Introduction
Cell therapy products have become more sophisticated both in type and manufacturing complexity (1-3). As clinicians' and researchers' understanding of disease and cell mechanisms of action continue to grow, cell therapy products are being increasingly used or considered for use in clinical trials (4-12). The safety and efficacy of cell therapy products have been reported within individual clinical trials and through review articles (13-19). These publications are often limited to an individual cell type and also differ by how the data are being collected and reported. The Health Resources and Services Administration (HRSA) has provided funds to study the use of cell therapy in the United States with a goal of building a database similar to that established and maintained by the bone marrow transplant community for years. The European Union has pursued a similar goal. Despite these efforts, there is limited published information that describes cell therapy product administration across both cell types and indications. To this end, The National Institutes of Health, National Heart, Lung and Blood Institute (NHLBI) Production Assistance for Cellular Therapies (PACT) program has collected data over the past 10 years on the administration of cell therapy products manufactured within the program.
The PACT program was formed in 2003 through funding from the NHLBI. The program provides clinical cell therapy product manufacturing support to investigators wishing to transition a novel cell therapy from the developmental stage to clinical applications within the purview of the NHLBI. PACT is not responsible for directly monitoring the clinical trials of investigators receiving PACT-manufactured products. PACT has required collection of standardized information on product manufacturing, transport, receipt, administration, and adverse reactions with product administration.
The purpose of this data collection is 1) to monitor administration of PACT cell therapy products, and 2) to build a product administration database to identify trends or safety concerns that may be associated with cell therapy product administrations. All clinical trials where PACT has provided manufacturing support were approved by local ethics committees.
Methods
Development of Data Collection Forms and Web-Based Data Collection System
The PACT Coordinating Center (CC), the EMMES Corporation, was responsible for development of the data collection forms in conjunction with the PACT cell processing facilities. These forms were used by the cell processing facility staff and clinical investigators. The forms were developed in conformance with Food and Drug Administration (FDA) regulations, and AABB and the Foundation for the Accreditation of Cellular Therapy (FACT) standards, as applicable. PACT also collaborated with the Center for International Blood and Marrow Transplant Research (CIBMTR), which also had a mandate to develop a database on the use of hematopoietic cell therapy products in clinical trials. Additionally, the forms' content was harmonized with the cell therapy collections forms used by the European Society for Blood and Marrow Transplantation (EBMT). PACT outcome data are collected at 3, 6, and 12 months after product administration in the clinical trial.
The following forms were used for clinical product data collection:
Product Manufacturing Information (including cell type)
Product Transport and Receipt (including fresh or cryopreserved)
Product Administration
Post-Product Administration (Outcome) Data (started in 2010; outcome data was not requested for product administrations prior to 2010)
On the Product Administration form, the data elements include:
Primary treatment indication (cardiovascular, pulmonary, hematology, BMT, or other)
Route of administration
Whether or not the subject experienced any adverse reactions associated with product administration
Type(s) of reactions, if applicable
Severity of reactions
On the Post-Product Administration Data form, the data elements include:
- Response to cellular therapy related to:
- Efficacy endpoint (achieved/not achieved, unknown, not evaluated, or N/A)
- Safety evaluation (no, mild, or severe toxicity, or unknown)
- Clinical response (none, partial, complete, or unknown)
Survival: survival status (alive, dead, or lost to follow up) and primary cause of death
Additionally, the PACT CC was responsible for developing the web-based data collection system. The PACT CC monitored the data for completeness, generated queries, and implemented database upgrades as needed.
Data Collection Procedures
The paper data collection forms, as well as the PACT web-based data collection system (through which data are submitted to the CC), are made accessible to the PACT cell processing facility staff through the PACT website. Applicable paper data collection forms are also provided to the clinical site investigator when his/her clinical trial commences.
Both the designated cell processing facility and the clinical site investigator are required to provide information in designated sections of the data collection forms. The paper forms are returned to the cell processing facilities for entry into the data system.
The number and status of clinical projects approved to receive PACT services are shown in Figure 1. Over 50 clinical projects have been approved. Of those, 37 have administered cell products. The clinical cell therapy product data collected to date are from 8 cell product types provided for 31 clinical projects. Six clinical projects were unable to provide clinical cell therapy product data at the time of product administration for a variety of logistical reasons. The specific cell product types that PACT has manufactured for clinical trials and the total number of data forms collected are listed in Table 1.
Figure 1.
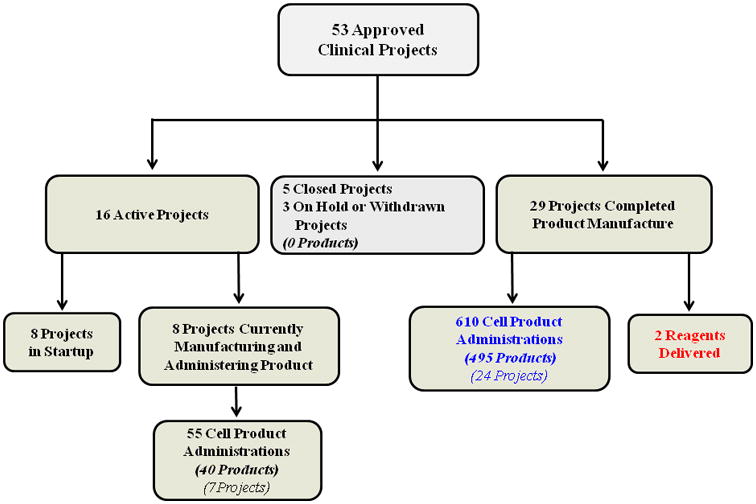
Number and status of clinical projects approved to receive PACT services. The number of products refers to the number of unique lots of a cell product manufactured by the cell processing facilities. Larger product lots (e.g., multiple vials) may have been used for multiple administrations.
Table 1.
Cell product types manufactured for clinical use and total number of product administration data forms collected.
A subset of the total number of administrations by each cell product type occurring since 2010, during which the request for outcome data collection began, is indentified in the table.
| Cell Product Type | Product Administration Forms (2003-2014) | Product Administration Forms (2010-2014)* | Post-Product Administration (Outcome) Data Forms | ||
|---|---|---|---|---|---|
| Month 3 | Month 6 | Month 12 | |||
| Bone marrow mononuclear cells (BM-MNCs) | 240 | 141 | 26 | 25 | 23 |
| Cytotoxic T lymphocytes (CTLs) | 209 | 132 | 9 | 8 | 1 |
| Natural killer (NK) cells | 77 | 15 | 13 | 6 | 1 |
| Dendritic cells (DCs) | 49 | 0 | 0 | 0 | 0 |
| T regulatory cells (Tregs) | 46 | 5 | 3 | 1 | 0 |
| Hematopoietic stem cells (HSCs) | 29 | 29 | 29 | 23 | 21 |
| Mesenchymal stem/stromal cells (MSCs) | 13 | 13 | 10 | 2 | 0 |
| CD133+ progenitor cells | 2 | 2 | 0 | 0 | 0 |
| Total forms collected | 665 | 337 | 90 | 65 | 46 |
Outcome data collection began in 2010
Data Receipt and Review
The CC provides administrative support and reviews the data for completeness, validity, and consistency. Any questions regarding anomalies or inconsistencies are distributed to the cell processing facilities and tracked at the CC until resolution is obtained. Reports are developed based on the collected data to provide metrics and facilitate monitoring by the PACT Steering Committee.
Results
Forms Collected
As of February 2014, PACT has received 665 Product Administration forms and 201 Post-Product Administration (Outcome) Data forms (Table 1). Of the five primary treatment indications, 232 Product Administration forms have been collected for cardiovascular-related, 127 forms for hematology-related, 11 for bone marrow transplant (BMT)-related, and 3 for pulmonary-related indications. Product Administration forms collected for other indications included viral infections (145), lymphoma (Hodgkin and non-Hodgkin) (92), diabetes (33), traumatic brain injury (10), multiple myeloma (9), and GVHD prevention (3).
By cell type, the 665 administrations include 240 bone marrow mononuclear cells (BM-MNCs), 209 cytotoxic T lymphocytes (CTLs), 77 natural killer (NK) cells, 49 dendritic cells (DCs), 46 T regulatory cells (Tregs), 29 hematopoietic stem cells (HSCs), 13 mesenchymal stem/stromal cells (MSCs), and 2 CD133+ progenitor cells (Table 1).
Post-Product Administration (Outcome) data form collection began in 2010. Since then, 337 product administrations have occurred. Forms were collected at 3, 6, and 12 month time points post product administration with 90, 65, and 46 forms collected, respectively, for a total of 201 forms (Table 1). The majority of these forms have been for products used in hematology-related indications (85), 74 for cardiovascular-related, 13 for BMT-related indications, and 29 related to other indications (data not shown).
Adverse Reactions with Product Administration
Adverse reactions post-administration were reported on 8.4% (56/665) of Product Administration forms. A total of 114 adverse reactions were reported from 56 product administrations, with one or more adverse reactions being reported with a single product administration. The three most common types of post-administration-related adverse reactions were chills 46% (26/56), hypertension 30% (17/56), and fever 21% (12/56) (Table 2). Nine of these 56 reactions (16%) occurred with administration of thawed cryopreserved products containing dimethyl sulfoxide (DMSO). This represents 3% (9/265) of the products in which DMSO was used as a cryopreservative.
Table 2. Summary of product administration data.
Each row represents cell product data collected from a unique PACT approved project or clinical trial having the unique attributes of cell product type, administration of fresh or cryopreserved cells, and route of administration unless as otherwise footnoted below. Data from the 3 most common post-administration adverse reaction types are provided separately and all others were grouped together. The product source is indicated in parentheses.
| Cell Product Type | Fresh/Cryopreserved (DMSO) | Route of Administration | Adverse Reaction | Reaction Type | ||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | Chills | Hyper-tension | Fever | Other | |||
| BM-MNCs (auto, BM-derived)a | Fresh | Intra-arterial | - | 164 | - | - | - | - |
| Fresh | Direct tissue injection | - | 41 | - | - | - | - | |
| Fresh | Intravenous | - | 35 | - | - | - | - | |
| CD133+ progenitor cells (auto, leukapheresis) | Fresh | Intramuscular | - | 2 | - | - | - | - |
| CTLs (auto, PB-derived) | Cryopreserved | Intravenous | - | 77 | - | - | - | - |
| CTLs (allo, BM-derived) | Cryopreserved | Intravenous | - | 12 | - | - | - | - |
| CTLs (allo, PB-derived)b | Cryopreserved | Intravenous | 8 | 112 | - | - | 3 | 5 |
| DCs (auto, leukapheresis) | Cryopreserved | Other: Intra-lymph node | - | 16 | - | - | - | - |
| DCs (auto, leukapheresis) | Cryopreserved | Intra-dermal | - | 33 | - | - | - | - |
| HSCs (allo, UCB-derived) | Fresh | Intravenous | 5 | 24 | - | 5 | - | 4 |
| MSCs (allo, BM-derived) | Fresh | Intravenous | 4 | 6 | 1 | 1 | - | 9 |
| MSCs (allo, BM-derived) | Cryopreserved | Intravenous | - | 3 | - | - | - | - |
| NK cells (allo, apheresis)c | Fresh | Intravenous | 26 | 26 | 16 | 9 | 5 | 34 |
| NK cells (allo, UCB-derived) | Fresh | Intravenous | 9 | 7 | 8 | 1 | 3 | 3 |
| NK cells (auto or allo, leukapheresis)d | Fresh | Intravenous | 1 | 4 | 1 | - | 1 | 1 |
| Cryopreserved | 1 | 3 | - | - | - | 1 | ||
| T regs (allo, PB-derived) | Fresh | Intravenous | - | 3 | - | - | - | - |
| T regs (allo, UCB-derived)e | Fresh | Intravenous | 2 | 41 | - | 1 | - | 2 |
| Total | 56 | 609 | 26 | 17 | 12 | 59 | ||
Source: auto = autologous; allo = allogeneic; BM-derived = bone marrow-derived; PB-derived = peripheral blood-derived; UCB-derived = umbilical cord blood-derived. Cell type: BM-MNCs = bone marrow mononuclear cells; CTLs = cytotoxic T lymphocytes; DCs = dendritic cells; MSCs = mesenchymal stem/stromal cells; NK = natural killer cells; Tregs = T regulatory cells; HSCs = hematopoietic stem cells.
Data collected from 1 single-site and 3 multi-site trials.
Data collected from 4 single-site trials, 1 multi-site trial, and from third party use under compassionate use INDs.
Data collected from 3 single-site trials.
This single-site trial performed 9 administrations and used either autologous (7) or allogeneic (2) NK cells that were administered either fresh (5) or upon thaw (4).
Data collected from 2 single-site trials.
Sixty-six percent (37/56) of reported adverse reactions followed administration of natural killer (NK) cells representing 48% (37/77) of the NK cell product administrations. The indications for use of NK cells included multiple myeloma, hematopoietic stem cell transplant (HSCT) for acute myeloid leukemia, cancer, and other hematological malignancies. Other adverse reactions were also seen after administration of CTLs - 4% (8/209), T reg cells - 4% (2/46), HSCs - 17% (5/29), and MSCs - 40% (4/10). No adverse reactions were reported with DCs, BM-MNCs, or CD133+ progenitor cells (Table 2).
Approximately 62% (409/665) of cell products were administered intravenously with adverse reactions occurring in 14% (56/409). No adverse reactions were reported for intra-arterial, -dermal, -muscular, or other administration routes which accounted for 38% (256/665) of product administrations. A summary of Product Administration data is provided in Table 2. Data on the severity of reported reactions were requested; however, severity data received from the clinical sites were often missing, and therefore only a binary presence or absence is reported.
Outcome Data
Since the request for outcome data began in 2010, only 27% (90/337) of product administrations reported had associated outcome data forms provided. Thus, information pertaining to clinical efficacy, safety, and clinical response is limited. An outcome form data entry field without a response was included in the “unknown” category for this analysis. At Month 3, 8 of the 90 forms reported that a clinical efficacy endpoint was achieved, 5 not achieved, 2 were not applicable, and the remaining 75 were unknown. There were no reported instances of severe toxicity at any time point (i.e., Months 3, 6, and 12), and only one reported instance of mild toxicity from a CTL product at Month 3. Complete and partial clinical responses were noted only for the CTL and NK cell product types at any time point (Month 6 and Month 12 data not shown).
Thirty-two deaths were reported on the Outcome data forms, with 20 of those deaths reported by Month 3. Primary causes of death were reported as either 1) disease relapse/progression/persistent disease or 2) other cause. No deaths were reported as related to the cellular therapy product. A summary of efficacy, safety and clinical response outcome data for the Month 3 time point is provided in Table 3.
Table 3. Summary of efficacy, safety, and clinical response outcome data collected at Month 3.
Each row represents cell product data collected from a unique PACT approved project or clinical trial having the unique attributes of cell product type, administration of fresh or cryopreserved cells, and route of administration unless as otherwise footnoted below. A form data entry field without a response was included in the ‘unknown’ category for this analysis. The product source is indicated in parentheses.
| Cell Product Type | Indication | Product Administrations (2010-2014) | Efficacy | Safety | Clinical Response | |||
|---|---|---|---|---|---|---|---|---|
| BM-MNCs (auto, BM-derived)a | Cardiovascular | 26 | Unknown | 26 | Unknown | 26 | Unknown | 26 |
| CTLs (allo, PB-derived)b | Other disease indication | 8 | Achieved | 6 | No toxicity | 6 | Complete | 2 |
| Unknown | 1 | Mild toxicity | 1 | Unknown | 1 | |||
| Not Applicable | 1 | Unknown | 1 | Partial | 3 | |||
| -- | -- | -- | -- | None | 2 | |||
| CTLs (allo, BM-derived) | Other disease indication | 1 | Achieved | 1 | No toxicity | 1 | Complete | 1 |
| HSCs (allo, UCB-derived) | Hematology | 29 | Unknown | 29 | Unknown | 29 | Unknown | 29 |
| MSCs (allo, BM-derived) | Hematology | 10 | Unknown | 10 | Unknown | 10 | Unknown | 10 |
| NK cells (auto and allo, leukapheresis) | Other disease indication | 7 | Achieved | 1 | No toxicity | 5 | None | 5 |
| Not Achieved | 5 | Unknown | 2 | Complete | 1 | |||
| Not Applicable | 1 | -- | -- | Unknown | 1 | |||
| NK cells (allo, apheresis) | BMT | 6 | Unknown | 6 | Unknown | 6 | Unknown | 6 |
| Tregs (allo, UCB-derived) | BMT | 3 | Unknown | 3 | Unknown | 3 | Unknown | 3 |
| Total product administrations with outcome data | 90 | |||||||
Source: auto = autologous; allo = allogeneic; BM-derived = bone marrow-derived; PB-derived = peripheral blood-derived; UCB-derived = umbilical cord blood-derived. BM-MNCs = bone marrow mononuclear cells; CTLs = cytotoxic T lymphocytes; DCs = dendritic cells; MSCs = mesenchymal stem/stromal cells; NK = natural killer cells; Tregs = T regulatory cells; UCB-derived HSCs = umbilical cord blood-derived hematopoietic stem cells.
Data collected from 2 single-site trials.
Data collected from 2 single-site trials, 1 multi-site trial, and from third party use under compassionate use INDs.
Discussion
Cellular therapy products are currently manufactured by biotechnology companies and academic institutions. These groups are largely invested in their products, and often maintain portfolios of intellectual property rights. As a result, data generated from these products, including specific product administration data, have largely been kept confidential. It has also not been possible to obtain standardized data from these entities. The PACT program has developed a central database for collection of cell therapy product administration and outcome data across a variety of cell types used for a number of clinical indications. PACT collects a standard data set from each clinical trial for which it has manufactured clinical product. The PACT program has achieved a level of operational efficiency among the five participating cell processing facilities such that obtaining central and consistent data related to the cell manufacturing process and administration is successful. The PACT program has done this without jeopardizing intellectual property. Of note, the primary point of data collection has been at the cell processing facility and not the clinical site.
There have been challenges, however, obtaining the data related to outcomes (at 3, 6, and 12 months) from the clinical sites. They include: 1) PACT does not have direct access to the clinical data; only the manufacturing data, 2) outcome data are often a part of the primary endpoint for the clinical trial and are, therefore, not available until results have been published well after the completion of the trial and without any involvement of the manufacturing facility, and 3) investigators have limited interest in devoting the time and resources to provide these data to a third party on an ongoing basis.
Based on the administration and outcome data that have been collected thus far, PACT-manufactured cell therapy products have been well tolerated. Responses to product administration have not been the same for all cell therapy products, as demonstrated by the higher rate of events noted with the administration of NK cells. This may be due to the nature of the cell or manufacturing method, e.g., incubation with cytokines such as IL-2 or IL-15 (20-22). Specifically, remaining IL-2 after the product is washed with 5% human serum albumin or cytokines produced by the NK cells as the cells sit for a few hours awaiting lot release testing prior to administration likely contribute to the reactions seen in subjects. Other possible factors such as administration volume, cell dose administered, or subject preparative/lymphodepletion regimens are less likely the cause. Administered volumes were well within the mid-range for cell therapies, and the dosage administered was not high. Additionally the preparative/lymphodepletion regimens implemented were not intensive (20). As noted previously, longer term data are not as easily obtained, making it difficult to analyze the clinical efficacy of the cell products. Additionally, there are a variety of cell types and disease areas represented in the individual clinical trials for which PACT has provided product (23). Large databases will need to be developed and populated to allow these types of analyses.
The field of cellular therapy is relatively new, yet basic and preclinical research and initial clinical trials have shown very promising results, stimulating interest in extending its clinical applications. The majority of current research, which covers a wide range of indications and cell types, is still at an early stage with only a small fraction of cellular therapies reaching Phase III clinical stage and even fewer achieving licensure. Data regarding different cell types and indications need to be centralized and analyzed in a uniform way.
As many of the indications for cellular therapies are directed at relatively rare genetic or immunologic indications, it becomes difficult to adequately assess the safety of a given cell type for one indication in a single clinical trial.
Therefore, there is a need in the cellular therapy community for collaboration and central data collection to enhance the ability to characterize the safety profile for a given cell product. Researchers and regulators could have more compelling uniform data to support, and possibly advance, research more quickly as safety profiles are established.
It would require effort and incentives well beyond the scope of the PACT program to advance this goal.
The PACT program has established a proof of concept that centralized data collection is possible across cell therapy manufacturing. The challenge is to the cell therapy community at large to promote establishing and committing to support this type of effort beyond NIH funding. The basic structure PACT established can be built upon as a model for future initiatives to meet this goal.
Acknowledgments
The PACT program is supported with federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services under contract numbers HHSN268201000006C, HHSN268201000007C, HHSN268201000008C, HHSN268201000009C, HHSN268201000010C, and HHSN268201000011C.
Footnotes
Conflict of Interest: Cliona M. Rooney PhD - board membership, Cell Medica; consultant, license fees, Celgene.
All other authors declare no conflict.
References
- 1.Rayment E, Williams D. Concise review: mind the gap: challenges in characterizing and quantifying cell- and tissue-based therapies for clinical translation. Stem Cells. 2010;28:996–1004. doi: 10.1002/stem.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belardelli F, Rizza P, Moretti F, Carella C, Gallia MC, Migliaccio G. Translational Research on Advanced Therapies. Ann Ist Super Sanita. 2011;47:72–78. doi: 10.4415/ANN_11_01_15. [DOI] [PubMed] [Google Scholar]
- 3.Giancola R, Bonfini T, Iacone A. Cell therapy: cGMP facilities and manufacturing. MLT Journal. 2012;2:243–47. [PMC free article] [PubMed] [Google Scholar]
- 4.Leventhal J, Miller J, Abecasssis M, Tollerud D, Ildstad S. Evolving approaches of hematopoietic stem cell-based therapies to induce tolerance to organ transplants: the long road to tolerance. Clin Pharmacol Ther. 2013;93:36–45. doi: 10.1038/clpt.2012.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trounson A. New perspectives in human stem cell therapeutic research. BMC Med. 2009;7:29. doi: 10.1186/1741-7015-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoyos V, Savoldo B, Dotti G. Genetic modification of human T lymphocytes for the treatment of hematologic malignancies. Haematologica. 2012;97:1622–31. doi: 10.3324/haematol.2012.064303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller J, Warren E, Brink M, Ritz J, Shlomchik W, Murphy W, et al. NCI first international workshop on the biology, prevention, and treatment of relapse after allogeneic hematopoietic stem cell transplantation: report from the committee on the biology underlying recurrence of malignant disease following allogeneic HSCT: graft-versus-tumor/leukemia reaction. Biol Blood Marrow Transplant. 2010;16:565–86. doi: 10.1016/j.bbmt.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Copland I. Mesenchymal stromal cells for cardiovascular disease. J Cardiovasc Dis Res. 2011;2:3–13. doi: 10.4103/0975-3583.78581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel D, Shah J, Srivastava A. Therapeutic potential of mesenchymal stem cells in regenerative medicine. Stem Cells Int. 2013 doi: 10.1155/2013/496218. Eepub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barcala Tabarrozzi AE, Castro CN, Dewey RA, Sogayar MC, Labriola L, Perone MJ. Cell-based interventions to halt autoimmunity in type 1 diabetes mellitus. Clin Exp Immunol. 2013;171:135–46. doi: 10.1111/cei.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang Q, Bluestone JA. Regulatory T-cell therapy in transplantation: moving to the clinic. Cold Spring Harb Perspect Med. 2013;3:a015552. doi: 10.1101/cshperspect.a015552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McMurchy A, Bushell A, Levings M, Wood K. moving to tolerance: clinical applications of T regulatory cells. Semin Immunol. 2011;23(4) doi: 10.1016/s.smim.2011.04.001. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lanzoni G, Roda G, Belluzzi A, Roda E, Bagnara GP. Inflammatory bowel disease: moving toward a stem cell-based therapy. World J Gastroenterol. 2008;14:4616–26. doi: 10.3748/wjg.14.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dalal J, Gandy K, Domen J. Role of mesenchymal stem cell therapy in crohn's disease. Pediatr Res. 2012;71:445–51. doi: 10.1038/pr.2011.56. [DOI] [PubMed] [Google Scholar]
- 15.Lalu M, McIntyre L, Pugliese C, Fergusson D, Winston B, Marshall J, et al. Safety of cell therapy with mesenchymal stromal cells (safecell): a systematic review and meta-analysis of clinical trials. PLOS ONE. 2012;7(10):e47559. doi: 10.1371/journal.pone.0047559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giannoukakis N, Phillips B, Finegold D, Harnaha J, Truco M. Phase I (safety) study of autologous tolerogenic dendritic cells in type 1 diabetic patients. Diabetes Care. 2011;34:2026–32. doi: 10.2337/dc11-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Ianni M, Falzetti F, Carotti A, Terenzi A, Castellino F, Bonifacio E, et al. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood. 2011;117:3921–28. doi: 10.1182/blood-2010-10-311894. [DOI] [PubMed] [Google Scholar]
- 18.Wernicke C, Grunewald T, Hendrik J, Kuci S, Kuci Z, Koehl U, et al. Mesenchymal stromal cells for treatment of steroid-refractory GVHD: a review of the literature and two pediatric cases. Int Arch Med. 2011;4:27. doi: 10.1186/1755-7682-4-27. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tibbetts MD, Samuel MA, Chang TS, Ho AC. Stem cell therapy for retinal disease. Curr Opin Ophthalmol. 2012;23:226–34. doi: 10.1097/ICU.0b013e328352407d.. [DOI] [PubMed] [Google Scholar]
- 20.Miller J, Soignier Y, Panoskaltsis-Mortari A, McNearney S, Yun G, Fautsch S, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–57. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 21.McKenna D, Sumstad D, Bostrom N, Kadidlo D, Fautsch S, McNearney S, et al. Good manufacturing practices production of natural killer cells for immunotherapy: a six-year single-institution experience. Transfusion. 2007;47:520–28. doi: 10.1111/j.1537-2995.2006.01145.x. [DOI] [PubMed] [Google Scholar]
- 22.Klingemann H, Grodman C, Cutler E, Duque M, Kadidlo D, Klein A, et al. Autologous stem cell transplant recipients tolerate haploidentical related-donor natural killer cell-enriched infusions. Transfusion. 2013;53:412–18. doi: 10.1111/j.1537-2995.2012.03764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wood D, Wesselschmidt R, Hematti P, Gee AP, Rooney C, Silberstein L, et al. An update from the United States National Heart, Lung, and Blood Institute-funded Production Assistance for Cellular Therapies (PACT) program: A decade of cell therapy. Clin Trans Sci. 2014;7(2):93–9. doi: 10.1111/cts.12148. Epub 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]


