 10.3 | 1.5 |
| AUC0–inf (ug·L−1·hr) | ND | ND | ND | ND | ||||
| (−)-EPI-Glucuronide | ||||||||
| Cmax (ug /L) | 344 | 48.7 | 100.2 | 70.4 | 85.4 | 16.3 | 108.2 | 80.2 |
| Tmax (hr)a | 1.0 | 1.0 | 1.0 | 0.5–2.0 | 1.0 | 0.5–1.0 | 1.5 | 0.5–2.0 |
| Kel (hr−1) | 0.591 | 0.055 | 0.577 | 0.099 | 0.413 | 0.036 | 0.482 | 0.052 |
| t1/2 (hr) | 1.2 | 0.10 | 1.2 | 0.2 | 1.7 | 0.18 | 1.5 | 0.17 |
| AUC0–6 (ug·L−1·hr) | 762.3 | 134.3 | 261.7.3 | 140 | 242.5 | 55.1 | 299.5 | 166.1 |
| AUC0–inf (ug·L−1·hr) | 792.3 | 137 | 272.4 | 142.5 | 268 | 61 | 319.6 | 166.1 |
| Methyl-(−)-EPI-Sulfate | ||||||||
| Cmax (ug /L) | 471.6 | 375.1 | 469.3 | 337.5 | 136 | 37.4 | 113 | 51.3 |
| Tmax (hr)a | 2.0 | 1.0–2.0 | 1.0 | 0.5–2.0 | 1.0 | 0.5–2.0 | 2.0 | 1.0–2.0 |
| Kel (hr−1) | 0.596 | 0.072 | 0.456 | 0.142 | 0.437 | 0.103 | 0.45 | 0.082 |
| t1/2 (hr) | 1.2 | 0.16 | 1.8 | 0.45 | 1.8 | 0.3 | 1.68 | 0.3 |
| AUC0–6 (ug·L−1·hr) | 1490 | 1121 | 1089 | 563.7 | 424.5 | 159.8 | 356.1 | 136.6 |
| AUC0–inf (ug·L−1·hr) | 1546 | 1165 | 1199 | 553.6 | 503.9 | 194.3 | 414.2 | 155.7 |
| (−)-EPI-Sulfate | ||||||||
| Cmax (ug /L) | 433.5 | 351.1 | 645.67 | 495.8 | 140.4 | 52 | 121.5 | 73.8 |
| Tmax (hr)a | 1.0 | 1.0–2.0 | 2.0 | 1.0–2.0 | 1.0 | 0.5–1.0 | 1.5 | 0.5–2.0 |
| Kel (hr−1) | 0.689 | 0.064 | 0.699 | 0.141 | 0.652 | 0.125 | 0.486 | 0.052 |
| t1/2 (hr) | 1.02 | 0.10 | 1.06 | 0.2 | 1.15 | 0.16 | 1.47 | 0.15 |
| AUC0–6 (ug·L−1·hr) | 1221 | 925.3 | 1311 | 825.4 | 407.8 | 190.5 | 363.6 | 181.3 |
| AUC0–inf (ug·L−1·hr) | 1246 | 943.5 | 1339 | 823 | 435.6 | 209.8 | 385.8 | 190.1 |
Data is expressed as mean and SEM.
Cmax, maximum plasma concentration; tmax, time to reach Cmax; AUC, area under the plasma concentration-time curve; Kel, terminal elimination constant; t1/2, terminal elimination half life; q.d., once a day; b.i.d., twice a day. ND, not determined.
Tmax is median and range.
Fig. 2.
(−)-Epicatechin ((−)-EPI) metabolite profile curves. (a) Concentration vs. time profile of (−)-EPI and its metabolites after administration of single 50 mg dose (n=7). (b) Area under the curve (AUC0–6) of the sum of (−)-EPI metabolites after the administration of 50, 100 and 200 mg (−)-EPI single doses in healthy individuals (n=3/dose). Methyl-(−)-EPI-Sulfate is the sum of five metabolites. Results are expressed as means with their standard errors.
Fig. 3.
Concentration vs. time curves for free (−)-Epicatechin ((−)-EPI) in plasma after administration of 50, 100 and 200 mg single doses. Results are expressed as means with their standard errors(n=3/dose).
3.3 Pharmacodynamics
PD endpoints measured at day 1 and 5 are reported in Table 4. There was a significant increase in plasma nitrite of 30% (p=0.02) and 24% (p=0.02) in subjects receiving 50 mg of (−)-EPI q.d. and b.i.d. respectively. No significant changes in plasma nitrate were observed. Plasma S-nitrosothiols (SNO) increased by 25% in subjects receiving b.i.d, although, the result was not significant (p=0.4). The analysis of platelet mitochondrial enzyme complexes I and IV and citrate synthase activities in the (−)-EPI q.d. group demonstrated a significant increase on day 5 vs. day 1 (table 4) (sample degradation prevented determinations in the b.i.d. group). As shown in Supplemental Figure S1, on day 1, following (−)-EPI administration, plasma follistatin levels shifted in a manner parallel to the time course of plasma (−)-EPI levels. On day 5, plasma follistatin at baseline was higher vs. day 1. After (−)-EPI administration, follistatin levels increased following a plasma concentration time profile similar to that of (−)-EPI. Average day 5 follistatin AUC levels (table 4) were ~ 2.5 fold higher (5105 pg/ml/hr) vs. day 1 AUC levels (1931 pg/ml/hr) suggesting a priming effect of long-term (−)-EPI dosing on follistatin production.
Table 4.
(−)-Epicatechin ((−)-EPI) effects on PD endpoints
| (−)-EPI 50 mg q.d. | |||||
|---|---|---|---|---|---|
| Parameters | Day 1
|
Day 5
|
p | ||
| Mean | SEM | Mean | SEM | ||
| Plasma Nitrite (uM) | 0.13 | 0.03 | 0.17 | 0.02 | 0.02 |
| Plasma Nitrate (uM) | 57.9 | 20.9 | 50 | 16.4 | 0.15 |
| Plasma SNO (nM) | 9.1 | 8.3 | 8.3 | 3.4 | 0.88 |
| Plasma Carbonyl (nmol/mg) | 0.79 | 0.11 | 0.69 | 0.09 | 0.02 |
| Complex I (nmol/min/mg) | 0.298 | 0.018 | 0.57 | 0.011 | 0.01 |
| Complex IV (k/min/mg) | 9.0 | 2.98 | 14.5 | 3.65 | 0.008 |
| Citrate Synthase (nmol/min/mg) | 0.72 | 0.09 | 0.78 | 0.09 | 0.043 |
| (−)-EPI 50 mg b.i.d. | |||||
| Plasma Nitrite (uM) | 0.21 | 0.07 | 0.26 | 0.08 | 0.02 |
| Plasma SNO (nM) | 9.06 | 2.1 | 11.3 | 1.75 | 0.4 |
| Plasma Carbonyl (nmol/mg) | 0.85 | 0.11 | 0.66 | 0.11 | .004 |
| Follistatin AUC0–6 (pg·ml−1·hr) | 1931 | 392.6 | 5105 | 1170 | |
Plasma SNO, plasma nitrosothiols. AUC, area under the plasma concentration-time curve.
q.d., once a day; b.i.d., twice a day.
3.4 Safety and tolerability
No adverse effects were reported or observed in any of the 9 subjects in the q.d. Group. Subjects denied symptoms such as light headedness, dizziness, fatigue, chest discomfort or changes in breathing during the observation period. Additionally, blood pressure (BP) recorded with a calibrated mercury sphygmomanometer in accordance to the recommendations of the American Heart Association showed no significant changes in systolic or diastolic BP after neither single nor q.d. and b.i.d. (−)-EPI dosing (see Supplemental Figure S2 and S3). Comparison of blood laboratory results at baseline and on day 5 are shown in Supplemental Table S2. Among subjects taking (−)-EPI b.i.d., there was a reduction in the platelet count on day 5 compared to day 1. However, the reduction was small and the absolute value of the platelet count remained within normal limits. There was also a small but statistically significant reduction in alkaline phosphatase levels of uncertain significance. There were no other changes in laboratory values.
4. Discussion and conclusions
In this study, we examined for the first time, initial safety, tolerability, PK and in vascular and skeletal muscle related PD endpoints of pure (−)-EPI in healthy human subjects. (−)-EPI was well tolerated and there were no reported or observed adverse effects on electrocardiogram, heart rate and BP at any of the (−)-EPI doses or dosing schedules studied. Results also demonstrate that (−)-EPI is rapidly absorbed and undergoes rapid phase 2 metabolism. The three most dominant groups of metabolites detected (by abundance) were sulfated, glucuronidated and methyl-sulfated (−)-EPI, which were detected in concentrations higher than free (−)-EPI. Measured plasma concentration of (−)-EPI and metabolites were proportional by a factor of ~2.7 to the administered dose over the range of 50–200 mg. The half-life of (−)-EPI and all of the metabolites was similar in all samples assayed and ranged between 1–3 h. There were no significant differences in the PK profile of 50 mg of (−)-EPI after 4 days of q.d. or b.i.d. compared to day 1. However, inter- and intra-individual variability in plasma (−)-EPI and metabolites were observed in the multiple dose study.
Our results are consistent with two of the most recent studies evaluating (−)-EPI PK and metabolism following cocoa product consumption 12, 13. In both studies, (−)-EPI-3′-β-O-glucuronide, (−)-EPI-3′-sulfate, and 3′-O-methyl-(−)-EPI (substituted in the 4′, 5, and 7 positions)-sulfate were the predominant plasma metabolites. In our study, we found (−)-EPI-glucuronide, (−)-EPI-sulfate and methyl-(−)-EPI-sulfate as the main metabolites. However, the exact position of the substituents cannot be determined due to the unavailability of pure standards. In this study for the first time, we report on the half-life of free (−)-EPI in plasma. Plasma levels of free (−)-EPI levels were higher at the 100 and 200 mg doses. Currently, it is unclear if the biological effects of (−)-EPI are mediated by the free molecule, any of its metabolites or both.
Laboratory assessment at baseline and on day 5 included complete blood count (CBC), electrolytes, lipids, renal and liver function. Platelet counts and alkaline phosphatase levels were the only endpoints that decreased both, within normal limits and have no known clinical significance. Our findings showing that (−)-EPI can be safely administered are consistent with extensive literature of human studies using high-flavanol cocoa or chocolate, indicating that flavanols can be administered at doses up to 1008 mg/day for 15 days and 444 mg flavanols/day for 6 weeks without adverse effects 14, 15. A safety study of a green tea extract containing ~124 mg (−)-EPI per dose showed that once a day dosing for 4 weeks yielded the same safety profile as placebo, with no significant differences in hematologic or clinical chemistry 16.
The mechanisms that mediate the reported beneficial effects of (−)-EPI on vasculature remain unclear. In order to indirectly assess the effects of (−)-EPI on NO metabolism we measured plasma nitrite, nitrate and SNO at baseline on day 1 and prior to the final dose of (−)-EPI on day 5. Nitrite increased significantly in the q.d. and b.i.d. groups. These findings are consistent with other recent studies and suggest an increase in NO production following (−)-EPI dosing 4, 5, 13. Interestingly, a meta-analysis of studies examining the effects of chocolate on BP found that dark chocolate significantly reduced BP relative to controls, but only in hypertensive or pre-hypertensive subgroups 17. BP was not significantly reduced in the normotensive subgroups, suggesting that flavanols including (−)-EPI, do not override control of normal BP and apparently do not pose a significant risk of producing hypotension consistent with our observations on the stability of vital signs in this study.
Improvements in mitochondrial structure and function have also been suggested as mechanisms for cocoa’s healthy effects 7, 8. The effects of q.d. (−)-EPI consumption on mitochondrial function were assessed by measuring platelet mitochondrial function. Results indicate a significant increase in complex I, IV and citrate synthase activities. These results parallel the positive effects that (−)-EPI has on skeletal muscle citrate synthase activity 6, 7. We have also previously reported increases in cardiac and skeletal muscle mitochondria cristae abundance, volume as well as complex I and IV protein abundance 7. It is possible that the effects noted on complex I, IV and citrate synthase activities may follow similar actions on platelets. Of interest is that we have reported 18 that increases in NO metabolites (nitrites) can stimulate mitochondrial biogenesis and the increases reported in this study for nitrite levels may partially account for the positive effects noted on platelet mitochondria function.
Plasma follistatin levels were measured in subjects receiving (−)-EPI q.d. for 5 days. At day 1, plasma levels averaged ~254 pg/ml and peaked following (−)-EPI to ~404 pg/ml. Following 5 days of (−)-EPI b.i.d., baseline values averaged ~881 pg/ml and peaked at ~1239 pg/ml. Follistatin AUC levels at day 5 were ~2.5 fold higher vs. day 1 indicating that repeated (−)-EPI dosing has a vigorous and sustained effect on follistatin production. The biological roles of follistatin are not completely understood but beneficial effects on muscle growth have been documented 19–21. The only known physiological inducer of increased plasma follistatin levels is physical exercise 22. To our knowledge, this is the first demonstration that a small molecule can increase follistatin plasma levels. Myostatin is the most effective inhibitor of muscle growth known to date 23, 24. Follistatin by binding to myostatin can promote muscle growth resulting from interfering with myostatin attaching to its receptor 25. The reported effects are in agreement with results obtained from the use of (−)-EPI-rich cocoa in heart failure patients 9.
In summary, results demonstrate that purified (−)-EPI is rapidly absorbed and modified by phase 2 metabolism. PK data indicates an average half-life of 2.5 h and 1.2–3.1 h for free (−)-EPI and metabolites, respectively. No adverse effects attributable to (−)-EPI were reported or observed. Additionally, our findings suggest that increases in NO metabolites, mitochondrial enzyme function and plasma follistatin levels may underlie some of the beneficial effects of cocoa products or (−)-EPI as reported in other studies. The fact that multiple and potentially independent blood “biomarkers” of (−)-EPI effects yielded positive signals is encouraging as future clinical trials may take advantage of these endpoints so as to document the effectiveness of specific dosing schemes on endpoints of interest.
Study limitations
This is an initial PK study with a small number of participants where all analyses and conclusions may not fully represent the characteristics of (−)-EPI behavior in the general population. The method used for (−)-EPI metabolite estimation was based on inferred values given the absence of available standards. Regarding PD outcomes, this study was implemented to analyze a limited number of vascular and blood related endpoints. More detailed studies will need to be implemented in the future in order to explore these and other endpoints using a larger number of participants. Changes in platelet numbers and alkaline phosphatase levels observed in the 5 day b.i.d. intake group (although within normal limits) may or may not represent adverse effects and will need to be monitored in future, longer term studies.
Supplementary Material
Acknowledgments
We wish to acknowledge the contributions of Rebecca Scherzer for statistical advice and Dr. Neil Benowitz. C. B., P.T, G.C., S.D., G.S., and F.V. contributed to study design. A.M.U. assisted with (−)-EPI analysis in plasma. S.S. was involved in plasma endpoints analysis. I.R.S. carried out follistatin determinations. S.Y. assisted in HPLC-MS analysis. All authors contributed to manuscript preparation. This study was supported by Cardero Therapeutics Inc (Los Altos Hills, CA). This project was supported by the National Center for Advancing Translational Sciences of the NIH, through UCSF-CTSI Grant UL1 TR000004 and DK092154. Dr. Barnett received research funding from Cardero Therapeutics. Drs. Taub and Villarreal are co-founders of Cardero Therapeutics. Dr. Schreiner is employed by Cardero Therapeutics and Dr. Ceballos is a stockholder of the company.
References
- 1.Katz DL, Doughty K, Ali A. Antioxid Redox Signal. 2011;15:2779–2811. doi: 10.1089/ars.2010.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buitrago-Lopez A, Sanderson J, Johnson L, Warnakula S, Wood A, Di Angelantonio E, Franco OH. BMJ. 2011;343:d4488. doi: 10.1136/bmj.d4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hooper L, Kay C, Abdelhamid A, Kroon PA, Cohn JS, Rimm EB, Cassidy A. Am J Clin Nutr. 2012;95:740–751. doi: 10.3945/ajcn.111.023457. [DOI] [PubMed] [Google Scholar]
- 4.Schroeter H, Heiss C, Balzer J, Kleinbongard P, Keen CL, Hollenberg NK, Sies H, Kwik-Uribe C, Schmitz HH, Kelm M. Proc Natl Acad Sci U S A. 2006;103:1024–1029. doi: 10.1073/pnas.0510168103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loke WM, Hodgson JM, Proudfoot JM, McKinley AJ, Puddey IB, Croft KD. Am J Clin Nutr. 2008;88:1018–1025. doi: 10.1093/ajcn/88.4.1018. [DOI] [PubMed] [Google Scholar]
- 6.Huttemann M, Lee I, Malek MH. FASEB J. 2012;26:1413–1422. doi: 10.1096/fj.11-196154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nogueira L, Ramirez-Sanchez I, Perkins GA, Murphy A, Taub PR, Ceballos G, Villarreal FJ, Hogan MC, Malek MH. J Physiol. 2011;589:4615–4631. doi: 10.1113/jphysiol.2011.209924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taub PR, Ramirez-Sanchez I, Ciaraldi TP, Perkins G, Murphy AN, Naviaux R, Hogan M, Maisel AS, Henry RR, Ceballos G, Villarreal F. Clin Transl Sci. 2012;5:43–47. doi: 10.1111/j.1752-8062.2011.00357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taub PR, Ramirez-Sanchez I, Ciaraldi TP, Gonzalez-Basurto S, Coral-Vazquez R, Perkins G, Hogan M, Maisel AS, Henry RR, Ceballos G, Villarreal F. Clin Sci (Lond) 2013;125:383–389. doi: 10.1042/CS20130023. [DOI] [PubMed] [Google Scholar]
- 10.Roura E, Andres-Lacueva C, Jauregui O, Badia E, Estruch R, Izquierdo-Pulido M, Lamuela-Raventos RM. J Agric Food Chem. 2005;53:6190–6194. doi: 10.1021/jf050377u. [DOI] [PubMed] [Google Scholar]
- 11.Mukherjee A, Srere PA, Frenkel EP. J Biol Chem. 1976;251:2155–2160. [PubMed] [Google Scholar]
- 12.Actis-Goretta L, Leveques A, Giuffrida F, Romanov-Michailidis F, Viton F, Barron D, Duenas-Paton M, Gonzalez-Manzano S, Santos-Buelga C, Williamson G, Dionisi F. Free Radic Biol Med. 2012;53:787–795. doi: 10.1016/j.freeradbiomed.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 13.Ottaviani JI, Momma TY, Kuhnle GK, Keen CL, Schroeter H. Free Radic Biol Med. 2012;52:1403–1412. doi: 10.1016/j.freeradbiomed.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 14.Grassi D, Desideri G, Necozione S, Lippi C, Casale R, Properzi G, Blumberg JB, Ferri C. J Nutr. 2008;138:1671–1676. doi: 10.1093/jn/138.9.1671. [DOI] [PubMed] [Google Scholar]
- 15.Farouque HM, Leung M, Hope SA, Baldi M, Schechter C, Cameron JD, Meredith IT. Clin Sci (Lond) 2006;111:71–80. doi: 10.1042/CS20060048. [DOI] [PubMed] [Google Scholar]
- 16.Chow HH, Cai Y, Hakim IA, Crowell JA, Shahi F, Brooks CA, Dorr RT, Hara Y, Alberts DS. Clin Cancer Res. 2003;9:3312–3319. [PubMed] [Google Scholar]
- 17.Ried K, Sullivan T, Fakler P, Frank OR, Stocks NP. BMC Med. 2010;8:39. doi: 10.1186/1741-7015-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mo L, Wang Y, Geary L, Corey C, Alef MJ, Beer-Stolz D, Zuckerbraun BS, Shiva S. Free Radic Biol Med. 2012;53:1440–1450. doi: 10.1016/j.freeradbiomed.2012.07.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Medeiros EF, Phelps MP, Fuentes FD, Bradley TM. Am J Physiol Regul Integr Comp Physiol. 2009;297:R235–242. doi: 10.1152/ajpregu.91020.2008. [DOI] [PubMed] [Google Scholar]
- 20.Matzuk MM, Lu N, Vogel H, Sellheyer K, Roop DR, Bradley A. Nature. 1995;374:360–363. doi: 10.1038/374360a0. [DOI] [PubMed] [Google Scholar]
- 21.Lee SJ, Lee YS, Zimmers TA, Soleimani A, Matzuk MM, Tsuchida K, Cohn RD, Barton ER. Mol Endocrinol. 2010;24:1998–2008. doi: 10.1210/me.2010-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hansen J, Brandt C, Nielsen AR, Hojman P, Whitman M, Febbraio MA, Pedersen BK, Plomgard P. Endocrinology. 2011;152:164–171. doi: 10.1210/en.2010-0868. [DOI] [PubMed] [Google Scholar]
- 23.McPherron AC, Lawler AM, Lee SJ. Nature. 1997;387:83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- 24.Lee SJ, McPherron AC. Proc Natl Acad Sci U S A. 2001;98:9306–9311. doi: 10.1073/pnas.151270098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cash JN, Rejon CA, McPherron AC, Bernard DJ, Thompson TB. EMBO J. 2009;28:2662–2676. doi: 10.1038/emboj.2009.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.