Abstract
Bone augmentation requires scaffold to promote forming of natural bone structure. Currently, most of the reported bone scaffolds are porous solids with uniform pores. The aim of the currentstudy is to evaluate the effect of a graded porous β-tricalcium phosphate scaffolds on alveolar bone augmentation. Three groups of scaffolds were fabricated by a template-casting method: (1) graded porous scaffolds with large pores in the center and small pores at theperiphery, (2) scaffolds with large uniform pores, and (3) scaffolds with small uniform pores. Bone augmentation on rabbit mandible wasinvestigated by microcomputed tomography, sequential fluorescentlabeling, and histologic examination 3 months after implantation.The result presents that all the scaffold groups maintain their augmented bone height after 3-month observation, whereas the autograftinggroup presents an obvious bone resorption. Microcomputed tomography reveals that the graded porous group has significantly greater volume of new bone (P < 0.05) and similar bone density compared with the uniform pores groups. Bone substance distributes unevenly in all the 3 experimental groups. Greater bone volume can be observed in the area closer to the bone bed. The sequential fluorescentlabeling observation reveals robust bone regeneration in the first month and faster bone growth in the graded porous scaffold group than that in the large porous scaffold group. Histologic examinationsconfirm bone structure in the aspect of distribution, activity, and maturity. We conclude that graded porous designed biodegradableβ-tricalcium phosphate scaffolds are beneficial to promote bone augmentation in the aspect of bone volume.
Key Words: Bone regeneration, graded pore size, β-TCP, bone augmentation
Titanium dental implants are rapidly becoming a routine procedurein today’s dentistry and are widely used for a variety of cases, which includes surgical replacement of lost teeth or restorationof oral function.1,2 A significant factor that limits the clinical application of a titanium dental implant is the restrictive necessity for alveolar bone mechanical support.3,4 New advances brought by bone tissue engineering, in particular, by bone grafts, may surmountthe current deficits of titanium dental implants.
One approach to designing bone grafts for alveolar bone augmentationor repair of critical size defects is biomimicking the chemical composition and structural features of natural bone, thus achieving similar regenerative properties.5–8 Scaffolds fabricated with calcium phosphate ceramics such as hydroxyapatites and beta-tricalcium phosphate (β-TCP) have been shown to repair bone defectsin clinical applications because of their biocompatibility and resemblance in chemistry composition to natural bone.9,10 In addition,an interconnected pore structure and high porosity are essentialfor scaffolds, as they permit the exchange of nutrient transport within the scaffold and facilitate the ingrowth of cells and tissues. Currently, most of the reported bone scaffolds are porous solids with uniform pores.11–13 However, in nature, the bone has a graded architecture.Both long bones and flat bones change spatially and gradually from a dense, stiff external structure (cortical bone) to a porous internal structure (cancellous bone).14 Therefore, adhering to the cues from nature, graded-based strategies can be used in the designand fabrication of scaffolds for mimicking bone structure to promote bone repair and regeneration.15,16
New research investigating the effect of assorted pore diameters in β-TCP scaffolds (150, 260, 510, and 1220 μm) has demonstrated an inherent variance in bone formation and resorption rate among each group, suggesting that the spatial distribution of different pore sizes directly affects bone structure of the engineered bone.17
Creating a suitable graded porous ceramic scaffold that has adequate pore spatial distribution and interconnected pore structure has been a challenge.16 We addressed this problem by developing atemplate-casting method to easily fabricate porous ceramic scaffoldswith controlled spatial variation of chemistry and interconnected pore structure.18 In addition, through this method, the desirable prerequisitesof shape customization and pore size distribution could be fulfilled to achieve a graded pore distribution in a spatial manner. Inthis study, we fabricated 3 groups of scaffolds—(1) graded porous scaffolds, (2) large porous scaffolds, and (3) small porous scaffolds—and evaluated the effect of the scaffolds with recombinant human bone morphogenetic protein-2 (rhBMP-2) on osteogenesisand vertical augmentation in a rabbit mandibular defect model. By analyzing the difference of generated bone in the aspects of bone volume (BV) and bone density among the groups after 3 months of observation, we tend to find a suitable design of bone substitute.
MATERIALS AND METHODS
Scaffold Preparation
The following 3 groups of scaffolds with completely interconnectedpores were prepared by our template-casting method18: (1) graded porous scaffolds (600- to 800-μm pores in the center and 350- to 500-μm pores at the periphery), (2) large porous scaffolds (600- to 800-μm uniform pores), and (3) small porous scaffolds (350- to 500-μm uniform pores). The fabrication process of the scaffolds included β-TCP slurry preparation, molding, casting,solidifying, drying, and sintering.19 Gross and microcomputed tomography (μCT) examinations of their transverse section were performed as described previously.20 The structural patterns of the β-TCP scaffolds were imaged as described in Figure 1. All the scaffolds were molded into cylinders with a height of 6 mm and a diameter of 8 mm. As for the graded scaffolds, the central region containing the larger pores was 4 mm in diameter, whereas the outer layer containing the smaller pores had a thickness of 4 mm.
FIGURE 1.
μCT images. A to C show images of the graded porous scaffolds (GP), the large pore scaffolds (LP), and the small pore scaffolds (SP). Scale bars = 1000 μm.
Scanning Electron Microscopy
Morphology of the scaffolds was observed using a scanning electron microscope (SEM; FEG-Philip XL30 ESEM, 2525 V). Before the observation, the samples were coated with a thin layer of Pt-Au.
In Vivo Study of Osteogenesis Ability
Surgical Process
A total of 10 male New Zealand rabbits (8 w, 2.5–3.0 kg) with 20 mandibular sites were used to evaluate the in vivo osteogenesisability of the 3 groups of β-TCP scaffolds (graded pores, large pores, and small pores) loaded with rhBMP-2, 1 positive controlgroup (autogenous graft) and 1 negative control group (identical surgery without implantation) (n = 4 per group). All animal studies were performed in accordance with procedures approved by the Animal Care and Ethics Committee of Peking University Health Science Center. The scaffolds were sterilized by gamma irradiation before rhBMP-2 was applied. The rhBMP-2 solution (Peprotech, 100 μL) (>95% of purity, 0.1 mg/mL, 10 μg per scaffold) was appliedto the scaffolds. Animals were quarantined for a minimum of1 week before experimentation and had ad libitum access to standard rabbit chow and water at all times.
To start the surgical process, the rabbits were anesthetized with sodium pentobarbital (30 mg/kg) by injection into the lateral ear vein. After hair shaving, a horizontal incision (25 mm) was made over the submandibular region to expose the buccal ramus of the mandible. A bone defect (8-mm diameter, 2-mm depth) was then created on the buccal ramus. Autogenous bone grafts from the iliac bone (8 mm in diameter, 4 mm in height) were fixed with titanium screws (1.5 mm in diameter, 8 mm in length) onto the mandible surface. The scaffolds (8 mm in diameter, 6 mm in height) embedded in the defect protruded the same height with the autografts did (4 mm) from the bone surface. All the blocks were covered by biodegradable membranes (Shenzhen Lando BiomaterialsCo, Ltd). For the negative control group, a bone defect was created, without material implanted. The entire procedure was performedunder sterile conditions. After the surgery, all rabbits receivedantibiotics (penicillin G, 200,000 units) intramuscularly daily for 3 days. Animals were housed individually, kept in a 12-hour light/dark cycle at controlled temperature (21°C) and fed ad libitum with standard laboratory diet.
The animals were killed 3 months after the surgery with an overdose of sodium pentobarbital, and the samples were prepared for examinations.
Fluorochrome Labeling and Observation
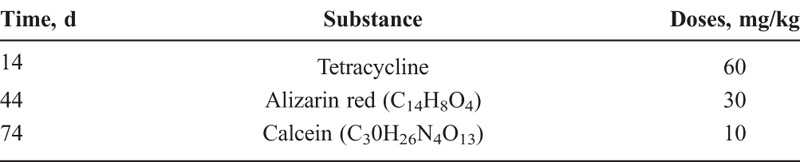
Sequential fluorochrome labeling was used to determine the onset time of mineralization and the speed of new bone (spicule) formation. Polyfluorochrome tracers (tetracycline, alizarin red, calcein) were subcutaneously injected as indicated in Table 1. After sample processing, polyfluorochrome tracers were identifiedby fluorescence microscopy. Imagepro-Plus 6.0 was used to analyze the proportion of each fluorochrome labeled area. Six regions of interest were selected from each sample according to different locations within the implanted scaffolds: outside-upper (O-U), outside-middle, outside-lower (O-L), inside-upper, inside-middle, and inside-lower. The level of mineralization of each region was blindly analyzed by an examiner who was not aware of the study design.
TABLE 1.
Injection Schedule and Dosage of Polyfluorochrome Markers
Histomorphometric Examination
After fixation with 10% of phosphate-buffered formalin, the samples of mandibles were cut into 2 halves. One half of each sample was dehydrated in ethanol and then embedded in acrylic resin. The embedded blocks were trimmed by cutter and further ground to a final thickness of approximately 40 μm. Then, the hard tissue slices were stained with toluidine blue and microscopically examined (Zeiss, LSM 5 EXCITER). The other half of each sample was decalcified with 5% of formic acid for 2 weeks21 and then embeddedin paraffin parallel to the sectioned surface. Serial cross-sections of decalcified samples were sectioned for hematoxylin and eosin (H&E) stain, toluidine blue staining, Masson staining, and osteocalcin(anti-osteocalcin antibody, ab13420; Abcam) with immunohistochemistry staining.
μCT Measurement
To quantify the bone regeneration in the implantation site, BV and bone mineral density (BMD) of each specimen were measuredby μCT scans and analysis. All specimens were cut into rectangularprisms (1 cm3) containing the tissue-engineered construct. Samples were examined with a Skyscan 1076 high-resolution desk-top μCT system (Skyscan, Kontich, Belgium). Taking into account the camera definition and the source-object-camera distance, two-dimensional images (pixel size, 8.665 μm) were obtained. The correspondingthree-dimensional images were produced by stacking all the two-dimensional cross-sections.
To study the scaffold pore size effect on bone formation, 6 regions of interest from different locations within the implanted blocks were selected, and each region was shaped into a cylinder (2 mm in diameter and 2 mm in length). After selection, upper and lower threshold values were decided to separate the bone from other tissues, and then the BV, tissue volume (TV), BV/TV, and bone density were calculated and analyzed using CTAn version 1.11.8.0+ (64 bits). The BV was defined as the volume of newly formed bone in the selected region with a threshold between 50 and 130, and the TV was defined as the overall volume in the selected region with a threshold below 130, which excluded the scaffold. We used the BV/TV as the index for new bone formation for comparison. The BMD was defined as the mineral density of the newly formed bone in pores of the scaffolds.
Statistical Analysis
To further compare the regeneration ability among different sites in the scaffolds, 6 locations in each sample were selected and analyzed. Both the BV and the BMD were calculated. Statistics of each scaffold groups were analyzed in SPSS 17.0 software (IL). Two-way analysis of variance with Tukey post hoc test for multiple comparisonswas used. The level for significance was set at P < 0.05.
RESULTS
SEM Examination
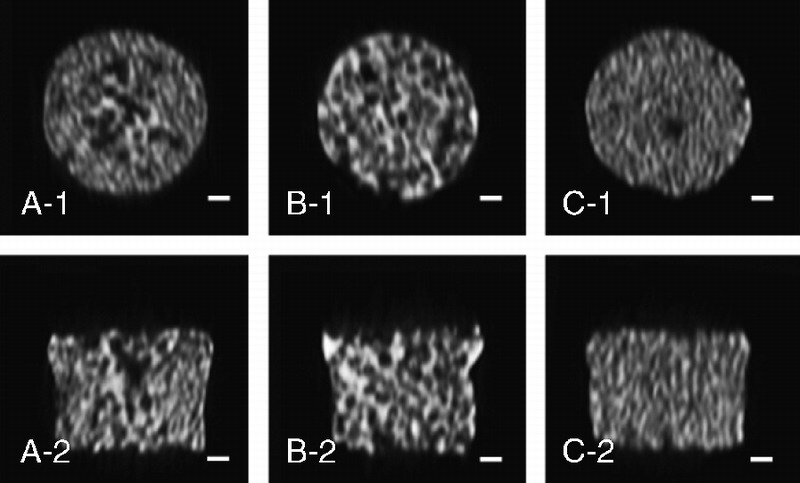
An SEM micrograph (Fig. 2) of the β-TCP porous scaffolds prepared from the template-casting method shows the interconnectedstructure of pores. Under low magnification, graded porous scaffolds showed the change of pore sizes, from central large pores (600–800 μm) to peripheral small pores (350–500 μm). No cracks or delamination was observed at the interface between zones in the samples. In uniform-size scaffolds, interconnected windows in the pores were seen. Under high magnification, micropores were observed in the struts of the 3 types of scaffolds.
FIGURE 2.
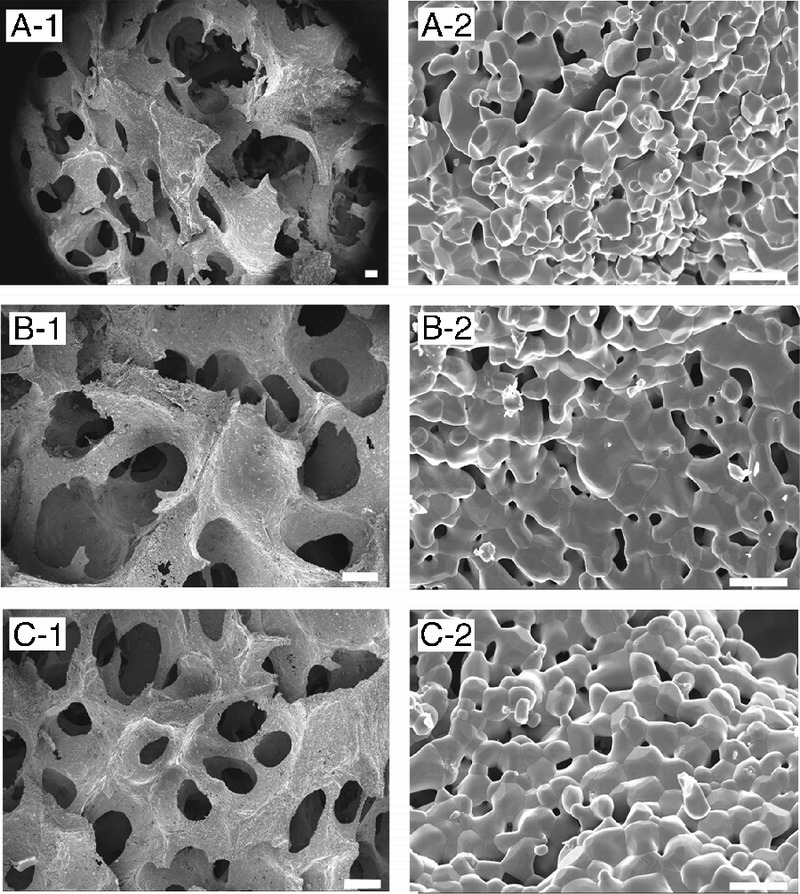
The SEM examination of the 3 kinds of scaffolds. A to C show images of GP, LP, and SP, respectively. A-1 shows the junction between large and small pore regions. A-2, B-2, and C-2 show high-magnification scans of their respective surfaces. Scale bars = 200 μm for A-1, B-1, and C-1; scale bars = 10 μm for A-2, B-2, and C-2.
In Vivo Osteogenic Ability
Polyfluorochrome Labeling on Nondecalcified Samples
The nondecalcified hard-tissue slices showed that the height of experimental groups was stable, and the scaffolds maintained their original shape (Figs. 3A–C). On the other hand, the shape of the transplanted iliac bone changed. Although the autogenous implantswere stable and integrated to the bone, protruding bone appeared as a hemisphere rather than a cylinderlike shape. Moreover,their height decreased (Fig. 3D). As for the negative control, no vertical augmentation was observed, but dense tissue was formed bridging the bone defect (Fig. 3E). The fluorescence of the slices showed continuous mineralization through the entire 3months aftersurgery. The blue, red, and green fluorescent colors indicate the newly formed bone within the first, second, and third months, respectively. The intensity and region of colors suggested the extent and location of the newly formed bone. In the scaffold groups, the areas of blue, red, and green color regions were different, whereas in the control groups, all colors covered the same area when superimposed.By comparing the 3 colored areas in the scaffold groups, we found that the blue regions (tetracycline-labeled for the newly formed bone within the first month) were the largest and that the red and green regions adjacent to the blue regions were much smaller. Our analysis showed that the tissue area labeled for the newly formed bone decreased over time (Fig. 4A), and no significant difference was found between the core area and the peripheral area. The newly formed bone areas labeled in the first month were significantly greater than those in the second and third months. Moreover, the bone areas colored in blue in the graded porous scaffolds were significantly larger than those in the large porous scaffolds (Fig. 4B) (P < 0.05).
FIGURE 3.
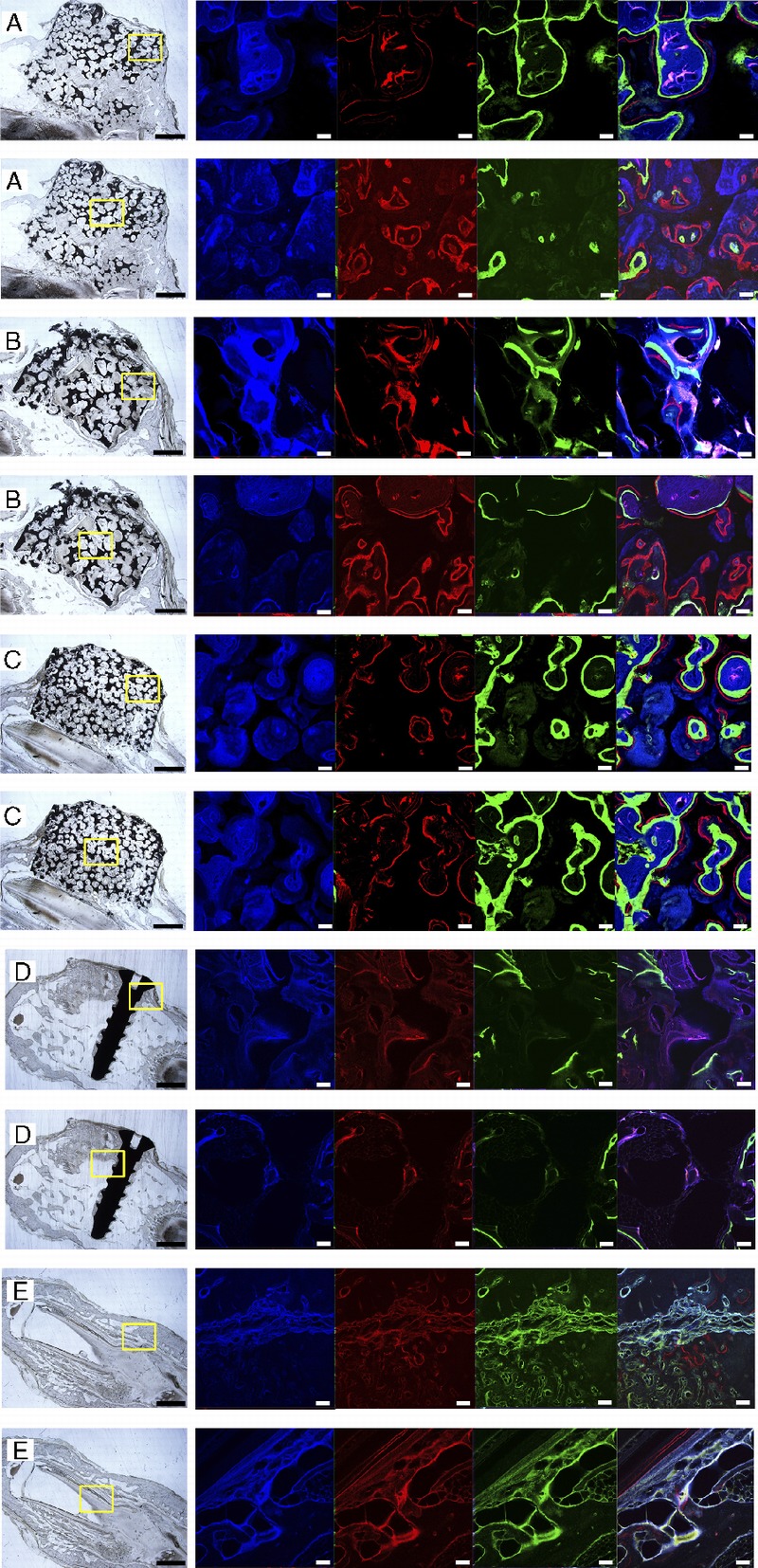
Fluorescent labeling of new bone formation over time. A to E show images of GP, LP, SP, the positive control group, and the negative control group. In each sample, 2 fluorescent labeling areas are presented: one in the center of the slices and the other in the outer ring of the slices. Each result includes a general view of the abrasive slice and a series of fluorescent-labeled images captured after excitation at different wavelengths. There are 4 fluorescent-labeled images in each series. Images in blue, red, and green present the calcified tissue in the first, second, and third months, respectively. The last column in each series shows the computer generated merged images. Scale bars = 1000 μm in abrasive slices; scale bars = 100 μm in fluorescent-labeled images.
FIGURE 4.
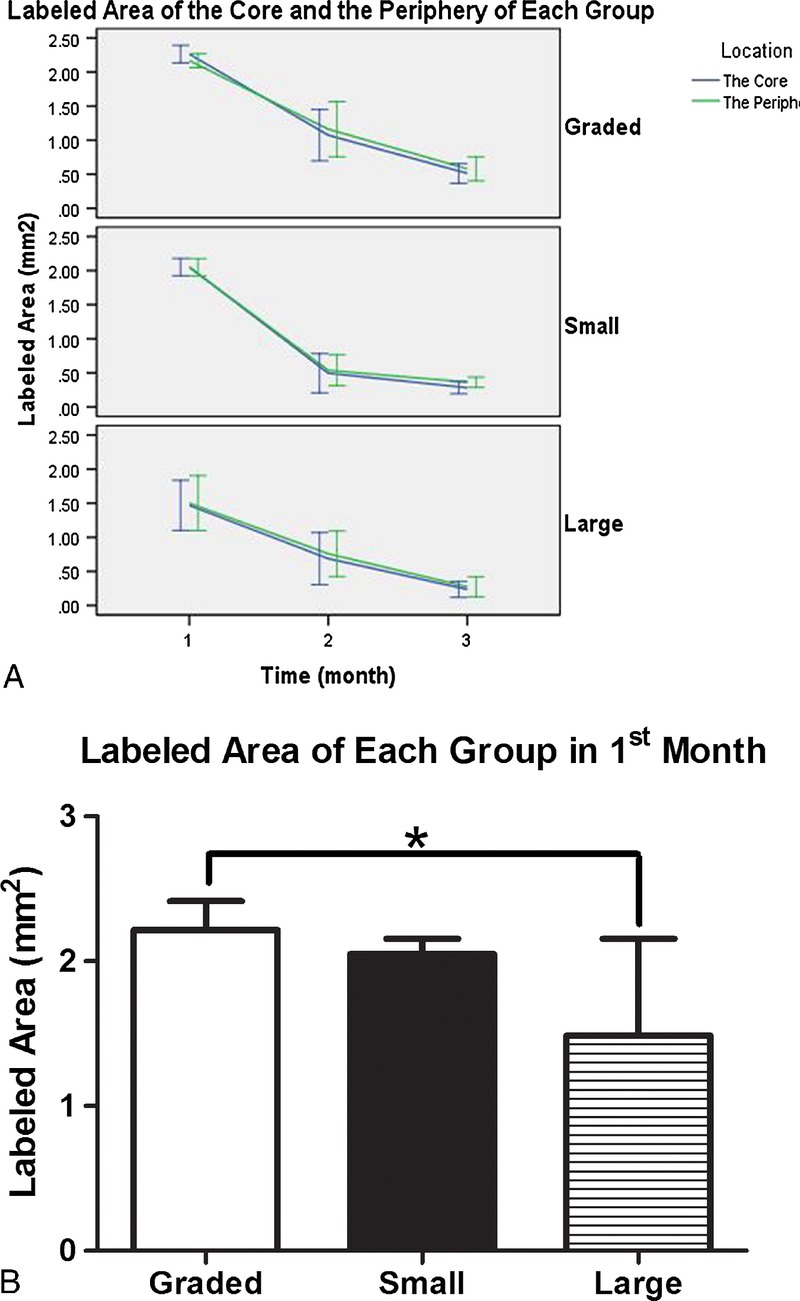
Quantitative analysis of fluorescent-labeled areas in each group (GP, SP, and LP). A shows the areas in the first, second, and third months. The violet lines present areas in the core of scaffolds (inside-upper, inside-middle, and inside-lower), whereas the green lines present the area in the periphery of the scaffolds (O–U, outside-middle, and O-L). B shows the statistic analysis result in the first month.
Histologic Evaluation
The H&E staining of the samples revealed that, in all the groups, implanted blocks were tightly bound and integrated to the mandible bed, and no inflammation was found in any of the samples after surgery (Fig. 5). From the general view, it was obvious that thedistribution of regenerated bone was uneven in the scaffolds. For all groups, BV near the bone bed seemed to be greater than those far from the base area. However, all the scaffold groups demonstrated a well-formed bone structure. At the magnification of 200×, both bone tissue and soft tissue could be detected. Empty spaces interspersedin the tissues indicate areas where the scaffold was before demineralization. Immunohistochemistry staining of osteocalcinrevealedactive bone mineralization in all the groups, although the ranges of brown-colored tissue were different in each group (Fig. 6). Toluidine blue staining of the hard tissue slices clearly demonstrated the distribution of bone tissue in blue and the scaffoldstructure in black (Fig. 7). The gross view of the toluidine blue staining was in accordance with that of H&E staining, characterized with uneven bone forming from the base to the top of the scaffolds. Images showed close integration between bone tissue and scaffolds.Ata high magnification of 100×, intimate integration was found both in the bone-scaffold interface and implant-bone interface. Masson trichrome staining of all the samples presented a red color forcytoplasm, scattered with bits of blue for the bone (Fig. 8), which indicated wide range of bone tissue in the form of collagen. Collagen was mostly scattered around the bone lacunae.
FIGURE 5.
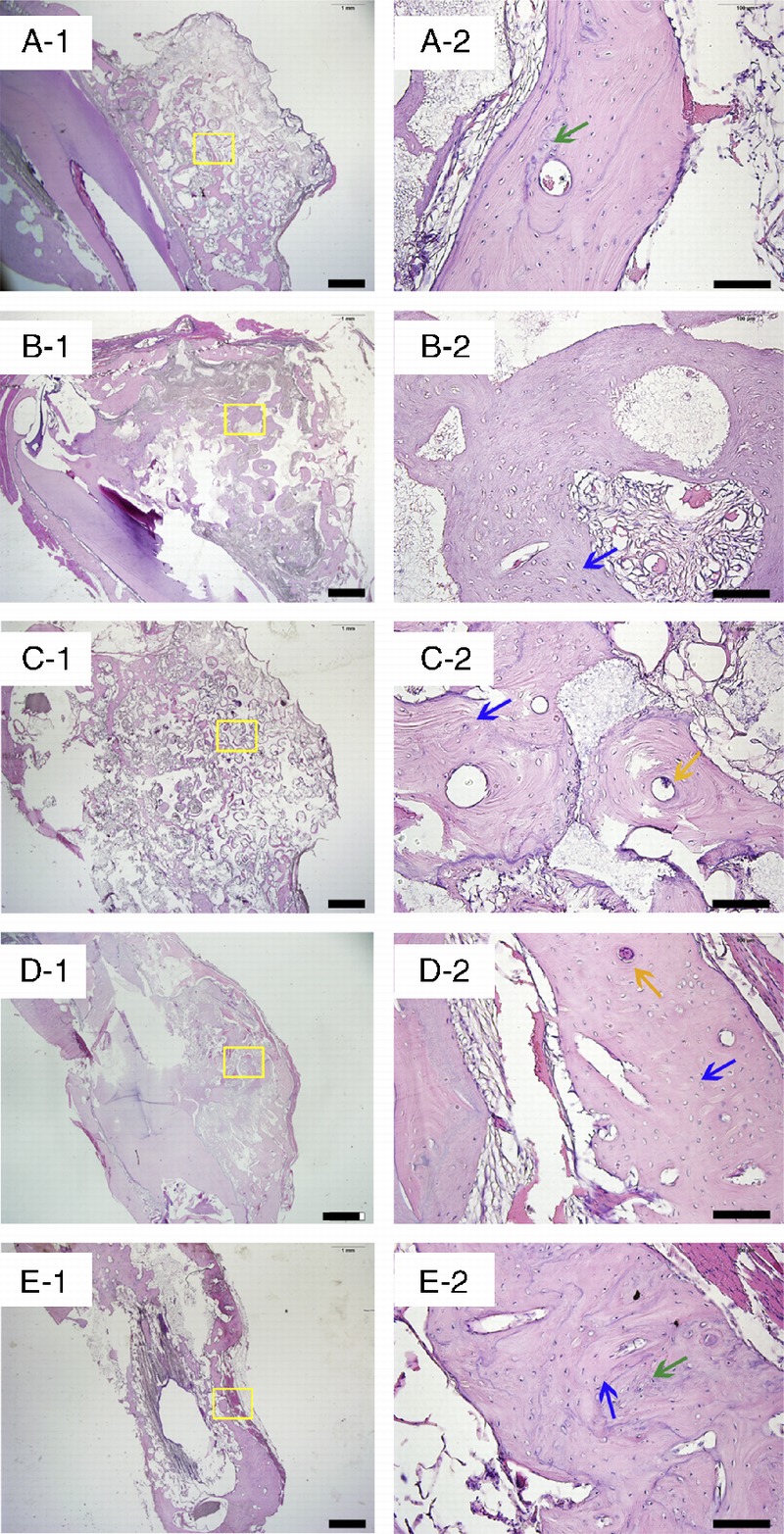
The H&E staining of the decalcified samples. A to E show images of GP, LP, SP, the positive control group, and the negative control group. Blue arrows indicate bone matrix spotted with osteocytes, green arrows indicate osteoid, and yellow arrows indicate Haversian canals. Scale bars = 1000 μm in A-1, B-1, C-1, D-1, and E-1; scale bars = 100 μm in A-2, B-2, C-2, D-2, and E-2.
FIGURE 6.
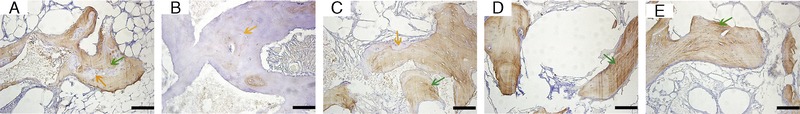
Immunohistochemistry staining of osteocalcin. A to E show images of GP, LP, SP, the positive control group, and the negative control group. Green arrows indicate the mineralized tissue, and yellow arrows indicate the tissue not mineralized. Scale bars = 100 μm.
FIGURE 7.
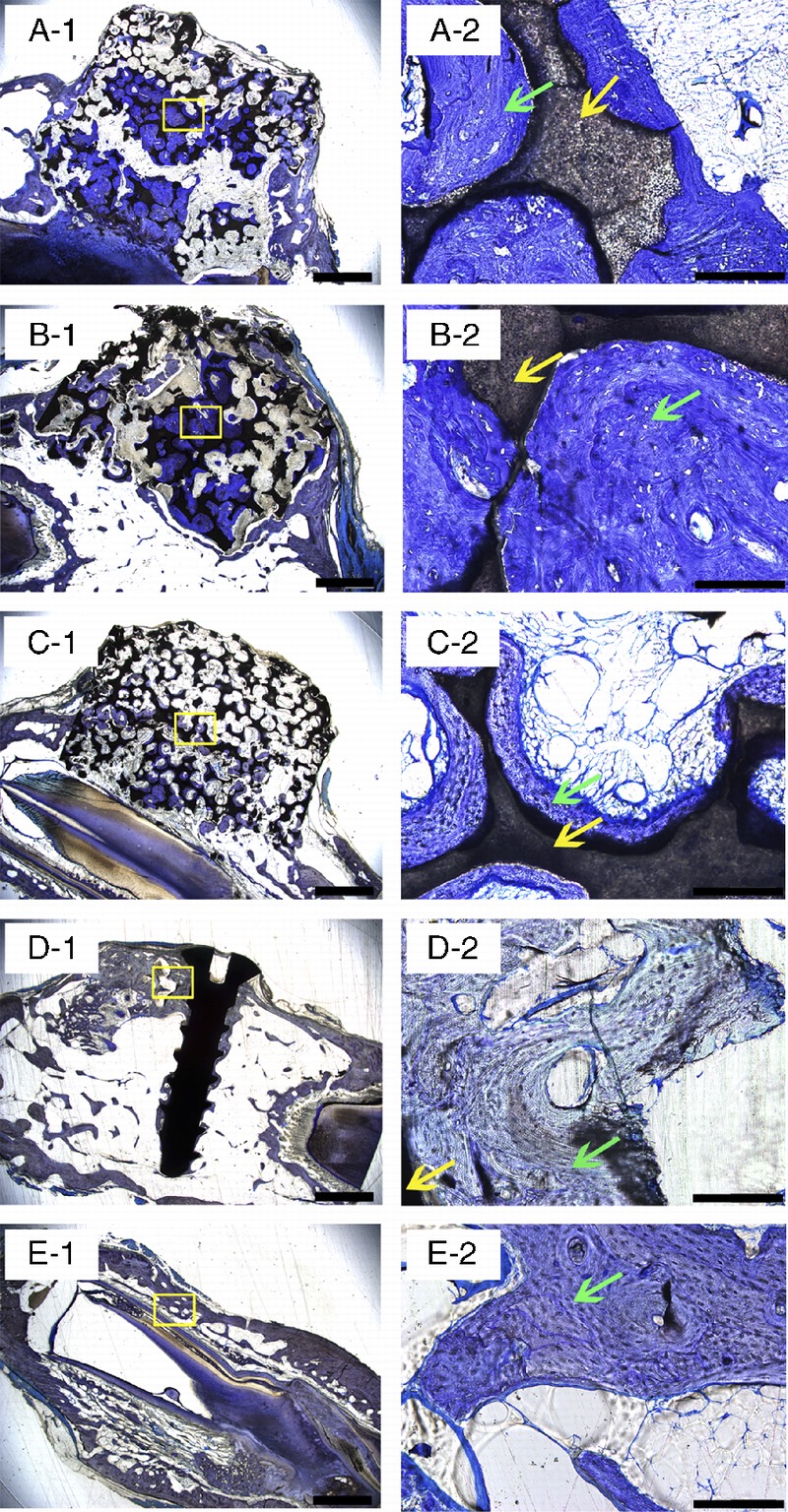
Toluidine blue staining of the hard tissue slices of all the samples. A to E show images of GP, LP, SP, the positive control group, and the negative control group. Green arrows indicate bone tissue, and yellow arrows indicate the scaffolds. Scale bars = 1000 μm in A-1, B-1, C-1, D-1, and E-1; scale bars = 100 μm in A-2, B-2, C-2, D-2, and E-2.
FIGURE 8.
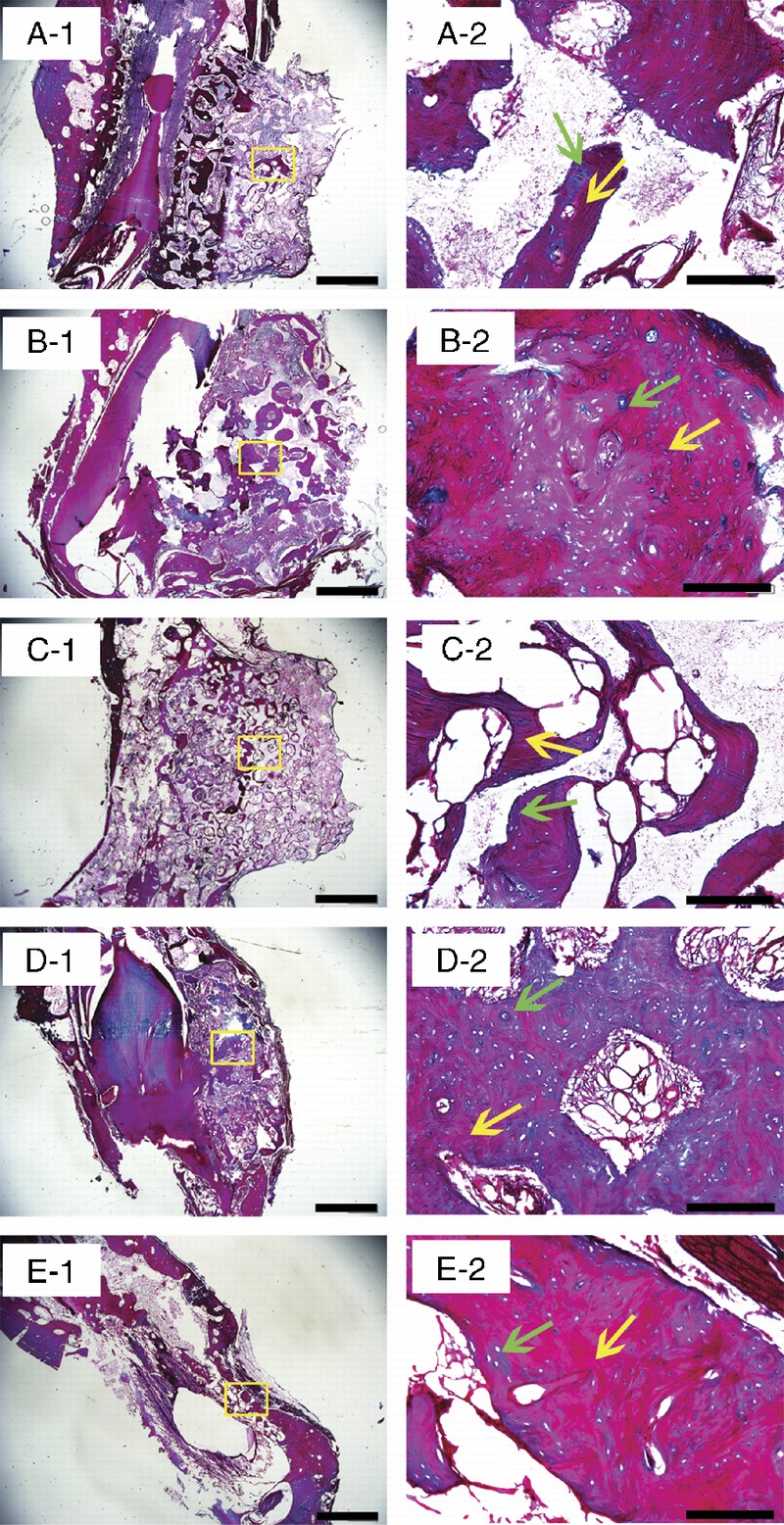
Masson trichrome staining of the samples. A to E show images of GP, LP, SP, the positive control group, and the negative control group. Green arrows indicate the immature bone tissue, and yellow arrows indicate the mature bone tissue. Scale bars = 1000 μm in A-1, B-1, C-1, D-1, and E-1; scale bars = 100 μm in A-2, B-2, C-2, D-2, and E-2.
μCT Observation and Measurements
Figure 9 shows two-dimensional profile μCT images of themandible explants. Comparing the coronal image series of each sample of scaffold groups, identifiable differences in pore patterns could be observed. For the graded porous scaffold group (Fig. 9D), the large pore scaffold group (Fig. 9E), and the small pore scaffold group (Fig. 9F), the difference in pore diameters was obvious as designed. High-density tissue deposition was observed in pores. The distribution pattern varies among different tomography slice levels.
FIGURE 9.
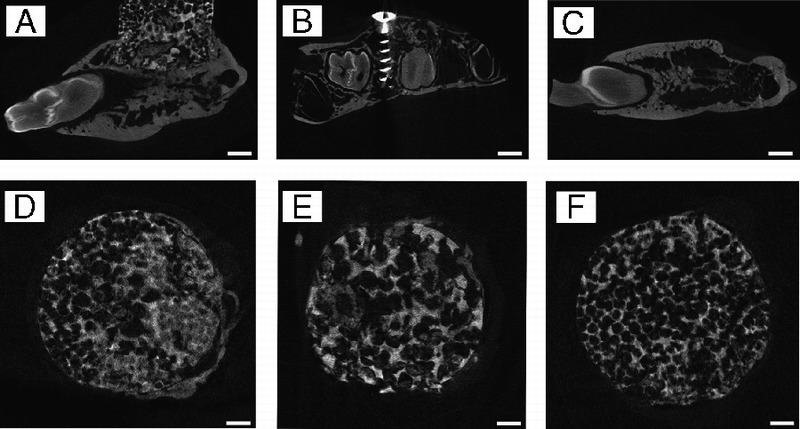
The μCT sagittal images of samples of augmented mandibles. A is the experimental group, B is the positive control group, and C is the negative control group. D to F are the μCT coronal images of the samples of the 3 experimental groups. D shows the GP group, E shows the LP group, and F shows the SP group. Scale bars = 1000 μm.
BV and TV Measurements
The BV/TV ratio was used as the most important index for new bone formation in this study. The BV/TV ratios were different among the 3 groups, with statistical significance as shown in Figure 10. The ratio of BV/TV in graded porous scaffolds was significantly higher than those with large pores (P < 0.01) and small pores (P < 0.05), suggesting much greater new bone formation. No statistical difference was found between the 2 groups with uniform pores (Fig. 10A). In consistence with H&E staining, the ratio of BV/TV of different regions presented a slight decreasing trend from bottom to top of the blocks. Statistical differences were found between not only the O-U and O-L areas but also the central-upper and O-L areas (Fig. 10C). The bone density showed no significant difference among the groups (Fig. 10B) or between different regions (Fig. 10D).
FIGURE 10.
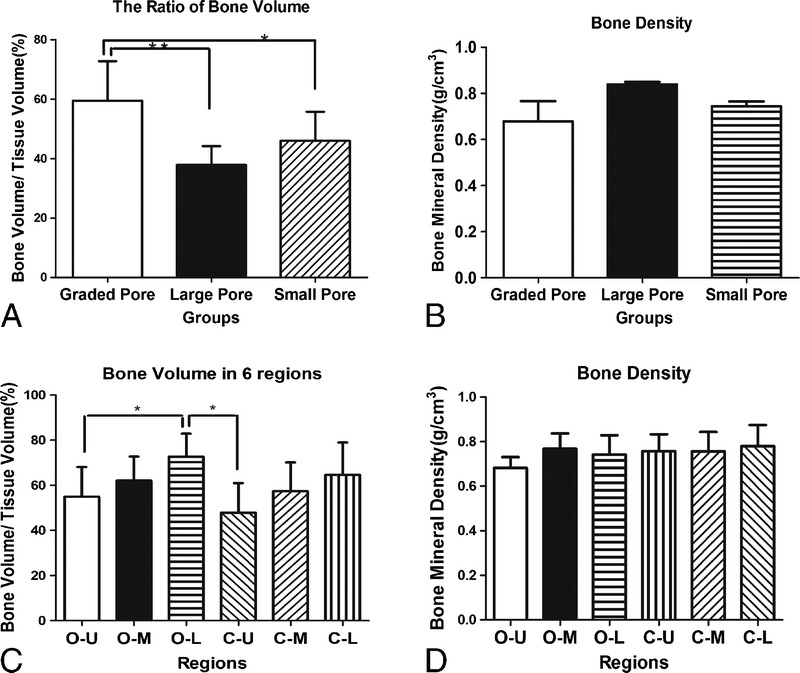
The μCT quantitative analysis of the newly formed bone. A and B show the statistical analysis of μCT measurements of the augmented mandibles in the 3 experimental groups for BV/TV and BMD. C and D show the statistical analysis of μCT measurement of the augmented mandibles in 6 different areas in the scaffolds.
DISCUSSION
With the purpose of improving the osteogenic potential of β-TCP porous ceramics, we evaluated bone enhancement of porous scaffold with the addition of growth factor in bone defect models.22 Porous scaffolds have been studied for decades, the uniform pore architectureof scaffolds faces a significant challenge in inducing the growth of new tissue in the way of natural bone acts.23–26 The architectural motif of natural bone can be described as having a stiff, dense externallayer of cortical bone, transitioning to a thin and porous cancellous bone internally.15 There have been attempts to establish a fabrication method to manufacture a kind of scaffold that is both graded and highly interconnected.27–30 Graded porous scaffolds have been proven to be advantageous compared with uniform pore designinboth ectopic and orthotopic31,32 osteogeneses, overcoming the current limitations associated with uniform pore design. The purpose of this study was to demonstrate the advantage of the designedgraded porous scaffolds fabricated by a template-casting technique on bone augmentation in orthotopic bone defect.
Our in vivo study with New Zealand rabbits confirmed our hypothesis that the graded porous scaffold loaded with rhBMP-2 promoted bone augmentation at a higher rate compared with scaffoldswith uniform pore sizes with the same loading of rhBMP-2 in orthotopic osteogenesis. The μCT analysis revealed superior BV augmentation in the graded porous scaffold group, with no decrease in BMD compared with uniform-size scaffolds, which excluded the possibility of impaired bone quality. The decreasing trend of BV fromthe base to the top of the scaffolds implied blood supply effectonbone regeneration. The obvious difference of augmented boneheight between the experimental groups and the positive control group further demonstrated the advantages of engineered bone over autogenous iliac grafts in the aspect of bone configuration, which proved that the mechanical support provided by the scaffold can withstand pressure from the surrounding soft tissue, maintaining a stable bone height during the 3-month implantation period, whereas iliac grafts underwent a dramatic reconstruction, which lead to a boneheight reduction. Although bone tissue in scaffolds presented an uneven distribution and different degrees of maturity, the structureof the mature part was familiar with that of the natural bone inthe positive control group, such as typical lamellar bone mingled with woven bone, spotted osteocytes, and Haversian canals. As for the immature part, experiments with longer observing period need to be conducted. Masson staining revealed the maturity of the engineeredtissue in most of the observed areas, whereas active osteogenesisseemed to be around the fringe, indicating the presence of a stable bone structure with active growth.
The polyfluorescent labeling is to visualize the process of new bone formation by identifying mineralized tissue during the drug injection period.33 Because the injecting time interval of the 3 labelingdrugs was the same, the larger the labeled areas are, the larger areathe mineralized bone occupied. In our experiments, the bone growth in the first month was the fastest. Moreover, the difference of regenerated bone between the graded porous scaffolds and the uniform scaffolds had already emerged during this time, which providesfurther proof of the improved bone regeneration in the graded porous scaffolds, although the difference is not as significant as the μCT examination due to the limitation of information sample slices could ever offer. During the following 2 months, the bone formation/augmentation declined. Similar observation was found in other ectopicand orthotopic animal models as well.34,35 The surgically created bone defect control groups showed little regeneration as evidenced by the lack of calcium ion deposition in the implantation area.
In our study, the intrinsic mechanism of the advantage of graded porous scaffolds seems to be various rather than single-effect. Becauseno significant difference was found between the core area and the peripheral area in this study, we believe that the graded scaffold act as a unique, single functional unit that the different configurationof the scaffold cause interstitial fluid diffusion changes and scaffold degradation profile changes, which lead to the different cell adhesion and different cell behavior as a response to the scaffold degradation. Considering that the living organism is such a complex system that a graded porous design is far from biomimicking, further investigations on scaffold development about scaffold degradation, growth factor release, and blood supply improvement should be considered. However, based on our study on the osteogenesis of porousscaffolds, the graded porous design could be proved to be beneficialin bone augmentation in orthotopic osteogenesis.
Footnotes
Supported by grants from Beijing Natural Science Foundation (7133255), NIH R01DE021468 (NIDCR), NIH R01AR057837 (NIAMS), and DOD W81XWH-10-1-0966 (PRORP).
The authors report no conflicts of interest.
REFERENCES
- 1. Shimono K, Oshima M, Arakawa H, et al. The effect of growth factors for bone augmentation to enable dental implant placement: a systematic review. Jpn Dent Sci Rev 2010; 46: 43– 53 [Google Scholar]
- 2. Davo R, Malevez C, Rojas J. Immediate function in the atrophic maxilla using zygoma implants: a preliminary study. J Prosthet Dent 2007; 97: S44– S51 [DOI] [PubMed] [Google Scholar]
- 3. Cawood JI, Howell RA. Reconstructive preprosthetic surgery: I. Anatomical considerations. Int J Oral Maxillofac Surg 1991; 20: 75– 82 [DOI] [PubMed] [Google Scholar]
- 4. McAllister BS, Haghighat K. Bone augmentation techniques. J Periodontol 2007; 78: 377– 396 [DOI] [PubMed] [Google Scholar]
- 5. Block MS, Kent JN. Long-term radiographic evaluation of hydroxylapative-augmented mandibular alveolar ridges. J Oral Maxillofac Surg 1984; 42: 793– 796 [DOI] [PubMed] [Google Scholar]
- 6. Jeon I-S, Heo M-S, Han K-H, et al. Vertical ridge augmentation with simultaneous implant placement using β-TCP and PRP: a report of two cases. J Oral Maxillofac Surg Med Pathol [Google Scholar]
- 7. Kent JN, Quinn JH, Zide MF, et al. Alveolar ridge augmentation using nonresorbable hydroxylapatite with or without autogenous cancellous bone. J Oral Maxillofac Surg 1983; 41: 629– 642 [DOI] [PubMed] [Google Scholar]
- 8. Wang S, Zhang Z, Zhao J, et al. Vertical alveolar ridge augmentation with β-tricalcium phosphate and autologous osteoblasts in canine mandible. Biomaterials 2009; 30: 2489– 2498 [DOI] [PubMed] [Google Scholar]
- 9. Chow LC. Calcium phosphate cements. Monogr Oral Sci 2001; 18: 148– 163 [DOI] [PubMed] [Google Scholar]
- 10. LeGeros RZ. Calcium phosphates in oral biology and medicine. Monogr Oral Sci 1991; 15: 1– 201 [PubMed] [Google Scholar]
- 11. Eggli PS, Muller W, Schenk RK. Porous hydroxyapatite and tricalcium phosphate cylinders with two different pore size ranges implanted in the cancellous bone of rabbits. A comparative histomorphometric and histologic study of bony ingrowth and implant substitution. Clin Orthop Relat Res 1988: 127– 138 [PubMed] [Google Scholar]
- 12. Schliephake H, Neukam FW, Klosa D. Influence of pore dimensions on bone ingrowth into porous hydroxylapatite blocks used as bone graft substitutes. A histometric study. Int J Oral Maxillofac Surg 1991; 20: 53– 58 [DOI] [PubMed] [Google Scholar]
- 13. Gauthier O, Bouler JM, Aguado E, et al. Macroporous biphasic calcium phosphate ceramics: influence of macropore diameter and macroporosity percentage on bone ingrowth. Biomaterials 1998; 19: 133– 139 [DOI] [PubMed] [Google Scholar]
- 14. Sah RL. Interface and bulk regions in the repair, regeneration, and replacement of articular cartilage. J Musculoskelet Neuronal Interact 2004; 4: 393– 395 [PubMed] [Google Scholar]
- 15. Pompe W, Worch H, Epple M, et al. Functionally graded materials for biomedical applications. Mater Sci Eng A 2003; 362: 40– 60 [Google Scholar]
- 16. Muthutantri A, Huang J, Edirisinghe M. Novel preparation of graded porous structures for medical engineering. J R Soc Interface 2008; 5: 1459– 1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. von Doernberg MC, von Rechenberg B, Bohner M, et al. In vivo behavior of calcium phosphate scaffolds with four different pore sizes. Biomaterials 2006; 27: 5186– 5198 [DOI] [PubMed] [Google Scholar]
- 18. Liu Y, Kim JH, Young D, et al. Novel template-casting technique for fabricating beta-tricalcium phosphate scaffolds with high interconnectivity and mechanical strength and in vitro cell responses. J Biomed Mater Res A 2010; 92: 997– 1006 [DOI] [PubMed] [Google Scholar]
- 19. Kang Y, Kim S, Khademhosseini A, et al. Creation of bony microenvironment with CaP and cell-derived ECM to enhance human bone-marrow MSC behavior and delivery of BMP-2. Biomaterials 2011; 32: 6119– 6130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jason A, Berdick RLM. Biomaterials for tissue engineering application: a review of the past and future trends. Biomater Tissue Eng 2012: 29 [Google Scholar]
- 21. Frank JD, Balena R, Masarachia P, et al. The effects of three different demineralization agents on osteopontin localization in adult rat bone using immunohistochemistry. Histochemistry 1993; 99: 295– 301 [DOI] [PubMed] [Google Scholar]
- 22. Lim HP, Mercado-Pagan AE, Yun KD, et al. The effect of rhBMP-2 and PRP delivery by biodegradable beta-tricalcium phosphate scaffolds on new bone formation in a non-through rabbit cranial defect model. J Mater Sci Mater Med 2013; 24: 1895– 1903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sanz-Herrera JA, Garcia-Aznar JM, Doblare M. On scaffold designing for bone regeneration: a computational multiscale approach. Acta Biomater 2009; 5: 219– 229 [DOI] [PubMed] [Google Scholar]
- 24. Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials 2003; 24: 4337– 4351 [DOI] [PubMed] [Google Scholar]
- 25. Alsberg E, Kong HJ, Hirano Y, et al. Regulating bone formation via controlled scaffold degradation. J Dent Res 2003; 82: 903– 908 [DOI] [PubMed] [Google Scholar]
- 26. Park SH, Gil ES, Kim HJ, et al. Relationships between degradability of silk scaffolds and osteogenesis. Biomaterials 2010; 31: 6162– 6172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hutmacher DW. Scaffold design and fabrication technologies for engineering tissues—state of the art and future perspectives. J Biomater Sci 2001; 12: 15. [DOI] [PubMed] [Google Scholar]
- 28. Freed LE, Vunjak-Novakovic G, Biron RJ, et al. Biodegradable polymer scaffolds for tissue engineering. Biotechnology 1994; 12: 5. [DOI] [PubMed] [Google Scholar]
- 29. Ma PX, Zhang R. Microtubular architecture of biodegradable polymer scaffolds. J Biomed Mater Res 2001; 56: 8. [DOI] [PubMed] [Google Scholar]
- 30. Mooney DJ, Organ G, Vacanti JP, et al. Design and fabrication of biodegradable polymer devices to engineer tubular tissues. Cell Transplant 1994; 3: 8. [DOI] [PubMed] [Google Scholar]
- 31. Kuboki Y, Jin Q, Takita H. Geometry of carriers controlling phenotypic expression in BMP-induced osteogenesis and chondrogenesis. J Bone Joint Surg 2001; 83: 11. [PubMed] [Google Scholar]
- 32. Lan Levengood SK, Polak SJ, Poellmann MJ, et al. The effect of BMP-2 on micro- and macroscale osteointegration of biphasic calcium phosphate scaffolds with multiscale porosity. Acta Biomater 2010; 6: 9. [DOI] [PubMed] [Google Scholar]
- 33. van Gaalen SM, Kruyt MC, Geuze RE, et al. Use of fluorochrome labels in in vivo bone tissue engineering research. Tissue Eng Part B Rev 2010; 16: 209– 217 [DOI] [PubMed] [Google Scholar]
- 34. Hernández A, Sánchez E, Soriano I, et al. Material-related effects of BMP-2 delivery systems on bone regeneration. Acta Biomater 2012; 8: 11. [DOI] [PubMed] [Google Scholar]
- 35. Patel ZS, Yamamoto M, Ueda H, et al. Biodegradable gelatin microparticles as delivery systems for the controlled release of bone morphogenetic protein-2. Acta Biomater 2008; 4: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]


